1. Introduction
Anaplastic lymphoma kinase (ALK) (
ALK)-gene rearrangements ALK
+ are oncogenic drivers found in 3–5% of patients with non-small-cell lung cancers (NSCLCs) [
1]. Patients with ALK
+ NSCLCs are generally younger and non or light smokers, with adenocarcinoma histology and a high risk of brain metastases at diagnosis and progression [
1,
2]. Brigatinib is a next-generation ALK inhibitor (ALKi) with enhanced brain activity and potent efficacy against many ALK-resistance mutations [
3,
4]. Brigatinib was initially developed for crizotinib-pretreated
ALK+ NSCLCs [
2,
5,
6].
More recently, following the results of the phase 3 trial—ALK in lung cancer trial of brigatinib in 1st line (ALTA-1L)—brigatinib was granted marketing authorization for the first-line treatment of advanced ALK
+ NSCLCs [
7,
8,
9,
10]. ALTA-1L results indicated superior brigatinib efficacy compared to crizotinib in patients with ALK tyrosine-kinase inhibitor (TKI)-naive ALK
+ advanced NSCLCs [
7,
8]: median progression-free survival (PFS) according to an independent review committee (primary endpoint) was 24.0 months with brigatinib vs. 11.1 months with crizotinib (HR 0.49 (95% confidence interval (CI) 0.35–0.68);
p < 0.0001). At the end of the study, respective 3-year PFS determined by a blinded independent review committee was 43% vs. 19%, with a median OS not reached (NR) for either group. At present, several alternatives to crizotinib are available [
10,
11,
12,
13,
14,
15], rendering real-life data essential [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27] to attempt to evaluate optimal therapeutic sequences [
28].
The national, retrospective, multicenter and real-world study, BrigALK, analyzed patients with ALK
+ advanced NSCLCs enrolled in the brigatinib French early-access program (FEAP). According to the interim BrigALK analysis, after the enrollment of 104 patients (data cut-off: 30 June 2018, median follow-up 6.7 months), the primary endpoint—invPFS from brigatinib initiation—was 6.6 months (95% CI 4.8–9.9) with median overall survival (OS) of 17.2 months (95% CI 11.0–NR) months [
19].
BrigALK2 covered the entire FEAP period; an updated analysis examined brigatinib efficacy. Subsequent treatments were collected, focusing on post-brigatinib lorlatinib efficacy.
2. Patients and Methods
2.1. Study Design and Patients
The objective of this non-interventional study was to evaluate, in the real-world setting, brigatinib efficacy in ALK+ advanced NSCLC. Inclusion criteria were: 18 years old; ALK+ advanced NSCLCs assessed by fluorescence in situ hybridization and/or immunohistochemistry or NGS in each participating center; prior treatment with 1 ALK inhibitor(s) (ALKi(s)), including crizotinib; FEAP-provided brigatinib (1 August 2016 to 21 January 2019).
2.2. Data Collection
Patient data, collected retrospectively from medical files, included demographics, NSCLC characteristics, numbers and localizations of metastatic sites, previous treatments, tumor response to brigatinib, resistance mutations before starting brigatinib or after progression and post-progression treatments. All consecutive patients meeting inclusion criteria were enrolled without selection in each participating center.
2.3. Endpoints
The primary endpoint was invPFS from brigatinib onset, i.e., from the first brigatinib dose to first documentation of objective disease progression or death from any cause. Secondary endpoints included objective response rate (ORR), median duration of treatment (DOT), median OS and analysis of subsequent treatment(s). Analysis focused particularly on lorlatinib efficacy (median DOT and OS from lorlatinib initiation) after progression on brigatinib.
2.4. Statistical Analyses
Comparisons between patient characteristics were performed using the chi-square test or Fisher’s exact test for discrete variable. The Kaplan–Meier method was used to estimate PFS and OS for the entire population and defined subgroups according to the number of treatment lines. The log-rank test compared survival by treatment category. Best response to treatment was assessed according to RECIST 1.1 criteria. Statistical analyses were computed with SAS v9.4 software (SAS Institute, Cary, NC, USA).
The study was conducted in accordance with French laws and regulations in force (law 78–17 of 6 January 1978 modified by laws 94–548 of 1 July 1994, 2002-303 of 4 March 2002, 2004-801 of 6 August 2004). The GFPC has committed to the CNIL (French National Commission for Data Protection and Liberties) to respect the MR-004 reference methodology for research not involving human subjects.
3. Results
During FEAP period, access to brigatinib was requested for 227 patients. Among them, 183 patients managed in 66 centers were included in the BrigALK2 study. The main reasons for patient exclusion from BrigALK2 analysis are summarized in the study flowchart (
Figure 1).
Patients’ characteristics before brigatinib treatment are summarized in
Table 1; 37.4% had Eastern Cooperative Oncology Group performance status (ECOG PS), more than half of them (55.3%) had ≥3 metastatic sites—with the brain being the most frequent site (71.6%)—and 7.1% of patients had leptomeningeal carcinomatosis. Median time from diagnosis of metastatic NSCLC to brigatinib initiation was 30.6 months (95% CI 17–34.6). Patients received a median of 3 (range 1–6) lines and were pretreated with a median of 2 ALKis (91.8% crizotinib, 85.3% ceritinib and 9.2% alectinib) (
Table 2). Brigatinib was given to 14 (7.7%) patients as second-line therapy, 44 (24%) as third line, 74 (40.4%) as fourth line and 51 (27.9%) as fifth line. Most often, therapeutic sequences comprised two (mainly, crizotinib then ceritinib,
n = 36 (19.7%)) or three lines (mainly, chemotherapy, crizotinib then ceritinib,
n = 49 (26.8%)); 129 (70.5%) patients had received chemotherapy before brigatinib (
Table S1: Supplementary Material). In addition, rebiopsies (tissu and ctDNA) were obtained from 51 patients (27.8%) who experienced disease progression before brigatinib treatment. Resistance-mutation genotyping was carried out for 28 patients (21 not carried out and 2 failures) and revealed a secondary mutation in 10 of them (35.7%). G1202R was the most frequently identified secondary mutations (7/10) with one case of compound mutation (F1174L/G1202R). (Type of mutation, best response to brigatinib and DOT with brigatinib are presented in
Table 3.)
The standard brigatinib dose (180 mg/day) was given to 175 (95.6%) patients with a 7-day induction of 90 mg/day; 37 (20.2%) patients required dose adjustments, without definitive interruption for intolerance or patient choice, and 19 (10.4%) discontinued brigatinib because of adverse events or patient choice.
At data cut-off (1 February 2021), median follow-up was 40.4 months (95% CI 38.4–42.4) (
Table 4); 21 (11.5%) patients were still on treatment. Median invPFS was 7.4 months (95% CI 5.9–9.6) and brigatinib median DOT was 7.3 months (95% CI 5.8–9.4) with 59.5% and 39% still on treatment at 6 and 12 months. For patients who received 1 (
n = 14), 2 or 3 (
n = 118) or >3 (
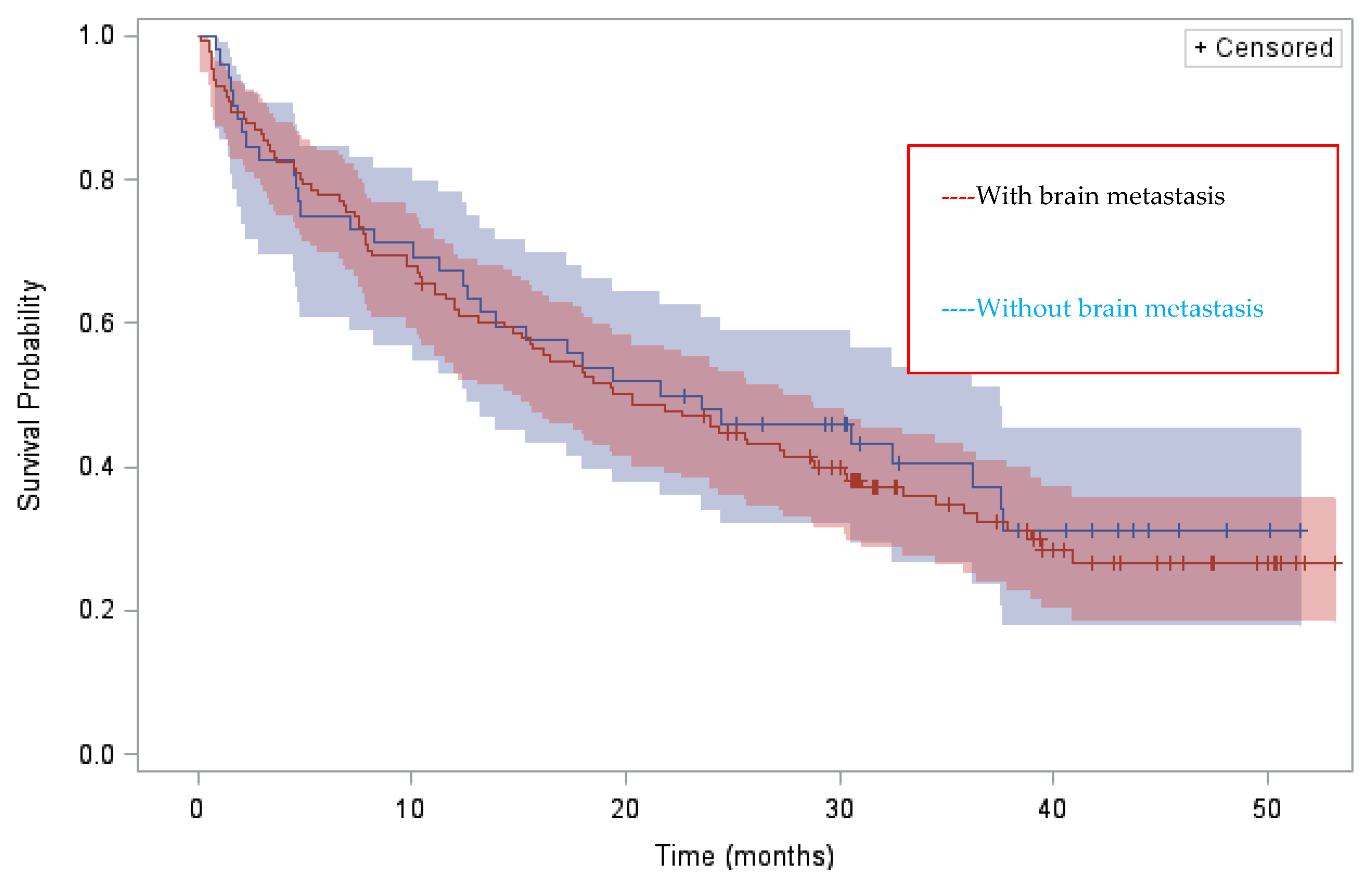
n = 51) ALKi(s) before brigatinib, median DOTs were 13.8 (95% CI 3.8–26.4), 7.4 (95% CI 5.6–9.9) and 4.9 (95% CI 1.7–9.3) months, respectively. Final ORR and disease-control rate (DCR) for the 169 assessable patients were 44.3% and 74.2%, respectively. Median OS from brigatinib initiation in the whole population was 20.3 months (95% CI 15.6–27.4), with 78% and 63.8% alive at 6 and 12 months. As for median DOT, median OS varied according to the number of pre-brigatinib ALKis: median OS from brigatinib initiation was 33 (95% CI 9.7–NR), 20.3 (95% CI 15.7–28.7) and 18.1 (95% CI 3.3–24.5) months, respectively, for patients pretreated with 1, 2 or 3 and >3 ALKi(s). There was no significant difference in median OS between patients with and without brain metastases (20.3 months (14.7–27.4) vs. 22.6 months (12.6–37.5) respectively) (
Figure 2). It should be noted that more than half of the patients had brain radiotherapy before brigatinib initiation regardless of treatment line.
At the time of the analysis, 112/183 patients (60.9%) had progressed on brigatinib. Progression involved known metastases for 110/112 (98.2%) patients (known brain lesions for 45) and new lesions for 47/112 (42%), 23 of which were cerebral. Post-brigatinib, new biopsies were obtained from 22 patients (19.6%). Genotyping, carried out for fifteen patients, identified a secondary mutation in six of them: G1202R was still the most identified secondary mutation (5/6), with another case of compound mutation (F1174V and D1203N).
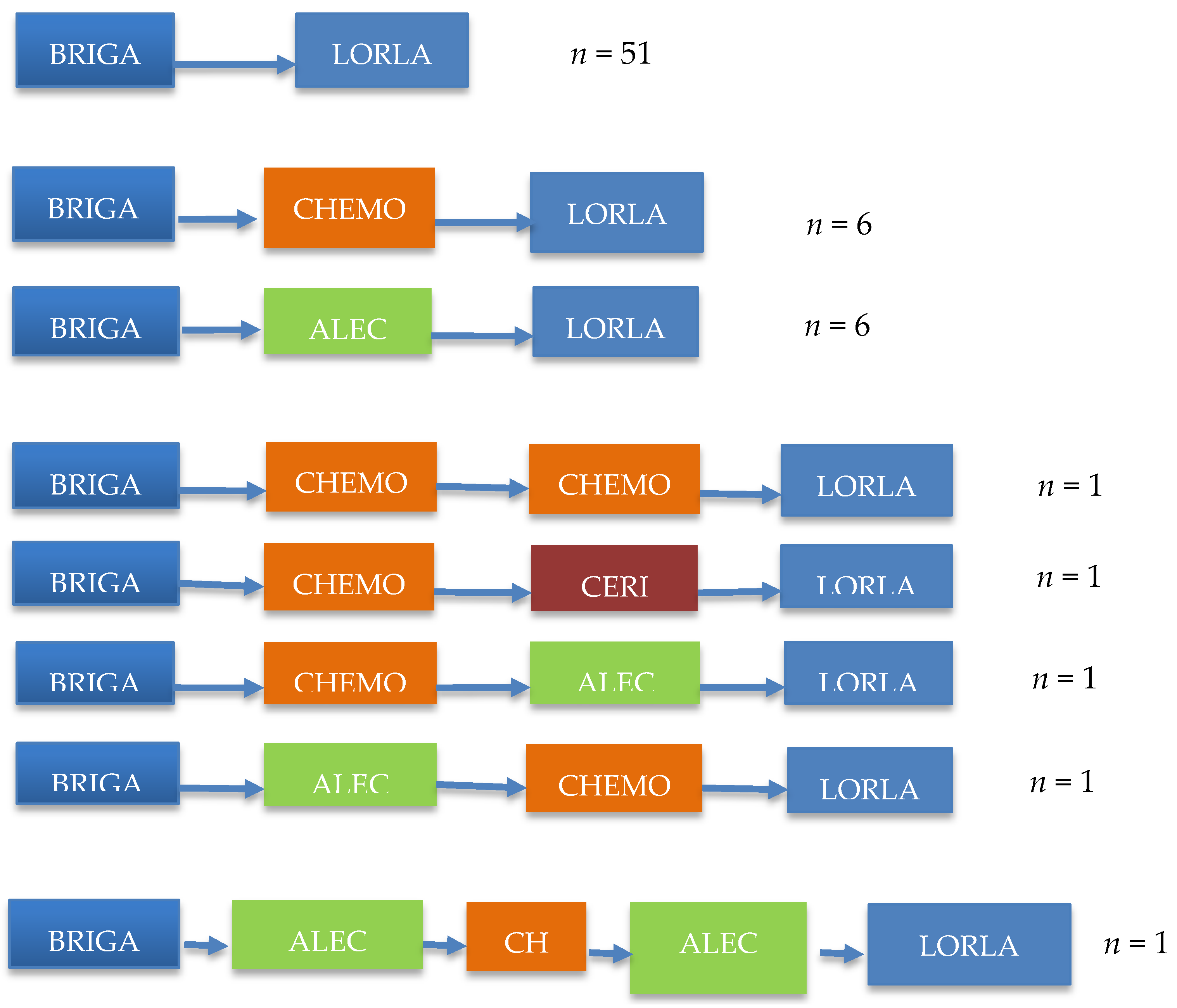
Among 106 (57.9%) patients receiving at least one therapy after brigatinib, clinical data could be obtained for 92 (86.8%) of them. A total of 68 (64.1%) received lorlatinib: 51 patients (75%) received it immediately after brigatinib; 17 received it after sequential administration of another second-generation ALKi + chemotherapy (
Figure 3). The interval between advanced NSCLC diagnosis and lorlatinib start was 52.8 months (95% CI 43.2–60.3). With a median follow-up of 29.9 months, the median lorlatinib DOT was 5.3 months (95% CI 3.6–7.6) and median OS from lorlatinib initiation was 14.1 months (95% CI 10.3–19.2).
Finally, the median OS from the initial NSCLC diagnosis was 75.3 months (95% CI 38.2–174.6), knowing that 86.4% of the patients had an advanced stage at diagnosis.
4. Discussion
With a median follow-up of 40.4 months, the results of this retrospective, multicenter, real-life study confirmed brigatinib efficacy at managing heavily pre-treated advanced ALK
+ NSCLCs with median invPFS and OS from brigatinib initiation at 7.4 and 20.3 months, respectively. As noted previously and in another analysis [
20,
21,
22], brigatinib effectiveness (DOT and OS) tends to decline, depending on the line at which it is used. Most patients were able to receive the standard regimen, with dose reduction for 20% of them and a 10% discontinuation rate, thereby confirming intermediate analysis findings [
19].
Several recent studies examined brigatinib efficacy in heavily pretreated patients ALK
+ advanced NSCLCs. According to a retrospective chart review of 104 brigatinib-treated patients in Italy, Norway, Spain and the UK, ORR was 39.8%, median PFS was 11.3 (95% CI 8.6–12.9) months and median OS lasted 23.3 (95% CI 16.0–NR) months [
22]. Based on 604 patients (from 21 countries), including those previously given next-generation ALKis, median brigatinib DOTs for patients with prior crizotinib, alectinib, ceritinib or lorlatinib were 10.0, 8.7, 10.3 or 7.5 months, respectively [
21]. Finally, a phase 2, single-arm study analyzed brigatinib efficacy and safety in 47 Japanese patients with
ALK+ advanced NSCLCs that progressed from previous alectinib or other ALKis [
20]. Their ORR and DCR were 34% (16/47) and 79% (37/47), respectively, with median PFS lasting 7.3 months. These summarized results differ slightly from ours and this difference is likely attributable, in part, to the limitations of retrospective chart data but also, and perhaps more importantly, to imbalances between the populations analyzed. Patients’ health status was better in the studies by Popat et al. [
22] and Lin et al. [
21], with respective ECOG PS 0/1 for 84% and 85.9%, and they were less intensively pretreated (median 2 lines and 1 TKI before brigatinib) in Popat et al.’s study [
22] compared to medians of 3 lines and 2 TKIs herein.
Access to treatment post-brigatinib progression was also an important factor: 58% of our patients had access to post-progression therapy. Among them, nearly two-thirds received the third-generation ALKi lorlatinib with a median DOT of 5.3 months and median OS from lorlatinib initiation of 14.1 months. These results are close to those of Popat et al., who reported that 69% (53/77) of their patients who had progressed at the time of the analysis had received systemic therapy after brigatinib, 42 with an ALKi. 34 treated with lorlatinib. The median DOT for evaluable patients was 2.57 months [
22].
Lorlatinib efficacy in this context was also analyzed in several studies [
23,
24,
25,
26]. Lorlatinib was accorded marketing authorization for second-line treatment after failure of a first-line second-generation TKI, regardless of the existence of a resistance mechanism. In a phase 2 trial, lorlatinib efficacy was evaluated in different populations based on treatment history before lorlatinib. Of the five cohorts, three included a second-generation TKI in their treatment sequence. Analysis of data from those three populations (comprised of 159 patients) showed an ORR of 39.6%, median PFS lasting 6.6 months and median OS 20.7 months after starting lorlatinib. These observations are quite similar to ours and are further supported by real-life data. In a multicenter retrospective analysis of lorlatinib in 37 heavily pretreated, ALK
+ advanced NSCLC patients, median lorlatinib DOT was 4.4 months, with 43.2% ORR and median OS from lorlatinib onset lasting 10.2 months [
24]. Another analysis of 22 patients in the same setting found 35.7% ORR and 64.3% DCR, with median PFS at 6.2 months. PFS was longer for patients who benefited from prior ALKi(s) than those who did not (6.5 vs. 3.5 months, respectively) [
24]. In a real-world analysis of 76
ALK+ NSCLC patients enrolled in early or expanded access programs for lorlatinib in Asia and the United States, respective ORR and median PFS for those treated with <2 previous TKIs, 2 previous TKIs and 3 previous TKIs, were 42% and NR months, 35% and 11.2 months and 18% and 6.5 months [
27].
There is a growing body of literature on the search for mechanisms of resistance to ALKi but this practice is not mandatory for progression management [
28,
29,
30]. In our retrospective series, rebiopsy was performed in approximately 25% and 20% of pre- and post-brigatinib patients. Though these rates may seem low, they represent an accurate reflection of a period of management of ALK+ aNSCLC that corresponds to the brigatinib EAP between August 2016 and January 2019. When genotyping was performed, it led to the identification of an ALK secondary mutation in 37.5% and 40%, pre- and post- brigatinib, respectively, but we could not know whether the identification of the mutations was considered for the therapeutic decision. These rates of resistance mutations are demonstrated in a series of patients pre-treated with a median of two pre-brigatinib TKIs, mainly the crizotinib-ceritinib sequence. They are quite similar to those reported by Gainor et al. [
31]: after two TKIs, including a second-generation TKI, secondary mutations were detected in approximately 50% of cases. Moreover, among mutations detected when patients experienced progression, G1202R was the most common. This is also consistent with previously known data that have shown the high frequency of the G1202R mutation after second-generation TKIs [
32].
Our study has several limitations. First of all, it should be noted that treatment sequences are currently different from those analyzed in the brigALK study, as most patients are treated with a second-generation TKI in the first line (alectinib or brigatinib). Other limitations are those inherent in this type of retrospective study without data monitoring. Treatment efficacy was assessed by the investigators, without any independent review committee. Investigator assessment bias cannot be excluded. In this real-life study, it was not always possible to obtain complete information from patients’ medical records. That was the case, for example, for patients with a dose reduction or those who discontinued brigatinib before disease progression. Another limitation to point out is the small number of events on lorlatinib that could explain the CI 95% of DOT and OS that are a little too wide. One of the strengths of the study is the absence of stringent criteria for study inclusion, meaning the population is representative of real-life, heavily treated patients with ALK+ advanced NSCLCs.
5. Conclusions
This analysis of FEAP data confirmed brigatinib effectiveness in a population of heavily pretreated ALK+ advanced NSCLCs and provided informative real-life observations about lorlatinib efficacy post-brigatinib.
Author Contributions
Conceptualization, R.D., J.-B.A. and C.C.; methodology, G.R.-B., D.P. and C.C.; validation, all the authors; formal analysis, R.D., G.R.-B. and C.C.; investigation, all the authors; data curation, all the authors; writing—original draft preparation, R.D. and C.C.; writing—review and editing, all the authors; funding acquisition, F.G., R.S., J.-B.A. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
Academic grant from Takeda. The sponsor does not have any role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
The study conformed to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines, and approved by: Ile de France II; Protocol: GFPC 02-2019, date of approval: 25 May 2020.
Informed Consent Statement
Patients received written and oral information on the study and gave their consent to participate in the study and for the use of their medical data for research purposes.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the investigators and staff of all participating centers: Cécile Dujon (Le Chesnay), Jeannick Madelaine (Caen), Gaelle Jeannin (Clermont-Ferrand), Olivier Bylicki (HIA Percy), Catherine Daniel (Institut Curie, Paris), Dominique Spaeth (Poyclinique de Gentilly, Nancy), Nicolas Cloarec (Avignon), Alain Vergnenègre (Limoges), Eric Jauffret (Boulogne-Billancourt), Hannah Ghalloussi (Nice), Nicolas Martin (Nice), Patricia Barré (Cahors), Laurent Greillier (Marseille), Pierre Fournel (Saint-Priest-en-Jarez), Olivier Molinier (Le Mans), Guillaume Leveillier (Saint-Brieuc), Haitham Oweis (Châlons-en-Champagne), Ahmed Azzedine (Montélimar), Marie Coudurier (Chambéry), Anne-Marie Chiappa (Quimper), Arnaud Bedin (Evreux), Etienne Devun (Evreux), Hugues Morel (Orléans), Romain Corre (Rennes), Corinne Lamour (Poitiers), Denis Morot-Sibilot (La Tronche), Bachar Chahine (Bois-Guillaume), Bruno Taviot (Givors), Violaine Giraud (Boulogne-Billancourt), Olivier Herreman (Romans), Laurence Bigay-Game (Toulouse), Anne-Sophie Bugnet (Thonon-les-Bains), Boris Duchemann (Bobigny), Hélène Doubre (Suresnes), Frédéric Bigot (Angers), Dominique Arpin (Villefranche-sur-Saone), Clarisse Audigier Valette (Toulon), Erick Chirat (Etampe), Chantal Decroisette (Annecy), Stéphane Culine (Paris), Michael Duruisseaux (Lyon), Robert Hervé (Papeete), Youssef Tazi (Strasbourg), Sylvie Demolombe (Lyon), Didier Debieuvre (Mulhouse), Anne-Sophie Blanchet (Lyon), Jean-Yves Tavernier (Douai), Nadia Munsch (Albi), Thierry Muron (Saint-Etienne), Jean-Marie Vantelon (Toulouse), Linda Sakhri (Grenoble), Mathieu Dusselier (Périgueux), Rémy Largillier (Mougins), Marie Marcq (La Roche-sur-Yon), Nicolas Paleiron (Toulon), Soizic Ferlandin and Mireille Cosquer.
Conflicts of Interest
R.D. reports personal fees from TAKEDA, personal fees from PFIZER, personal fees from NOVARTIS, personal fees from ROCHE, outside the submitted work. M.P. reports grants and other from ROCHE, other from LILLY, other from PFIZER, other from BOEHRINGER, other from MSD, other from BMS, grants and other from ASTRA ZENECA, grants from CHUGAI, from TAKEDA, other from NOVARTIS, other from PIERREFABRE, other from CLOVIS, during the conduct of the study. D.P. reports personal fees from ASTRAZENECA, personal fees from Boehringer, personal fees from BMS, personal fees from MSD, personal fees from NOVARTIS, personal fees from PFIZER, personal fees from CELGENE, personal fees from ROCHE, personal fees from PRIME ONCOLOGY, personal fees from PEER CME, outside the submitted work. B.M. reports personal fees from PFIZER, personal fees from ROCHE, personal fees from NOVARTIS, outside the submitted work. M.W. reports personal fees from BOERINGHER INGELHEIM, personal fees and non-financial support from ROCHE, personal fees and non-financial support from MSD, personal fees from BMS, personal fees from ASTRAZENECA, personal fees from AMGEN, outside the submitted work. J.C. reports honoraria from AstraZeneca/MedImmune, Bristol Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Boehringer Ingelheim and consulting or advisory Rôle for AstraZeneca/MedImmune, Roche/Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Takeda, Merck Sharp & Dohme, Pfizer, Lilly, Novartis. A.B.C. reports grant support, advisory board fees, lecture fees, and travel support from Novartis, advisory board fees, lecture fees, and travel support from Pfizer, AstraZeneca, and Takeda, grant support, advisory board fees, lecture fees, consulting fees, and travel support from Roche, and advisory board fees and lecture fees from Boehringer Ingelheim and MSD. F.G reports personal fees and non-financial support from BMS, personal fees from MSD/MERCK US, personal fees from ASTRAZENECA, personal fees from ROCHE, grants and personal fees from BOEHRINGER INGELHEIM, non-financial support from CHUGAI, non-financial support from PFIZER, outside the submitted work. J.-B.A. reports personal fees and non-financial support from ASTRAZENECA, personal fees and non-financial support from ROCHE, non-financial support from MSD, non-financial support from BOEHRINGER, personal fees and non-financial support from BMS, outside the submitted work. C.C. reports grants and personal fees from TAKEDA, grants from ROCHE, grants and personal fees from PFIZER, grants and personal fees from NOVARTIS, outside the submitted work. G.R.-B., L.G., P.D., P.S., É.D., M.G. and J.A. have nothing to disclose.
References
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Anjum, R.; Squillace, R.; Nadworny, S.; Zhou, T.; Keats, J.; Ning, Y.; Wardwell, S.D.; Miller, D.; Song, Y.; et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin. Cancer Res. 2016, 22, 5527–5538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoy, S.M. Brigatinib: A Review in ALK-Inhibitor Naïve Advanced ALK-Positive NSCLC. Drugs 2021, 81, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Tiseo, M.; Ahn, M.-J.; Reckamp, K.L.; Hansen, K.H.; Kim, S.-W.; Huber, R.M.; West, H.L.; Groen, H.J.; Hochmair, M.J.; et al. Brigatinib in Patients with Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2017, 35, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Camidge, D.R.; Tiseo, M.; Reckamp, K.L.; Hansen, K.H.; Kim, S.-W.; Huber, R.M.; West, H.J.; Groen, H.J.; Hochmair, M.J.; et al. Brigatinib (BRG) in crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): Updates from ALTA, A Pivotal Randomized Phase 2 Trial. J. Clin. Oncol. 2017, 35, e20503. [Google Scholar] [CrossRef]
- Huber, R.M.; Hansen, K.H.; Rodríguez, L.P.-A.; West, H.L.; Reckamp, K.L.; Leighl, N.B.; Tiseo, M.; Smit, E.F.; Kim, D.-W.; Gettinger, S.N.; et al. Brigatinib in Crizotinib Refractory ALK+ NSCLC: 2-Year Follow-up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J. Thorac. Oncol. 2020, 15, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in ALK Inhibitor Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 2021, 16, 2091–2108. [Google Scholar] [CrossRef]
- Bronte, G.; Cafaro, A.; Pasini, L.; Priano, I.; Andrikou, K.; Cravero, P.; Burgio, M.A.; Delmonte, A.; Crinò, L. Brigatinib in the first-line treatment of ALK+ metastatic NSCLC: Safety and efficacy. Expert Rev. Anticancer Ther. 2021, 21, 809–817. [Google Scholar] [CrossRef]
- Campelo, M.R.G.; Lin, H.M.; Zhu, Y.; Pérol, M.; Jahanzeb, M.; Popat, S.; Zhang, P.; Camidge, D.R. Health-related quality of life in the randomized phase III trial of brigatinib vs crizotinib in advanced ALK inhibitor-naïve ALK+ non-small cell lung cancer (ALTA-1L). Lung Cancer 2021, 155, 68–77. [Google Scholar] [CrossRef]
- Reckamp, K.; Lin, H.M.; Huang, J.; Proskorovsky, I.; Reichmann, W.; Krotneva, S.; Kerstein, D.; Huang, H.; Lee, J. Comparative efficacy of brigatinib versus ceritinib and alectinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive n-small cell lung cancer. Curr. Med. Res. Opin. 2019, 354, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Gandhi, L.; Gadgeel, S.; Riely, G.J.; Cetnar, J.; West, H.; Camidge, D.R.; Socinski, M.A.; Chiappori, A.; Mekhail, T.; et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016, 17, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.-H.I.; Ahn, J.S.; De Petris, L.; Govindan, R.; Yang, J.C.-H.; Hughes, B.; Lena, H.; Moro-Sibilot, D.; Bearz, A.; Ramirez, S.V.; et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J. Clin. Oncol. 2016, 34, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.-C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Duruisseaux, M.; Besse, B.; Cadranel, J.; Pérol, M.; Mennecier, B.; Bigay-Game, L.; Descourt, R.; Dansin, E.; Audigier-Valette, C.; Moreau, L.; et al. Overall survival with crizotinib and next generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): A French nationwide cohort retrospective study. Oncotarget 2017, 8, 21903–21917. [Google Scholar] [CrossRef] [Green Version]
- Heredia, D.; Barrón, F.; Cardona, A.F.; Campos, S.; Rodriguez-Cid, J.; Martinez-Barrera, L.; Alatorre, J.; Salinas, M.Á.; Lara-Mejia, L.; Flores-Estrada, D.; et al. Brigatinib in ALK-positive non-small cell lung cancer: Real-world data in the Latin American population (Bri-world extend CLICaP). Future Oncol. 2021, 17, 169–181. [Google Scholar] [CrossRef]
- Lin, H.M.; Pan, X.; Hou, P.; Allen, S.; Baumann, P.; Hochmair, M.J. Real-world treatment duration in ALK-positive non-small-cell lung cancer patients receiving brigatinib through the early access program. Future Oncol. 2020, 16, 1031–1041. [Google Scholar] [CrossRef]
- Descourt, R.; Perol, M.; Rousseau-Bussac, G.; Planchard, D.; Mennecier, B.; Wislez, M.; Cortot, A.; Guisier, F.; Galland, L.; Dô, P.; et al. Brigatinib in patients with ALK positive advanced non-small-cell lung cancer pretreated with sequential ALK inhibitors: A multicentric real-world study (BRIGALK study). Lung Cancer 2019, 136, 109–114. [Google Scholar] [CrossRef]
- Nishio, M.; Yoshida, T.; Kumagai, T.; Hida, T.; Toyozawa, R.; Shimokawaji, T.; Goto, K.; Nakagawa, K.; Ohe, Y.; Seto, T.; et al. Brigatinib in Japanese patients with ALK positive NSCLC previously treated with alectinib and other tyrosine kinase inhibitors: Outcomes of the phase 2 J-ALTA trial. J. Thorac. Oncol. 2021, 16, 452–463. [Google Scholar] [CrossRef]
- Lin, J.J.; Zhu, V.W.; Schoenfeld, A.; Yeap, B.Y.; Saxena, A.; Ferris, L.A.; Dagogo-Jack, I.; Farago, A.F.; Taber, A.; Traynor, A.; et al. Brigatinib in Patients with Alectinib-Refractory ALK-Positive NSCLC. J. Thorac. Oncol. 2018, 13, 1530–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popat, S.; Brustugun, O.T.; Cadranel, J.; Felip, E.; Garassino, M.C.; Griesinger, F.; Åslaug, H.; Hochmair, M.; Pérol, M.; Bent-Ennakhil, N.; et al. Real-world treatment outcomes with brigatinib in patients with pretreated ALK+ metastatic non-small cell lung cancer. Lung Cancer 2021, 157, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hochmair, M.; Fabikan, H.; Illini, O.; Weinlinger, C.; Setinek, U.; Krenbek, D.; Prosch, H.; Rauter, M.; Schumacher, M.; Wöll, E.; et al. Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis. Pharmaceuticals 2020, 13, 371. [Google Scholar] [CrossRef]
- Lee, P.-H.; Chen, K.-C.; Hsu, K.-H.; Huang, Y.-H.; Tseng, J.-S.; Yang, T.-Y.; Chang, G.-C. Real-world efficacy and safety of lorlatinib in treating advanced ALK-positive non-small cell lung cancer patients. Anticancer Drugs 2021, 32, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, C.; Addeo, A.; Rechsteiner, M.; Delaloye, R.; Früh, M.; Metro, G.; Banini, M.; Gautschi, O.; Rothschild, S.I.; Wild, P.J.; et al. Real-World Treatment Patterns and Survival Outcome in Advanced Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small-Cell Lung Cancer Patients. Front. Oncol. 2020, 10, 1299. [Google Scholar] [CrossRef]
- Zhu, V.W.; Lin, Y.-T.; Kim, D.-W.; Loong, H.H.; Nagasaka, M.; To, H.; Ang, Y.L.-E.; Ock, C.-Y.; Tchekmedyian, N.; Ou, S.-H.I.; et al. An International Real-World Analysis of the Efficacy and Safety of Lorlatinib Through Early or Expanded Access Programs in Patients with Tyrosine Kinase Inhibitor-Refractory ALK-Positive or ROS1-Positive NSCLC. J. Thorac. Oncol. 2020, 15, 1484–1496. [Google Scholar] [CrossRef]
- Song, L.; Xu, Q.; Lizaso, A.; Zhang, Y. Brigatinib After Progression from Alectinib or Crizotinib: Paving the Way for Treatment Sequencing of ALK Inhibitors in ALK-Positive NSCLC. J. Thorac. Oncol. 2021, 16, 349–351. [Google Scholar] [CrossRef]
- Haratake, N.; Toyokawa, G.; Seto, T.; Tagawa, T.; Okamoto, T.; Yamazaki, K.; Takeo, S.; Mori, M. The mechanisms of resistance to second- and third-generation ALK inhibitors and strategies to overcome such resistance. Expert Rev. Anticancer Ther. 2021, 21, 975–988. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Besse, B.; Bauer, T.M.; Lin, C.-C.; Soo, R.A.; Riely, G.J.; Ou, S.-H.I.; Clancy, J.S.; Li, S.; et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 1370–1379. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Chiang, C.-L.; Hung, J.-Y.; Lee, M.-H.; Su, W.-C.; Wu, S.-Y.; Wei, Y.-F.; Lee, K.-Y.; Tseng, Y.-H.; Su, J.; et al. Resistance profiles of anaplastic lymphoma kinase tyrosine kinase inhibitors in advanced non-small-cell lung cancer: A multicenter study using targeted next-generation sequencing. Eur. J. Cancer 2021, 156, 1–11. [Google Scholar] [CrossRef]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagogo-Jack, I.; Rooney, M.; Lin, J.J.; Nagy, R.J.; Yeap, B.Y.; Hubbeling, H.; Chin, E.; Ackil, J.; Farago, A.F.; Hata, A.N.; et al. Treatment with next-generation ALK inhibitors fuels plasma ALK muttion diversity. Clin. Cancer Res. 2019, 25, 6662–6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).