Multiparametric Magnetic Resonance Imaging Grades the Aggressiveness of Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting, and Participants
2.2. MpMRI Technique and Evaluation
2.3. Prostate Biopsy Procedure
2.4. Pathologic Analysis and csPCa Definition
2.5. Measurement of PCa Aggressiveness
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.; Talala, K.M.; et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drazer, M.W.; Huo, D.; Eggener, S.E. National Prostate Cancer Screening Rates after the 2012 US Preventive Services Task Force Recommendation Discouraging Prostate-Specific Antigen–Based Screening. J. Clin. Oncol. 2015, 33, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Bergh, R.C.V.D.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Drost, F.-J.H.; Osses, D.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Roobol, M.J.; Schoots, I.G. Prostate Magnetic Resonance Imaging, with or without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 78–94. [Google Scholar] [CrossRef]
- Van Poppel, H.; Hogenhout, R.; Albers, P.; van den Bergh, R.C.N.; Barentsz, J.O.; Roobol, M.J. A European Model for an Organised Risk-stratified Early Detection Programme for Prostate Cancer. Eur. Urol. Oncol. 2021, 5, 731–793. [Google Scholar] [CrossRef]
- Mazzone, E.; Gandaglia, G.; Ploussard, G.; Marra, G.; Valerio, M.; Campi, R.; Mari, A.; Minervini, A.; Serni, S.; Moschini, M.; et al. Positive Predictive Value of Prostate Imaging Reporting and Data System Version 2 for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2021, 7, 697–713. [Google Scholar] [CrossRef]
- Schoots, I.G. MRI in early prostate cancer detection: How to manage indeterminate or equivocal PI-RADS 3 lesions? Transl. Androl. Urol. 2018, 7, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Osses, D.F.; Roobol, M.J.; Schoots, I.G. Prediction Medicine: Biomarkers, Risk Calculators and Magnetic Resonance Imaging as Risk Stratification Tools in Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2019, 20, 1637. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Abreu-Gomez, J.; Wu, M.; McInnes, M.D.F.; Thornhill, R.E.; Flood, T.A.; Schieda, N. Shape Analysis of Peripheral Zone Observations on Prostate DWI: Correlation to Histopathology Outcomes After Radical Prostatectomy. Am. J. Roentgenol. 2020, 214, 1239–1247. [Google Scholar] [CrossRef]
- Boschheidgen, M.; Schimmöller, L.; Arsov, C.; Ziayee, F.; Morawitz, J.; Valentin, B.; Radke, K.L.; Giessing, M.; Esposito, I.; Albers, P.; et al. MRI grading for the prediction of prostate cancer aggressiveness. Eur. Radiol. 2021, 32, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; De Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Tsuruta, C.; Hirata, K.; Kudo, K.; Masumori, N.; Hatakenaka, M. DWI-related texture analysis for prostate cancer: Differences in correlation with histological aggressiveness and data repeatability between peripheral and transition zones. Eur. Radiol. Exp. 2022, 6, 1. [Google Scholar] [CrossRef]
- Kozminski, M.A.; Tomlins, S.; Cole, A.; Singhal, U.; Lu, L.; Skolarus, T.A.; Palapattu, G.S.; Montgomery, J.S.; Weizer, A.Z.; Mehra, R.; et al. Standardizing the definition of adverse pathology for lower risk men undergoing radical prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 415.e1–415.e6. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Dominique, G.; Brisbane, W.G.; Reiter, R.E. The utility of prostate MRI within active surveillance: Description of the evidence. World J. Urol. 2022, 40, 71–77. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.H.; Kim, J.Y. Dynamic contrast-enhanced magnetic resonance imaging for risk stratification in patients with prostate cancer. Quant. Imaging Med. Surg. 2022, 12, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Pooli, A.; Johnson, D.C.; Shirk, J.; Markovic, D.; Sadun, T.Y.; Sisk, A.E.; Bajgiran, A.M.; Mirak, S.A.; Felker, E.R.; Hughes, A.K.; et al. Predicting Pathological Tumor Size in Prostate Cancer Based on Multiparametric Prostate Magnetic Resonance Imaging and Preoperative Findings. J. Urol. 2021, 205, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Rahota, R.; Salin, A.; Gautier, J.; Almeras, C.; Garnault, V.; Tollon, C.; Loison, G.; Beauval, J.; Ploussard, G. Pathological features of PI-RADS 3 MRI lesions in biopsy and radical prostatectomy specimens. BJU Int. 2021. [Google Scholar] [CrossRef]
- De Luca, L.; Crocetto, F.; Barone, B.; Creta, M.; Pesce, S.; Aveta, A.; Campanino, M.R.; Imbimbo, C.; Longo, N. Granulomatous prostatitis mimicking prostate cancer in a patient with psoriatic arthritis: A case report. Future Sci. 2020, 6, FSO591. [Google Scholar] [CrossRef]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef]
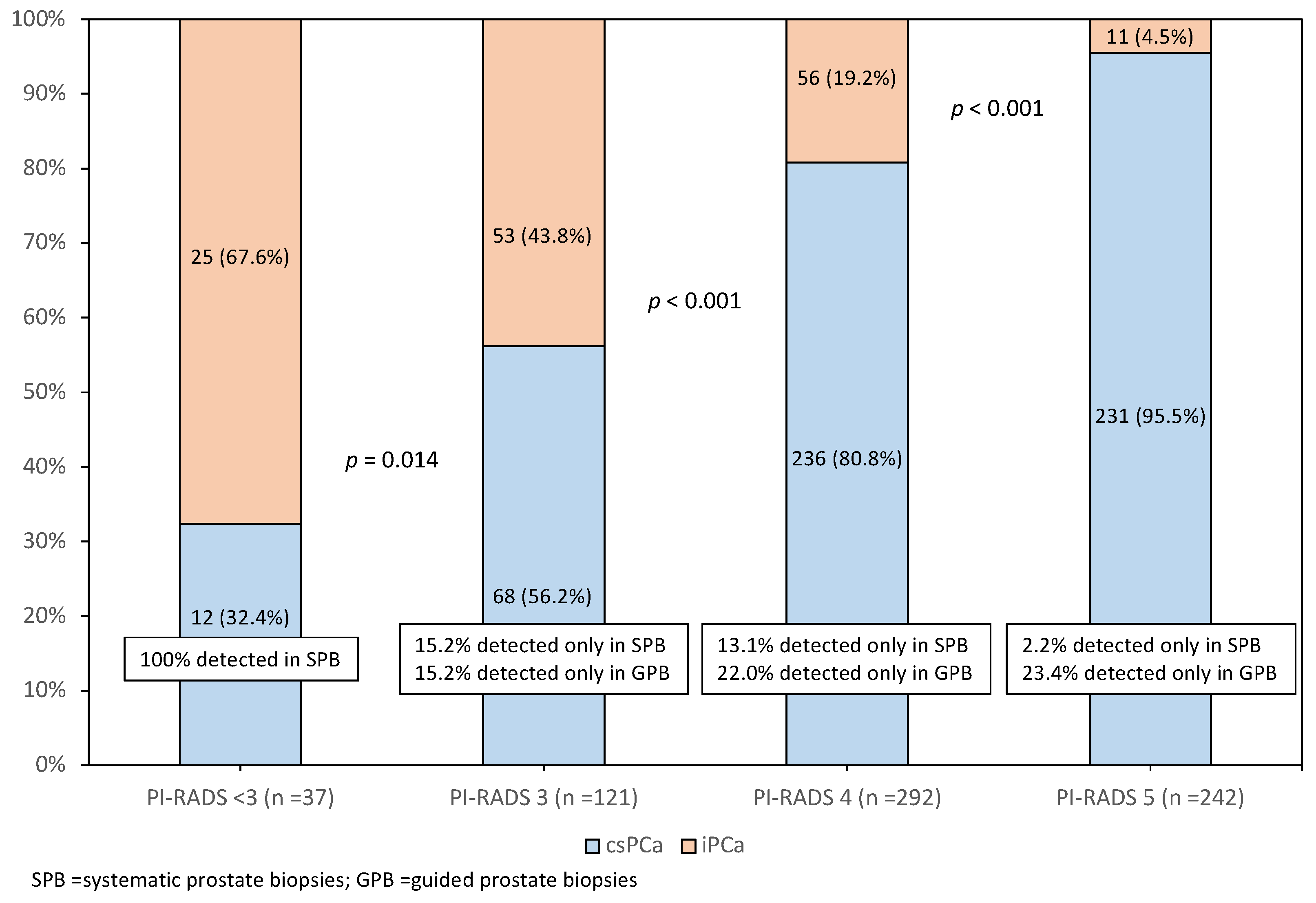
| Characteristic | Value |
|---|---|
| Number of cases | 1486 |
| Median age, years (IQR) | 69 (62–74) |
| Median total PSA, ng/mL (IQR) | 6.0 (4.3–9.2) |
| Abnormal DRE, n (%) | 109 (19.2) |
| Median prostate volume, mL (IQR) | 55 (40–76) |
| Median PSA density, ng/mL/cc (IQR) | 0.11 (0.07–0.18) |
| Repeat biopsy, n (%) | 133 (23.5) |
| Family history of PCa, n (%) | 48 (8.6%) |
| PI-RADS, n (%) | |
| 1–2 | 315 (21.2) |
| 3 | 444 (29.9) |
| 4 | 450 (30.3) |
| 5 | 277 (18.6) |
| Overall PCa detection, n (%) | 692 (46.6) |
| csPCa detection, n (%) | 547 (36.8) |
| iPCa detection, n (%) | 145 (9.8) |
| PI-RADS | Grade Group | p Value | Mean GG (95% CI) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | All | ||||
| 1–2, n (%) | 25 (67.6) | 8 (21.6) | 1 (2.7) | 3 (1.7) | 0 (0) | 37 (5.3) | - | 1.5 (1.2–1.8) | - |
| 3, n (%) | 53 (43.8) | 41 (33.9) | 18 (14.9) | 8 (7.0) | 1 (0.8) | 121 (17.5) | <0.001 | 1.9 (1.7–2.0) | =0.017 |
| 4, n (%) | 56 (19.2) | 118 (40.4) | 69 (23.6) | 36 (12.3) | 13 (4.5) | 292 (42.2) | <0.001 | 2.4 (2.3–2.6) | <0.001 |
| 5, n (%) | 11 (4.5) | 45 (18.6) | 61 (25.2) | 67 (27.7) | 58 (24.0) | 242 (35.0) | <0.001 | 3.5 (3.3–3.6) | <0.001 |
| All | 145 (21.0) | 212 (30.6) | 149 (21.5) | 114 (16.5) | 72 (10.4) | 692 (100) | - | 2.5 (2.5–2.6) | - |
| Aggressiveness of PCa, n (%) | PI-RADS | All | |||
|---|---|---|---|---|---|
| 1–2 | 3 | 4 | 5 | ||
| Clinical stage (n = 692), p = 0.012 | |||||
| Localised | 37 (100) | 121 (100) | 286 (97.9) | 180 (74.4) | 624 (90.2) |
| Locally advanced | 0 (0) | 0 (0) | 2 (0.7) | 39 (16.1) | 41 (5.9) |
| Metastatic | 0 (0) | 0 (0) | 4 (1.4) | 23 (9.5) | 27 (3.9) |
| EAU risk of localised tumours (n = 624), p < 0.001 | |||||
| Low | 29 (80.0) | 61 (50.4) | 79 (27.6) | 11 (6.1) | 180 (28.9) |
| Intermediate | 6 (13.3) | 47 (38.8) | 115 (40.2) | 98 (54.4) | 266 (42.6) |
| High | 2 (5.4) | 13 (10.7) | 92 (32.2) | 71 (39.5) | 178 (28.5) |
| Type of pathology in surgical specimens (n = 234), p < 0.001 | |||||
| Favourable | 6 (100) | 27 (53.6) | 54 (36.0) | 3 (10.0) | 90 (38.5) |
| Unfavourable | 0 (0) | 21 (43.8) | 96 (64.0) | 27 (90.0) | 144 (61.5) |
| Criteria of Aggressiveness | PI-RADS | Odds Ratio (95% CI) | p Value | |
|---|---|---|---|---|
| 1–3 | 4–5 | |||
| Grade group > 3, n (%) | 12/158 (7.6) | 174/534 (32.6) | 4.881 (3.177–6.885) | <0.001 |
| EAU high-risk localised PCa | 15/158 (9.5) | 163/466 (35.0) | 3.681 (2.032–6.785) | =0.001 |
| Advanced PCa, n (%) | 0/158 (0) | 68/534 (12.7) | 1.139 (1.086–1.196) | <0.001 |
| Unfavourable pathology | 21/54 (38.9) | 123/180 (68.3) | 1.867 (1.137–8.112) | =0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morote, J.; Borque-Fernando, A.; Triquell, M.; Celma, A.; Regis, L.; Mast, R.; de Torres, I.M.; Semidey, M.E.; Santamaría, A.; Planas, J.; et al. Multiparametric Magnetic Resonance Imaging Grades the Aggressiveness of Prostate Cancer. Cancers 2022, 14, 1828. https://doi.org/10.3390/cancers14071828
Morote J, Borque-Fernando A, Triquell M, Celma A, Regis L, Mast R, de Torres IM, Semidey ME, Santamaría A, Planas J, et al. Multiparametric Magnetic Resonance Imaging Grades the Aggressiveness of Prostate Cancer. Cancers. 2022; 14(7):1828. https://doi.org/10.3390/cancers14071828
Chicago/Turabian StyleMorote, Juan, Angel Borque-Fernando, Marina Triquell, Anna Celma, Lucas Regis, Richard Mast, Inés M. de Torres, María E. Semidey, Anna Santamaría, Jacques Planas, and et al. 2022. "Multiparametric Magnetic Resonance Imaging Grades the Aggressiveness of Prostate Cancer" Cancers 14, no. 7: 1828. https://doi.org/10.3390/cancers14071828
APA StyleMorote, J., Borque-Fernando, A., Triquell, M., Celma, A., Regis, L., Mast, R., de Torres, I. M., Semidey, M. E., Santamaría, A., Planas, J., Esteban, L. M., & Trilla, E. (2022). Multiparametric Magnetic Resonance Imaging Grades the Aggressiveness of Prostate Cancer. Cancers, 14(7), 1828. https://doi.org/10.3390/cancers14071828







