PGC-1α Regulates Cell Proliferation, Migration, and Invasion by Modulating Leucyl-tRNA Synthetase 1 Expression in Human Colorectal Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Materials
2.3. RNA Isolation and First-Strand cDNA Synthesis
2.4. Annealing Control Primer (ACP)-Based GeneFishing PCR
2.5. Generation of Stable PGC-1α-Overexpressing SW480 and PGC-1α shRNA-Knocked Down SW620 Cell Line
2.6. Generation of Stable LARS1-Overexpressing SW480 and LARS1 shRNA-Knocked Down SW620 Cell Line
2.7. RNA Extraction and Real-Time Quantitative Reverse Transcriptase–Polymerase Chain Reaction (qRT-PCR)
2.8. Cell Counting
2.9. MTT Assay
2.10. Transwell Migration and Invasion Assays
2.11. Western Blot Analysis
2.12. Immunofluorescence Staining and Fluorescence Quantification
2.13. LARS1 Expression and LARS1 shRNA Expression Vector Transfection
2.14. PGC-1α Expression and PGC-1α shRNA Expression Vector Transfection
2.15. Statistical Analysis
3. Results
3.1. Overexpression of PGC-1α Leads to Upregulation of LARS1 in Human Embryonic Kidney 293 (HEK293) Cells
3.2. LARS1 Overexpression Enhances Cell Proliferation, Migration, and Invasion of SW480 Cells
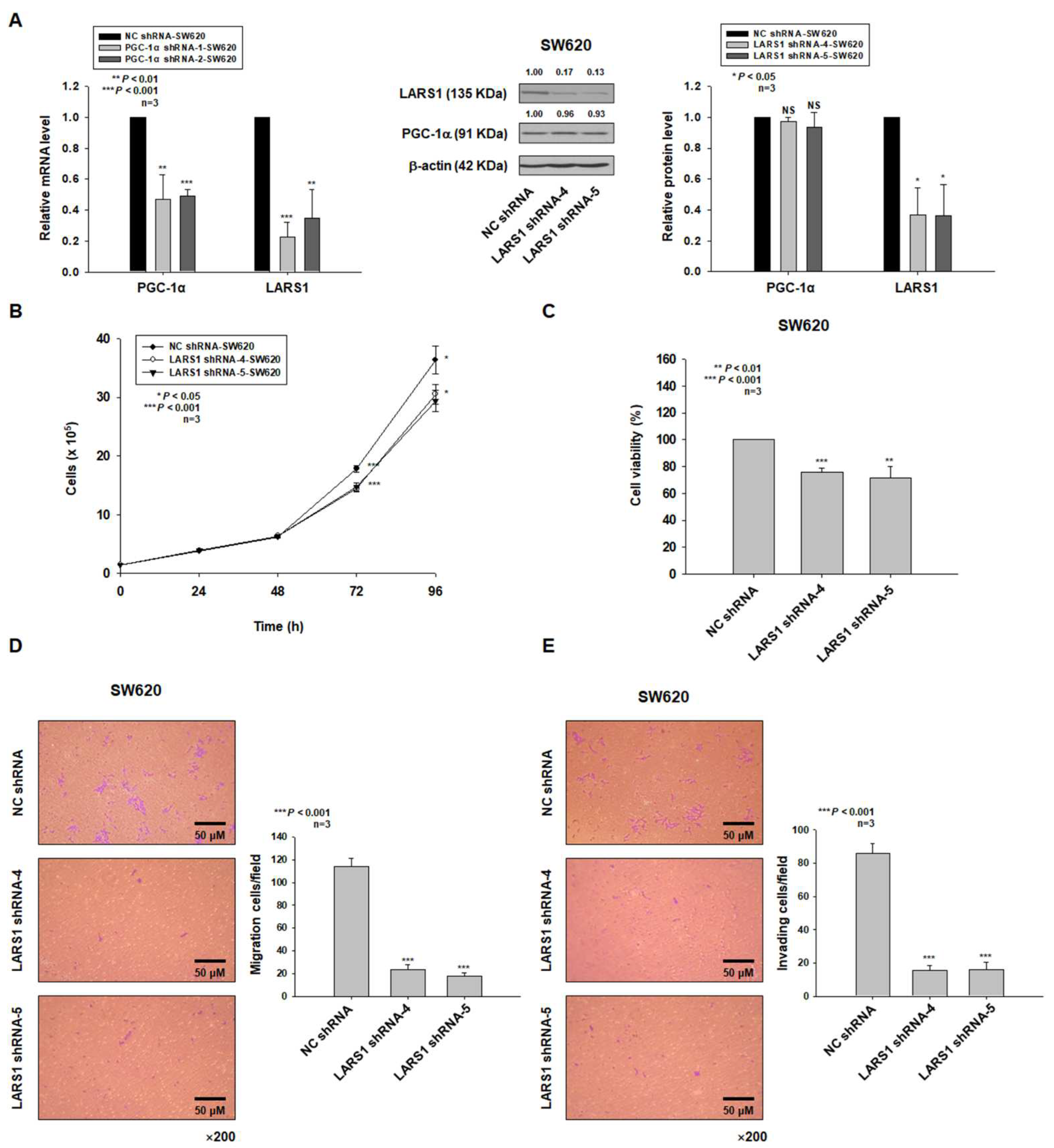
3.3. LARS1 Knockdown Reduces Cell Proliferation, Migration, and Invasion of SW620 Cells
3.4. PGC-1α Regulates Cell Proliferation, Migration, and Invasion of PGC-1α-HEK293, SW480, and SW620 Cells by Regulating LARS1 Expression
3.5. PGC-1α Regulates Cell Proliferation, Migration, and Invasion through LARS1/AKT/GSK-3β/β-Catenin Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, A.W.; Yao, Z.; Vicencio, J.M.; Karkucinska-Wieckowska, A.; Szabadkai, G. PGC-1 family coactivators and cell fate: Roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signaling. Mitochondrion 2012, 12, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Widlund, H.; Puigserver, P. PGC-1 coactivators: Shepherding the mitochondrial biogenesis of tumors. Trends Cancer 2016, 2, 619–631. [Google Scholar] [CrossRef]
- Yun, S.H.; Han, S.H.; Park, J.I. Peroxisome proliferator-activated receptor γ and PGC-1α in cancer: Dual actions as tumor promoter and suppressor. PPAR Res. 2018, 2018, 6727421. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, I.; Salvatore, L.; Murzilli, S.; Sasso, G.L.; Latorre, D.; Martelli, N.; Egorova, A.; Polishuck, R.; Madeyski-Bengston, K.; Lelloitt, C.; et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc. Natl. Acad. Sci. USA 2011, 108, 6603–6608. [Google Scholar] [CrossRef]
- Torrano, V.; Valcarcel-Jimenez, L.; Cortazar, A.R.; Liu, X.; Urosevic, J.; Castillo-Martin, M.; Fernández-Ruiz, S.; Morciano, G.; CaroMaldonado, A.; Guiu, M.; et al. The metabolic co-regulator PGC1alpha suppresses prostate cancer metastasis. Nat. Cell. Biol. 2016, 18, 645–656. [Google Scholar] [CrossRef]
- Bhalla, K. PGC-1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011, 71, 6888–6898. [Google Scholar] [CrossRef]
- Shin, S.W.; Yun, S.H.; Park, E.S.; Jeong, J.S.; Kwak, J.Y.; Park, J.I. Overexpression of PGC-1α enhances cell proliferation and tumorigenesis of HEK293 cells through the upregulation of Sp1 and Acyl-CoA binding protein. Int. J. Oncol. 2015, 46, 1328–1342. [Google Scholar] [CrossRef]
- Yun, S.H.; Shin, S.W.; Park, J.I. Expression of fatty acid synthase is regulated by PGC-1α and contributes to increased cell proliferation. Oncol. Rep. 2017, 38, 3497–3506. [Google Scholar] [CrossRef][Green Version]
- Yun, S.H.; Park, J.I. PGC-1α regulates cell proliferation and invasion via AKT/GSK-3β/β-catenin pathway in human colorectal cancer SW620 and SW480 cells. Anticancer Res. 2020, 40, 653–664. [Google Scholar] [CrossRef]
- Schimmel, P. Aminoacyl tRNA synthetases: General scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Ann. Rev. Biochem. 1987, 56, 125–158. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Bonfils, G.; Jaquenoud, M.; Bontron, S.; Ostrowicz, C.; Ungermann, C.; De Virgilio, C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol. Cell. 2012, 46, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Guan, K.L. Nutrient signaling to mTOR and cell growth. Trends. Biochem. Sci. 2013, 38, 233–242. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Leucine and protein synthesis: mTOR and beyond. Nutr. Rev. 2007, 65, 122–129. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nature Rev. Mol. Cell. Biol. 2009, 29, 32–38. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, H.S.; Jung, S.H.; Xu, H.D.; Jeong, Y.B.; Chung, Y.G. Implication of leucyl-tRNA synthetase 1 (LARS1) over-expression in growth and migration of lung cancer cells detected by siRNA targeted knock-down analysis. Exp. Mol. Med. 2008, 40, 229–236. [Google Scholar] [CrossRef]
- Gao, G.; Yao, Y.; Li, K.; Mashausi, D.S.; Li, D.; Negi, H.; Kamle, S.; Chen, H.; Wu, Z.; Zhou, H.; et al. A human leucyl-tRNA synthetase as an anticancer target. Onco. Targets Ther. 2015, 8, 2933–2942. [Google Scholar]
- Kim, E.Y.; Lee, J.G.; Lee, J.M.; Kim, A.; Yoo, H.C.; Kim, K.; Lee, M.; Lee, C.; Han, G.; Han, J.M.; et al. Therapeutic effects of the novel leucyl-tRNA synthetase inhibitor BC-LI-0186 in non-small cell lung cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846798. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, J.H.; Yoon, I.; Kim, C.; Kim, S.E.; Koh, Y.; Jeong, S.J.; Lee, J.; Kim, S.; Lee, J. Discovery of (S)-4-isobutyloxazolidin-2-one as a novel leucyl-tRNA synthetase (LRS)-targeted mTORC1 inhibitor. Bioorg. Med. Chem. Lett. 2016, 26, 3038–3041. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, J.H.; Kim, S.E.; Kim, C.; Tran, P.T.; Ann, J.; Koh, Y.; Kim, S.; Moon, H.S.; Kim, W.K.; et al. Discovery of leucyladenylate sulfamates as novel leucyl-tRNA synthetase (LRS)-targeted mammalian target of rapamycin complex 1 (mTORC1) inhibitors. J. Med. Chem. 2016, 59, 10322–10328. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, J.H.; Koh, Y.; Tran, P.T.; Ann, J.; Yoon, I.; Jang, J.; Kim, W.K.; Lee, S.; Lee, K.; et al. Discovery of simplified leucyladenylate sulfamates as novel leucyl-tRNA synthetase (LRS)-targeted mammalian target of rapamycin complex 1 (mTORC1) inhibitors. Bioorg. Med. Chem. 2017, 25, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Zuo, D.; Kim, J.H.; Yoon, I.; Ann, J.; Kim, S.E.; Cho, D.; Kim, W.K.; Lee, S.; Lee, J.; et al. Discovery of novel leucyladenylate sulfamate surrogates as leucyl-tRNA synthetase (LRS)-targeted mammalian target of rapamycin complex 1 (mTORC1) inhibitors. Bioorg. Med. Chem. 2018, 26, 4073–4079. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, S.E.; Kim, J.H.; Yoon, I.; Tran, P.T.; Ann, J.; Kim, C.; Byun, W.S.; Lee, S.; Kim, S.; et al. Structure-activity relationship of leucyladenylate sulfamate analogues as leucyl-tRNA synthetase (LRS)-targeting inhibitors of mammalian target of rapamycin complex 1 (mTORC1). Bioorg. Med. Chem. 2019, 27, 1099–1109. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwak, C.I.; Gu, Y.Y.; Hwang, I.T.; Chun, J.Y. Annealing control primer system for identification of differentially expressed genes on agarose gels. Biotechniques 2004, 36, 424–430. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell. Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef]
- Pόpulo, H.; Lopes, J.M.; Soares, P. The mTOR signaling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Chen, W.; Lin, Y.; Jiang, M.; Wang, Q.; Shu, Q. Identification of LARS as an essential gene for osteosarcoma proliferation through large-scale CRISPR-Cas9 screening database and experimental verification. J. Transl. Med. 2022, 20, 355. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, J.H. Leucyl-tRNA synthetase 1 is required for proliferation of TSC-null cells. Biochem. Biophys. Res. Commun. 2021, 571, 159–166. [Google Scholar] [CrossRef]
- Passarelli, M.C.; Pinzaru, A.M.; Asgharian, H.; Liberti, M.V.; Heissel, S.; Molina, H.; Goodarzi, H.; Tavazoie, S.F. Leucyl-tRNA synthetase is a tumour suppressor in breast cancer and regulates codon-dependent translation dynamics. Nat. Cell. Biol. 2022, 24, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Kishida, S.; Yamamoto, H.; Murai, H.; Koyama, S.; Kikuchi, A. Avin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998, 17, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell. Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chuang, W.M.; Sun, Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J. Biol. Chem. 2002, 277, 30935–30941. [Google Scholar] [CrossRef]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef]
- Son, K.; You, J.S.; Yoon, M.S.; Dai, C.; Kim, J.H.; Khanna, N.; Banerjee, A.; Martinis, S.A.; Han, G.; Han, J.M.; et al. Nontranslational function of leucyl-tRNA synthetase regulates myogenic differentiation and skeletal muscle regeneration. J. Clin. Investig. 2019, 129, 2088–2093. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.G.; Park, S.-J.; Han, S.-H.; Park, J.-I. PGC-1α Regulates Cell Proliferation, Migration, and Invasion by Modulating Leucyl-tRNA Synthetase 1 Expression in Human Colorectal Cancer Cells. Cancers 2023, 15, 159. https://doi.org/10.3390/cancers15010159
Cho JG, Park S-J, Han S-H, Park J-I. PGC-1α Regulates Cell Proliferation, Migration, and Invasion by Modulating Leucyl-tRNA Synthetase 1 Expression in Human Colorectal Cancer Cells. Cancers. 2023; 15(1):159. https://doi.org/10.3390/cancers15010159
Chicago/Turabian StyleCho, Jun Gi, Su-Jeong Park, Sang-Heum Han, and Joo-In Park. 2023. "PGC-1α Regulates Cell Proliferation, Migration, and Invasion by Modulating Leucyl-tRNA Synthetase 1 Expression in Human Colorectal Cancer Cells" Cancers 15, no. 1: 159. https://doi.org/10.3390/cancers15010159
APA StyleCho, J. G., Park, S.-J., Han, S.-H., & Park, J.-I. (2023). PGC-1α Regulates Cell Proliferation, Migration, and Invasion by Modulating Leucyl-tRNA Synthetase 1 Expression in Human Colorectal Cancer Cells. Cancers, 15(1), 159. https://doi.org/10.3390/cancers15010159




