The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets
Abstract
Simple Summary
Abstract
1. Introduction
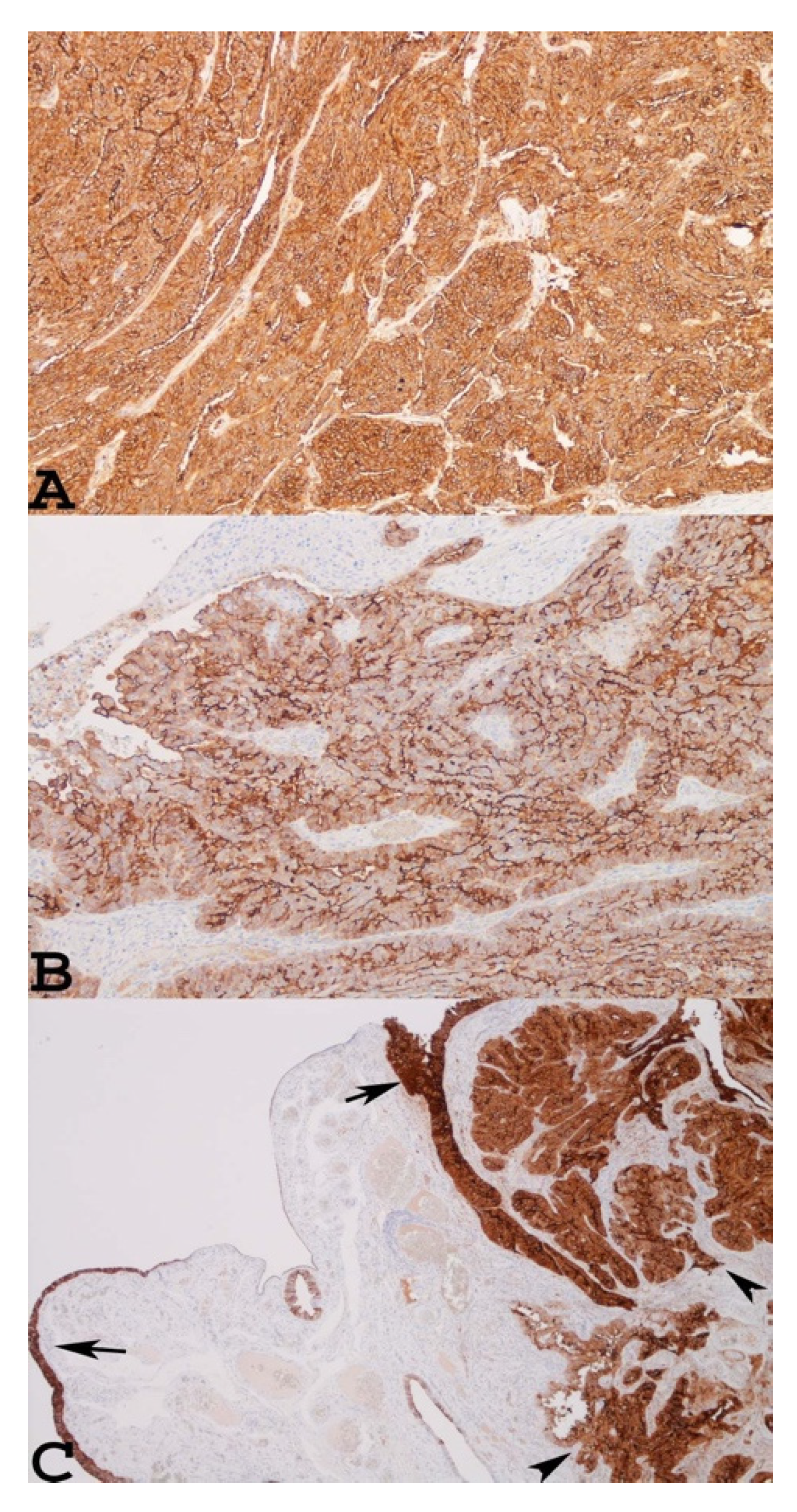
2. Mesothelin as a New Cancer Biomarker for the Diagnosis and Prognosis of Ovarian Carcinomas
| Authors | Case Number | Techinique Used for the Study | Prognosistic Data and Outcome |
|---|---|---|---|
| Ordóñez, N. [22] | 14 | Immunohistochemistry of formalin-fixed, paraffin-embedded neoplastic tissue | NR |
| Weidemann, S., et al. [36] | 386 | Immunohistochemistry of formalin-fixed, paraffin-embedded neoplastic TMA | No statistical association betwen MSLN expression and prognosis |
| Yildiz, Y., et al. [38] | 42 advanced stage of HSOC | Immunohistochemistry of formalin-fixed, paraffin-embedded neoplastic tissue | High staining associated with platinum chemoresistence and worse OS |
| Cheng, et al. [37] | 86 | MSLN mRNA by RT-PCR on frozen neoplastic tissue | Positive MSLN expression correlated with chemoresistant and worse OS |
| Yen, M.J., et al. [39] | 105 advanced stage of HSOC | Immunohistochemistry of formalin-fixed, paraffin-embedded neoplastic tissue and RT-PCR on eight cases | MSLN expression associated with prolonged survival |
| Magalhaes, I., et al. [42] | 107 | Immunohistochemistry of formalin-fixed, paraffin-embedded neoplastic TMA | No significant correaltion between positive MSLN and MSLN negative expression with OS in primary neoplasm and in the metastatic sites |
3. The Impacts of CA125, Other Molecules, and CA125–Mesothelin Binding on the Spread and Neoplastic Progression of HSOC
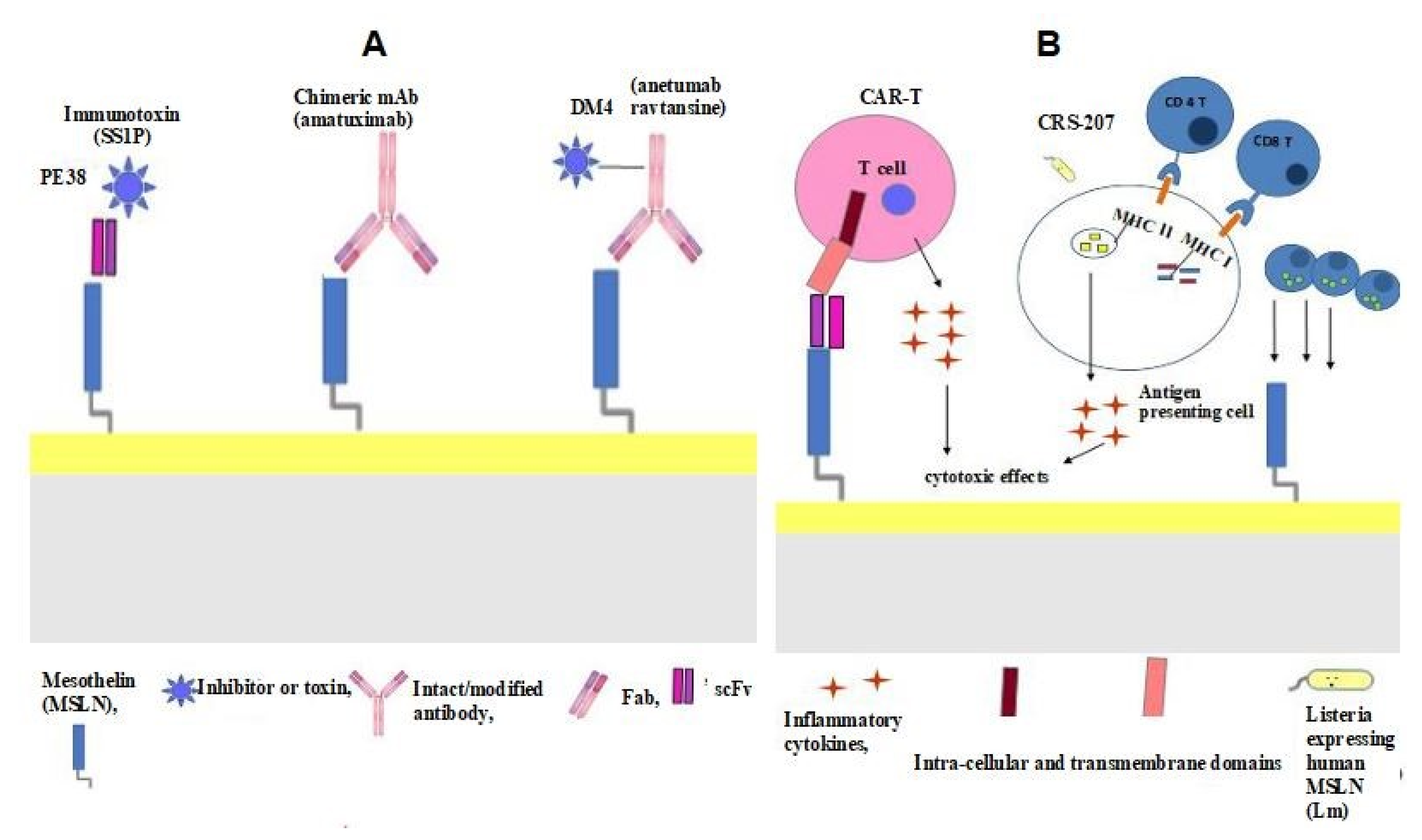
4. Mesothelin as a Therapeutic Target
4.1. Anti-Mesothelin Immunotoxin SS1P
4.2. MORAb-009 (Chimeric Anti-Mesothelin mAb)
4.3. Anti-Mesothelin Antibody–Drug Conjugate (BAY-94 9343)
4.4. Chimeric Antigen Receptor T cell (CAR T) Therapy
4.5. Vaccines
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, K.; Pastan, I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Oh-eda, M.; Hattori, K.; Taniguchi, Y.; Tamura, M.; Ochi, N.; Yamaguchi, N. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J. Biol. Chem. 1995, 270, 21984–21990. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Pastan, I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell. Biol. 2000, 20, 2902–2906. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Qian, M.; Ho, M. The role of mesothelin in tumor progression and targeted therapy. Anti-Cancer Agents Med. Chem. 2013, 13, 276–280. [Google Scholar] [CrossRef]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef]
- Cheng, W.F.; Hung, C.F.; Chai, C.Y.; Chen, C.A.; Lee, C.N.; Su, Y.N.; Tseng, W.Y.; Hsieh, C.Y.; Shih, I.M.; Wang, T.L.; et al. Generation and characterization of an ascitogenic mesothelin-expressing tumor model. Cancer 2007, 110, 420–431. [Google Scholar] [CrossRef]
- Chang, C.L.; Wu, T.C.; Hung, C.F. Control of human mesothelin-expressing tumors by DNA vaccines. Gene Ther. 2007, 14, 1189–1198. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2012. [Google Scholar]
- Cristaudo, A.; Foddis, R.; Vivaldi, A.; Guglielmi, G.; Dipalma, N.; Filiberti, R.; Neri, M.; Ceppi, M.; Paganuzzi, M.; Ivaldi, G.P.; et al. Clinical significance of sera mesothelin in patients with mesothelioma and lung cancer. Clin. Cancer Res. 2007, 13, 5076–5081. [Google Scholar] [CrossRef]
- Berruti, A.; Tampellini, M.; Torta, M.; Buniva, T.; Gorzegno, G.; Dogliotti, L. Prognostic value in predicting overall survival of two mucinous markers: CA 15-3 and CA 125 in breast cancer patients at first relapse of disease. Eur. J. Cancer 1994, 30, 2082–2084. [Google Scholar] [CrossRef]
- Norum, L.F.; Erikstein, B.; Nustad, K. Elevated CA125 in breast cancer a sign of advanced disease. Tumour Biol. 2000, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hedman, M.; Arnberg, H.; Wernlund, J.; Riska, H.; Brodin, O. Tissue polypeptide antigen (TPA), hyaluronan and CA 125 as sera markers in malignant mesothelioma. Anticancer Res. 2003, 23, 531–536. [Google Scholar] [PubMed]
- Bairey, O.; Blickstein, D.; Stark, P.; Prokocimer, M.; Nativ, H.M.; Kirgner, I.; Shaklai, M. Serum CA 125 as a prognostic factor in non-Hodgkin’s lymphoma. Leuk. Lymphoma 2003, 44, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Burney, I.A.; Siddiqui, T.; Siddiqui, I. Serum CA 125 is of clinical value in the staging and follow-up of patients with non- Hodgkin’s lymphoma: Correlation with tumor parameters and disease activity. Cancer 1999, 85, 755–756. [Google Scholar] [CrossRef]
- Zidan, J.; Hussein, O.; Basher, W.; Zohar, S. Serum CA 125: A tumor marker for monitoring response to treatment and follow-up in patients with non-Hodgkin’s lymphoma. Oncologist 2004, 9, 417–421. [Google Scholar] [CrossRef]
- Yamamoto, M.; Baba, H.; Toh, Y.; Okamura, T.; Maehara, Y. Peritoneal lavage CEA/CA125 is a prognostic factor for gastric cancer patients. J. Cancer Res. Clin. Oncol. 2007, 33, 471–476. [Google Scholar] [CrossRef]
- Jacobs, I.; Bast, R.C., Jr. The CA 125 tumour-associated antigen: A review of the literature. Hum. Reprod. 1989, 4, 1–12. [Google Scholar] [CrossRef]
- Zurawski, V.R.; Knapp, R.C.; Einhorn, N.; Kenemans, P.; Mortel, R.; Ohmi, K.; Bast, R.C., Jr.; Ritts, R.E., Jr.; Malkasian, G. An initial analysis of pre-operative serum CA125 levels in patients with early stage ovarian carcinoma. Gynecol. Oncol. 1998, 30, 7–14. [Google Scholar] [CrossRef]
- Einhorn, N. Ovarian cancer: Early diagnosis and screening. Hematol. Oncol. Clin. N. Am. 1992, 6, 843–850. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef]
- Scholler, N.; Fu, N.; Yang, Y.; Ye, Z.; Goodman, G.E.; Hellström, K.E.; Hellström, I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc. Natl. Acad. Sci. USA 1999, 96, 11531–11536. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, D.; Liu, L.; Liu, B.; Liang, H.; Yang, B. Serum soluble mesothelin-related peptide (SMRP): A potential diagnostic and monitoring marker for epithelial ovarian cancer. Arch. Gynecol. Obstet. 2014, 289, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Okła, K.; Surówka, J.; Frąszczak, K.; Czerwonka, A.; Kaławaj, K.; Wawruszak, A.; Kotarski, J.; Wertel, I. Assessment of the clinicopathological relevance of mesothelin level in plasma, peritoneal fluid, and tumor tissue of epithelial ovarian cancer patients. Tumour Biol. 2018, 40, 1010428318804937. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Cheng, W.F.; Lee, C.N.; Su, Y.N.; Chien, S.C.; Tzeng, Y.L.; Hsieh, C.Y.; Chen, C.A. Serum mesothelin in epithelial ovarian carcinoma: A new screening marker and prognostic factor. Anticancer Res. 2006, 26, 4721–4728. [Google Scholar] [PubMed]
- Badgwell, D.; Lu, Z.; Cole, L.; Fritsche, H.; Atkinson, E.N.; Somers, E.; Allard, J.; Moore, R.G.; Lu, K.H.; Bast, R.C., Jr. Urinary mesothelin provides greater sensitivity for early stage ovarian cancer than serum mesothelin, urinary hCG free beta subunit and urinary hCG beta core fragment. Gynecol. Oncol. 2007, 106, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, I.; Hellstrom, K.E. Two novel biomarkers, mesothelin and HE4, for diagnosis of ovarian carcinoma. Expert Opin. Med. Diagn. 2011, 5, 227–240. [Google Scholar] [CrossRef]
- Sandow, J.J.; Rainczuk, A.; Infusini, G.; Makanji, M.; Bilandzic, M.; Wilson, A.L.; Fairweather, N.; Stanton, P.G.; Garama, D.; Gough, D.; et al. Discovery and validation of novel protein biomarkers in ovarian cancer patient urine. Proteom. Clin. Appl. 2018, 12, e1700135. [Google Scholar] [CrossRef]
- Hollevoet, K.; Speeckaert, M.M.; Decavele, A.S.; Vanholder, R.; van Meerbeeck, J.P.; Delanghe, J.R. Mesothelin levels in urine are affected by glomerular leakage and tubular reabsorption. Clin. Lung Cancer 2012, 13, 470–474. [Google Scholar] [CrossRef]
- Prantner, A.M.; Turini, M.; Kerfelec, B.; Joshi, S.; Baty, D.; Chames, P.; Scholler, N. Anti-mesothelin nanobodies for both conventional and nanoparticle-based biomedical applications. J. Biomed. Nanotechnol. 2015, 11, 1201–1212. [Google Scholar] [CrossRef]
- Prantner, A.M.; Yin, C.; Kamat, K.; Sharma, K.; Lowenthal, A.C.; Madrid, P.B.; Scholler, N. Molecular imaging of mesothelin-expressing ovarian cancer with a human and mouse cross reactive nanobody. Mol. Pharm. 2018, 15, 1403–1411. [Google Scholar] [CrossRef]
- Scales, S.J.; Gupta, N.; Pacheco, G.; Firestein, R.; French, D.M.; Koeppen, H.; Rangell, L.; Barry-Hamilton, V.; Luis, E.; Chuh, J.; et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol. Cancer Ther. 2014, 13, 2630–2640. [Google Scholar] [CrossRef]
- Lamberts, L.E.; Menke-van der Houven van Oordt, C.W.; ter Weele, E.J.; Bensch, F.; Smeenk, M.M.; Voortman, J.; Hoekstra, O.S.; Williams, S.P.; Fine, B.M.; Maslyar, D.; et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin. Cancer Res. 2016, 22, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- van Scheltinga, A.G.T.; Ogasawara, A.; Pacheco, G.; Vanderbilt, A.N.; Tinianow, J.N.; Gupta, N.; Li, D.; Firestein, R.; Marik, J.; Scales, S.J.; et al. Preclinical efficacy of an antibody-drug conjugate targeting mesothelin correlates with quantitative 89Zr-ImmunoPET. Mol. Cancer Ther. 2017, 16, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kanner, W.A.; Galgano, M.T.; Stoler, M.H.; Mills, S.E.; Atkins, K.A. Distinguishing breast carcinoma from Müllerian serous carcinoma with mammaglobin and mesothelin. Int. J. Gynecol. Pathol. 2008, 27, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, S.; Gagelmann, P.; Gorbokon, N.; Lennartz, M.; Menz, A.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Blessin, N.C.; Fraune, C.; et al. Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors. Biomedicines 2021, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.F.; Huang, C.Y.; Chang, M.C.; Hu, Y.H.; Chiang, Y.C.; Chen, Y.L.; Hsieh, C.Y.; Chen, C.A. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br. J. Cancer 2009, 100, 1144–1153. [Google Scholar] [CrossRef]
- Yildiz, Y.; Kabadayi, G.; Yigit, S.; Kucukzeybek, Y.; Alacacioglu, A.; Varol, U.; Taskaynatan, H.; Salman, T.; Oflazoglu, U.; Akyol, M.; et al. High expression of mesothelin in advanced serous ovarian cancer is associated with poor prognosis. J. BUON 2019, 24, 1549–1554. [Google Scholar]
- Yen, M.J.; Hsu, C.Y.; Mao, T.L.; Wu, T.C.; Roden, R.; Wang, T.L.; Shih, I.M. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin. Cancer Res. 2006, 12, 827–831. [Google Scholar] [CrossRef]
- Thomas, A.; Chen, Y.; Steinberg, S.M.; Luo, J.; Pack, S.; Raffeld, M.; Abdullaev, Z.; Alewine, C.; Rajan, A.; Giaccone, G.; et al. High mesothelin expression in advanced lung adenocarcinoma is associated with KRAS mutations and a poor prognosis. Oncotarget 2015, 6, 11694–11703. [Google Scholar] [CrossRef]
- Einama, T.; Kamachi, H.; Nishihara, H.; Homma, S.; Kanno, H.; Takahashi, K.; Sasaki, A.; Tahara, M.; Okada, K.; Muraoka, S.; et al. Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas 2011, 40, 1276–1282. [Google Scholar] [CrossRef]
- Magalhaes, I.; Fernebro, J.; Abd Own, S.; Glaessgen, D.; Corvigno, S.; Remberger, M.; Mattsson, J.; Dahlstrand, H. Mesothelin Expression in Patients with High-Grade Serous Ovarian Cancer Does Not Predict Clinical Outcome But Correlates with CD11c+ Expression in Tumor. Adv. Ther. 2020, 37, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Einama, T.; Homma, S.; Kamachi, H.; Kawamata, F.; Takahashi, K.; Takahashi, N.; Taniguchi, M.; Kamiyama, T.; Furukawa, H.; Matsuno, Y.; et al. Luminal membrane expression of mesothelin is a prominent poor prognostic factor for gastric cancer. Br. J. Cancer 2012, 107, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, F.; Kamachi, H.; Einama, T.; Homma, S.; Tahara, M.; Miyazaki, M.; Tanaka, S.; Kamiyama, T.; Nishihara, H.; Taketomi, A.; et al. Intracellular localization of mesothelin predicts patient prognosis of extrahepatic bile duct cancer. Int. J. Oncol. 2012, 41, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamagishi, Y.; Einama, T.; Koiwai, T.; Yamasaki, T.; Fukumurakoga, M.; Ishibashi, Y.; Takihata, Y.; Shiraishi, T.; Miyata, Y.; et al. Membrane mesothelin expression positivity is associated with poor clinical outcome of luminal-type breast cancer. Oncol. Lett. 2020, 20, 193. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, S.; Miyamoto, M.; Einama, T.; Matsuura, H.; Iwahashi, H.; Ishibashi, H.; Sakamoto, T.; Hada, T.; Takano, M. Co-Expression of Mesothelin and CA125 Is Associated with the Poor Prognosis of Endometrial Serous Carcinoma and Mixed Carcinomas Including Serous Carcinoma. Pathol. Oncol. Res. 2020, 26, 2299–2306. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar] [CrossRef]
- Casey, M.J.; Synder, C.; Bewtra, C.; Narod, S.A.; Watson, P.; Lynch, H.T. Intra-abdominal carcinomatosis after prophylactic oophorectomy in women of hereditary breast ovarian cancer syndrome kindreds associated with BRCA1 and BRCA2 mutations. Gynecol. Oncol. 2005, 97, 457–467. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Lynch, H.T.; Neuhausen, S.L.; Narod, S.A.; Van’t Veer, L.; Garber, J.E.; Evans, G.; Isaacs, C.; Daly, M.B.; Matloff, E.; et al. Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N. Engl. J. Med. 2002, 346, 1616–1622. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Zhang, L.; Tian, S.; Yang, T.; Wang, L.; Zhao, M.; Yang, Q.; Wang, Y.; Yang, X. Evaluation of the Efficacy and Safety of PARP Inhibitors in Advanced-Stage Epithelial Ovarian Cancer. Front. Oncol. 2020, 10, 954. [Google Scholar] [CrossRef]
- Crum, C.P.; Drapkin, R.; Miron, A.; Ince, T.A.; Muto, M.; Kindelberger, D.W.; Lee, Y. The distal fallopian tube: A new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 2007, 19, 3–9. [Google Scholar] [CrossRef]
- Patrono, M.G.; Corzo, C.; Iniesta, M.; Ramirez, P.T. Management of Preinvasive Lesions. Clin. Obstet. Gynecol. 2017, 60, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Medeiros, F.; Kindelberger, D.; Callahan, M.J.; Muto, M.G.; Crum, C.P. Advances in the recognition of tubal intraepithelial carcinoma: Applications to cancer screening and the pathogenesis of ovarian cancer. Adv. Anat. Pathol. 2006, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scholler, N.; Garvik, B.; Hayden-Ledbetter, M.; Kline, T.; Urban, N. Development of a CA125-mesothelin cell adhesion assay as a screening tool for biologics discovery. Cancer Lett. 2007, 247, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bruney, L.; Conley, K.C.; Moss, N.M.; Liu, Y.; Stack, M.S. Membrane-type I matrixmetalloproteinase-dependent ectodomain shedding of mucin16/CA-125 on ovarian cancer cells modulates adhesion and invasion of peritoneal mesothelium. Biol. Chem. 2014, 395, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Alvarez, V.; et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 2003, 348, 403–413, Erratum in N. Engl. J. Med. 2005, 353, 2827. [Google Scholar] [CrossRef]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Rynne-Vidal, Á.; Pérez-Lozano, M.L.; Gilsanz, Á.; Ruiz-Carpio, V.; Reyes, R.; García-Bordas, J.; Stamatakis, K.; Dotor, J.; et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J. Pathol. 2013, 231, 517–531. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Jin, H.; Yu, Y.; Zhang, T.; Zhou, X.; Zhou, J.; Jia, L.; Wu, Y.; Zhou, B.P.; Feng, Y. Snail is critical for tumor growth and metastasis of ovarian carcinoma. Int. J. Cancer 2010, 126, 2102–2111. [Google Scholar] [CrossRef]
- Carey, P.; Low, E.; Harper, E.; Stack, M.S. Metalloproteinases in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 3403. [Google Scholar] [CrossRef]
- Yuan, Q.; Song, J.; Yang, W.; Wang, H.; Huo, Q.; Yang, J.; Yu, X.; Liu, Y.; Xu, C.; Bao, H. The effect of CA125 on metastasis of ovarian cancer: Old marker new function. Oncotarget 2017, 8, 50015–50022. [Google Scholar] [CrossRef][Green Version]
- Furuya, M.; Masuda, H.; Hara, K.; Uchida, H.; Sato, K.; Sato, S.; Asada, H.; Maruyama, T.; Yoshimura, Y.; Katabuchi, H.; et al. ZEB1 expression is a potential indicator of invasive endometriosis. Acta Obstet. Gynecol. Scand. 2017, 96, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Hosono, S.; Kajiyama, H.; Terauchi, M.; Shibata, K.; Ino, K.; Nawa, A.; Kikkawa, F. Expression of Twist increases the risk for recurrence and for poor survival in epithelial ovarian carcinoma patients. Br. J. Cancer 2007, 96, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, Y.; Ng, S.K.; Huang, K.C.; Ni, X.; Choi, P.W.; Hasselblatt, K.; Muto, M.G.; Welch, W.R.; Berkowitz, R.S.; et al. Characterization of MicroRNA-200 pathway in ovarian cancer and serous intraepithelial carcinoma of fallopian tube. BMC Cancer 2017, 17, 422. [Google Scholar] [CrossRef] [PubMed]
- Wimberger, P.; Hauch, S.; Kimmig, R.; Kuhlmann, J.D. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 2017, 8, 48820–48831. [Google Scholar] [CrossRef]
- Huo, Q.; Xu, C.; Shao, Y.; Yu, Q.; Huang, L.; Liu, Y.; Bao, H. Free CA125 promotes ovarian cancer cell migration and tumor metastasis by binding Mesothelin to reduce DKK1 expression and activate the SGK3/FOXO3 pathway. Int. J. Biol. Sci. 2021, 17, 574–588. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Chen, Y.F.; Pandey, S.; Day, C.H.; Chen, Y.F.; Jiang, A.Z.; Ho, T.J.; Chen, R.J.; PadmaViswanadha, V.; Kuo, W.W.; Huang, C.Y. Synergistic effect of HIF-1alpha and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J. Cell. Physiol. 2017, 233, 3660–3671. [Google Scholar] [CrossRef]
- Gilley, J.; Coffer, P.J.; Ham, J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 2003, 162, 613–622. [Google Scholar] [CrossRef]
- McClelland Descalzo, D.L.; Satoorian, T.S.; Walker, L.M.; Sparks, N.R.; Pulyanina, P.Y.; Zur Nieden, N.I. Glucose-induced oxidative stress reduces proliferation in embryonic stem cells via FOXO3A/beta-catenin-dependent transcription of p21(cip1). Stem Cell Rep. 2016, 7, 55–68. [Google Scholar] [CrossRef]
- McGowan, S.E.; McCoy, D.M. Platelet-derived growth factor-a regulates lung fibroblast S-phase entry through p27(kip1) and FoxO3a. Respir. Res. 2013, 14, 68. [Google Scholar] [CrossRef]
- Joseph, J.; Ametepe, E.S.; Haribabu, N.; Agbayani, G.; Krishnan, L.; Blais, A.; Sad, S. Inhibition of ROS and upregulation of inflammatory cytokines by FoxO3a promotes survival against Salmonella typhimurium. Nat. Commun. 2016, 7, 12748. [Google Scholar] [CrossRef] [PubMed]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Ye, L.; Liu, G.; An, J.; Sehouli, J.; Sun, P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: An updated systematic review and meta-analysis. Onco Targets Ther. 2018, 11, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Gourley, C.; Balmaña, J.; Ledermann, J.A.; Serra, V.; Dent, R.; Loibl, S.; Pujade-Lauraine, E.; Boulton, S.J. Moving from poly (ADP-Ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. J. Clin. Oncol. 2019, 37, 2257–2269. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin therapy of cancer. Nat. Rev. Cancer 2006, 6, 559–565. [Google Scholar] [CrossRef]
- Hassan, R.; Lerner, M.R.; Benbrook, D.; Lightfoot, S.A.; Brackett, D.J.; Wang, Q.C.; Pastan, I. Antitumor activity of SS(dsFv)PE38 and SS1(dsFv)PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin. Cancer Res. 2002, 8, 3520–3526. [Google Scholar]
- Hassan, R.; Sharon, E.; Thomas, A.; Zhang, J.; Ling, A.; Miettinen, M.; Kreitman, R.J.; Steinberg, S.M.; Hollevoet, K.; Pastan, I. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014, 120, 3311–3319. [Google Scholar] [CrossRef]
- Hassan, R.; Miller, A.C.; Sharon, E.; Thomas, A.; Reynolds, J.C.; Ling, A.; Kreitman, R.J.; Miettinen, M.M.; Steinberg, S.M.; Fowler, D.H.; et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci. Transl. Med. 2013, 23, 208ra147. [Google Scholar] [CrossRef]
- Hassan, R.; Ebel, W.; Routhier, E.L.; Patel, R.; Kline, J.B.; Zhang, J. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007, 7, 20. [Google Scholar]
- Hassan, R.; Schweizer, C.; Lu, K.F.; Schuler, B.; Remaley, A.T.; Weil, S.C. Inhibition of mesothelin-CA-125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: Implications for cancer therapy. Lung Cancer 2010, 68, 455–459. [Google Scholar] [CrossRef]
- Hassan, R.; Cohen, S.J.; Phillips, M.; Pastan, I.; Sharon, E.; Kelly, R.J.; Schweizer, C.; Weil, S.; Laheru, D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin expressing cancers. Clin. Cancer Res. 2010, 16, 6132–6138. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Kindler, H.L.; Jahan, T.; Bazhenova, L.; Reck, M.; Thomas, A.; Pastan, I.; Parno, J.; O’Shannessy, D.J.; Fatato, P.; et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin. Cancer Res. 2014, 20, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, Z.; Arnst, K.E.; Miller, D.D.; Li, W. Tubulin Inhibitor-Based Antibody-Drug Conjugates for Cancer Therapy. Molecules 2017, 22, 1281. [Google Scholar] [CrossRef]
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 20. [Google Scholar] [CrossRef]
- Quanz, M.; Hagemann, U.B.; Zitzmann-Kolbe, S.; Stelte-Ludwig, B.; Golfier, S.; Elbi, C.; Mumberg, D.; Ziegelbauer, K.; Schatz, C.A. Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expression human ovarian cancer models. Oncotarget 2018, 9, 34103–34121. [Google Scholar] [CrossRef]
- Hung, C.F.; Xu, X.; Li, L.; Ma, Y.; Jin, Q.; Viley, A.; Allen, C.; Natarajan, P.; Shivakumar, R.; Peshwa, M.V.; et al. Development of Anti-Human Mesothelin-Targeted Chimeric Antigen Receptor Messenger RNA-Transfected Peripheral Blood Lymphocytes for Ovarian Cancer Therapy. Hum. Gene Ther. 2018, 29, 614–625. [Google Scholar] [CrossRef]
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, D.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014, 2, 112–120. [Google Scholar] [CrossRef]
- Carpenito, C.; Milone, M.C.; Hassan, R.; Simonet, J.C.; Lakhal, M.; Suhoski, M.M.; Varela-Rohena, A.; Haines, K.M.; Heitjan, D.F.; Albelda, S.M.; et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA. 2009, 106, 3360–3365. [Google Scholar] [CrossRef]
- Sandaltzopoulos, R.; Scholler, N.; Powell, D.J., Jr. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol. Ther. 2012, 20, 633–643. [Google Scholar] [CrossRef]
- Banville, A.C.; Wouters, M.C.A.; Oberg, A.L.; Goergen, K.M.; Maurer, M.J.; Milne, K.; Ashkani, J.; Field, E.; Ghesquiere, C.; Jones, S.J.M.; et al. Co-expression patterns of chimeric antigen receptor (CAR)-T cell target antigens in primary and recurrent ovarian cancer. Gynecol. Oncol. 2021, 160, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Huang, K.; Zhang, Y.; Kupfer, G.; Zhao, Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Eppolito, C.; Lele, S.; Shrikant, P.; Matsuzaki, J.; Odunsi, K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015, 6, 27359–27377. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [PubMed]
- Schoutrop, E.; El-Serafi, I.; Poiret, T.; Zhao, Y.; Gultekin, O.; He, R.; Moyano-Galceran, L.; Carlson, J.W.; Lehti, K.; Hassan, M.; et al. Mesothelin-Specific CAR T Cells Target Ovarian Cancer. Cancer Res. 2021, 81, 3022–3035. [Google Scholar] [CrossRef]
- Frey, N.; Porter, D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2019, 25, e123–e127. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.L.; Stashwick, C.; Plesa, G.; Morgan, M.A.; Porter, D.; Maus, M.V.; June, C.H. Possible Compartmental Cytokine Release Syndrome in a Patient With Recurrent Ovarian Cancer After Treatment With Mesothelin-targeted CAR-T Cells. J. Immunother. 2017, 40, 104–107. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, J.; Wu, Z.; Yu, J.; Cui, Q.; Pu, C.; Liang, B.; Luo, Y.; Shi, J.; Jin, A.; et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J. Hematol. Oncol. 2016, 9, 70. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224ra25. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Oladejo, M.; Paterson, Y.; Wood, L.M. Clinical Experience and Recent Advances in the Development of Listeria-Based Tumor Immunotherapies. Front. Immunol. 2021, 12, 642316. [Google Scholar] [CrossRef]
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin. Cancer Res. 2012, 18, 858–868. [Google Scholar] [CrossRef]
- Golub, S.H.; O’Connell, T.X.; Morton, D.L. Correlation of in vivo and in vitro assays of immunocompetence in cancer patients. Cancer Res. 1974, 34, 1833–1837. [Google Scholar]
- Flickinger, J.C., Jr.; Rodeck, U.; Snook, A.E. Listeria monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines 2018, 6, 48. [Google Scholar] [CrossRef]

| Clinical Trials gov Identifier | Agent | Phase | Status | Disease Setting | Recruiting Centers |
|---|---|---|---|---|---|
| NCT00066651 | SS1P | I | Completed | Advanced Cervical, ovarian Fallopian tube, pacreatic, peritoneal, lung, head and neck cancer | Unites States |
| NCT01521325 | MORAb-009 (Chimeric Anti-Mesothelin mAb) amatuximab | I | Completed | Ovarian carcinoma, Mesothelioma, Pancreatic Cancer, Non Small Cell Lung | United States |
| NCT01413451 | MORAb-009 (Chimeric Anti-Mesothelin mAb) amatuximab | Early Phase I | Terminated without efficacy in patienets with Ovarian Carcinoma | Ovarian carcinoma Mesothelioma, Pancreatic Cancer, Non Small Cell Lung cancer expressing mesothelin | United States |
| NCT01439152 | BAY-94 9343 (Anti-MesothelinAntibody Drug Conjugate) Anetumab ravtansine | I | Completed | Invasive epithelial ovarian, primary serous peritoneal fallopian tube cancer | United States |
| NCT02751918 | BAY-94 9343 (Anti-MesothelinAntibody Drug Conjugate) Anetumab ravtansine + pegyleted liposomal doxorubicin | Ib | Completed | Invasive or metastatic, predominantly epithelial platinum-resistant ovarian, fallopian tube, or primary serous peritoneal cancer | United States Belgium Moldova Spain |
| NCT03814447 | CAR-T-meso | Early Phase I | Recruitment | Refractory-Relapsed Ovarian Cancer | China |
| NCT03608618 | CAR-T-meso+ intraperitoneal MCY-M11 | Early Phase I | Recruitment | Advanced Ovarian cancer and mesthelioma | United States |
| NCT00585845 | CRS-207 | I | Terminated | Ovarian Carcinoma, Mesothelioma, Non small-cell Lung carcinoma, Pancratic carcinoma, who have failed or who are not candidates for standard treatments | United States |
| NCT02575807 | CRS-207 + alfaPD-1 + IDO1 inhibitor (Epacadostat) | I /II | Terminated low enrollment and lack of cinical activity | Platinum-resistant ovarian, fallopian or seous peritoneal cancer | United States |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, G.; Ferioli, E.; Tafuni, A. The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets. Cancers 2022, 14, 2283. https://doi.org/10.3390/cancers14092283
Giordano G, Ferioli E, Tafuni A. The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets. Cancers. 2022; 14(9):2283. https://doi.org/10.3390/cancers14092283
Chicago/Turabian StyleGiordano, Giovanna, Elena Ferioli, and Alessandro Tafuni. 2022. "The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets" Cancers 14, no. 9: 2283. https://doi.org/10.3390/cancers14092283
APA StyleGiordano, G., Ferioli, E., & Tafuni, A. (2022). The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets. Cancers, 14(9), 2283. https://doi.org/10.3390/cancers14092283






