Preoperative Osteopenia Is Associated with Significantly Shorter Survival in Patients with Perihilar Cholangiocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Detection of Osteopenia
2.3. Definition of Sarcopenia
2.4. Measurement of NLR and PNI
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
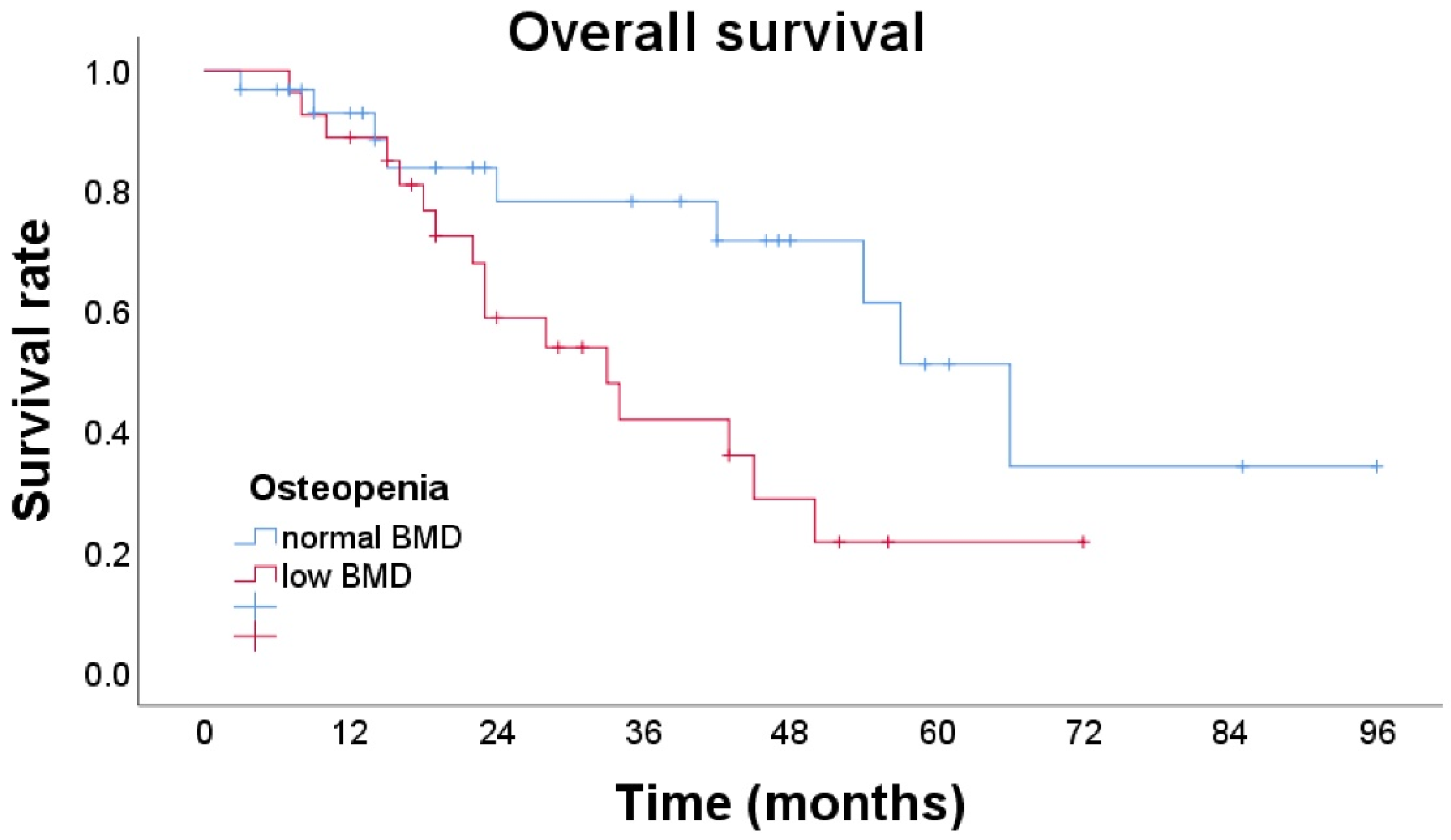
3.2. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Qin, Y.; Cui, Y.; Chen, H.; Hao, X.; Li, Q. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: A Chinese experience. Dig. Surg. 2011, 28, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.J.; Zhou, R.X.; Shrestha, A.; Tan, Y.Q.; Ma, W.J.; Yang, Q.; Lu, J.; Wang, J.K.; Zhou, Y.; Li, F.-Y. Relationship of tumor size with pathological and prognostic factors for hilar cholangiocarcinoma. Oncotarget 2017, 8, 105011–105019. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; van Vugt, J.L.A.; Gaspersz, M.P.; Coelen, R.J.S.; Roos, E.; Labeur, T.A.; Margonis, G.A.; Ethun, C.G.; Maithel, S.K.; Poultsides, G.; et al. Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastases. HPB 2017, 19, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Sandroussi, C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB 2013, 15, 492–503. [Google Scholar] [CrossRef]
- Lafaro, K.; Buettner, S.; Maqsood, H.; Wagner, D.; Bagante, F.; Spolverato, G.; Xu, L.; Kamel, I.; Pawlik, T.M. Defining Post Hepatectomy Liver Insufficiency: Where do We stand? J. Gastrointest. Surg. 2015, 19, 2079–2092. [Google Scholar] [CrossRef]
- Wang, A.; He, Z.; Cong, P.; Qu, Y.; Hu, T.; Cai, Y.; Sun, B.; Chen, H.; Fu, W.; Peng, Y. Controlling nutritional status (CONUT) score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to NLR and PNI: A single-center retrospective study. Front. Oncol. 2021, 10, 593452. [Google Scholar] [CrossRef]
- Tangvik, R.J.; Tell, G.S.; Eisman, J.A.; Guttormsen, A.B.; Henriksen, A.; Nilsen, R.M.; Øyen, J.; Ranhoff, A.H. The nutritional strategy: Four questions predict morbidity, mortality and health care costs. Clin. Nutr. 2014, 33, 634–641. [Google Scholar] [CrossRef]
- Sungurtekin, H.; Sungurtekin, U.; Balci, C.; Zencir, M.; Erdem, E. The influence of nutritional status on complications after major intraabdominal surgery. J. Am. Coll. Nutr. 2004, 23, 227–232. [Google Scholar] [CrossRef]
- Ardito, F.; Lai, Q.; Rinninella, E.; Mimmo, A.; Vellone, M.; Panettieri, E.; Adducci, E.; Cintoni, M.; Mele, M.C.; Gasbarrini, A.; et al. The impact of personalized nutritional support on postoperative outcome within the enhanced recovery after surgery (ERAS) program for liver resections: Results from the NutriCatt protocol. Updates Surg. 2020, 72, 681–691. [Google Scholar] [CrossRef]
- Schwegler, I.; von Holzen, A.; Gutzwiller, J.P.; Schlumpf, R.; Mühlebach, S.; Stanga, Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. J. Br. Surg. 2010, 97, 92–97. [Google Scholar] [CrossRef]
- Abe, T.; Nakata, K.; Kibe, S.; Mori, Y.; Miyasaka, Y.; Ohuchida, K.; Ohtsuka, T.; Oda, Y.; Nakamura, M. Prognostic value of preoperative nutritional and immunological factors in patients with pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Parikh, N.D.; Yu, J.; Barman, P.; Derstine, B.A.; Sonnenday, C.J.; Wang, S.C.; Su, G.L. Bone mineral density predicts posttransplant survival among hepatocellular carcinoma liver transplant recipients. Liver Transpl. 2016, 22, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Eguchi, H.; Iwagami, Y.; Mukai, Y.; Hashimoto, Y.; Asaoka, T.; Noda, T.; Kawamoto, K.; Gotoh, K.; Kobayashi, S.; et al. Patients treated with preoperative chemoradiation for pancreatic ductal adenocarcinoma have impaired bone density, a predictor of distant metastasis. Ann. Surg. Oncol. 2017, 24, 3715–3724. [Google Scholar] [CrossRef]
- Motomura, T.; Uchiyama, H.; Iguchi, T.; Ninomiya, M.; Yoshida, R.; Honboh, T.; Sadanaga, N.; Akashi, T.; Matsuura, H. Impact of osteopenia on oncologic outcomes after curative resection for pancreatic cancer. In Vivo 2020, 34, 3551–3557. [Google Scholar] [CrossRef]
- Yao, S.; Kaido, T.; Okumura, S.; Iwamura, S.; Miyachi, Y.; Shirai, H.; Kobayashi, A.; Hamaguchi, Y.; Kamo, N.; Uozumi, R.; et al. Bone mineral density correlates with survival after resection of extrahepatic biliary malignancies. Clin. Nutr. 2019, 38, 2770–2777. [Google Scholar] [CrossRef]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. International Union against Cancer (UICC): TNM Classification of Malignant Tumours. In Oesophagus including Oesophagogastric Junction, 7th ed.; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Abe, K.; Furukawa, K.; Okamoto, T.; Matsumoto, M.; Futagawa, Y.; Haruki, K.; Shirai, Y.; Ikegami, T. Impact of osteopenia on surgical and oncological outcomes in patients with pancreatic cancer. Int. J. Clin. Oncol. 2021, 26, 1929–1937. [Google Scholar] [CrossRef]
- Toshima, T.; Yoshizumi, T.; Kosai-Fujimoto, Y.; Inokuchi, S.; Yoshiya, S.; Takeishi, K.; Itoh, S.; Harada, N.; Ikegami, T.; Soejima, Y.; et al. Prognostic impact of osteopenia in patients who underwent living donor liver transplantation for hepatocellular carcinoma. World J. Surg. 2020, 44, 258–267. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef]
- Liu, F.; Luo, H.; Zhu, Z.; Zhu, P.; Huang, J. Prognostic significance of peripheral blood-derived neutrophil/lymphocyte ratio in patients with digestive cancer. J. Cell. Physiol. 2019, 234, 22775–22786. [Google Scholar] [CrossRef] [PubMed]
- Akgül, Ö.; Bagante, F.; Olsen, G.; Cloyd, J.M.; Weiss, M.; Merath, K.; Alexandrescu, S.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; et al. Preoperative prognostic nutritional index predicts survival of patients with intrahepatic cholangiocarcinoma after curative resection. J. Surg. Oncol. 2018, 118, 422–430. [Google Scholar] [PubMed]
- Yoo, J.; Kim, J.H.; Bae, J.S.; Kang, H.J. Prediction of prognosis and resectability using MR imaging, clinical, and histopathological findings in patients with perihilar cholangiocarcinoma. Abdom. Radiol. 2021, 46, 4159–4169. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Watanabe, J.; Saitsu, A.; Miki, A.; Kotani, K.; Sata, N. Prognostic value of preoperative low bone mineral density in patients with digestive cancers: A systematic review and meta-analysis. Arch. Osteoporos. 2022, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef]
- Langen, R.C.; Schols, A.M.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001, 15, 1169–1180. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A.L. Muscle wasting in fasting requires activation of NF-κB and Inhibition of AKT/Mechanistic target of rapamycin (mTOR) by the protein acetylase, GCN5. J. Biol. Chem. 2015, 290, 30269–30279. [Google Scholar] [CrossRef]
- Zhang, K.; Zhaos, J.; Liu, X.; Yan, B.; Chen, D.; Gao, Y.; Hu, X.; Liu, S.; Zhang, D.; Zhou, C. Activation of NF-B upregulates Snail and consequent repression of E-cadherin in cholangiocarcinoma cell invasion. Hepatogastroenterology 2011, 58, 1–7. [Google Scholar]
- Srikoon, P.; Kariya, R.; Kudo, E.; Goto, H.; Vaeteewoottacharn, K.; Taura, M.; Wongkham, S.; Okada, S. Diethyldithiocarbamate suppresses an NF-kappaB dependent metastatic pathway in cholangiocarcinoma cells. Asian Pac. J. Cancer Prev. 2013, 14, 4441–4446. [Google Scholar] [CrossRef][Green Version]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.S.; Pfeiffer, R.M.; Gabbi, C.; Anderson, L.; Gadalla, S.M.; Koshiol, J. Menopausal hormone therapy and risk of biliary tract cancers. Hepatology 2022, 75, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Horne, A.M.; Mihov, B.; Stewart, A.; Garratt, E.; Wong, S.; Wiessing, K.R.; Bolland, M.J.; Bastin, S.; Gamble, G.D. Fracture prevention with zoledronate in older women with osteopenia. N. Engl. J. Med. 2018, 379, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Meng, X.; Feng, H.; Zhuang, S.; Liu, Z.; Zhu, T.; Ye, K.; Xing, Y.; Sun, C.; Zhou, F.; et al. Estimation and projection about the standardized prevalence of osteoporosis in mainland China. Arch. Osteoporos. 2019, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Zain, N.M.; Seriramulu, V.P.; Chelliah, K.K. Bone mineral density and breast cancer risk factors among premenopausal and postmenopausal women a systematic review. Asian Pac. J. Cancer Prev. 2016, 17, 3229–3234. [Google Scholar] [PubMed]
- van der Klift, M.; de Laet, C.E.; Coebergh, J.W.; Hofman, A.; Pols, H.A. Bone mineral density and the risk of breast cancer: The Rotterdam Study. Bone 2003, 32, 211–216. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Zhao, Z.; Wei, K.; Meng, W.; Li, X. The clinicopathological factors associated with prognosis of patients with resectable perihilar cholangiocarcinoma: A systematic review and meta-analysis. Medicine 2018, 97, e11999. [Google Scholar] [CrossRef]
- Baton, O.; Azoulay, D.; Adam, D.V.; Castaing, D. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: Prognostic factors and longterm outcomes. J. Am. Coll. Surg. 2007, 204, 250–260. [Google Scholar] [CrossRef]
- Dumitrascu, T.; Chirita, D.; Ionescu, M.; Popescu, I. Resection for hilar cholangiocarcinoma: Analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J. Gastrointest. Surg. 2013, 17, 913–924. [Google Scholar] [CrossRef]
- Neuhaus, P.; Thelen, A.; Jonas, S.; Puhl, G.; Denecke, T.; Veltzke-Schlieker, W.; Seehofer, D. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann. Surg. Oncol. 2012, 19, 1602–1608. [Google Scholar] [CrossRef]
- de Jong, M.C.; Marques, H.; Clary, B.M.; Bauer, T.W.; Marsh, J.W.; Ribero, D.; Majno, P.; Hatzaras, I.; Walters, D.M.; Barbas, A.S.; et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: A multi-institutional analysis of 305 cases. Cancer 2012, 118, 4737–4747. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Prasad, K.R.; Toogood, G.J.; Lodge, J.P. Surgical treatment of hilar cholangiocarcinoma in a new era: Comparison among leading Eastern and Western centers, Leeds. J. Hepatobiliary Pancreat. Sci. 2010, 17, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.-N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Hau, H.M.; Meyer, F.; Jahn, N.; Rademacher, S.; Sucher, R.; Seehofer, D. Prognostic Relevance of the Eighth Edition of TNM Classification for Resected Perihilar Cholangiocarcinoma. J. Clin. Med. 2020, 9, 3152. [Google Scholar] [CrossRef] [PubMed]
- Farges, O.; Regimbeau, J.M.; Fuks, D.; Le Treut, Y.P.; Cherqui, D.; Bachellier, P.; Mabrut, J.Y.; Adham, M.; Pruvot, F.R.; Gigot, J.F. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. J. Br. Surg. 2013, 100, 274–283. [Google Scholar] [CrossRef]
- Nuzzo, G.; Giuliante, F.; Ardito, F.; Giovannini, I.; Aldrighetti, L.; Belli, G.; Bresadola, F.; Calise, F.; Dalla Valle, R.; D’Amico, D.F.; et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: Results of an Italian multicenter analysis of 440 patients. Arch. Surg. 2012, 147, 26–34. [Google Scholar] [CrossRef]
- Kenjo, A.; Miyata, H.; Gotoh, M.; Kitagawa, Y.; Shimada, M.; Baba, H.; Tomita, N.; Kimura, W.; Sugihara, K.; Mori, M. Risk stratification of 7732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J. Am. Coll. Surg. 2014, 218, 412–422. [Google Scholar] [CrossRef]
- Kondo, S.; Hirano, S.; Ambo, Y.; Tanaka, E.; Okushiba, S.; Morikawa, T.; Katoh, H. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: Results of a prospective study. Ann. Surg. 2004, 240, 95–101. [Google Scholar] [CrossRef]
- Olthof, P.B.; Miyasaka, M.; Koerkamp, B.G.; Wiggers, J.K.; Jarnagin, W.R.; Noji, T.; Hirano, S.; van Gulik, T.M. A comparison of treatment and outcomes of perihilar cholangiocarcinoma between Eastern and Western centers. HPB 2019, 21, 345–351. [Google Scholar] [CrossRef]
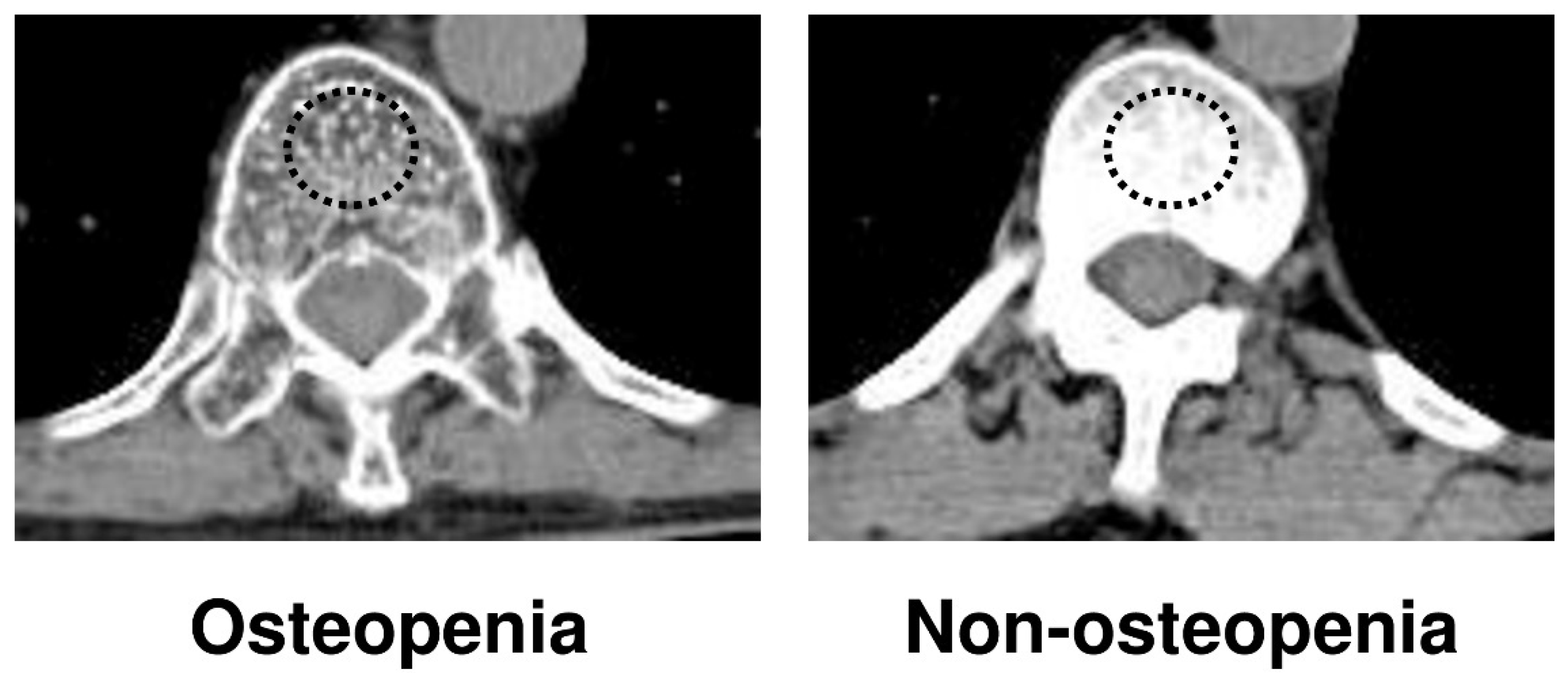
| Variables | Osteopenia N = 27 | Non-Osteopenia N = 31 | p-Value |
|---|---|---|---|
| Variables | |||
| Age (y), mean ± SD | 70.3 ± 7.2 | 69.0 ± 9.4 | 0.544 |
| Gender (male/female), N | 16/11 | 26/5 | 0.036 * |
| BMI (kg m−2), mean ± SD | 22.3 ± 3.4 | 22.2 ± 3.2 | 0.991 |
| ASA-PS ≥ 3, N | 4/23 | 6/25 | 0.653 |
| PMI (cm2/m2), mean ± SD | 3.67 ± 1.4 | 3.99 ± 1.6 | 0.423 |
| White blood cell (/mm3), mean ± SD | 5767 ± 2560 | 5674 ± 1378 | 0.868 |
| Serum albumin (g/dL), mean ± SD | 3.69 ± 0.6 | 3.66 ± 0.6 | 0.829 |
| PNI, mean ± SD | 42.4 ± 6.9 | 44.1 ± 7.1 | 0.355 |
| NLR, mean ± SD | 2.67 ± 1.7 | 2.61 ± 2.4 | 0.921 |
| CEA (mg/dL), mean ± SD | 5.87 ± 14.0 | 2.87 ± 4.6 | 0.295 |
| CA19-9 (IU/mL), mean ± SD | 228 ± 505 | 447 ± 1141 | 0.346 |
| Adjuvant chemotherapy (yes/no), N | 5/22 | 4/27 | 0.556 |
| Clavien–Dindo classification ≥ 3, N | 15/11 | 17/14 | 0.829 |
| Operative factor | |||
| Operation time (min), mean ± SD | 543 ± 111 | 569 ± 135 | 0.440 |
| Intraoperative bleeding (mL), mean ± SD | 1604 ± 1210 | 1337 ± 912 | 0.354 |
| Procedure (right/left hepatectomy/others) | 11/14/2 | 17/14/0 | 0.220 |
| Pathological factor | |||
| Maximum tumor size (mm), mean ± SD | 36.1 ± 25.3 | 38.5 ± 25.9 | 0.751 |
| Primary tumor stage (T3, T4/Tis, T1, T2), N | 10/17 | 9/22 | 0.517 |
| Lymph node metastases (yes/no) | 10/17 | 8/23 | 0.504 |
| R0 resection (yes/no) | 13/14 | 14/17 | 0.820 |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR | p Value | |
| Age (≥65) | 0.92 (0.40–2.11) | 0.846 | ||
| Gender (M/F) | 1.05 (0.42–2.65) | 0.923 | 2.85 (0.95–8.55) | 0.062 |
| BMI (≥25) | 1.71 (0.70–4.17) | 0.242 | ||
| ASA-PS (≥3) | 1.87 (0.62–5.60) | 0.265 | ||
| PMI (low) | 1.96 (0.26–14.6) | 0.511 | ||
| PNI (<40) | 1.15 (0.48–2.79) | 0.755 | ||
| NLR (>3.37) | 1.76 (0.40–7.78) | 0.455 | ||
| CEA (>5) | 2.36 (0.92–6.06) | 0.074 | ||
| CA19-9 (>37) | 1.58 (0.67–3.70) | 0.295 | ||
| Adjuvant chemotherapy (yes) | 1.90 (0.64–5.66) | 0.250 | ||
| Osteopenia (BMD < 160) | 2.42 (1.04–5.60) | 0.040 * | 2.57 (1.06–6.28) | 0.038 * |
| Operative factor | ||||
| Bleeding (≥1000 mL) | 1.82 (0.75–4.41) | 0.183 | ||
| Operation time (≥500 min) | 1.36 (0.54–3.42) | 0.508 | ||
| R0 resection (no) | 1.45 (0.65–3.22) | 0.363 | ||
| CD classification (≥3) | 1.39 (0.57–3.38) | 0.465 | ||
| Pathological factor | ||||
| Tumor size (≥50 mm) | 0.44 (0.10–1.89) | 0.269 | ||
| Primary tumor stage T3–4 | 2.43 (1.07–5.50) | 0.033 * | 3.08 (1.21–7.90) | 0.019 * |
| Lymph node metastases (yes) | 1.25 (0.55–2.87) | 0.596 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, J.; Miki, A.; Sakuma, Y.; Shimodaira, K.; Aoki, Y.; Meguro, Y.; Morishima, K.; Endo, K.; Sasanuma, H.; Lefor, A.K.; et al. Preoperative Osteopenia Is Associated with Significantly Shorter Survival in Patients with Perihilar Cholangiocarcinoma. Cancers 2022, 14, 2213. https://doi.org/10.3390/cancers14092213
Watanabe J, Miki A, Sakuma Y, Shimodaira K, Aoki Y, Meguro Y, Morishima K, Endo K, Sasanuma H, Lefor AK, et al. Preoperative Osteopenia Is Associated with Significantly Shorter Survival in Patients with Perihilar Cholangiocarcinoma. Cancers. 2022; 14(9):2213. https://doi.org/10.3390/cancers14092213
Chicago/Turabian StyleWatanabe, Jun, Atsushi Miki, Yasunaru Sakuma, Kentaro Shimodaira, Yuichi Aoki, Yoshiyuki Meguro, Kazue Morishima, Kazuhiro Endo, Hideki Sasanuma, Alan Kawarai Lefor, and et al. 2022. "Preoperative Osteopenia Is Associated with Significantly Shorter Survival in Patients with Perihilar Cholangiocarcinoma" Cancers 14, no. 9: 2213. https://doi.org/10.3390/cancers14092213
APA StyleWatanabe, J., Miki, A., Sakuma, Y., Shimodaira, K., Aoki, Y., Meguro, Y., Morishima, K., Endo, K., Sasanuma, H., Lefor, A. K., Teratani, T., Fukushima, N., Kitayama, J., & Sata, N. (2022). Preoperative Osteopenia Is Associated with Significantly Shorter Survival in Patients with Perihilar Cholangiocarcinoma. Cancers, 14(9), 2213. https://doi.org/10.3390/cancers14092213







