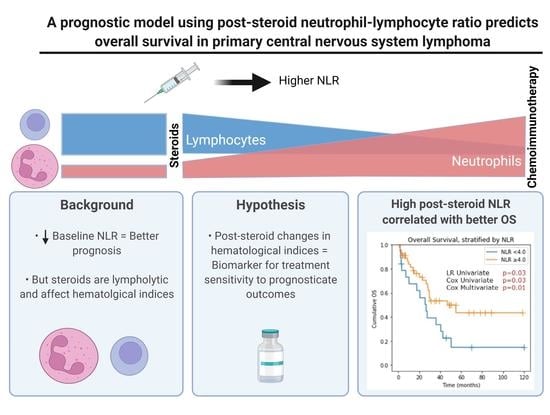
A Prognostic Model Using Post-Steroid Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Primary Central Nervous System Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
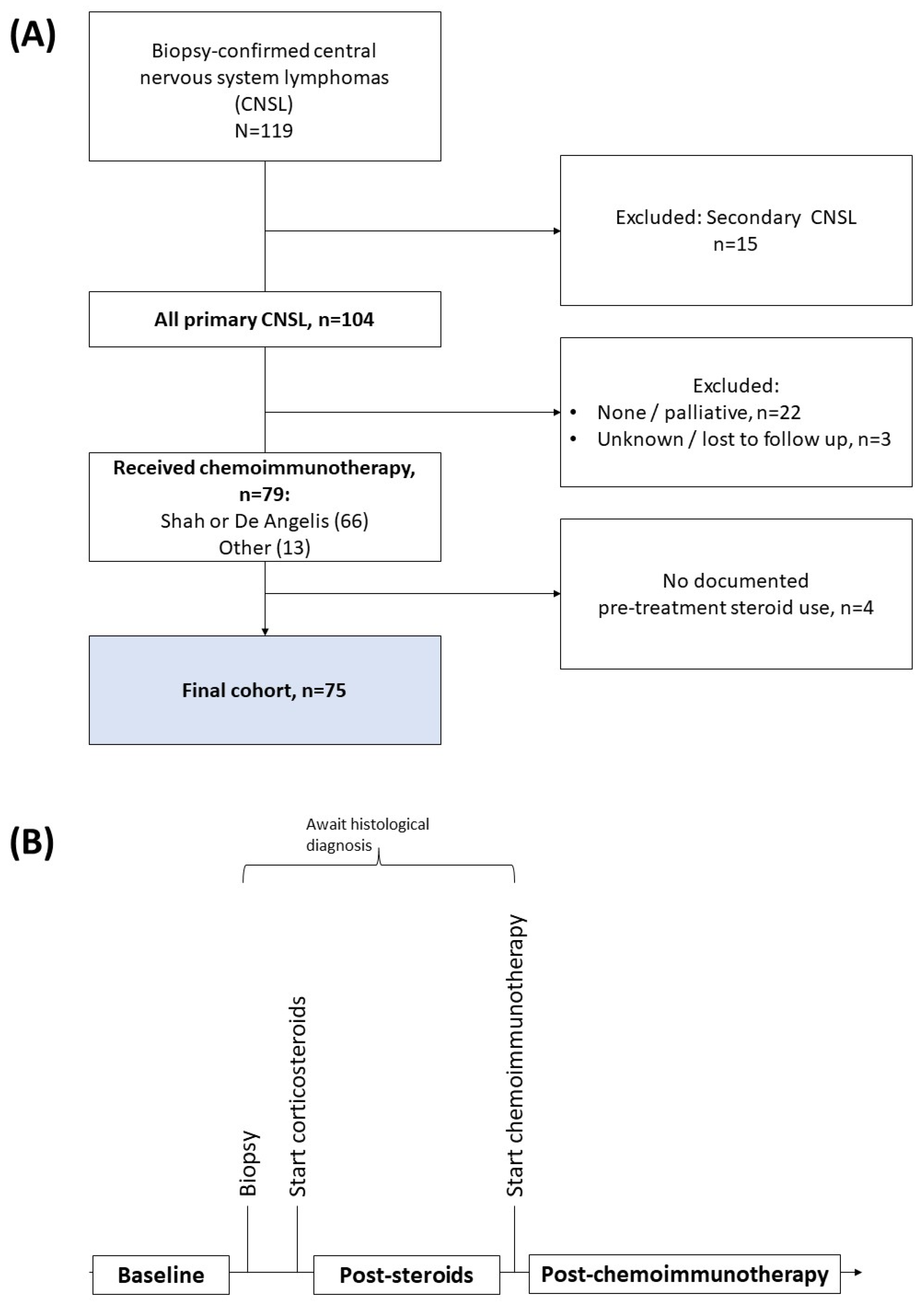
2.1. Patient Selection
2.2. Data Collection
2.3. Hematological Indices (HI)
2.4. Outcomes
2.5. Trend and Correlation Analyses
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
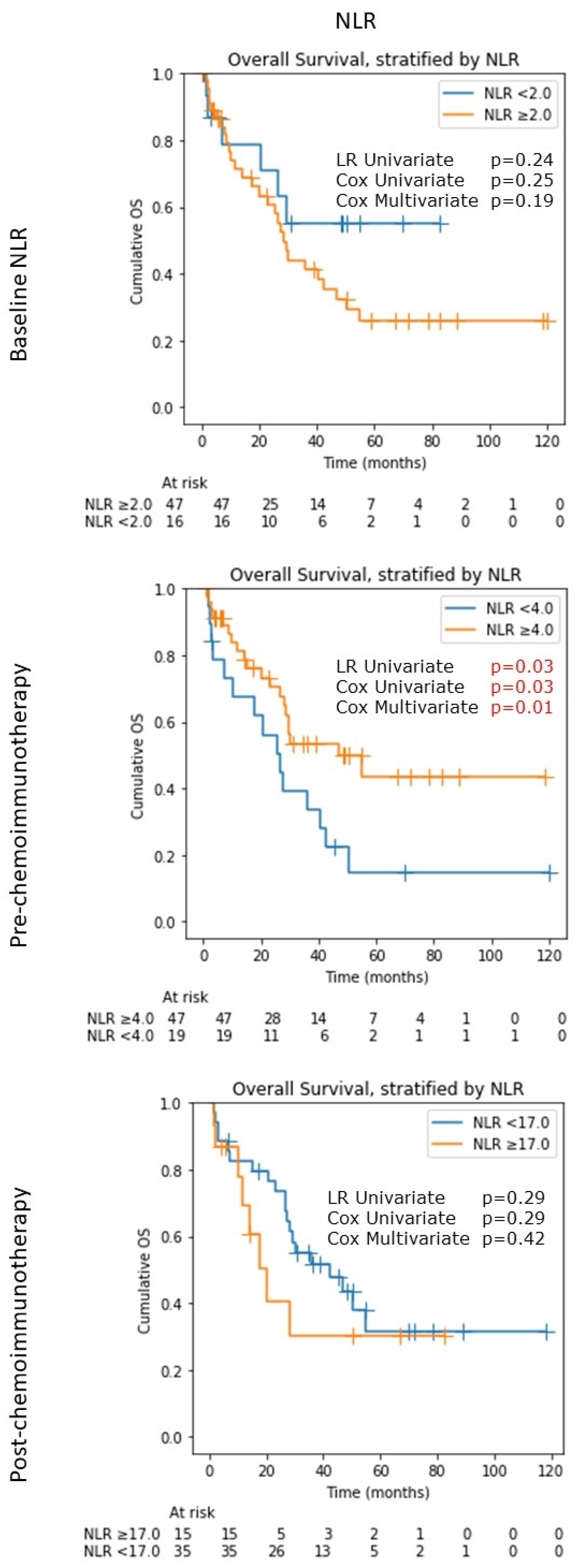
3.2. Neutrophil-Lymphocyte Ratio (NLR)
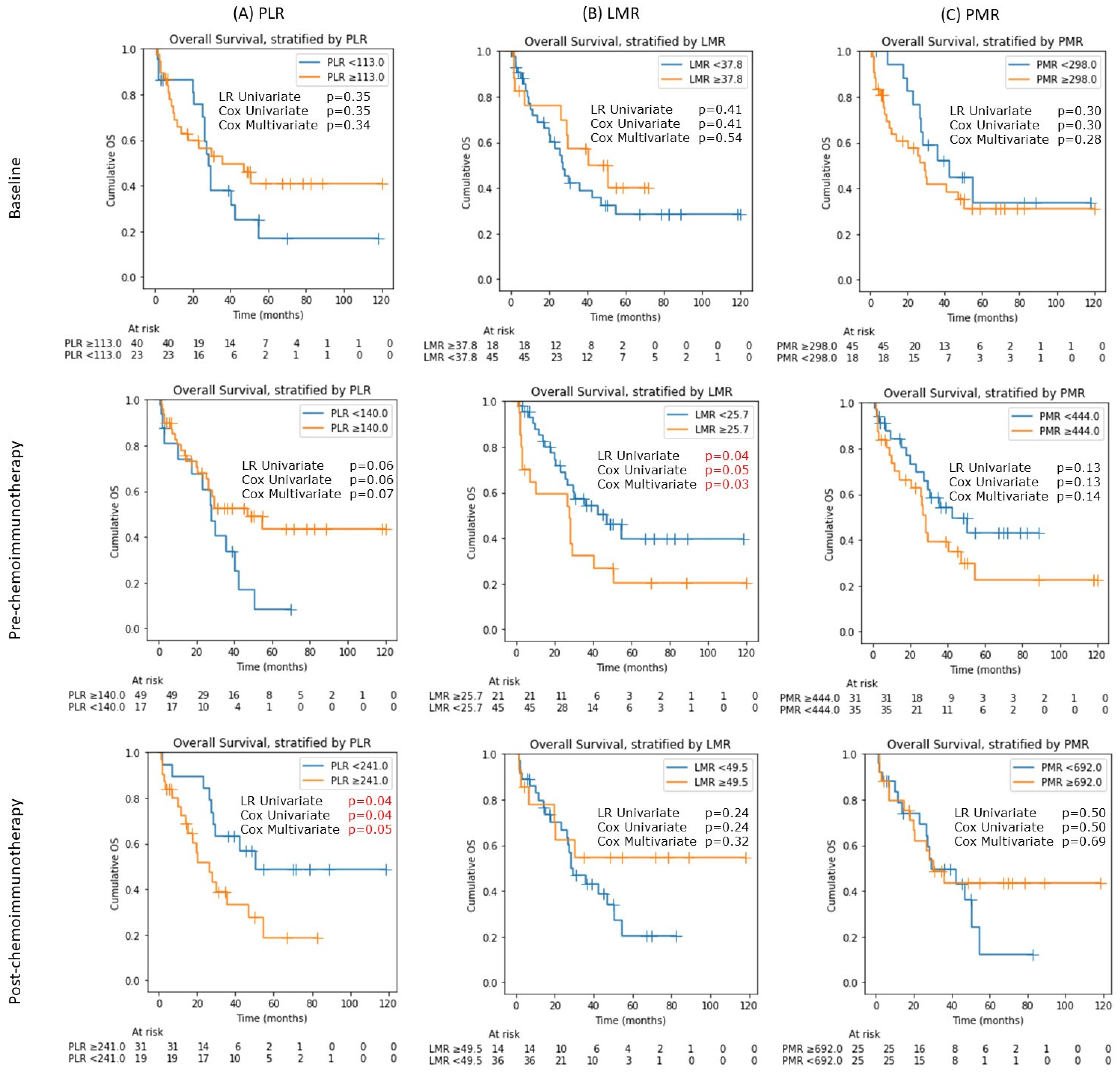
3.3. Platelet-Lymphocyte Ratio (PLR), Lymphocyte-Monocyte Ratio (LMR) and Platelet-Monocyte Ratio (PMR)
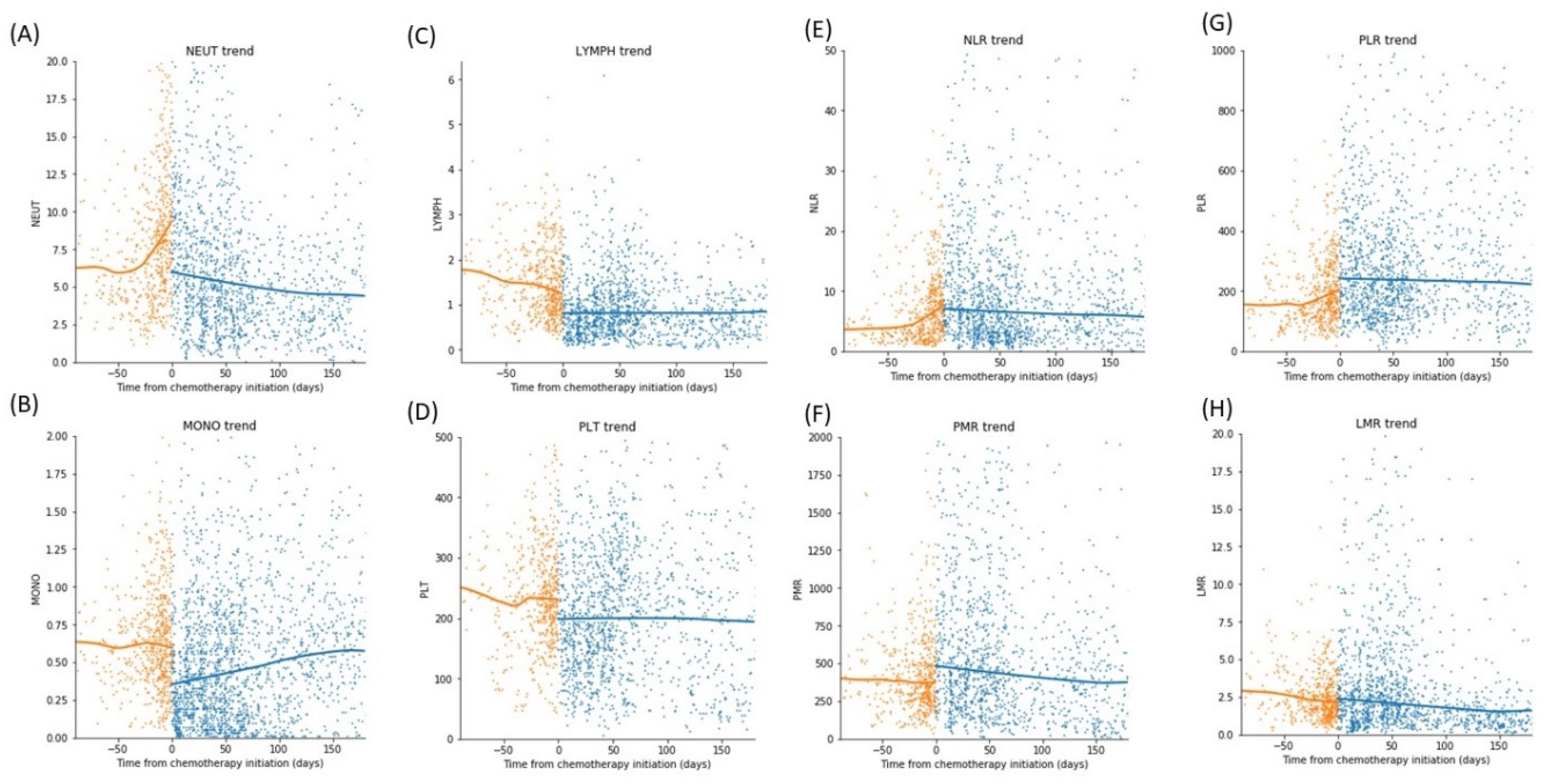
3.4. Trend of Differential Counts and Hematological Indices (HI) over Time
3.5. Comparison of the Change in Absolute Blood Differential Count and Hematological Indices Pre and Post Steroid
3.6. Comparison of the Change in Absolute Blood Differential Count and Hematological Indices Pre and Post Chemoimmunotherapy
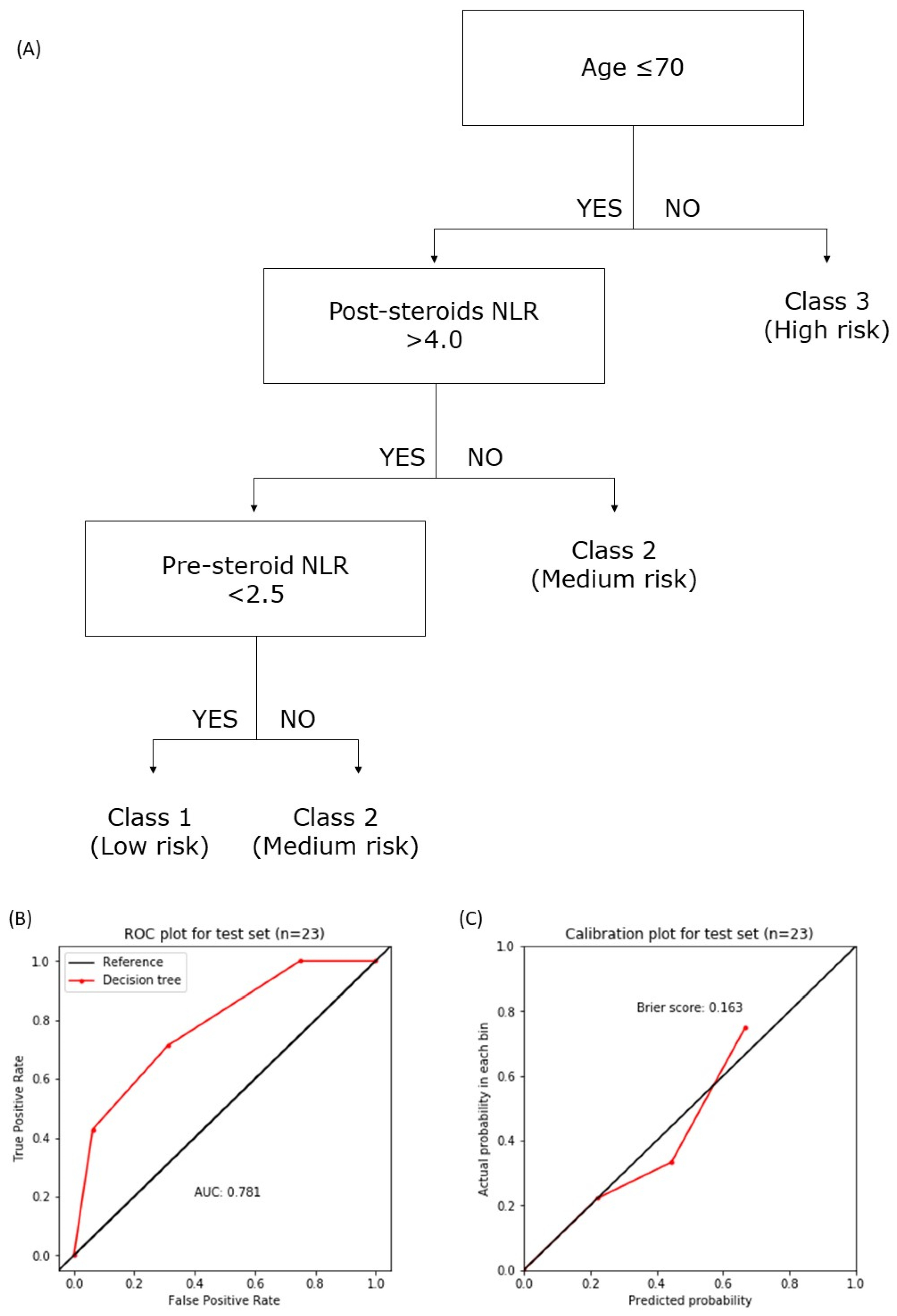
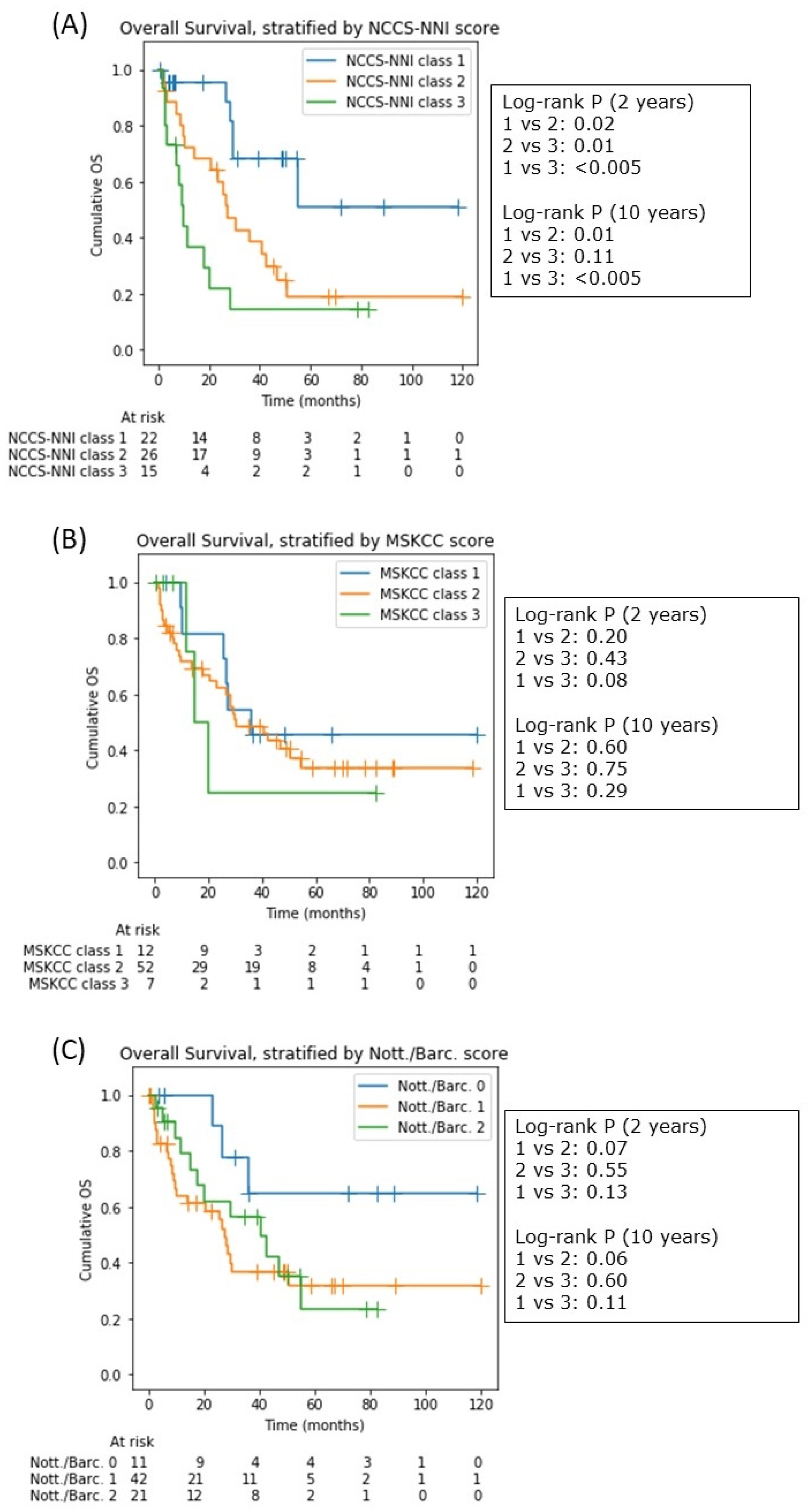
3.7. The National Cancer Center Singapore—National Neuroscience Institute (NCCS-NNI) Prognostic Model
4. Discussion
4.1. Relationship of NLR and Survival
4.2. The Effect of Corticosteroids on Blood Cells
4.3. NCCS-NNI Prognostic Model
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, J.; Lee, H.; Yun, T.; Lee, E.; Moon, H.; Joo, J.; Park, W.S.; Choi, M.; Lee, J.O.; Lee, J.S.; et al. Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Patients with Primary Central Nervous System Lymphoma. Oncotarget 2017, 8, 74975–74986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, A. Primary Cns Lymphoma: Neutrophil To Lymphocyte Ratio Predictive of Survival. Indian J. Appl. Res. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- Lo, Y.T.; Ang, Y.L.S.; Yang, V.S.; Kanavathy, D.T.; Liang, S.; Lee, L. Motor Deficits at Presentation and Predictors of Overall Survival in Central Nervous System Lymphomas. J. Neuro-Oncol. 2021, 151, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Nakaya, A.; Fujita, S.; Satake, A.; Nakanishi, T.; Tsubokura, Y.; Saito, R.; Konishi, A.; Hotta, M.; Yoshimura, H.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Fails to Predict Outcome of Diffuse Large B Cell Lymphoma. Leuk. Res. Rep. 2019, 12, 100173. [Google Scholar] [CrossRef]
- DeAngelis, L.M.; Seiferheld, W.; Clifford Schold, S.; Fisher, B.; Schultz, C.J. Combination Chemotherapy and Radiotherapy for Primary Central Nervous System Lymphoma: Radiation Therapy Oncology Group Study 93-10. J. Clin. Oncol. 2002, 20, 4643–4648. [Google Scholar] [CrossRef]
- Shah, G.D.; Yahalom, J.; Correa, D.D.; Lai, R.K.; Raizer, J.J.; Schiff, D.; LaRocca, R.; Grant, B.; DeAngelis, L.M.; Abrey, L.E. Combined Immunochemotherapy with Reduced Whole-Brain Radiotherapy for Newly Diagnosed Primary CNS Lymphoma. J. Clin. Oncol. 2007, 25, 4730–4735. [Google Scholar] [CrossRef] [Green Version]
- Ronchetti, S.; Ricci, E.; Migliorati, G.; Gentili, M.; Riccardi, C. How Glucocorticoids Affect the Neutrophil Life. Int. J. Mol. Sci. 2018, 19, 4090. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, M.; Terashima, T.; D’yachkova, Y.; Bondy, G.P.; Hogg, J.C.; van Eeden, S.F. Glucocorticoid-Induced Granulocytosis: Contribution of Marrow Release and Demargination of Intravascular Granulocytes. Circulation 1998, 98, 2307–2313. [Google Scholar] [CrossRef] [Green Version]
- Shoenfeld, Y.; Gurewich, Y.; Gallant, L.A.; Pinkhas, J. Prednisone-Induced Leukocytosis. Influence of Dosage, Method and Duration of Administration on the Degree of Leukocytosis. Am. J. Med. 1981, 71, 773–778. [Google Scholar] [CrossRef]
- Heckmann, J.G.; Bockhorn, J.; Stolte, M.; Druschky, A.; Neundörfer, B. An Instructive False Diagnosis: Steroid-Induced Complete Remission of a CNS Tumor—Probably Lymphoma. Neurosurg. Rev. 1998, 21, 48–51. [Google Scholar] [CrossRef]
- Vaquero, J.; Martinez, R.; Rossi, E.; Lopez, R. Primary Cerebral Lymphoma: The “Ghost Tumor”. Case Report. J. Neurosurg. 1984, 60, 174–176. [Google Scholar] [CrossRef]
- Önder, E.; Arikök, A.T.; Önder, S.; Han, Ü.; Sorar, M.; Kertmen, H.; Yilmaz, E.D.; Fesli, R.; Alper, M. Corticosteroid Pre-Treated Primary CNS Lymphoma: A Detailed Analysis of Stereotactic Biopsy Findings and Consideration of Interobserver Variability. Int. J. Clin. Exp. Pathol. 2015, 8, 7798–7808. [Google Scholar]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; la Rosée, P.; Schorb, E.; et al. Chemoimmunotherapy with Methotrexate, Cytarabine, Thiotepa, and Rituximab (MATRix Regimen) in Patients with Primary CNS Lymphoma: Results of the First Randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) Phase 2 Trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary Central Nervous System Lymphoma: The Memorial Sloan-Kettering Cancer Center Prognostic Model. J. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef]
- Bessell, E.M.; Graus, F.; Lopez-Guillermo, A.; Lewis, S.A.; Villa, S.; Verger, E.; Petit, J. Primary Non-Hodgkin’s Lymphoma of the CNS Treated with CHOD/BVAM or BVAM Chemotherapy before Radiotherapy: Long-Term Survival and Prognostic Factors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Lausen, B. On the Exact Distribution of Maximally Selected Rank Statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Davidson-Pilon, C. Lifelines: Survival Analysis in Python. J. Open Source Softw. 2019, 4, 1317. [Google Scholar] [CrossRef] [Green Version]
- Schemper, M.; Smith, T.L. A Note on Quantifying Follow-up in Studies of Failure Time. Controll. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, C.; Fu, C.; Wu, W.; Wei, Y.; Zou, L. Prognostic Role of Blood Markers in Primary Central Nervous System Lymphoma Patients Treated With High-Dose Methotrexate-Based Therapy. Front. Oncol. 2021, 11, 639644. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S. Mechanisms of Corticosteroid Action on Lymphocyte Subpopulations. II. Differential Effects of in Vivo Hydrocortisone, Prednisone and Dexamethasone on in Vitro Expression of Lymphocyte Function. Clin. Exp. Immunol. 1976, 24, 54–62. [Google Scholar] [PubMed]
- Schmidt, S.; Rainer, J.; Ploner, C.; Presul, E.; Riml, S.; Kofler, R. Glucocorticoid-Induced Apoptosis and Glucocorticoid Resistance: Molecular Mechanisms and Clinical Relevance. Cell Death Differ. 2004, 11, S45–S55. [Google Scholar] [CrossRef] [Green Version]
- Gaynon, P.S.; Carrel, A.L. Glucocorticosteroid Therapy in Childhood Acute Lymphoblastic Leukemia. Adv. Exp. Med. Biol. 1999, 457, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Schabet, M.; Eichhorn, M.; Petersen, D.; Grote, E.H.; Meyermann, R.; Dichgans, J. Prolonged Corticosteroid-Lnduced Remission in Primary Central Nervous System Lymphoma: Report of a Case and Review of the Literature. Eur. Neurol. 1996, 36, 241–243. [Google Scholar] [CrossRef]
- Pirotte, B.; Levivier, M.; Goldman, S.; Brucher, J.M.; Brotchi, J.; Hildebrand, J. Glucocorticoid-Induced Long-Term Remission in Primary Cerebral Lymphoma: Case Report and Review of the Literature. J. Neuro-Oncol. 1997, 32, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ilizaliturri, F.J.; Jupudy, V.; Ostberg, J.; Oflazoglu, E.; Huberman, A.; Repasky, E.; Czuczman, M.S. Neutrophils Contribute to the Biological Antitumor Activity of Rituximab in a Non-Hodgkin’s Lymphoma Severe Combined Immunodeficiency Mouse Model. Clin. Cancer Res. 2003, 9, 5866–5873. [Google Scholar] [PubMed]
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 163. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Final Cohort (n = 75) |
|---|---|
| Age, median (range) | 61 (21–82) |
| Male gender, n (%) | 41 (55) |
| KPS, median (range) | 80 (20–100) |
| CCI, median (range) | 2 (0–6) |
| MSKCC prognostic score, n (%) | |
| Class 1 | 12 (16) |
| Class 2 | 52 (69) |
| Class 3 | 7 (9) |
| Pre-biopsy dexamethasone use, n (%) | 36 (48) |
| Post-biopsy dexamethasone use, n (%) | |
| None | 4 (5) |
| Low-dose (<12 mg/day) or delayed | 8 (11) |
| High-dose (≥12 mg/day) | 63 (84) |
| Chemoimmunotherapy regime, n (%) | |
| Shah (MTX with Rituximab) | 28 (37) |
| De Angelis (MTX without Rituximab) | 35 (47) |
| Other (e.g., MATRIX) | 12 (16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.T.; Lim, V.Y.; Ng, M.; Tan, Y.H.; Chiang, J.; Chang, E.W.Y.; Chan, J.Y.; Poon, E.Y.L.; Somasundaram, N.; Bin Harunal Rashid, M.F.; et al. A Prognostic Model Using Post-Steroid Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Primary Central Nervous System Lymphoma. Cancers 2022, 14, 1818. https://doi.org/10.3390/cancers14071818
Lo YT, Lim VY, Ng M, Tan YH, Chiang J, Chang EWY, Chan JY, Poon EYL, Somasundaram N, Bin Harunal Rashid MF, et al. A Prognostic Model Using Post-Steroid Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Primary Central Nervous System Lymphoma. Cancers. 2022; 14(7):1818. https://doi.org/10.3390/cancers14071818
Chicago/Turabian StyleLo, Yu Tung, Vivian Yujing Lim, Melissa Ng, Ya Hwee Tan, Jianbang Chiang, Esther Wei Yin Chang, Jason Yongsheng Chan, Eileen Yi Ling Poon, Nagavalli Somasundaram, Mohamad Farid Bin Harunal Rashid, and et al. 2022. "A Prognostic Model Using Post-Steroid Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Primary Central Nervous System Lymphoma" Cancers 14, no. 7: 1818. https://doi.org/10.3390/cancers14071818
APA StyleLo, Y. T., Lim, V. Y., Ng, M., Tan, Y. H., Chiang, J., Chang, E. W. Y., Chan, J. Y., Poon, E. Y. L., Somasundaram, N., Bin Harunal Rashid, M. F., Tao, M., Lim, S. T., & Yang, V. S. (2022). A Prognostic Model Using Post-Steroid Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Primary Central Nervous System Lymphoma. Cancers, 14(7), 1818. https://doi.org/10.3390/cancers14071818