Simple Summary
Nerve fibers in the microenvironment have shown notable prognostic potential in various malignancies; however, its role in hepatocellular carcinoma remains to be elucidated. Therefore, the impact of nerve fibers on oncological survival was investigated in a large European cohort of patients with hepatocellular carcinoma who underwent curative-intent liver resection. By means of univariate and multivariate statistics as well as group comparisons of patients with and without nerve fibers, the presence of nerve fibers itself, as well as the corresponding density, was not shown to be associated with survival or the risk of tumor recurrence. Despite being of major prognostic value in various cancer types, nerve fibers in the microenvironment of hepatocellular carcinoma could not be used as a prognostic biomarker in these patients.
Abstract
It has been shown that the presence and density of nerve fibers (NFs; NFD) in the tumor microenvironment (TME) may play an important prognostic role in predicting long-term oncological outcomes in various malignancies. However, the role of NFD in the prognosis of hepatocellular carcinoma (HCC) is yet to be explored. To this end, we aimed to investigate the impact of NFs on oncological outcomes in a large European single-center cohort of HCC patients. In total, 153 HCC patients who underwent partial hepatectomy in a curative-intent setting between 2010 and 2021 at our university hospital were included in this study. Group comparisons between patients with and without NFs were conducted and the association of recurrence-free survival (RFS) and overall survival (OS) with the presence of NFs and other clinico-pathological variables were determined by univariate and multivariable Cox regression models. Patients with NFs in the TME presented with a median OS of 66 months (95% CI: 30–102) compared to 42 months (95% CI: 20–63) for patients without NFs (p = 0.804 log-rank). Further, RFS was 26 months (95% CI: 12–40) for patients with NFs compared to 18 months (95% CI: 9–27) for patients without NFs (p = 0.666 log-rank). In a subgroup analysis, patients with NFD ≤ 5 showed a median OS of 54 months (95% CI: 11–97) compared to 48 months (95% CI: 0–106) for the group of patients with NFD > 5 (p = 0.787 log-rank). Correspondingly, the RFS was 26 months (95% CI: 10–42) in patients with NFD ≤ 5 and 29 months (95% CI: 14–44) for the subcohort with NFD > 5 (p = 0.421 log-rank). Further, group comparisons showed no clinico-pathological differences between patients with NFs (n = 76) and without NFs (n = 77) and NFs were not associated with OS (p = 0.806) and RFS (p = 0.322) in our Cox regression models. In contrast to observations in various malignancies, NFs in the TME and NFD are not associated with long-term oncological outcomes in HCC patients undergoing surgery.
1. Introduction
Hepatocellular carcinoma (HCC) is unquestionably a significant health burden as the third most frequent cause of cancer-related mortality globally [1,2]. Liver resection (LR) remains the primary treatment for patients with early HCC and with an increasingly progressive surgical approach nowadays, resection is often considered in selected patients with advanced tumor stages [3,4,5]. Especially in individuals with limited disease and preserved function of the liver remnant, 10-year survival rates above 50% have been reported in selected cohorts [6]. However, as HCC arises on a background of chronic liver disease, tumor recurrence in the remnant liver is reported in up to 80% of patients, even after complete initial tumor clearance and R0 resection [7]. Based on this, liver transplantation might be the best option for HCC patients as it addresses both the underlying parenchymal liver disease leading to cancer and the oncological disease itself; however, its utilization in this setting is strongly limited by the scarcity of liver allografts from deceased donors [8].
Prognostic biomarkers are important for the development of prognostic models which are suitable to predict clinical prognosis in oncological patients and guide treatment decisions in complex clinical situations [9]. In Europe, HCC treatment is most often guided by the Barcelona Clinic Liver Cancer (BCLC) staging system, which summarizes key prognostic characteristics, such as tumor burden, the extent of liver dysfunction, and the general performance status of the patient [10,11]. However, the overall validity of the rather conservative BCLC staging system has been critically discussed, as some reports indicate a significant survival benefit after liver resection over other interventional or palliative treatment modalities even in higher preoperative BCLC stages [4,5]. Thus, in surgical candidates with HCC, identifying novel prognostic biomarkers and the development of accurate prognostic models are of utmost scientific importance with practice-changing potential.
Our group has recently shown the notable prognostic value of nerve fibers (NFs) in the tumor microenvironment (TME) in cholangiocarcinoma (CCA), which is the second most common primary liver cancer [12,13]. As these NFs have a small diameter (diameters of <100 μm) and are usually not visible on routine H&E staining and require additional immunohistochemical staining (Figure 1). NFs and their respective count (nerve fiber density, NFD) have shown to have a prognostic significance in other malignancies as well (e.g., pancreatic ductal adenocarcinoma (PDAC) and gastric or colorectal cancer) [14,15,16,17], however, their exact role in HCC patients has not been investigated before. Therefore, in this study, we subsequently investigated NFs as a prognostic marker in a large European cohort of HCC patients undergoing hepatectomy with a curative intent.
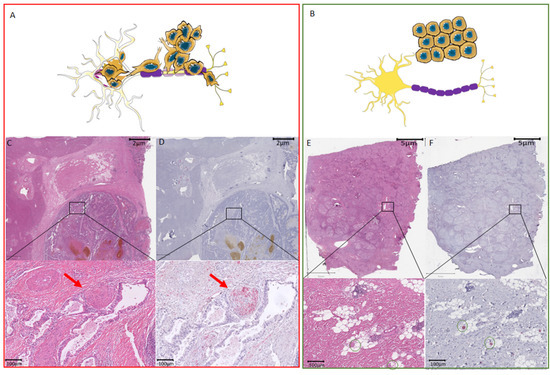
Figure 1.
Difference between perineural invasion and nerve fiber density with respect to hepatocellular carcinoma. (A) Schematic overview of tissue with cancers cells invading the nerve. (B) Schematic overview of tissue with NFs in the TME. (C) Routine HE staining showing of HCC. The black box illustrates the zoomed-in area shown underneath with perineural invasion by cancer cells invading a large nerve trunk (red arrow). (D) Consecutive slide used for immunohistochemistry with the neuronal marker PGP9.5. The black box illustrates the zoomed-in area shown underneath with perineural invasion by cancer cells invading a large nerve trunk (red arrow). The nerve trunk is illustrated in red. (E) Routine HE staining showing of HCC. Routine HE staining with the black box indicates the localization of the small nerve fibers that are not visible on the HE staining. Green circles in the zoomed-in image mark the regions where the small nerve fibers are found by immunohistochemistry. (F) Consecutive slide used for immunohistochemistry with the neuronal marker PGP9.5. The black box illustrates the zoomed-in area shown underneath with the presence of small nerve fibers without cancer invasion. These small nerve fibers are illustrated in red and marked by green circles. HCC, hepatocellular carcinoma; NF, nerve fibers; TME, tumor microenvironment.
2. Materials and Methods
2.1. Patients
All consecutive patients who underwent surgical resection for HCC between 2010 and 2020 at the University Hospital RWTH Aachen (UH-RWTH) were considered for inclusion in this study. Of these patients (n = 212), 59 individuals were ultimately excluded (this includes: n = 49 with missing NF data; n = 10 cases of perioperative mortality), resulting in a study cohort of 153 patients. The study was approved by the institutional Ethical Review Board (EK 106/18) and was carried in line with the good clinical practice guidelines (ICH-GCP) and the principles of the Declaration of Helsinki.
2.2. Staging and Surgical Technique
All individuals treated for HCC in our institution underwent a detailed clinical workup as previously described [2,18]. Magnetic resonance imaging (MRI) or computed tomography (CT) was utilized to assess the number, size, and location of tumor nodules as well as the presence of distant metastases. The preoperative risk assessment was based on the American society of anesthesiologists—(ASA) performance status, preoperative calculation of the future liver remnant (FLR) as well as an evaluation of the parenchymal liver function (standard laboratory parameters and the LiMAx test (Humedics® GmbH, Berlin, Germany)) [19]. Patients staged Barcelona Clinic Liver Cancer (BCLC) A to C without any evidence of extrahepatic spread and compensated liver function were considered candidates for surgery as primary treatment. The final decision for surgery was made by a dedicated hepatobiliary surgeon and approved by the local interdisciplinary tumor board for every HCC patient. Liver resection was carried out in line with our clinical standards [2,18]. Briefly, an intraoperative ultrasound was performed regularly to detect other suspicious lesions and visualize the local tumor spread. The decision for either non-anatomic atypical wedge resections with an adequate resection margin or anatomic resections or was based on the surgeon’s preference. Parenchymal transection was carried out with the Cavitron Ultrasonic Surgical Aspirator (CUSA®, Integra LifeSciences®, Plainsboro, NJ, USA) in open hepatectomy, while in laparoscopic resection, parenchymal transection was commonly performed by Harmonic Ace® (Ethicon Inc., Somerville, NJ, USA), Thunderbeat ® (Olympus K.K., Tokyo, Japan) or laparoscopic CUSA (Integra Life Sciences, Princeton, NJ, USA) in combination with polymer clips (Teleflex Inc., Wayne, PA, USA) or vascular staplers (Echelon, Ethicon, Somerville, NJ, USA). Intermittent Pringle maneuvers were used if necessary. The anesthesiologic management was based on a restrictive fluid intervention strategy ensuring a low central venous pressure (CVP) during parenchymal dissection.
2.3. Assessment of Nerve Fibers
The formalin-fixed paraffin-embedded (FFPE) blocks were retrieved from the archive of the local Institute of Pathology. Slides (2.5 μm sections) were cut to conduct immunohistochemistry staining with the neuronal marker PGP9.5. Before this, the tissue was deparaffinized in xylene and rehydrated in graded alcohols. Subsequently, the tissue was heated in citrate buffer (pH 6.0) at 95–100 °C for 5 min and cooled down for 20 min. The immunostaining anti-rabbit PGP9.5 (Dako antibody 1:100) was incubated overnight at 4 °C. A Ventana digital scanner was used to digitalize all slides. The digital image was processed in Qupath 0.1.6. The nerve fiber count was analyzed by a trained pathologist who was blinded to the clinical outcomes in every case. The presence of nerve fascicles with diameters of <100 μm in 20 continuous non-overlapping visual fields at ×200 magnification was subsequently assessed [12,16].
2.4. Statistical Analysis
The primary endpoint of this analysis was recurrence-free survival (RFS), which was defined as the period from surgery to the date of the first recurrence. The secondary endpoint was overall survival (OS), which was defined as the time period between the date of resection and the date of death of any cause. Patients not displaying tumor recurrence were censored at the time of death or the last follow-up. Perioperative mortality was defined as in-hospital mortality. Group comparisons were conducted by the chi-squared test, Fisher’s exact test, or linear-by-linear association for categorical variables and by the Mann–Whitney-U-Test in case of continuous variables. The associations of the endpoints with clinico-pathological variables were assessed by a univariate and multivariable Cox regression analyses in a forward selection model. Survival curves were generated according to the Kaplan–Meier method and compared with the log-rank test. Median follow-up was calculated according to the reverse Kaplan–Meier method. The level of significance was p < 0.05 and p-values were considered for two-sided testing. Analyses were performed using SPSS Statistics 24 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Patient Cohort
The study group consisted of 105 men (68.6%) and 48 women (31.4%) with a median age of 69 years. Most individuals were assessed as ASA (American Society of Anesthesiologists classification) III or higher (66.0%, 101/153). Non-alcoholic fatty liver disease (NAFLD, 60/153, 39.2%) was the most common disease etiology, followed by viral hepatitis (39/153, 25.5), alcoholic liver disease (ALD, 34/153, 22.2%), and cryptogenic or other diseases (20/153; 20). Liver function was mainly compensated, with most individuals being diagnosed as Child-Pugh A patients (139/153, 90.85). Most of the patients underwent minor liver resection (95/153, 62.1%). Accordingly, R0 resection was achieved in 96.1% (147/153) of the overall cohort. NFs in the TME were present in 76 individuals (49.7%). Major complications as defined by Clavien-Dindo ≥ IIIa were observed in 21.6% (33/153) of the patients. Patients decreasing due to postoperative complications were excluded from the analysis as stated above. Further details, including preoperative imaging characteristics and clinico-pathological details, are displayed in Table 1.

Table 1.
Patients’ characteristics.
3.2. Survival Analysis with Respect to the Presence of Nerve Fibers in the Tumor Microenvironment and Nerve Fiber Density
The median follow-up was calculated to be 48 months for the analysis. The median OS of the overall cohort was 54 months (95% confidence interval (CI): 33–74) and the median RFS 23 months (95% CI: 16–30, Figure 2A,B). A Kaplan–Meier analysis with respect to NFs showed a median OS of 66 months (95% CI: 30–102) in patients with NFs compared to 42 months (95% CI: 20–63) in patients without NFs (p = 0.804 log-rank, Figure 2C). Further, RFS was 26 months (95% CI: 12–40) in patients with NFs compared to 18 months (95% CI: 9–27) patients without NFs (p = 0.666 log-rank, Figure 2D). Further, a quantitative analysis of patients with NFs was carried out by dividing this subgroup into patients demonstrating an NFD of ≤5 compared to >5. Here the median OS was 54 months (95% CI: 11–97) in patients with NFD ≤ 5 compared to 48 months (95% CI: 0–106) in patients with NFD > 5 (p = 0.787 log-rank, Figure 2E). Correspondingly, the RFS was 26 months (95% CI: 10–42) in patients with NFD ≤ 5 and 29 months (95% CI: 14–44) patients with NFD > 5 (p = 0.421 log-rank, Figure 2F).
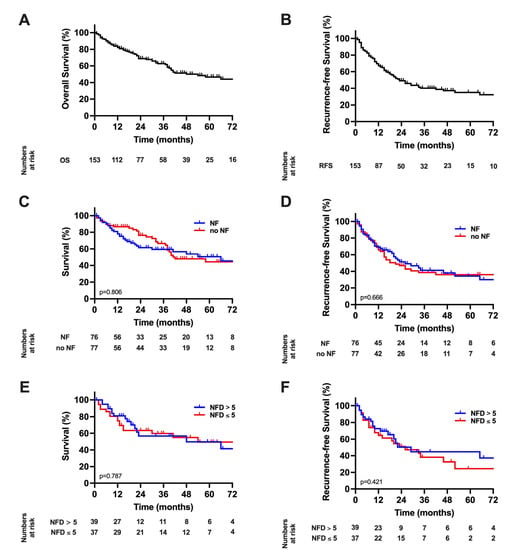
Figure 2.
Long-term outcome in hepatocellular carcinoma; (A) Overall survival. The median OS of the study cohort was 54 months; (B) Recurrence-free survival. The median RFS was 23 months; (C) Overall survival stratified by nerve fibers. The median OS of 66 months in patients with NF compared to 42 months (p = 0.804 log-rank). (D) Recurrence-free survival stratified by nerve fibers. The median RFS was 26 months in patients with NF compared to 18 months in patients without NF (p = 0.666 log-rank). (E) Overall survival in patients with nerve fibers survival stratified by nerve fiber density. The median OS was 54 months in patients with NFD ≤ 5 compared to 48 months in patients with NFD > 5 (p = 0.787 log-rank). (F) Recurrence-free survival in patients with nerve fibers survival stratified by nerve fiber density. The RFS was 26 months in patients with NFD ≤ 5 and 29 months patients with NFD > 5 (p = 0.421 log-rank). RFS, recurrence-free survival; OS, overall survival.
To further investigate patients displaying tumor recurrence, we separately analyzed patients with early recurrence (RFS < 24 months, n = 66) and late recurrence (RFS ≥ 24 months, n = 14). Here no difference in the likelihood of having NFs in the TME was shown between patients with early (31/66, 47.0%) or late (9/14, 64.3%) recurrence (p = 0.239). Moreover, separate survival analyses in patients with early and late recurrence showed no influence of the presence of NFs on OS (p = 0.182 log-rank; p = 0.867 log-rank) or RFS (p = 0.800 log-rank; p = 0.697 log-rank) in either of the subgroups (Supplementary Figure S1).
In another sub-analysis, we investigated the role of NFs in different disease etiologies. Here no difference in the likelihood of having NFs in the TME was shown between patients with alcoholic (15/34, 44.1%), non-alcoholic fatty (28/60, 46.7%), viral (23/39, 59.0%), and cryptogenic/other liver disease (10/20, 50.0%, p = 0.575). Moreover, separate survival analyses for each disease entity showed no influence of the presence of NFs on OS or RFS in either of the underlying liver diseases (Supplementary Figure S2).
3.3. Cox Regression Analysis of the Overall Cohort
As neither the presence of NFs in the whole cohort nor NFD within patients displaying NFs in the TME were found to be of prognostic value for OS and RFS in the Kaplan-Meier analysis, Cox regressions were used to determine risk factors for inferior oncological outcomes.
In univariate analysis, gender (p = 0.035), ASA score (p = 0.011), MELD (p = 0.017) AFP, p = 0.002) as well as a variety of other liver function parameters and preoperative imaging features, R1 resection (p = 0.003), pT category (p < 0.001), microvascular invasion (MVI, p < 0.001) and the duration of hospitalization (p = 0.003) gained statistical significance for OS (Table 2). These variables were transferred to a multivariable Cox regression model. Here, MELD score (p = 0.032), number of nodules (p = 0.010), preoperative ascites (p = 0.022), R1 resection (p = 0.002) and MVI (p < 0.001) were identified as independent predictors of OS (Table 2). The presence of NFs was not associated with OS in this analysis (p = 0.806). A similar approach was conducted for RFS. As for OS a variety of liver function parameters and variables regarding preoperative imaging as well as R1 resection (p < 0.001), pT category (p < 0.001) and MVI (p < 0.001) were significantly associated with RFS (Table 3). These variables were subsequently transferred to a multivariable Cox regression model. Here, aspartate aminotransferase (AST, p = 0.005), portal vein invasion (p = 0.030), pT category (p < 0.001 were independently prognostic for RFS (Table 3). Again, the presence of NFs was not associated with RFS in this analysis (p = 0.322).

Table 2.
Univariate and multivariable analysis of overall survival in hepatocellular carcinoma.

Table 3.
Univariate and multivariable analysis of recurrence-free survival in hepatocellular carcinoma. Various parameters are prognostic for recurrence-free survival.
3.4. Comparative Analysis of the Overall Patient Cohort with Respect to Nerve Fibers
To ensure that the presence of NFs was not unequally distributed among other oncological risk factors, a comparative analysis of patients with and without NFs was conducted (Table 1). This comparative analysis revealed no statistical significance in any characteristic and especially no difference was observed in the oncological risk factors of the overall cohort as determined by the univariate or multivariate Cox regression analysis.
3.5. Histological Characteristics
H&E and PGP9.5 scans were descriptively analyzed. The tumor region on the H&E staining was also identified on the PGP immunostaining (Figure 1). Nerve fibers in the TME were detected and counted as described previously [12,13].
4. Discussion
Chronic liver disease accounts for over 2 million deaths yearly, while HCC is one of the major oncological burdens from a global perspective and is projected to be responsible for more than 1 million annual deaths by 2030 [22]. With a poor overall 5-year survival of less than 20%, it belongs to the most lethal oncological diseases [9]. In this context, biomarkers with strong prognostic value and validity are under the spotlight of clinical and scientific interest as they might help to guide clinical decisions. Therefore, here we analyzed the prognostic value of the novel biomarker NFD within a large European single-center cohort of HCC patients undergoing curative-intent surgery. However, our data did not show any prognostic value for the presence of NFs in the TME or NFD in quantitative or qualitative analysis.
NFs play an integral role in the intense crosstalk of cancer-associated fibroblasts (CAF) or immune cells, or with tumor cells [23,24,25,26]. This inter-cellular crosstalk is partly based on released neurotransmitters of cancer cells binding to receptors of NFs and vice versa [27,28,29,30]. CAF triggers remodeling of the extracellular matrix, resulting in further neuronal growth, ultimately enhancing these effects [23,24]. It should be noted that these particular NFs are speculated to have a parasympathetic origin and must be differentiated from larger preexisting nerve trunks used to define classical PNI [12,12,31,32]. The underlying role of the nervous system in tumorigenesis and disease progression remains to be unraveled. However, some basic research findings did suggest certain antitumoral effects exerted by the parasympathetic system leading to decreased local tumor progression and attenuation of the development of distant metastases [33,34]. Considering these effects, NFs and NFD have been investigated in various oncological diseases and clinical settings, and so far, their prognostic value in predicting oncological outcomes has been reported in gastric and colorectal adenocarcinomas, breast cancer, PDAC as well as in intrahepatic and perihilar CCA [12,12,13,14,15,16,17,35]. Interestingly, seemingly there are disease-related differences regarding the exact role NFs are playing in outcomes. While a high NFD was shown to be associated with an impaired outcome in gastric and colorectal adenocarcinomas, it seems to be protective in terms of long-term survival in PDAC and both subtypes of CCA [12,12,13,14,15,16,17,35]. There are further heterogeneities even in entities with a positive correlation between NFD and oncological endpoints and cut-off values ideally used for differentiation between low-risk and high-risk patients vary between different tumors. While the mere presence or absence of NFs in intrahepatic CCA (NFD > 0) was defined as the best cut-off for defining prognosis, NFD ≥ 10 was determined for perihilar CCA and NFD > 7 for PDAC [12,13,16].
Given the significant prognostic value of NFD in CCA as the second most common liver tumor, we hypothesized that NF or NFD might also be associated with outcomes in HCC. However, our detailed analysis in this study could not find any difference in OS for HCC patients. The underlying mechanistic explanation for this observation is beyond the scope of this study and should be addressed in the future. One might argue that OS in HCC is also heavily influenced by the progression of the underlying liver disease and cirrhosis-associated complications, e.g., hepatic decompensations, malnutrition, sarcopenia, bacterial infections, or variceal bleedings [2,36]. This is an important cofounder which might influence our results as NFs are obviously associated with oncological prognosis in other tumors but not with the severity of cirrhosis. Thus, we decided to define RFS as the primary endpoint of the study and found no association between RFS and NFs, supporting the assumption that NFs might not be of prognostic value in HCC. As stated above, the definition of ideal cut-offs is a topic of ongoing debate and might be different among oncological entities [12,13,16]. Therefore, we conducted a secondary analysis exclusively for patients with NFs in the TME and stratified this subcohort based on the median NFD of these individuals. As we could not detect any tendency for improved OS or RFS in patients with high NFD, we waived a receiver operating characteristic (ROC)-based approach for optimal cut-off detection, which we previously utilized for CCA [12,13]. To also ensure that oncological risk factors of the analyzed study cohort were not unequally distributed between patients with and without NFs, we conducted Cox regression analyses to identify prognostic variables of the cohort and compared patients with NFs to patients without NFs within a group comparison. Here, no between-group differences were detected and no association in the Cox regressions were observed.
As stated above, tumor recurrence might be more suited to identify oncological risk factors as a large set of patients decease due to progression or complications of the underlying liver disease [2,36]. Recurrence patterns of HCC vary among patients, with individuals displaying an early recurrence (usually due to initial multicentric carcinogenesis or early metastatic recurrence) and others suffering from late tumor recurrence (de-novo HCC due to the carcinogenic potential of the underlying liver disease). To back up our observations, we conducted survival analyses for each of these recurrence sub-groups, and neither found a difference in the likelihood of having NFs in the TME between the sub-groups nor found an influence of the presence of NFs on OS or RFS in either of the sub-groups. Thus, we believe that different recurrence patterns do not affect our overall finding that NFs in the TME are not associated with oncological endpoints in HCC.
While our multivariable model identified MELD score, multifocal disease, preoperative ascites, R1 resections, and MVI as independent prognostic characteristics for OS; AST, portal vein invasion, and pT category were identified as independent predictors of RFS. MVI invasion as well as MELD score are known risk factors in HCC and are associated with poor OS [37,38]. Nodule count and vascular invasion are also the mainstays of the Milan criteria, which are used for therapy selection in these patients [39,40]. All parameters shown to be of significant prognostic value are well-known for their respective role in HCC. This underlines the validity of our cohort [10]. While our cohort certainly covers a representative spectrum of surgical candidates with HCC, including patients staged BCLC 0 to C, multifocal disease in one-third of all individuals, different stages of liver dysfunction, and various underlying liver diseases, e.g., viral, ALD and NAFLD, one might argue that our sample size might not be large enough to detect the effects of NFs in HCC. In the previous literature, cohorts of similar sizes were used to assess the prognostic potentials of NFs. Zhao et al. investigated NFs in breast cancer in 144 patients, Iwasaki et al. researched NFD in 256 PDAC patients and our group reported the role of NF in 101 patients with extrahepatic CCA and 95 intrahepatic CCA [12,13,16,35]. Given our sample size of 153 HCC cases and our detailed analysis, we, therefore, consider NFs in the TME and NFD as the corresponding number count not to be associated with OS and RFS in our cohort of HCC patients undergoing liver resection.
The underlying reason for this negative observation remains to be investigated. CCA and PDAC are reported to be neurotropic cancers with a high rate of PNI [41]. PNI can also be observed in colorectal and breast cancer [42,43], while HCC is not a disease to commonly show this histological feature. In neurotropic cancers, nerves are considered a potential pathway for cancer cell dissemination and metastasis in the same way as it is known for the vascular and lymphatic channels [44]. However, as stated above, the nerve fibers considered in determining NFD are independent of the large nerve trunks used to define PNI and require additional staining (PGP9.5) to be revealed. It is speculative whether and how these “protective” NFs interact with the larger nerve trunks, which are prone to tumor infiltration. Moreover, for example, PDAC and CCA are characterized by a prominent desmoplastic and hypovascularized stroma contributing to the unique TME of these tumor entities, which is not seen in HCCs [45]. NF directly regulates stromal compartments in the TME [46]. Here NFs promote angiogenesis and interact with immune cells in the TME on multiple levels [46]. Therefore, it seems plausible that the “protective” effect of NFs requires tumors with large stroma parts, e.g., CCA, to interact with stromal compartments to manifest oncological effects. However, exploring the role of the nervous system in tumorigenesis, cancer progression, and long-term outcome is still in its infancy [46]. The same accounts for our descriptive data and clinical observation, which warrant further translational and preclinical research to explore the underlying mechanism behind the oncological effects of NFs in the TME to explain why our study has failed to show an association between NFs and outcome in HCC.
As with all observational clinical studies‚ our analysis has some inherent limitations. All patients treated for HCC in this study underwent surgery and the corresponding treatment in a monocentric setting, reflecting the authors’ distinct approach to this malignancy. Further, our data is retrospective in nature as this study was not carried out in a controlled clinical trial setting. Moreover, histological data were not available for every consecutive patient of our hepatobiliary center, limiting the final dataset. This limited data set did not allow one to conduct a sub-analysis for histological subtypes. However, our analysis included a distinct analysis with respect to disease etiology, which might indicate some known histological subtypes, e.g., steatohepatitic HCC in NAFLD/non-alcoholic steatohepatitis (NASH) or macrotrabecular HCC in viral hepatitis. Future research regarding NFs in the TME in HCC should further explore the role in various histological subtypes and should also comprise molecular characteristics. As this kind of analysis requires a significant dataset, it is likely that a multicentric approach will be necessary. Another limitation might be that only one pathologist scored the tissue slides for NFs in the TME.
5. Conclusions
Notwithstanding the above-mentioned limitations, we have demonstrated for the first time that NFs in the TME of HCC are not associated with long-term outcomes in HCC. These observations underline a major difference compared to studies on other gastrointestinal cancers and warrant further basic research. Large-scale multi-center studies are required to validate and confirm our findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14092237/s1, Figure S1: Outcome in hepatocellular carcinoma with respect to early and late tumor recurrence. Figure S2: Outcome in hepatocellular carcinoma with respect disease etiology.
Author Contributions
Conceptualization, J.B. and L.R.H.; methodology, J.B., U.P.N. and L.R.H.; formal analysis, J.B., X.T., Z.C., G.W., R.D.B., P.B., S.A.L., T.F.U., U.P.N. and L.R.H.; investigation, J.B., X.T., Z.C., G.W., R.D.B., P.B., S.A.L., T.F.U., U.P.N. and L.R.H.; resources, L.R.H. and U.P.N.; data curation, J.B., X.T., Z.C. and L.R.H.; writing—original draft preparation, J.B., X.T. and L.R.H.; writing—review and editing, Z.C., G.W., R.D.B., P.B., S.A.L., T.F.U. and U.P.N.; supervision, U.P.N. and L.R.H.; project administration, U.P.N. and L.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This particular research received no external funding. X.T. received a scholarship from the China Scholarship Council (CSC Grant No: 201806210074). This project was supported by the German Research Foundation (SFB-CRC 1382-A01).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of RWTH Aachen University (protocol code: EK 106/18; date of approval: 22 November 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics for Hispanics/Latinos. CA Cancer J. Clin. 2012, 62, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Lurje, G.; Bednarsch, J.; Czigany, Z.; Amygdalos, I.; Meister, F.; Schoning, W.; Ulmer, T.F.; Foerster, M.; Dejong, C.; Neumann, U.P. Prognostic factors of disease-free and overall survival in patients with hepatocellular carcinoma undergoing partial hepatectomy in curative intent. Langenbeck’s Arch. Surg./Dtsch. Ges. Chir. 2018, 403, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [Green Version]
- Vitale, A.; Burra, P.; Frigo, A.C.; Trevisani, F.; Farinati, F.; Spolverato, G.; Volk, M.; Giannini, E.G.; Ciccarese, F.; Piscaglia, F.; et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: A multicentre study. J. Hepatol. 2015, 62, 617–624. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.W.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Chen, P.H.; Yeh, J.H.; Hsiao, P.; Lo, G.H.; Tan, T.; Cheng, P.N.; Lin, H.Y.; Chen, Y.S.; Hsieh, K.C.; et al. Clinical outcomes of surgical resection versus radiofrequency ablation in very-early-stage hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2021, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Mehrabi, A.; Mollberg, N.M.; Muller, S.A.; Koch, M.; Buchler, M.W.; Weitz, J. Hepatocellular carcinoma: Current management and perspectives for the future. Ann. Surg. 2011, 253, 453–469. [Google Scholar] [CrossRef]
- Nault, J.C.; Villanueva, A. Biomarkers for Hepatobiliary Cancers. Hepatology 2021, 73 (Suppl. 1), 115–127. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Bednarsch, J.; Czigany, Z.; Heise, D.; Joechle, K.; Luedde, T.; Heij, L.; Bruners, P.; Ulmer, T.F.; Neumann, U.P.; Lang, S.A. Prognostic evaluation of HCC patients undergoing surgical resection: An analysis of 8 different staging systems. Langenbeck’s Arch. Surg./Dtsch. Ges. Chir. 2021, 406, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Kather, J.; Tan, X.; Sivakumar, S.; Cacchi, C.; Wiltberger, G.; Czigany, Z.; Ulmer, F.; Neumann, U.P.; Heij, L.R. Nerve Fibers in the Tumor Microenvironment as a Novel Biomarker for Oncological Outcome in Patients Undergoing Surgery for Perihilar Cholangiocarcinoma. Liver Cancer 2021, 10, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Tan, X.; Czigany, Z.; Liu, D.; Lang, S.A.; Sivakumar, S.; Kather, J.N.; Appinger, S.; Rosin, M.; Boroojerdi, S.; et al. The Presence of Small Nerve Fibers in the Tumor Microenvironment as Predictive Biomarker of Oncological Outcome Following Partial Hepatectomy for Intrahepatic Cholangiocarcinoma. Cancers 2021, 13, 3661. [Google Scholar] [CrossRef] [PubMed]
- Albo, D.; Akay, C.L.; Marshall, C.L.; Wilks, J.A.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Ayala, G.E. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011, 117, 4834–4845. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, T.; Hiraoka, N.; Ino, Y.; Nakajima, K.; Kishi, Y.; Nara, S.; Esaki, M.; Shimada, K.; Katai, H. Reduction of intrapancreatic neural density in cancer tissue predicts poorer outcome in pancreatic ductal carcinoma. Cancer Sci. 2019, 110, 1491–1502. [Google Scholar] [CrossRef]
- Heij, L.R.; Tan, X.; Kather, J.N.; Niehues, J.M.; Sivakumar, S.; Heussen, N.; van der Kroft, G.; Damink, S.W.M.O.; Lang, S.; Aberle, M.R.; et al. Nerve Fibers in the Tumor Microenvironment Are Co-Localized with Lymphoid Aggregates in Pancreatic Cancer. J. Clin. Med. 2021, 10, 490. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Trautwein, C.; Ludde, T.; Strnad, P.; Gaisa, N.T.; Barabasch, A.; Bruners, P.; Ulmer, T.; et al. Intraoperative Transfusion of Fresh Frozen Plasma Predicts Morbidity Following Partial Liver Resection for Hepatocellular Carcinoma. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2020, 25, 1212–1223. [Google Scholar] [CrossRef]
- Buechter, M.; Thimm, J.; Baba, H.A.; Bertram, S.; Willuweit, K.; Gerken, G.; Kahraman, A. Liver Maximum Capacity: A Novel Test to Accurately Diagnose Different Stages of Liver Fibrosis. Digestion 2019, 100, 45–54. [Google Scholar] [CrossRef]
- Balzan, S.; Belghiti, J.; Farges, O.; Ogata, S.; Sauvanet, A.; Delefosse, D.; Durand, F. The “50-50 criteria” on postoperative day 5: An accurate predictor of liver failure and death after hepatectomy. Ann. Surg. 2005, 242, 824–828, discussion 828–829. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. Reply. N. Engl. J. Med. 2019, 381, e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, M.; Sherman, L.S. Neural stem cell niches: Roles for the hyaluronan-based extracellular matrix. Front. Biosci. 2011, 3, 1165–1179. [Google Scholar] [CrossRef] [Green Version]
- Gritsenko, P.G.; Ilina, O.; Friedl, P. Interstitial guidance of cancer invasion. J. Pathol. 2012, 226, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Silva, C.; Cardoso, F.; Veiga-Fernandes, H. Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu. Rev. Immunol. 2019, 37, 19–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Sivakumar, S.; Bednarsch, J.; Wiltberger, G.; Kather, J.N.; Niehues, J.; de Vos-Geelen, J.; Valkenburg-van Iersel, L.; Kintsler, S.; Roeth, A.; et al. Nerve fibers in the tumor microenvironment in neurotropic cancer-pancreatic cancer and cholangiocarcinoma. Oncogene 2020, 40, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Tay, S.S.; Leong, S.; Schemann, M. Colocalization of ChAT, DbetaH and NADPH-d in the pancreatic neurons of the newborn guinea pig. Cell Tissue Res. 1998, 294, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.; Meng, X.; Song, H. Nicotinic acetylcholine receptors and cancer. Biomed. Rep. 2016, 4, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Sha, M.; Cao, J.; Sun, H.Y.; Tong, Y.; Xia, Q. Neuroendocrine regulation of cholangiocarcinoma: A status quo review. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 66–73. [Google Scholar] [CrossRef]
- Franchitto, A.; Onori, P.; Renzi, A.; Carpino, G.; Mancinelli, R.; Alvaro, D.; Gaudio, E. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann. Transl. Med. 2013, 1, 27. [Google Scholar] [CrossRef]
- Shirai, K.; Ebata, T.; Oda, K.; Nishio, H.; Nagasaka, T.; Nimura, Y.; Nagino, M. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J. Surg. 2008, 32, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.B.; Patel, S.H.; Kooby, D.A.; Weber, S.; Bloomston, M.; Cho, C.; Hatzaras, I.; Schmidt, C.; Winslow, E.; Staley, C.A., 3rd; et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: A multi-institution analysis. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2012, 14, 514–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partecke, L.I.; Kading, A.; Trung, D.N.; Diedrich, S.; Sendler, M.; Weiss, F.; Kuhn, J.P.; Mayerle, J.; Beyer, K.; von Bernstorff, W.; et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFalpha in a murine pancreatic cancer model. Oncotarget 2017, 8, 22501–22512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Y.; Liang, X.; Du, G.; Liu, L.; Lu, L.; Dong, J.; Han, H.; Zhang, G. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer 2014, 14, 484. [Google Scholar] [CrossRef] [Green Version]
- Meister, F.A.; Lurje, G.; Verhoeven, S.; Wiltberger, G.; Heij, L.; Liu, W.J.; Jiang, D.; Bruners, P.; Lang, S.A.; Ulmer, T.F.; et al. The Role of Sarcopenia and Myosteatosis in Short- and Long-Term Outcomes Following Curative-Intent Surgery for Hepatocellular Carcinoma in a European Cohort. Cancers 2022, 14, 720. [Google Scholar] [CrossRef]
- Andreou, A.; Bahra, M.; Schmelzle, M.; Ollinger, R.; Sucher, R.; Sauer, I.M.; Guel-Klein, S.; Struecker, B.; Eurich, D.; Klein, F.; et al. Predictive factors for extrahepatic recurrence of hepatocellular carcinoma following liver transplantation. Clin. Transplant. 2016, 30, 819–827. [Google Scholar] [CrossRef]
- Shimozawa, N.; Hanazaki, K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J. Am. Coll. Surg. 2004, 198, 356–365. [Google Scholar] [CrossRef]
- Yau, T.; Tang, V.Y.; Yao, T.J.; Fan, S.T.; Lo, C.M.; Poon, R.T. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014, 146, 1691–1700.e3. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, B.Y.; Zhou, B.; Zhu, C.Z.; Sun, L.Q.; Feng, Y.J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar] [PubMed]
- Karak, S.G.; Quatrano, N.; Buckley, J.; Ricci, A., Jr. Prevalence and significance of perineural invasion in invasive breast carcinoma. Connect. Med. 2010, 74, 17–21. [Google Scholar]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the nervous system in cancers: A review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E.; Gores, G.J. Desmoplastic stroma and cholangiocarcinoma: Clinical implications and therapeutic targeting. Hepatology 2014, 59, 2397–2402. [Google Scholar] [CrossRef] [Green Version]
- Kepper, M.; Keast, J. Immunohistochemical properties and spinal connections of pelvic autonomic neurons that innervate the rat prostate gland. Cell Tissue Res. 1995, 281, 533–542. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).