Simple Summary
Transplantation of adult hematopoietic stem cells is an important therapeutic tool to help patients suffering from diverse hematological disorders. All types of blood cells can develop from a single hematopoietic stem cell underlining their enormous potential. Intense efforts are ongoing to generate “engraftable” human hematopoietic stem cells to treat hematopoietic diseases and to understand the molecular machinery driving them. Leukemic stem cells represent a low frequency subpopulation of leukemia cells that possess stem cell properties. They can instigate, maintain, and serially propagate leukemia in vivo, while they retain the capacity to differentiate into committed progenitors. Leukemic stem cells are unaffected by many therapeutic strategies and represent the major cause of relapse. We here describe all methods to maintain and expand murine and human hematopoietic cells in culture and describe their specific advantages. These methods are also employed to understand the biology of leukemic stem cells and to identify novel therapeutic strategies.
Abstract
Hematopoietic stem cells (HSCs) are rare, self-renewing cells that perch on top of the hematopoietic tree. The HSCs ensure the constant supply of mature blood cells in a tightly regulated process producing peripheral blood cells. Intense efforts are ongoing to optimize HSC engraftment as therapeutic strategy to treat patients suffering from hematopoietic diseases. Preclinical research paves the way by developing methods to maintain, manipulate and expand HSCs ex vivo to understand their regulation and molecular make-up. The generation of a sufficient number of transplantable HSCs is the Holy Grail for clinical therapy. Leukemia stem cells (LSCs) are characterized by their acquired stem cell characteristics and are responsible for disease initiation, progression, and relapse. We summarize efforts, that have been undertaken to increase the number of long-term (LT)-HSCs and to prevent differentiation towards committed progenitors in ex vivo culture. We provide an overview and compare methods currently available to isolate, maintain and enrich HSC subsets, progenitors and LSCs and discuss their individual advantages and drawbacks.
1. The Adult HSC—A Rare, Self-Renewing Cell
Bone marrow (BM) cell transplantation studies and ex vivo colony formation assays identified the presence of HSCs in the 1950s and 1960s [1,2,3]. Hematopoietic stem cells were functionally defined by their ability to serially engraft transplanted recipients and to replenish all myelolymphoid lineages, through their ability to self-renew and differentiate [4]. These transplantation studies revealed the heterogeneity of the HSC compartment, as HSC subclones were able to repopulate lethally irradiated mice ranged from short term (weeks to months, referred as short term (ST-) HSCs) to long term (more than 6 months, referred as LT-HSCs) [4,5]. Long term HSCs are predominantly in a quiescent/dormant state reflecting their steady-state conditions in the BM [6]. Short term HSCs possess the ability to switch from an active, ready-to-proliferate state back to dormancy [6] (Figure 1).
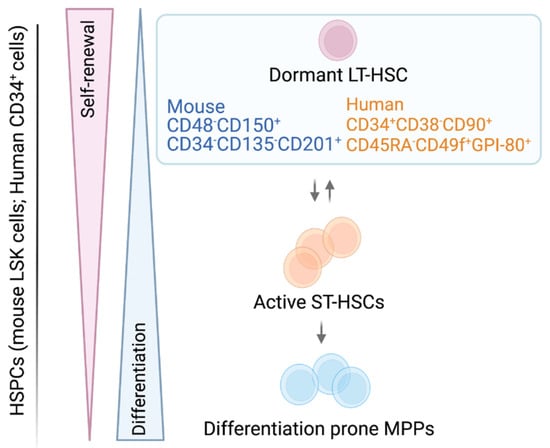
Figure 1.
Schematic model of hematopoietic stem and progenitor cells. Surface marker nomenclature for murine/human LT-HSCs cell populations are depicted. The LT-HSCs are activated and transit to ST-HSCs, which in turn gradually commit to more differentiation-prone progenitors. Self-renewal and differentiation are strictly balanced in stem and progenitor cells.
Hematopoietic stem cells are rare and represent only 0.005% to 0.01% of all nucleated BM cells. They are isolated based on the expression of a distinct pattern of cell surface markers. Long term HSCs are immune-phenotypically well characterized, while the heterogeneous ST-HSC/(multipotent progenitor)MPP pool lacks a widely used, standardized surface marker expression scheme [7]. Integrating current knowledge, murine HSCs are most commonly first negatively selected for lineage-specific markers (lin−), combined with positive selection for cKIT and SCA-1 surface expression by flow cytometry. This procedure results in a cell population called (lin− SCA-1+ c-KIT+ (LSK)) cells. Within the LSK cell pool, long-term multilineage reconstituting LT-HSCs are highly enriched in the LSK CD48− CD150+ CD34− or low CD135− CD201+ fraction, while metabolically active ST-HSCs mainly reside in the LSK CD48− or low CD150− CD34+ CD135− CD201− fraction. The situation is more complex for the heterogeneous MPPs, which express distinct lineage biases while retaining a degree of plasticity. Different combinations of surface marker expressions and naming strategies exist (LSK CD48− or + CD150− or + CD34+ CD135− or + CD201−) (Figure 1) [6,8,9,10,11,12,13,14,15,16,17] (Table 1). Further dissection and definition of the ST-HSC/MPP pool, aiming to standardize immune-phenotypic and functional characteristic of the distinct MPP populations were recently reviewed by Challen et al. [7].
In human BM and cord blood (CB), a lin− CD49f+ CD90+ CD45RA− CD34+ CD38− CD133+ CD201+ GPI-80+ surface profile is most commonly used to define LT-HSCs with multilineage reconstitution potential, while an universal definition for human MPPs is still lacking [18,19,20,21,22,23,24] (Table 1, Figure 1).
With the technological breakthrough of single-cell transcriptomics, it has become evident that predefined flow sorted stem and progenitor populations are only snapshots of HSC differentiation, which is rather a continuous process consisting of metastable cell states. Individual HSCs gradually acquire linage biases without passing through discrete hierarchically organized progenitor populations, suggesting that there is no obvious boundary between LT-, ST-HSCs and MPPs [25,26,27].

Table 1.
Cell surface markers of murine or human LT- and ST-HSCs and progenitors; some markers can be used for both species.
Table 1.
Cell surface markers of murine or human LT- and ST-HSCs and progenitors; some markers can be used for both species.
| HSC Surface Markers | |||||
|---|---|---|---|---|---|
| Murine | Refs. | Human | Refs. | ||
| CD19, CD45R CD11b, Ly-6G CD3 | Lineage negative selection (Lin−) | [28,29,30] | CD45RA | CD45 isoform with specific molecular weight | [31] |
| CD117 | Type III transmembrane tyrosine kinase receptor (c-KIT) | [29,32,33,34] | CD38 | Cyclic ADP ribose hydrolase | [31,35] |
| SCA-1 | Lymphocyte activation protein-6A (Ly-6A/E) | [29,30,32,33] | CD49f | Integrin α-6 | [20,31] |
| CD48, CD150 | Signaling lymphocyte activation molecule (SLAM) family protein | [9,11,12,16] | CD90 | Thy1 | [30,31,36] |
| CD34 | Transmembrane phosphor-glycoprotein | [9,16,33,37] | CD34 | Transmembrane phosphor-glycoprotein | [29,36,38,39] |
| CD201 | Endothelial protein C receptor (EPCR) | [40,41] | CD201 | Endothelial protein C receptor (EPCR) | [41,42] |
| CD135 | Fms-like tyrosine kinase 3 receptor (FLT3-R); FLK2 | [9,16,33] | CD133 | AC133, Prominin-1 | [43,44] |
| GPI-80 | Glycosylphosphatidyl Inositol-Anchored Protein GPI-80 | [21,23] | |||
2. Leukemic Stem Cells (LSCs)—Villain to Its Heathy Counterpart
Certain leukemias are hierarchically organized with LSCs being on the top, analogous to the hematopoietic tree. Like HSCs, LSCs possess typical stem cell characteristics including the ability for dormancy or self-renewal [45]. The LSCs are responsible for the initiation, progression and relapse of acute myeloid leukemia (AML) or chronic myeloid leukemia (CML) [45,46,47]. Their disease-initiating capacity term them also ‘leukemia-initiating cells’ (LICs). In AML and CML, LSCs can switch back to a quiescent state to evade chemotherapeutics [46,48,49,50]. This leaves LSCs frequently unaffected by therapeutic strategies and may cause relapse [51,52,53].
In AML, in vivo leukemia initiation studies are the gold-standard functional assays to define LSCs. Leukemia development directly correlates with the number of AML-LSCs in the primary sample and predicts clinical outcome [54,55,56]. This functional criterion is not shared by CML-LSCs, as a vast majority of primary samples from chronic phase (CP)-CML patients do not engraft in immunocompromised mice. This may be related to the lower mutational burden in CP-CML, compared to blast phase (BP)-CML, which mirrors an acute leukemia [57,58,59].
The LSCs predominantly belong to the CD34+ CD38− compartment [46,47,60,61]. The availability of severely immunocompromised mouse strains unveiled the potential of leukemia initiation of AML-LSCs. The LICs are not restricted to the CD34+ CD38− cells but are also found in the CD34− fractions [62,63]. Transcriptome analysis and studies of the differentiation capacities of CD34+ and CD34− AML LSCs revealed that progenitor as well as mature cells may serve as the origin of LSCs, through an acquired ability to self-renew [64,65,66,67]. In this respect, the more advanced stage of BP-CML mirrors an AML, in which LSC activity is found within CD34+ and CD34− populations [64,68,69]. In contrast, LSCs in CP-CML arise from cells with high inherent self-renewal, such as CD34+ CD38− HSCs, as the driver mutation (BCR–ABL1) impairs self-renewal [70]. Besides CD34 and CD38, a growing list of AML and CML LSC-selective cell surface markers were identified, enabling classification of LSCs (Table 2).

Table 2.
LSC-specific cell surface markers present both in AML and CML cells.
3. BM Niche of LT/ST-HSCs and LSCs
The LT/ST-HSCs reside in specialized niches that in the cavities of the trabecular regions in long bones. Multiple cellular types, soluble factors and components of the extracellular matrix form these niches [90,91]. The endosteal niche, located at the endosteal surface of the bone is characterized by bone regenerating osteoblasts (OBs) [92]. The OB cells regulate LT-HSCs quiescence by producing CXC motif chemokine 12 ligand (CXCL12), transforming growth factor β (TGFβ) and angiopoitin-1 (ANG-1) [90]. The arteriolar niche is located aside vascular structures and brings LT/ST-HSCs in close proximity to endothelial cells (ECs) and mesenchymal stromal/stem cells (MSCs) [93]. The ECs regulate dormancy and self-renewal via cell-cell contacts (e.g., via E-selectin) and by expression of stem cell factor (SCF), CXCL12 and Notch ligands [11,90,94,95,96]. The MSCs play a dominant part and are subdivided into Leptin+, CXCL12 abundant reticular (CAR) or Nestin+ cells, which provide soluble factors for HSC maintenance including SCF, thrombopoietin (TPO), CXCL12, fibroblast growth factor 2 (FGF-2) and WNT ligands [90,92,94,97,98,99]. The peripheral sympathetic nerves, ensheathed by non-myelinating Schwann cells (NMSCs), constitute another component of the arteriolar niche. Circadian adrenergic signals from nerve terminals regulate the production of CXCL12 in Nestin+ MSCs. Approximately 20% of HSCs are in direct contact with NMSCs, which are maintaining LT-HSC quiescence by producing TGF-β [100]. When activated, ST-HSCs relocate to the Leptin+ MSCs containing perisinusoidal area [100]. Adipocytes, pericytes, fibroblasts, macrophages and megakaryocytes are also part of the BM niche and modulate functions of HSCs [90].
The LT/ST-HSCs and niche cells can interact through juxtacrine signaling (or contact-dependent signaling) via N-cadherin, vascular cell adhesion molecule 1 (VCAM1)/very late antigen-4 (VLA-4), cKIT/membrane bound SCF or NOTCH receptor/ligand [101,102,103]. Niche cells provide extracellular matrix (ECM) proteins including glycoproteins (e.g., fibronectin), glycosaminoglycans (e.g., hyaluronic acid), collagen IV and matrix remodeling enzymes. The LT/ST-HSCs bind ECM proteins mainly via integrins, which triggers intracellular signals. Of note, the extent of signaling may be modulated by the “stiffness” of the surrounding via mechanotransduction. Stiffness, composition and location of the niche are crucial to modulate HSC behavior [104,105,106,107]. Besides signaling through integrins, ECM embeds LT/ST-HSCs in their niche and provides a reservoir for soluble factors. These factors and cell-matrix interactions impact the balance between stemness and differentiation [97].
In AML and CML, the BM is tightly packed with malignant hematopoietic cells causing disturbed niche structures and hematopoiesis [108,109]. The leukemic blasts occupy and rearrange the BM niches to establish self-protective niches. This process is regulated by stromal-secreted chemokines, CXCL12 and the CXCR4 receptor and creates a “reduced” version of the conventional BM niche [110]. It impairs normal homeostasis and promotes disease progression [45,109,111]. In this protective environment LICs/LSCs are less amenable to chemotherapeutics [45,73,108,112]. Attempts to mimic the BM niche in vitro by 2-3 D techniques try to reduce the transitional gap between in vitro and in vivo research [113,114].
4. Essential Factors for HSC Quiescence and Self-Renewal—Cell Cycle Components as Mediators
The LT/ST-HSCs are capable of self-renewal or differentiation. Under homeostatic conditions, most LT-HSCs exist in the G0 phase of the cell cycle and are considered “quiescent”. Signals from the BM preserve the quiescent state and protect from cell damage and exhaustion [115]. Quiescent HSCs are fundamental for transplantation and provide the potential for long-term engraftment [116].
Symmetric cell division results in two identical daughter cells, that either keep stem cell properties or differentiate. During asymmetric cell division, one daughter cell preserves stem cell characteristics, while the other one undergoes differentiation. During asymmetric division, cell fate determinants are unequally distributed, like tyrosine-protein kinase receptor 2 (TIE2) or NOTCH1. Similarly, active mitochondria, lysosomes and autophagosomes are unevenly shared. This procedure gives rise to a metabolically active, differentiated and short-lived progenitor cell and a metabolically less active, undifferentiated and long-lived HSC progeny [117,118,119,120].
Cell cycle regulators are critical factors in maintaining HSC quiescence and re-inducing proliferation. The cyclin C/cyclin dependent kinase 3 (CDK3) complex regulates G0 phase, while G1 is controlled by cyclin D/CDK4 and CDK6 complexes [121,122]. The balance of CDKs and cyclin dependent kinase inhibitors (CDKIs) control the transition from G0 to G1.
The G1 -specific CDKIs comprise of two families. The CIP/KIP family consists of p21 (p21CIP, CDKN1A), p27 (p27KIP, CDKN1B) and p57 (p57KIP2, CDKN1C). The second CDKI family includes four INK4 members: p15, p16, p18 and p19 (CDKN2A-D). The INK4 family members bind to CDK4/CDK6 and inhibit their kinase activities by interfering with their association with D-type cyclins. In contrast, the CIP/KIP family members bind and inhibits both cyclin and CDK subunits [121,123]. In the absence of CDK4/6 kinase activity, retinoblastoma (RB) proteins (RB, p107, p130) remain under-phosphorylated. Thus, they bind to E2F transcription factors and prevent them from being active. Entry into S phase and cell cycle progression are inhibited [121]. Among all CDKIs, p57 has the highest expression in LT-HSCs. Members p57 and p27 maintain HSC quiescence by preventing nuclear translocation of HSC70/cyclin D1 and consequent activation of CDK4/6 [124]. From the INK4 family, p15 and p18 have been found to negatively regulate HSC ex vivo expansion [125,126,127]. During genotoxic stress states and aging, p19 preserves HSCs in a quiescent state protecting them from apoptosis [128].
Pathways triggered by extracellular signals frequently converge on cell cycle regulators (cyclins, CDKs, CDKIs, transcription factors, microRNA, etc.) [115,129,130]. The SCF/c-KIT, TPO/c-MPL and NOTCH ligands/NOTCH1-4 signaling support HSC survival, self-renewal and regeneration [131,132,133,134]. The CXCL12/CXCR4, TGF-β/TGFβR and ANG-1/TIE1/2 signaling is essential to maintain HSCs in a quiescent state [100,135,136,137] (Figure 2).
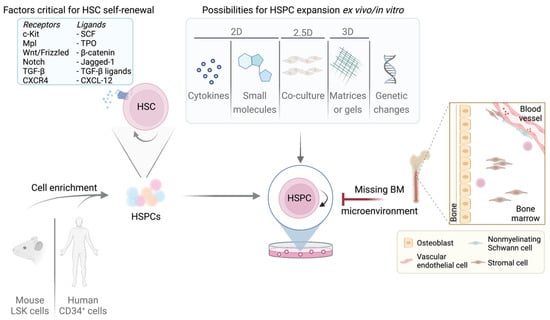
Figure 2.
Overview of murine/human LT/ST-HSCs expansion possibilities and limitations. Self-renewal in LT/ST-HSCs is strictly regulated by multiple factors. The most important receptors and their corresponding ligands are listed. Purified LT/ST-HSCs can be cultured by various methods summarized in the box. Adult LT-HSCs reside in the BM niche which is absent upon cultivation, presenting the greatest limitation and challenge.
Stimulation of c-KIT and MPL activates signal transducers and activators of transcription (STAT), mitogen activated protein kinase (MAPK) and phosphoinositide 3-kinases (PI3K)/Akt pathways to enhance HSC survival and expansion ex vivo [138,139,140,141,142,143,144,145]. The TPO drives the expression of two negative cell-cycle regulators (p57, p19) and the transcription factor homeobox B4 (HOXB4), a potent promoter of LT-HSC self-renewal [146,147].
Activation by STAT5 typically drives cell survival and proliferation but may also mediate quiescence through driving the expression of TIE2, p21 and p57 [148,149]. The hypoxia-inducible factor 2 alpha (HIF2α) is a direct STAT5 target gene, which upregulates c-MYC, VEGF and glucose metabolism. Under hypoxia, STAT5 can impose a long-term proliferative advantage on the CD34+/CD38− HSC population, but not on progenitors [150]. Thus, depending on niche signals, STAT5 links self-renewal to a quiescence-typical metabolic profile and cell cycle arrest.
NOTCH signaling promotes LT-HSC self-renewal and inhibits differentiation [95,151]. Unlike secreted niche factors, NOTCH signaling is a juxtacrine communication pathway between NOTCH ligands (Dll1, Dll4, Jagged1, or Jagged2) and NOTCH receptors (NOTCH1-4) expressing cells. Ligand-bound NOTCH receptor is cleaved, releasing the NOTCH intracellular domain, which translocates to the nucleus and alters gene transcription. NOTCH signaling provides direct transcriptional downregulation of p57 and upregulation of several genes that are important for HSC activation, including HES1, GATA2, cMYC [152,153,154,155].
Many important signaling pathways, such as WNT/beta-catenin, MAPK, PI3K/AKT/GSK-3 and JAK/STAT regulate expression of c-MYC. As a transcription factor, c-MYC antagonizes p21 and p27 activity by inducing the expression of D-type cyclins, thus enabling the formation of cyclin D-CDK4/6 complexes [156,157]. In fact, WNT signaling has been mostly characterized in dividing cells. However, LT-HSC appear to have the highest percentage of WNT activity, whereas MPPs have the lowest. This indicates that WNT signaling enforces quiescence, probably by upregulation of p21 [158,159].
The CXCL12 (also termed stromal cell-derived factor 1, SDF1) has pleiotropic effects. It is not only a chemoattractant for HSC homing, but also a regulator of HSC quiescence as it upregulates p57 and limits generation of reactive oxygen species (ROS) and genotoxic stress [136,160]. Similarly, TGF-β potently inhibits HSC proliferation, regulates quiescence, and protects HSCs from excessive differentiation signals. It does so by downregulating cyclin D2 and upregulating p15, p21, p27 and p57 [125,137,161,162,163,164,165]. The P53 is highly expressed in LT-HSCs. It regulates quiescence by inducing p21 expression and driving the expression of the transcriptional repressors GfI-1 and Necdin [166,167]. Necdin directly inhibits E2F1, while GFI-1 decreases the expression of inhibitor of DNA binding and differentiation-2 (ID2), an inhibitor of RB and repressor of CDKI p21 and p27 [168,169,170]. Upon activation, p53 is repressed by the ETS family transcription factor, MEF/ELF4, enabling entry into the cell cycle [171].
Among other transcriptional regulators, MEF/ELF4 and CDK6 regulate the exit from dormancy, while PBX-1 and EVI-1 maintain LT-HSC self-renewal [171,172,173,174,175].
Abnormal activation of HSC signaling pathways induce cell cycling, exhaustion or development of leukemia [176]. Hematopoietic challenges such as inflammation, BM transplantation or oncogenic transformation also trigger activation and proliferation of LT/ST-HSCs [177,178].
5. Signaling and Metabolic Changes in LSCs
Malignant transformation is caused by genetic or epigenetic alterations due to hereditary or environmental conditions. The fusion protein BCR–ABL1, a constitutively active tyrosine kinase, is the unique hallmark and main driver of CML leukemic cells and LSCs, as it is present in >90% of the patients [179]. Both BP-CML and AML are genetically more complex and heterogeneous with subgroups showing individual mutations. Early mutations improve the potential to self-renew and may impair differentiation leading to heterogeneously expanded clones of pre-leukemic HSCs in patients [180,181,182]. Late co-expressing mutations occur in signaling pathways (e.g., FLT3), promote proliferation and enhance block of differentiation [183,184]. The molecular defects underlying AML are complex with at least 24 different genetically defined subtypes [185]. Expression of AML-associated mutations and fusion genes involve transcription factors or epigenetic regulators, such as DNMT3A, IDH1/2 and TET2 mutations or MLL-, NUP98- and AML1-fusions to name few. Co-expression of mutations in tyrosine kinases and other signaling mediators, such as FLT3- N/K-RAS-, KIT- mutations support proliferation leading to a more aggressive disease progression [186].
Common mRNA and epigenetic signatures are found in AML LSCs irrespective of the oncogenic driver or immunophenotype. Ng and colleagues identified a panel of 17 genes (called LSC17), which are highly expressed in LSCs (relative to the bulk of AML cells). High LSC17 expression reflects stemness properties of LSCs and resistance to standard AML therapy. The LSC signature genes include GPR56, AKR1C3, CD34, EMP1, SMIM24, SOCS2, CPXM1, CDK6, KIAA0125, DPYSL3, MMRN1, LAPTM4B, ARHGAP22, NYNRIN, ZBTB46, DNMT3B and are predictive and/or prognostic biomarkers [187,188].
Sachs et al. demonstrated that transcriptional profiles of self-renewal and proliferation are distinct in AML LSCs. LSC-specific self-renewal signature (CD69, S100A4, MYB, ADA, MRI1, CKS2) and proliferation genes (H2AFZ, BCL2A1D, CD36) were identified based on high expression in AML LSCs relative to normal hematopoietic stem/progenitor cells (HSPC)s. Using cell surface markers, CD69 and CD36 allowed the isolation of different subsets of LSCs. The CD69high LSCs were capable of self-renewal and poorly proliferative, whereas the CD36high LSCs did not inflict leukemia and were highly proliferative [89]. These genes were not found as a signature in normal HSPCs and may represent a unifying feature for the identification of LSCs [62,89,187,189].
The HSCs rely on glycolysis in the hypoxic BM microenvironment, rather than oxidative phosphorylation (OXPHOS) [190]. In contrast, AML and CML LSCs have higher mitochondrial mass and an increased oxygen consumption rate with a greater dependency on mitochondrial function and OXPHOS [191]. Mitochondrial respiration generates high levels of ROS in bulk CML and AML blasts relative to LT/ST-HSCs [192,193]. High ROS levels can induce oxidative DNA damage, high mutational burden and genomic instability, which may impair stem cell function [194,195,196,197]. Quiescent AML LSCs generally have low ROS levels compared to cycling LSCs and bulk AML cells [198]. These cells may revert to glycolysis or using mitophagy to reduce their dependency on mitochondrial respiration [199,200]. Quiescent low-ROS AML LSCs frequently overexpress BCL-2 and are dependent on amino acid uptake and OXPHOS [198,200]. Besides mitochondrial respiration and ROS amounts, levels of glucose, amino acid and free fatty acid are altered in LSCs [191,198,201]. Therefore, ex vivo expansion of LSCs requires specific conditions to allow biomarker discovery, drug development, identification of resistance mechanisms and combination treatments.
6. Major Challenges Culturing LT-HSCs
Maintaining and expanding LT/ST-HSCs ex vivo are required for curative transplantation therapies and to allow the study of molecular mechanisms [202,203]. The long-term goal is to further optimize application methods for clinical hematopoietic stem cell transplantation (HSCT).
The effects of cell culture stress on HSCs induces several changes including loss of polarization, accumulation of reactive oxygen species, endoplasmic reticulum stress, genotoxic stress, replicative stress, disturbed protein homeostasis and ultimately loss of HSC functions [204,205,206,207,208,209,210,211]. As a result of these cell intrinsic changes occurring during ex vivo culture, the number of LT/ST-HSCs declines over time accompanied by an increase of myeloid potential [206,207,211]. To reduce environmental stress and preserve stem cell functions, enhanced proteostasis, a dynamic maintenance of proteome integrity, is particularly important in cultured HSCs [207]. Defining culture conditions favoring expansion of LT/ST-HSCs while maintaining their fitness, still represent a major hurdle.
7. Maintaining Quiescent LT-HSCs
The BM has limited oxygen supply; the most quiescent LT-HSCs reside in a “hypoxic niche” where blood perfusion and oxygen tension are low [212,213,214,215]. In vitro hypoxic cultures with 1–3% oxygen enhance LT/ST-HSC expansion and subsequent engraftment [216,217,218,219]. Under normoxia (20% O2), the maintenance of self-renewal requires the presence of high cytokine concentrations. Still, normoxia favors differentiation over self-renewal [220].
Kobayashi et al. defined a minimal set of factors that mimic the physiological conditions in the BM microenvironment and maintain LT-HSCs in a quiescent, but still engraftable state for 1 month. Murine LT-HSCs need low concentrations of cytokines (3 ng/mL SCF, 0.1 ng/mL TPO), hypoxia (1% O2) and 4% bovine serum albumin (BSA). Supplementing fatty-acids in an albumin-bound form is crucial to avoid intrinsic fatty acid synthesis, which is triggered by hypoxia and low cytokine concentrations [220]. Intrinsic fatty acid synthesis would interfere with HSC survival [221]. Under low cytokine, hypoxia, 4% BSA conditions, differentiation is suppressed and more than 60% of cells remain functional CD150+ CD48− LT-HSCs. A gradual decrease of HSC markers including CD150 and EVI1 was only observed after a month of culture. Human LT-HSCs were also maintained under comparable hypoxic conditions in 4% BSA with low cytokines, supplemented with fatty acids and cholesterol. ~90% of the cells exhibited a CD34+ CD38− marker phenotype and 40% a CD90+ CD45RA− phenotype, reflecting minimal differentiation [220].
Retinoic acid (RA) signaling is high in dormant LT-HSCs compared to ST-HSCs and MPPs [9]. Retinoic acid is produced by two sequential oxidation steps from dietary vitamin A (retinol). The biological active derivative ATRA signals through the retinoic acid receptor (RAR) and the retinoid X receptor (RXR) families [222,223]. The ATRA restrains c-MYC expression, and inhibition of MYC activity partially mimics the preservation of dormancy by ATRA. Treatment with ATRA, retinol or MYC inhibitor retains LT-HSC quiescence ex vivo in serum free, cytokine supplemented (SCF, TPO, FLT3L) media by downregulating G2M checkpoints, E2F targets, ROS species and c-MYC targets compared to untreated cells [224].
Recently, purified single mouse (CD45+ EPCR+ CD48− CD150+ SCA-1high) and human (CD34+ CD38− CD90+ CD45RA− CD19− CD49f+) LT-HSCs were maintained in a hibernated (hibHSC), non-proliferative state under minimal cytokine conditions (only interleukin-11 (IL-11)) over a 7 days period [225,226,227]. Large proportions of hibHSCs survived without dividing and retained their functional properties, as determined by single-cell transplantation. Stress response pathways together with glycolysis, fatty acid biosynthesis, cAMP and mTOR signaling pathways were upregulated in hibHSCs, most presumably because of nutrient withdrawal and limited cytokine availability [225]. The development of a “quiescent LT-HSC ex vivo system” will open an avenue to study steady-state LT-HSC properties and effects of targeted manipulation.
8. Expansion Techniques to Retain LT/ST-HSC Phenotype Ex Vivo
8.1. 2D Methods
8.1.1. Suspension Culture of Murine LT/ST-HSCs
Ex vivo culture approaches try to mimic physiological conditions in the BM and provide growth factors and cytokines to maintain quiescence or induce proliferation. Early attempts did not include any supporting BM cells but relied on media supplements like cytokines and growth factors (Figure 2).
Later, in the 1990s, 5-fluorouracil (5-FU) treated mice were used as a source for collecting and culturing LT/ST-HSCs. They were kept in 20% fetal calf serum (FCS) (or without serum), 1% BSA, SCF, FLT3/FLK-2 ligand (FLT3L) and IL-11 containing media [228]. Frequently, IL-11 is replaced by IL-6 or IL-12 [229]. Further supplements included ITS-X (insulin, transferrin, selenium, ethanolamine) or low-density lipoproteins [229,230,231,232]. Under these conditions, the LT/ST-HSCs maintained their ability to reconstitute lethally irradiated recipient mice for up to three weeks but lost it upon addition of IL-3 and/or IL-1 to the culture medium [228,232,233,234]. In contrast, addition of TPO to mouse BM cells enhanced the number of LT/ST-HSCs and increased the efficiency of BM reconstitution [235].
The advanced knowledge about HSC self-renewal and its regulation by niche components allowed for the development of novel ex vivo expansion approaches using different combinations of supplements [236,237,238]. The BM niche cells (e.g., ECs) stimulate self-renewal of LT/ST-HSCs by inducing NOTCH signaling [151,239]. Using the engineered NOTCH ligand Delta1ext-IgG, mouse LSK cells were successfully cultured in 20% FBS, SCF, FLT3L, IL-6 and IL-11 supplemented media up to 42 days investigated [240,241]. The immobilized Delta1ext-IgG (composed of the extracellular domain of Delta1 joined to a Fc part of human immunoglobulin G1) accelerates the expansion of LSKs and the rate of T-cell reconstitution after transplantation [241,242].
Ieyashu et al. showed that interleukin-1α (IL-1α) and hemopexin (HPX) in serum-free, but BSA-containing medium supports the maintenance of LT/ST-HSCs [231]. The heme-binding plasma glycoprotein HPX is expressed on NMSCs in the BM niche and prevents heme-mediated oxidative stress and dampens intracellular ROS levels [243]. The use of SCF and TPO together with IL-1α and HPX provides a highly reproducible ex vivo mouse LT/ST-HSC expansion culture system [231].
One of the most frequently used media to enrich LT/ST-HSCs was developed by the group of Lodish. It includes serum-free medium with SCF, TPO, FGF-1, IGF-2, and heparin, resulting in an 8-fold increase of mouse LT-HSCs in three weeks of culture [145,230]. Addition of angiopoietin-like proteins (ANGPTLs: ANGPTL2, ANGPTL3) to the mixture of SCF, TPO, IGF-2 and FGF-1 revealed a roughly 50-fold increase in numbers of repopulating mouse LT-HSCs [230,244]. Although previous protocols used similar media reagents, Lodish’s specific combinations favored proliferation of LT-HSCs over ST-HSCs and prevented them from being outcompeted during long-term culturing.
Another critical feature for successful maintenance of LT/ST-HSCs ex vivo is the use of appropriate BSA, which is a component of most protocols containing fetal bovine serum (FBS). The quality and composition of FBS varies between batches, which leads to differences in culture conditions and affects self-renewal [231,245]. In general, differences in serum and BSA concentrations modulate the bio-availability of cytokines and growth factors based on binding to serum albumin [246]. To standardize culture conditions, BSA was replaced by polyvinyl alcohol (PVA), a synthetic amphiphilic polymer, which stabilizes cytokines. In the presence of PVA, 100 ng/mL TPO and 10 ng/mL SCF are considered optimal for murine LT/ST-HSC culture [226]. This represents a significant improvement and enables long term culture (1- to 2-month) with an up to 900-fold expansion of functional lin− c-KIT+ SCA-1+ CD150+ CD34− LT-HSCs [145,226,227,228,232,247,248].
8.1.2. Suspension Culture of Human LT/ST-HSCs
Human cord blood CB-derived hematopoietic stem cells (CB-HSCs) are an important source for HSC transplantations. Their numbers are low in vivo, which requires expanding CB-HSCs ex vivo while preserving their stemness properties for effective application in transplantation and gene therapy. Several promising protocols for serum-free cultivation of human LT/ST-HSCs using combinations of cytokines or small molecules have been described (Figure 2).
Commonly used cytokines for expansion of CD34+ HSCs include SCF, TPO, FLT3L, granulocyte colony-stimulating factor (G-CSF), IL-6 IL-3 and FGF-1 [249,250,251]. Using a cytokine cocktail (SCF, TPO, FLT3L and FGF-1) supplemented with insulin-like growth factor-binding protein 1/2 (IGFBP1/2) and ANGPTL5, increased the number of human CD34+ CD38− CD90+ CD133+ CB stem cells. These cells repopulate NOD-SCID mice with a ∼20-fold higher efficiency than non-cultured HSCs [249,251].
Developmental regulators such as NOTCH ligand Delta-1, pleiotrophin, StemReginin-1, UM171, resveratrol, nicotinamide and valproic acid (VPA) were reported to further enhance CD34+ HSPCs expansion over 50 fold [250,252].
The FDA approved small molecular weight compounds StemReginin 1 (SR-1) or UM171 are used in addition to cytokines (SCF, TPO, FLT3L and IL-6) to expand human CD34+ HSCs ex vivo [253,254,255]. The SR-1 antagonizes the aryl hydrocarbon receptor (AHR), which regulates hematopoiesis through regulation of HES-1, c-MYC, C/EBP, PU.1, β-catenin and CXCR4 [256]. The Ahr knockout mice have increased numbers of HSCs with a higher proliferative rate and accumulation of plasmacytoid dendritic cells (pDCs) [256,257]. In line, antagonizing AHR via SR-1 ex vivo enhanced the frequency of CD34+ HSCs and induced differentiation of myeloid mDCs and pDCs [255]. In a clinical trial SR–1 has induced a 330-fold expansion of CD34+ cells and resulted in fast engraftment of neutrophils and platelets in patients. Neutrophil recovery response has been viewed as a surrogate marker of host immunity [258]. The AHR is also antagonized by Resveratrol, a naturally occurring polyphenol. Resveratrol binds receptors involved in HSC activity including AHR and integrin αvβ3 [259,260]. Addition of Resveratrol to a cytokine-containing (SCF, TPO, FLT3-L, IL-6) medium represents a robust ex vivo method for the expansion of functional CD34+ CB HSCs. The UM171 also inhibits LSD1 (H3K4me1/2 demethylase) and HDAC1/2 (e.g., H3K27ac deacetylase), leading to the re-establishment of H3K4me2 and H3K27ac epigenetic marks, which normally rapidly decrease in human LT/ST-HSCs ex vivo [261,262,263]. Of note, when UM171 is combined with SR-1 and cytokines (SCF, TPO, FLT3L, IL-6) the efficiency of expansion is further increased [263].
As a suppressor of SIRT1 deacytelase, nicotinamide inhibits differentiation and enhances expansion of CD34+ CB-HSCs [264]. The SIRT1 deacetylates and thereby deactivates p53 protein [265]. Proof for SIRT1´s ability to maintain stemness potential comes from a phase I clinical trial. The surrogate marker, median neutrophil recovery rate, was significantly increased in individuals, who had received nicotinamide treated CD34+ CB-HSCs [266].
A screen of CD34+ CB-HSCs identified histone deacetylase inhibitor (HDACI) VPA as a promising candidate for LT-HSC expansion. Adding VPA to SCF, TPO, FLT3L and IL-3 in serum free media enhanced expressions of CD90, c-KIT, CXCR4 and integrin α6 (CD49f), increased activation of p53 and reduced ROS levels. The VPA induced HSC expansion by reprogramming CD34+ CD90− cells to CD34+ CD90+ HSCs, accompanied by increased proliferation. The VPA-expanded peripheral blood (PB) cells and BM HSCs established unbiased multilineage human hematopoietic-cell chimerism in NSG mice at 16 weeks post-transplantation [267,268].
The major side effect of allogeneic transplantations is the development of graft-versus-host disease (GvHD). Compared to BM HSCs, the transplantation of CB-derived CD34+ cells with up to two human leukocyte antigen mismatches revealed a lower risk of GvHD [269]. Due to the low numbers of CB-CD34+ cells from a single donor cord, the hematopoietic and immunological recovery of the recipients may be delayed, causing higher infection rates and transplant-related mortality. In the clinics, SR-1, Notch-ligand, UM171 and nicotinamide-based methods have been associated with improved neutrophil recovery early after transplantation, reducing side effects of HSCT [258,266,270]. In spite of major developments in ex vivo expansion of CB-HSCs, the efficiency of their long-term engraftment is still inadequate [271]. To overcome this limitation, double CB transplantations, with nonexpanded CB-HSCs of a second donor, were used to engraft. In this case, a higher risk of GvHD was reported [272]. Thus, enhancing the availability of functional CD34+ CB-HSCs would give an excellent opportunity to improve therapeutic applications.
8.1.3. Suspension Culture of Human LSCs
Several patient-derived AML and CML cell lines are available. They are easy to culture but acquire multiple cytogenetic aberrations upon prolonged culture. This aspect needs to be considered when comparing experiments from different laboratories that may significantly differ [273]. In contrast to primary AML cells, which are genetically and functionally heterogenous (consisting of LSCs and differentiated cells), cell lines are homogeneous, favoring proliferation of the most aggressive clones [274,275,276,277]. Culture systems for primary AML samples that preserve clonal heterogeneity are required to mimic the situation in patients.
To selectively culture CD34+ HSPCs from CML/AML samples, a feeder cell-free and serum-free liquid culture system containing FLT3L, SCF, IL-3, IL-6, and TPO has been established. The outcome is highly patient-dependent and shows a great variability [276,278].
The AML specimens share the common feature of high AHR activation in vitro, which provokes differentiation. The AHR agonist SR-1 and other small molecular weight compounds (UM171 and its derivative UM729), counteract differentiation. No stromal co-culture is required but multiple cytokines are necessary, like BIT (BSA, insulin, transferrin), SCF, FLT3L, IL-3 and G-CSF [279,280]. It is currently unclear how the pyrimido-indole UM729 enhances CD34+ cell expansion which is AHR independent [280].
Inhibiting GSK-3 and mTORC1 also maintains self-renewing capacity of hematopoietic cells from healthy donors or AML patients [281]. The GSK3 inhibitors (GSK3i) prevent β-catenin degradation and activate WNT target genes, which is essential for long-term HSC self-renewal. The activation of mTORC1 drives HSC proliferation and differentiation, leading to HSC exhaustion [281]. As GSK3-inhibition activates mTORC1 a combined inhibition of GSK3i and mTORC1 is required to maintain self-renewing abilities [282].
8.2. 2.5D Methods
8.2.1. Co-Culturing LT/ST-HSCs
The balance between quiescence and activation of LT/ST-HSCs is tightly regulated by the BM microenvironment [90]. The absence of a BM niche leads to a gradual loss of the LT-HSC status and induces differentiation towards lineage-committed progenitors. Co-culture options were developed to mimic the BM microenvironment (reviewed in [283], Figure 2).
“2.5D co-culture” methods use supporting stromal or endothelial cells to simulate the BM niche. LT/ST-HSCs or LSCs grow on top of adherent cells, either in direct contact (contact culture) or separated by filters (trans-well culture). Trans-well cultures circumvent the need to separate HSC cells from the supporting cells, but are less efficient than cultures that allow for direct stroma contact [284,285]. The MSCs are most commonly used as they express high levels of HSC-supporting factors and significantly improve engraftment [286,287].
Primary MSCs downregulate niche factors (Scf, Cxcl12 and Vcam1) upon culture and their ability to maintain HSCs declines over time. Nakahara et al. identified five transcription factors (Klf7, Ostf1, Xbp1, Irf3 and Irf7) that restored HSC niche function in cultured MSCs. Overexpression of these factors revitalized MSCs (rMSCs). The rMSCs expanded cells showed a seven-fold higher efficiency in expansion of functional LT-HSCs in a setting where lineage-depleted mouse BM cells or CD34+ CBs were co-cultured in the presence of SCF and TPO [288].
Aside from MSCs, ECs serve as a supporting cell layer. The BM-derived ECs secrete self-renewal supporting angiocrine growth factors, such as VEGF-A and NOTCH ligands [289]. Long-term maintenance of primary ECs involves loss of their angiogenic properties. To preserve them and initiate immortalization, E4orf1 has been introduced. E4orf1 is an adenoviral E4 gene product which confers long-term survival through tonic phosphorylation of AKT [290]. Immortalization of ECs by E4orf1 allows long-term cultures and efficiently supports mouse LT-HSCs when in direct contact in serum-free, SCF supplemented co-culture [289]. The support is mainly provided by the initiation of Notch signaling. In analogy, human fetal liver (FL) sinusoidal ECs engineered and immortalized by E4orf1 (hFLSECs-E4orf1) are used for long-term culture of CD34+ CB cells. The hFLSECs-E4orf1 cells also provide activation of NOTCH signaling, mimic the vascular niche and prevent LT-HSC exhaustion [291].
8.2.2. Co-Culturing LSCs
Primary AML cells differentiate and/or undergo apoptosis in culture indicating that most AML cells depend on signals from the microenvironment [280,292,293,294,295]. Co-culture with BM stromal cells expands AML LICs, but frequently selects for a specific subpopulation [296,297,298]. Immortalized mouse BM mesenchymal or endothelial stromal cell lines, such as MS-5, FBMD-1, OP9, HS-5, HS-27 or HUVEC are used as supporting cells for primary AML cultures [114,296,297,299]. The mesenchymal nestin+ MS-5 murine BM stromal cell line efficiently maintains functional, chemo-resistant human LSCs ex vivo over 3 weeks [297,298]. The MS5 cells secrete CXCL12, ANGPT1, MCP-1 and HGF; MS5/LIC cocultures were further supplemented with IL-3, G-CSF and TPO and kept at 3% O2, which provides a niche-like milieu [300]. The hypoxia signaling pathway triggers LIC maintenance in vivo [301].
Primary MSCs isolated from AML patients have an impaired ability to support normal HSPCs, but an enhanced ability to maintain LSCs compared to MSCs from healthy donors [292,302,303]. Leukemic BM niche MSCs have different morphology and growth rate, altered osteogenic or adipogenic differentiation capacity and changed methylation signatures [292,302,303,304,305,306,307]. These co-culture conditions are labor-intensive and require careful standardization. Among the critical factors is the cell density of the feeder cells, which must be in a cell cycle arrested state. Passage restrictions of the feeder cells are required to avoid stromal cell line exhaustion and ensure comparable experimental conditions [297,308].
8.3. 3D Methods
8.3.1. 3D Culture of LT/ST-HSCs
The 3D culture methods attempt to mimic the spatial structure of the BM microenvironment by providing cell-to-cell or cell-to-biomimetic matrix contacts (Figure 2). Cell spheroids or aggregates are grown on a matrix, or in a scaffold-free suspension. Commonly used scaffold/matrix materials include natural polymers, such as alginate, Matrigel™ (basement membrane matrix), agarose and bacterial nanocellulose, hyaluronic acid or synthetic-based polymer materials [296,309,310]. Polyethylene glycol (PEG), PVA, poly (lactic-co-glycolic acid) (PLGA), poly (lactic acid) (PLA) and poly (ε-caprolactone) (PCL) are common materials used to form synthetic scaffolds hydrogels [311].
To generate scaffold-free spheroids, different cell types (e.g., MSCs and LT-/ST-HSCs) are cultured on hanging drops, microplates, non-adhesive surfaces or via a forced floating method (magnetic levitation or agitation-based approaches) to initiate aggregation and to avoid attachment to a culture dish [309,312,313,314,315,316]. Spheroids have some advantages compared to 2.5D cultures; (i) higher levels of hematopoietic niche factors provided by MSCs, (ii) the maintenance of the cell shape and (iii) improved signaling by cell-cell contacts increased ex vivo LT-/ST-HSC expansion compared to 2D and 2.5D methods [312,317].
The LT-/ST-HSCs cells can also be encapsulated into a natural or synthetic polymer solution that is cross-linked to form a hydrogel. Hydrogels are biocompatible, retain large amounts of water and provide excellent permeability [318,319]. Hydrogels are mixed with ECM proteins such as fibronectin, collagen, laminin or glycosaminoglycans to allow attachment of LT-/ST-HSCs [309,318]. Cytokines, small molecules or other factors can be incorporated depending on the aim of the research project [320].
Compared to a PEG scaffold, zwitterionic hydrogels are super-hydrophilic and are more resistant to non-specific protein binding [319,321]. Bai et al. set up a zwitterionic hydrogel system to ex vivo culture BM and CB LT-/ST-HSCs, using a metalloproteinase-cleavable zwitterionic peptide to reversibly crosslink the gels. The LT-/ST-HSCs-secreted metalloproteinases gradually cleave peptide crosslinker, allowing cells to actively shape their environment [320]. This improved cell migration, cell-cell contacts and stemness of the HSCs; and proved to be an efficient method to expand functional CD34+ LT-HSCs based on reduced differentiation, diminished reactive oxygen species (ROS) production and a low metabolic rate [319,320,321].
A disadvantage of encapsulation is the homogenous matrix, unlike the porous sponge-like structure of the BM. Although cells have full contact to the hydrogel/ECM matrix, a direct cell-cell contact may be missing. To circumvent this problem, porous scaffolds were generated (e.g., by salt leaching, freeze-drying or 3D printing), where MSCs and CD34+ CB-HSCs can form close cell-cell contacts, improving ex vivo expansion [322,323,324]. Alternatively, Wharton’s jelly, can be employed as a 3D matrix. Wharton’s jelly is a mucoid connective tissue surrounding the umbilical cord vessels to confer a mechanical protection in the womb. Decellularized Wharton’s jelly matrix (DWJM) serves as ECM scaffold and is mixed with primary human MSCs to maintain ex vivo CD34+ CB-HSCs cells. The DWJM shares many components of the BM ECM including collagens, fibronectin, tenascin-C, hyaluronic acid and numerous sulfated glycosaminoglycans possessing many unique biochemical characteristics [291,325,326].
Currently, self-sustained 3D applications like small-scale microfluidic devices and large-scale bioreactors are developed. Bioreactor is an engineered system that allows culturing large amounts of CD34+ CB-HSCs through automated control over medium supply, waste removal and agitation [327]. Microfluidic devices mimic the vascular niche by continuously supplying nutrients and oxygen, while generating gradients (e.g., oxygen, calcium ions, cytokines and small molecules) [328,329]. Bone marrow-on-a-chip is an even more complex microfluidic system, where the colonization of the 3D matrix by niche cells takes place in the BM in vivo [330,331].
8.3.2. 3D Culture of LSCs
In an effort to model the BM microenvironment for ex vivo leukemia studies, stiff and porous 3D scaffolds have been used [332,333,334,335,336,337]. Leukemia research is inclined to find strategies to disrupt the protective effect of the niche cells to improve therapeutic strategies. Bray et al. co-seeded ECs and MSCs with primary AML cells in matrix metalloproteinase–sensitive PEG-heparin hydrogels, supplemented with growth factors (VEGF, FGF-2, stromal cell-derived factor 1 (SCD1)). The protective effect of leukemic-vascular interactions increased chemoresistance of cancer cells in 3D compared to 2D [334].
The first bioreactor system was recently developed to maintain malignant CD34+ cells from AML and myeloproliferative neoplasm (MPN) patients for up to 3 weeks. The system contained human osteoblastic BM niches, engineered by MSCs in 3D porous scaffolds under perfusion flow [113].
The 3D methods represent the future to expand LT/ST-HSCs in large quantities for transplantations, but are less suitable for individual research-purposes, as they are technically challenging and labor-intensive. The use of 3D techniques is limited when it comes to high-throughput multi-well experiments, such as drug-screening, but represents the best choice for any validation experiments.
9. Immortalized Hematopoietic Stem/Progenitor Cell Lines
Genetic manipulation of self-renewal pathways by transgene overexpression may provide a suitable method for HSPC expansion and the establishment of cell lines. Successful HSPC immortalization was achieved by overexpression of embryonic developmental genes including HOXB, RARα and Lhx2 to enforce cell renewal and arrest cell differentiation [338,339,340] (Figure 3).
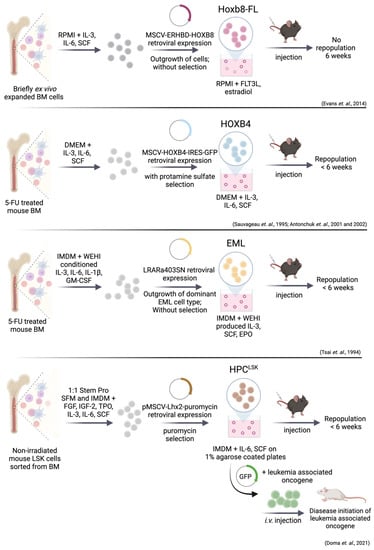
Figure 3.
Simplified visualization of unique cell system methods for combined in vitro and in vivo studies of genetically modified HSPCs. Retroviral overexpression of Hoxb8-FL, HOXB4, RARα403 and Lhx2 enforces HSC self-renewal and proliferation. Addition of cytokines is a key factor for long-term culture of HSPCs. The HPCLSK cell lines can be transformed with oncogenes including BCR/ABLp210, FLT3-ITD;NRASG12D and MLL/AF9. One critical step for generation of a HSPC cell line includes the engraftment for more than 6 weeks. Hoxb8-FL cells do not fulfill this requirement of reconstitution capacity.
Bulk- or LSK-sorted mouse BM cells were isolated and cultured in serum, growth factor and cytokine supplemented media. Dividing cells were immortalized through retroviral expression of HOXB4 [146,341], HOXB8 [342], truncated RARα, (RARα403) [343] or Lhx2 [339] and resulted in rapid and extensive ex vivo expansion of HSPC populations for more than 9 weeks (Figure 3). Exogenous growth factors are still required to induce proliferation. This process raised long-term expanded, progenitor cell lines that remained multipotent as demonstrated by their ability to fully repopulate lympho-myeloid lineages in primary and secondary recipients. All transplanted mice remained healthy and without manifestations of hematopoietic disorders after one year of observation [146,339,341,342,343].
9.1. Immortalization via HoxB8 and HoxB4
The HOX proteins are a family of evolutionary conserved transcription factors. In mammals, 39 HOX genes are organized into four distinct clusters: HOXA, HOXB, HOXC and HOXD [344]. A considerable amount of data link HOXA and HOXB genes to cell renewal and the arrest of cell differentiation [338,345]. These functions were exploited experimentally to establish stably growing, homogenous hematopoietic progenitor cell lines through retroviral expression of HOX genes (HOXB4 and HOXB8). Expression confers growth advantage but does not elicit leukemic potential [146,341,342].
Downregulation of Prdm16 might be involved in preventing leukemia in HOXB4 overexpressing LT/ST-HSCs transplanted mice [346]. The transcription regulator PRDM16 is associated with AML, causes oncogenic fate conversion from megakaryocyte-erythroid progenitors (MEPs) to LSCs, by interacting with super enhancers and activating myeloid master regulators, including PU.1 [347]. The PRDM16 was markedly repressed by HOXB4, but upregulated by HOXA9 and HOXA10 [346]. This may explain why HOXB4 and probably also HOXB8 lacks the leukemogenic potential seen with other oncogenic HOX factors such as HOXA9 and HOXA10.
In Hoxb8–FL cell lines, the hormone binding domain of the estrogen receptor (Erhbd) is fused to the coding sequence of Hoxb8, leading to the activation of Hox genes by estrogen. Estrogen withdrawal provokes differentiation into dendritic cells (DCs). Besides estrogen, growth and survival of these cells strictly depends on FLT3L [342] (Figure 3). Hoxb8–FL cells have both myeloid and lymphoid potential but lack any megakaryocyte and erythroid capacities and closely resemble MPP4 cells (lin− SCA-1+ cKIT+ CD48+ CD150− CD34+ CD135+) [6,342,348]. One month after transplantation, Hoxb8–FL cells are no longer detectable in the BM, but are still present in spleen, peripheral blood, and thymus. This observation suggests a compromised capacity to self-renew. The Hoxb8–FL–derived T–cells reached merely about 10–30% of physiological T–cell numbers in the thymus, but were absent in the periphery [342]. Within the four HSPC lines discussed here, Hoxb8–FL shoes the least multipotency.
The Hox factor HOXB4 is a key regulator of LT-HSCs self-renewal but its enforced expression allows differentiation when transplanted [341,349]. The factor HOXB4 drives proliferation by upregulating AP-1 complexes with subsequent enhanced cyclin D1 levels [350]. Combination of overexpression of HOXB4 with deletion of the HOX cofactor Pbx1 (pre-B-cell leukemia transcription factor 1) or expression of NUP98-HOXB4 fusion protein further enhances ex vivo expansion of LT-/ST-HSCs [351]. Both approaches (HOXB4/Pbx1 KO and NUP98-HOXB4) reconstituted myeloid and lymphoid populations in vivo without inducing leukemia [351,352,353,354]. Recent findings indicate that HOXB4 may also reprogram induced pluripotent stem cells (iPSCs) cells into long-term repopulating HSCs, opening new avenues for human therapeutic possibilities [355,356].
9.2. EML (Erythroid, Myeloid, and Lymphocytic) Cell Line
The EML cells originally emerged as an in vitro model to study self-renewal and lineage commitment. The EML cells express a truncated, dominant-negative form of the human RAR (RARα403) [343,357] (Figure 3). The RARα403 outcompetes the endogenous RAR in the formation of biologically active RAR/RXR complexes, leading to c-MYC upregulation and proliferation. As mentioned above, ectopic RA signaling maintains LT/ST-HSC quiescence, while inhibition leads to stem and progenitor proliferation [224,343].
The EML cells generate large numbers of B-lymphoid and erythroid progenitors at the expense of progenitors for the neutrophil and macrophage lineages. This may be overridden by a combination of IL-3 and high concentration of RA, which increases the number of myeloid progenitors [343]. One advantage of this cell system is the ability for in vitro T-cell differentiation (upon co-culture with murine OP9-DL1 stromal cells in the presence of SCF, IL-7, and FLT3). OP9-DL1 cells express delta-like-1, a NOTCH ligand which drives human and murine HSPCs into T-cells in vitro [358,359].
The main limitation of EML cells cultivated with SCF is their functional heterogeneity containing CD34/SCA-1low and high populations. Each subpopulation expresses a distinct pattern of HSPC markers and transcription factors, different multilineage differentiation potential and proliferation kinetics [357]. CD34/SCA-1high EML cells exhibit low GATA1 and high PU.1 level, which predisposes them to the myeloid lineage. In contrast, CD34/SCA-1low, GATA1 high, PU.1 low cells are erythroid-prone [360]. Besides GATA1, other erythroid genes including α- and β-hemoglobin, Epor, Eraf (erythroid associated factor) and mast cell proteases are expressed at high levels in the CD34/SCA-1low EML cells [361]. The EML cells serve as a model for cell intrinsic and extrinsic pathways that regulate plasticity among multipotent hematopoietic cells.
Ectopic expression of HOXB4 in EML cells allows HSC self-renewal through upregulation of stemness-related genes, such as Laptm4b, Gp49a, Sox4, and CD34. HOXB4 downregulates erythroid and B cell lineage-specific genes to keep cells in a primitive state [362]. This cell system extends our knowledge on molecular and functional properties of HSPCs but lacks the ability of long-term cultivation without differentiation.
9.3. Immortalization via Lhx2—HPCLSK Cell Lines
Retroviral transduction of the LIM-homeodomain (LIM-HD) transcription factor Lhx2 was used to generate multipotent HPCLSK cell lines [339,363,364] (Figure 3). The LHX2 has a critical role in hematopoiesis and Lhx2-null embryos die in utero with severe anemia [365,366]. The critical role of LHX2 in hematopoiesis was underlined by forced expression in embryonic stem (ES) cells, which resulted in the outgrowth of multipotent SCF-dependent progenitor cells [363]. The LHX2 upregulates hematopoietic self-renewal genes and inhibits differentiation of mouse ES derived LSK cells [367].
The SCF/IL-6 dependent HPCLSK cells are kept on agarose-coated plates to prevent adherence-induced myeloid differentiation. The HPCLSK cells efficiently home to the BM, blood, spleen, and thymus and can differentiate into myeloid and lymphoid lineages in vitro and in vivo. Numbers of HPCLSKs -derived myeloid and lymphoid progenitors in the BM and differentiated blood cells are comparable to BM-injected mice [339]. Transcriptome analysis of HPCLSKs showed a ST-HSC/MPP1 (lin− SCA-1+ cKIT+ CD48− CD150− CD34+ CD135−) signature, which corresponds to the earliest proliferating stem/progenitor cells despite expression of CD48 and CD150 [6,339,348]. The absence of any cell feeder layer or extensive amounts of cytokines makes them a robust and low-cost model system that guarantees long-term multipotency.
9.4. Leukemic Stem Cell Lines Using the HPCLSK System
The HPCLSK cells can be genetically modified e.g., by retroviral transduction to generate LSCs lines harboring hematopoietic stem cell oncogenes including BCR/ABL, MLL-AF9 or FLT3-ITD;NRASG12D [141,339,368,369] (Figure 3). The SCF/IL-6 dependency can be overcome by HPCLSK BCR/ABLp210+ cell lines, which grow cytokine-independently.
Injection of transformed HPCLSK-LSCs (BCR/ABLp210+, MLL-AF9, FLT3/NRASG12D) lines in immunocompromised mice induced myeloid leukemia. All diseased animals displayed elevated WBC counts, blast-like cells in the blood and suffered from splenomegaly [339]. The HPCLSK BCR/ABL lines demonstrated similar transcriptional and phospho-signaling signatures compared to BCR/ABL CML patients [368]. Another advantage of the HPCLSK system is the ability to rapidly generate cells lines from transgenic mouse models. The HPCLSKs lines have been generated from Cdk6−/−, Stat5a−/− and Stat5b−/− transgenic mice [141,339]. When HPCLSK BCR/ABLp210+ Cdk6−/− cells were transplanted, loss of CDK6 was associated with a reduced incidence of leukemia, mimicking the effects of published primary BM transplantation assays [175,339]. These data verified the potential of the HPCLSK system to generate diverse leukemia models.
Recently, a unique role for STAT5B in driving self-renewal of HSCs/LSCs was described, additionally using the HPCLSK system to underline the in vivo/ex vivo results. The factor STAT5B, but not STAT5A, is predominantly present in the nucleus of HPCLSK cells stimulated with cytokines (TPO, EPO, GM-CSF) or transformed cells [141]. These assays underline the broad application of the HPCLSK cell lines for functional assays that require high cell numbers. These findings support the relevance of HPCLSK cell system that represents a unique tool to compare LSCs to non-transformed HPCLSK cells in vitro and in vivo, which can be adapted for high-scale preclinical compound screening.
10. Conclusions
We here summarize the current knowledge on methods for ex vivo cultivating primary LT/ST-HSCs, LSCs, and generating in vitro HSPCs cell lines. In general, enhanced self-renewal ability comes at the expense of differentiation. Self-renewal is orchestrated by BM niche signals, including cytokines, cell-ECM, and cell-cell interactions while LSCs are additionally affected by the aberrant expression of oncogenic drivers.
Several approaches are available to promote self-renewal including co-cultivation on a stromal feeder layer or embedding in a BM-mimicking matrix, in the presence of cytokines. Alternatively, self-renewal may also be maintained via genetic modifications.
Murine and human primary HSCs cultures serve distinct purposes. Human LT/ST-HSC expansion aims primarily at improving conditions for transplantation settings in personalized medicine. Most preclinical studies use murine LT/ST-HSCs and ex vivo expanded primary LSCs as they are a versatile system to address research questions. Murine systems are instrumental in understanding the pathways regulating HSC quiescence and allow new medical perspectives through drug screening and biomarker discovery approaches.
Despite advances, culturing, isolating, and maintaining primary stem cells are still challenging. It is a labor-intensive process resulting in low numbers of cells, which prevents the conduction of high throughput techniques. Murine HPCLSK cells and HPCLSKs derived LSCs provide a solution, as they can be expanded indefinitely and facilitate basic and mechanistic studies. The HPCLSK lines thus represent a quick, reliable, and reproducible tool to study hematopoietic malignancies and large-scale drug sensitivity/resistant assays.
Despite encouraging results and novel in vitro/ex vivo assays, strategies to selectively target quiescent LSCs, the key drivers of relapse, are still elusive. Their identification will pave the way towards the development of new treatment strategies for AML and CML patients.
Author Contributions
All authors made substantial, direct, and intellectual contributions to the work. I.M.M. and E.D. reviewed the literature and wrote the manuscript. A.H.-K. corrected and edited the final version. V.S. corrected, edited, and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program grant agreement no. 694354.
Acknowledgments
The authors thank Sebastian Kollmann for continuous discussion. Graphics were created with BioRender.com (24 March 2022). Open Access Funding by the University of Veterinary Medicine Vienna.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Becker, A.J.; McCulloch, E.A.; Till, J.E. Cytological Demonstration of the Clonal Nature of Spleen Colonies Derived from Transplanted Mouse Marrow Cells. Nature 1963, 197, 452–454. [Google Scholar] [CrossRef]
- Cole, L.J.; Fishler, M.C.; Bond, V.P.S. Subcellular Fractionation of Mouse Spleen Radiation Protection Activity. Proc. Natl. Acad. Sci. USA 1953, 39, 759–772. [Google Scholar] [CrossRef]
- Till, J.E.; McCulloch, E.A. A Direct Measurement of the Radiation Sensitivity of Normal Mouse Bone Marrow Cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef]
- Morrison, S.J.; Weissman, I.L. The Long-Term Repopulating Subset of Hematopoietic Stem Cells Is Deterministic and Isolatable by Phenotype. Immunity 1994, 1, 661–673. [Google Scholar] [CrossRef]
- Bartelmez, S.H.; Andrews, R.G.; Bernstein, I.D. Uncovering the Heterogeneity of Hematopoietic Repopulating Cells. Exp. Hematol. 1991, 19, 861–862. [Google Scholar]
- Pietras, E.M.; Reynaud, D.; Kang, Y.-A.; Carlin, D.; Calero-Nieto, F.J.; Leavitt, A.D.; Stuart, J.M.; Göttgens, B.; Passegué, E. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell 2015, 17, 35–46. [Google Scholar] [CrossRef]
- Challen, G.A.; Pietras, E.M.; Wallscheid, N.C.; Signer, R.A.J. Simplified Murine Multipotent Progenitor Isolation Scheme: Establishing a Consensus Approach for Multipotent Progenitor Identification. Exp. Hematol. 2021, 104, 55–63. [Google Scholar] [CrossRef]
- Busch, K.; Klapproth, K.; Barile, M.; Flossdorf, M.; Holland-Letz, T.; Schlenner, S.M.; Reth, M.; Höfer, T.; Rodewald, H.-R. Fundamental Properties of Unperturbed Haematopoiesis from Stem Cells In Vivo. Nature 2015, 518, 542–546. [Google Scholar] [CrossRef]
- Cabezas-Wallscheid, N.; Klimmeck, D.; Hansson, J.; Lipka, D.B.; Reyes, A.; Wang, Q.; Weichenhan, D.; Lier, A.; von Paleske, L.; Renders, S.; et al. Identification of Regulatory Networks in HSCs and Their Immediate Progeny via Integrated Proteome, Transcriptome, and DNA Methylome Analysis. Cell Stem Cell 2014, 15, 507–522. [Google Scholar] [CrossRef]
- Kent, D.G.; Copley, M.R.; Benz, C.; Wöhrer, S.; Dykstra, B.J.; Ma, E.; Cheyne, J.; Zhao, Y.; Bowie, M.B.; Zhao, Y.; et al. Prospective Isolation and Molecular Characterization of Hematopoietic Stem Cells with Durable Self-Renewal Potential. Blood 2009, 113, 6342–6350. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, Ö.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef]
- Morita, Y.; Ema, H.; Nakauchi, H. Heterogeneity and Hierarchy within the Most Primitive Hematopoietic Stem Cell Compartment. J. Exp. Med. 2010, 207, 1173–1182. [Google Scholar] [CrossRef]
- Oguro, H.; Ding, L.; Morrison, S.J. SLAM Family Markers Resolve Functionally Distinct Subpopulations of Hematopoietic Stem Cells and Multipotent Progenitors. Cell Stem Cell 2013, 13, 102–116. [Google Scholar] [CrossRef]
- Rabe, J.L.; Hernandez, G.; Chavez, J.S.; Mills, T.S.; Nerlov, C.; Pietras, E.M. CD34 and EPCR Coordinately Enrich Functional Murine Hematopoietic Stem Cells under Normal and Inflammatory Conditions. Exp. Hematol. 2020, 81, 1–15.e6. [Google Scholar] [CrossRef]
- Sawai, C.M.; Babovic, S.; Upadhaya, S.; Knapp, D.J.H.F.; Lavin, Y.; Lau, C.M.; Goloborodko, A.; Feng, J.; Fujisaki, J.; Ding, L.; et al. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity 2016, 45, 597–609. [Google Scholar] [CrossRef]
- Wilson, A.; Laurenti, E.; Oser, G.; van der Wath, R.C.; Blanco-Bose, W.; Jaworski, M.; Offner, S.; Dunant, C.F.; Eshkind, L.; Bockamp, E.; et al. Hematopoietic Stem Cells Reversibly Switch from Dormancy to Self-Renewal during Homeostasis and Repair. Cell 2008, 135, 1118–1129. [Google Scholar] [CrossRef]
- Yamamoto, R.; Morita, Y.; Ooehara, J.; Hamanaka, S.; Onodera, M.; Rudolph, K.L.; Ema, H.; Nakauchi, H. Clonal Analysis Unveils Self-Renewing Lineage-Restricted Progenitors Generated Directly from Hematopoietic Stem Cells. Cell 2013, 154, 1112–1126. [Google Scholar] [CrossRef]
- Fares, I.; Chagraoui, J.; Lehnertz, B.; MacRae, T.; Mayotte, N.; Tomellini, E.; Aubert, L.; Roux, P.P.; Sauvageau, G. EPCR Expression Marks UM171-Expanded CD34+ Cord Blood Stem Cells. Blood 2017, 129, 3344–3351. [Google Scholar] [CrossRef]
- Gordon, P.R.; Leimig, T.; Babarin-Dorner, A.; Houston, J.; Holladay, M.; Mueller, I.; Geiger, T.; Handgretinger, R. Large-Scale Isolation of CD133+ Progenitor Cells from G-CSF Mobilized Peripheral Blood Stem Cells. Bone Marrow Transplant. 2003, 31, 17–22. [Google Scholar] [CrossRef][Green Version]
- Notta, F.; Doulatov, S.; Laurenti, E.; Poeppl, A.; Jurisica, I.; Dick, J.E. Isolation of Single Human Hematopoietic Stem Cells Capable of Long-Term Multilineage Engraftment. Science 2011, 333, 218–221. [Google Scholar] [CrossRef]
- Prashad, S.L.; Calvanese, V.; Yao, C.Y.; Kaiser, J.; Wang, Y.; Sasidharan, R.; Crooks, G.; Magnusson, M.; Mikkola, H.K.A. GPI-80 Defines Self-Renewal Ability in Hematopoietic Stem Cells during Human Development. Cell Stem Cell 2015, 16, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Radtke, S.; Pande, D.; Cui, M.; Perez, A.M.; Chan, Y.-Y.; Enstrom, M.; Schmuck, S.; Berger, A.; Eunson, T.; Adair, J.E.; et al. Purification of Human CD34+CD90+ HSCs Reduces Target Cell Population and Improves Lentiviral Transduction for Gene Therapy. Mol. Ther.-Methods Clin. Dev. 2020, 18, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Sumide, K.; Matsuoka, Y.; Kawamura, H.; Nakatsuka, R.; Fujioka, T.; Asano, H.; Takihara, Y.; Sonoda, Y. A Revised Road Map for the Commitment of Human Cord Blood CD34-Negative Hematopoietic Stem Cells. Nat. Commun. 2018, 9, 2202. [Google Scholar] [CrossRef] [PubMed]
- Zonari, E.; Desantis, G.; Petrillo, C.; Boccalatte, F.E.; Lidonnici, M.R.; Kajaste-Rudnitski, A.; Aiuti, A.; Ferrari, G.; Naldini, L.; Gentner, B. Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy. Stem Cell Rep. 2017, 8, 977–990. [Google Scholar] [CrossRef]
- Dussiau, C.; Boussaroque, A.; Gaillard, M.; Bravetti, C.; Zaroili, L.; Knosp, C.; Friedrich, C.; Asquier, P.; Willems, L.; Quint, L.; et al. Hematopoietic Differentiation Is Characterized by a Transient Peak of Entropy at a Single-Cell Level. BMC Biol. 2022, 20, 60. [Google Scholar] [CrossRef]
- Laurenti, E.; Göttgens, B. From Haematopoietic Stem Cells to Complex Differentiation Landscapes. Nature 2018, 553, 418–426. [Google Scholar] [CrossRef]
- Velten, L.; Haas, S.F.; Raffel, S.; Blaszkiewicz, S.; Islam, S.; Hennig, B.P.; Hirche, C.; Lutz, C.; Buss, E.C.; Nowak, D.; et al. Human Haematopoietic Stem Cell Lineage Commitment Is a Continuous Process. Nat. Cell Biol. 2017, 19, 271–281. [Google Scholar] [CrossRef]
- Morrison, S.; Lagasse, E.; Weissman, I. Demonstration That Thy(Lo) Subsets of Mouse Bone Marrow That Express High Levels of Lineage Markers Are Not Significant Hematopoietic Progenitors. Blood 1994, 83, 3480–3490. [Google Scholar] [CrossRef]
- Osawa, M.; Hanada, K.; Hamada, H.; Nakauchi, H. Long-Term Lymphohematopoietic Reconstitution by a Single CD34-Low/Negative Hematopoietic Stem Cell. Science 1996, 273, 242–245. [Google Scholar] [CrossRef]
- Spangrude, G.J.; Heimfeld, S.; Weissman, I.L. Purification and Characterization of Mouse Hematopoietic Stem Cells. Science 1988, 241, 58–62. [Google Scholar] [CrossRef]
- Majeti, R.; Park, C.Y.; Weissman, I.L. Identification of a Hierarchy of Multipotent Hematopoietic Progenitors in Human Cord Blood. Cell Stem Cell 2007, 1, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Nakauchi, H.; Nagayoshi, K.; Nishikawa, S.; Miura, Y.; Suda, T. In Vivo and in Vitro Stem Cell Function of C-Kit- and Sca-1-Positive Murine Hematopoietic Cells. Blood 1992, 80, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bryder, D.; Adolfsson, J.; Nygren, J.; Månsson, R.; Sigvardsson, M.; Jacobsen, S.E.W. Identification of Lin–Sca1+kit+CD34+Flt3– Short-Term Hematopoietic Stem Cells Capable of Rapidly Reconstituting and Rescuing Myeloablated Transplant Recipients. Blood 2005, 105, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.; Copley, M.; Benz, C.; Dykstra, B.; Bowie, M.; Eaves, C. Regulation of Hematopoietic Stem Cells by the Steel Factor/KIT Signaling Pathway. Clin. Cancer Res. 2008, 14, 1926–1930. [Google Scholar] [CrossRef]
- Deaglio, S.; Mehta, K.; Malavasi, F. Human CD38: A (r)Evolutionary Story of Enzymes and Receptors. Leuk. Res. 2001, 25, 1–12. [Google Scholar] [CrossRef]
- Baum, C.M.; Weissman, I.L.; Tsukamoto, A.S.; Buckle, A.M.; Peault, B. Isolation of a Candidate Human Hematopoietic Stem-Cell Population. Proc. Natl. Acad. Sci. USA 1992, 89, 2804–2808. [Google Scholar] [CrossRef]
- Matsubara, A.; Iwama, A.; Yamazaki, S.; Furuta, C.; Hirasawa, R.; Morita, Y.; Osawa, M.; Motohashi, T.; Eto, K.; Ema, H.; et al. Endomucin, a CD34-like Sialomucin, Marks Hematopoietic Stem Cells throughout Development. J. Exp. Med. 2005, 202, 1483–1492. [Google Scholar] [CrossRef]
- Civin, C.I.; Strauss, L.C.; Brovall, C.; Fackler, M.J.; Schwartz, J.F.; Shaper, J.H. Antigenic Analysis of Hematopoiesis. III. A Hematopoietic Progenitor Cell Surface Antigen Defined by a Monoclonal Antibody Raised against KG-1a Cells. J. Immunol. 1984, 133, 157–165. [Google Scholar]
- Tjrannfjord, G.E.; Steen, R.; Egeland, T. Characterization of CD34+ Peripheral Blood Cells from Healthy Adults Mobilized by Recombinant Human Granulocyte Colony-Stimulating Factor. Blood 1994, 84, 2795–2801. [Google Scholar] [CrossRef]
- Balazs, A.B.; Fabian, A.J.; Esmon, C.T.; Mulligan, R.C. Endothelial Protein C Receptor (CD201) Explicitly Identifies Hematopoietic Stem Cells in Murine Bone Marrow. Blood 2006, 107, 2317–2321. [Google Scholar] [CrossRef]
- Fukudome, K.; Esmon, C.T. Molecular Cloning and Expression of Murine and Bovine Endothelial Cell Protein C/Activated Protein C Receptor (EPCR). J. Biol. Chem. 1995, 270, 5571–5577. [Google Scholar] [CrossRef]
- Martin, G.H.; Park, C.Y. EPCR: A Novel Marker of Cultured Cord Blood HSCs. Blood 2017, 129, 3279–3280. [Google Scholar] [CrossRef]
- Gallacher, L.; Murdoch, B.; Wu, D.M.; Karanu, F.N.; Keeney, M.; Bhatia, M. Isolation and Characterization of Human CD34−Lin− and CD34+Lin− Hematopoietic Stem Cells Using Cell Surface Markers AC133 and CD7. Blood 2000, 95, 2813–2820. [Google Scholar] [CrossRef]
- Shmelkov, S.V.; St.Clair, R.; Lyden, D.; Rafii, S. AC133/CD133/Prominin-1. Int. J. Biochem. Cell Biol. 2005, 37, 715–719. [Google Scholar] [CrossRef]
- Vetrie, D.; Helgason, G.V.; Copland, M. The Leukaemia Stem Cell: Similarities, Differences and Clinical Prospects in CML and AML. Nat. Rev. Cancer 2020, 20, 158–173. [Google Scholar] [CrossRef]
- Holyoake, T.; Jiang, X.; Eaves, C.; Eaves, A. Isolation of a Highly Quiescent Subpopulation of Primitive Leukemic Cells in Chronic Myeloid Leukemia. Blood 1999, 94, 2056–2064. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A Cell Initiating Human Acute Myeloid Leukaemia after Transplantation into SCID Mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Guan, Y.; Gerhard, B.; Hogge, D.E. Detection, Isolation, and Stimulation of Quiescent Primitive Leukemic Progenitor Cells from Patients with Acute Myeloid Leukemia (AML). Blood 2003, 101, 3142–3149. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Farley, T.K.; Richoz, N.; Yao, C.; Shih, H.-Y.; Petermann, F.; Zhang, Y.; Sun, H.-W.; Hayes, E.; Mikami, Y.; et al. Retinoic Acid Receptor Alpha Represses a Th9 Transcriptional and Epigenomic Program to Reduce Allergic Pathology. Immunity 2019, 50, 106–120.e10. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Ho, Y.; Li, M.; Marcucci, G.; Tong, W.; Bhatia, R. Heterogeneity of Leukemia-Initiating Capacity of Chronic Myelogenous Leukemia Stem Cells. J. Clin. Investig. 2016, 126, 975–991. [Google Scholar] [CrossRef]
- Holyoake, T.L.; Vetrie, D. The Chronic Myeloid Leukemia Stem Cell: Stemming the Tide of Persistence. Blood 2017, 129, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Jordan, C.T. Therapeutic Targeting of Acute Myeloid Leukemia Stem Cells. Blood 2017, 129, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Majeti, R. Biology and Relevance of Human Acute Myeloid Leukemia Stem Cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Griessinger, E.; Vargaftig, J.; Horswell, S.; Taussig, D.C.; Gribben, J.; Bonnet, D. Acute Myeloid Leukemia Xenograft Success Prediction: Saving Time. Exp. Hematol. 2018, 59, 66–71.e4. [Google Scholar] [CrossRef]
- Pearce, D.J.; Taussig, D.; Zibara, K.; Smith, L.-L.; Ridler, C.M.; Preudhomme, C.; Young, B.D.; Rohatiner, A.Z.; Lister, T.A.; Bonnet, D. AML Engraftment in the NOD/SCID Assay Reflects the Outcome of AML: Implications for Our Understanding of the Heterogeneity of AML. Blood 2006, 107, 1166–1173. [Google Scholar] [CrossRef]
- Ran, D.; Schubert, M.; Pietsch, L.; Taubert, I.; Wuchter, P.; Eckstein, V.; Bruckner, T.; Zoeller, M.; Ho, A.D. Aldehyde Dehydrogenase Activity among Primary Leukemia Cells Is Associated with Stem Cell Features and Correlates with Adverse Clinical Outcomes. Exp. Hematol. 2009, 37, 1423–1434. [Google Scholar] [CrossRef]
- Gerber, J.M.; Qin, L.; Kowalski, J.; Smith, B.D.; Griffin, C.A.; Vala, M.S.; Collector, M.I.; Perkins, B.; Zahurak, M.; Matsui, W.; et al. Characterization of Chronic Myeloid Leukemia Stem Cells. Am. J. Hematol. 2011, 86, 31–37. [Google Scholar] [CrossRef]
- Hehlmann, R. How I Treat CML Blast Crisis. Blood 2012, 120, 737–747. [Google Scholar] [CrossRef]
- Tanizaki, R.; Nomura, Y.; Miyata, Y.; Minami, Y.; Abe, A.; Hanamura, A.; Sawa, M.; Murata, M.; Kiyoi, H.; Matsushita, T.; et al. Irrespective of CD34 Expression, Lineage-Committed Cell Fraction Reconstitutes and Re-Establishes Transformed Philadelphia Chromosome-Positive Leukemia in NOD/SCID/IL-2Rγc−/− Mice. Cancer Sci. 2010, 101, 631–638. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human Acute Myeloid Leukemia Is Organized as a Hierarchy That Originates from a Primitive Hematopoietic Cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Hope, K.J.; Jin, L.; Dick, J.E. Acute Myeloid Leukemia Originates from a Hierarchy of Leukemic Stem Cell Classes That Differ in Self-Renewal Capacity. Nat. Immunol. 2004, 5, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem Cell Gene Expression Programs Influence Clinical Outcome in Human Leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Taussig, D.C.; Vargaftig, J.; Miraki-Moud, F.; Griessinger, E.; Sharrock, K.; Luke, T.; Lillington, D.; Oakervee, H.; Cavenagh, J.; Agrawal, S.G.; et al. Leukemia-Initiating Cells from Some Acute Myeloid Leukemia Patients with Mutated Nucleophosmin Reside in the CD34(−) Fraction. Blood 2010, 115, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Goardon, N.; Marchi, E.; Atzberger, A.; Quek, L.; Schuh, A.; Soneji, S.; Woll, P.; Mead, A.; Alford, K.A.; Rout, R.; et al. Coexistence of LMPP-like and GMP-like Leukemia Stem Cells in Acute Myeloid Leukemia. Cancer Cell 2011, 19, 138–152. [Google Scholar] [CrossRef]
- Kirstetter, P.; Schuster, M.B.; Bereshchenko, O.; Moore, S.; Dvinge, H.; Kurz, E.; Theilgaard-Mönch, K.; Månsson, R.; Pedersen, T.Å.; Pabst, T.; et al. Modeling of C/EBPα Mutant Acute Myeloid Leukemia Reveals a Common Expression Signature of Committed Myeloid Leukemia-Initiating Cells. Cancer Cell 2008, 13, 299–310. [Google Scholar] [CrossRef]
- Somervaille, T.C.P.; Matheny, C.J.; Spencer, G.J.; Iwasaki, M.; Rinn, J.L.; Witten, D.M.; Chang, H.Y.; Shurtleff, S.A.; Downing, J.R.; Cleary, M.L. Hierarchical Maintenance of MLL Myeloid Leukemia Stem Cells Employs a Transcriptional Program Shared with Embryonic Rather Than Adult Stem Cells. Cell Stem Cell 2009, 4, 129–140. [Google Scholar] [CrossRef]
- Somervaille, T.C.P.; Cleary, M.L. Identification and Characterization of Leukemia Stem Cells in Murine MLL-AF9 Acute Myeloid Leukemia. Cancer Cell 2006, 10, 257–268. [Google Scholar] [CrossRef]
- Ishikawa, F.; Yoshida, S.; Saito, Y.; Hijikata, A.; Kitamura, H.; Tanaka, S.; Nakamura, R.; Tanaka, T.; Tomiyama, H.; Saito, N.; et al. Chemotherapy-Resistant Human AML Stem Cells Home to and Engraft within the Bone-Marrow Endosteal Region. Nat. Biotechnol. 2007, 25, 1315–1321. [Google Scholar] [CrossRef]
- Quek, L.; Otto, G.W.; Garnett, C.; Lhermitte, L.; Karamitros, D.; Stoilova, B.; Lau, I.-J.; Doondeea, J.; Usukhbayar, B.; Kennedy, A.; et al. Genetically Distinct Leukemic Stem Cells in Human CD34− Acute Myeloid Leukemia Are Arrested at a Hemopoietic Precursor-like Stage. J. Exp. Med. 2016, 213, 1513–1535. [Google Scholar] [CrossRef]
- Huntly, B.J.P.; Shigematsu, H.; Deguchi, K.; Lee, B.H.; Mizuno, S.; Duclos, N.; Rowan, R.; Amaral, S.; Curley, D.; Williams, I.R.; et al. MOZ-TIF2, but Not BCR-ABL, Confers Properties of Leukemic Stem Cells to Committed Murine Hematopoietic Progenitors. Cancer Cell 2004, 6, 587–596. [Google Scholar] [CrossRef]
- Sadovnik, I.; Hoelbl-Kovacic, A.; Herrmann, H.; Eisenwort, G.; Cerny-Reiterer, S.; Warsch, W.; Hoermann, G.; Greiner, G.; Blatt, K.; Peter, B.; et al. Identification of CD25 as STAT5-Dependent Growth Regulator of Leukemic Stem Cells in Ph+ CML. Clin. Cancer Res. 2016, 22, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Sadovnik, I.; Herrmann, H.; Eisenwort, G.; Blatt, K.; Hoermann, G.; Mueller, N.; Sperr, W.R.; Valent, P. Expression of CD25 on Leukemic Stem Cells in BCR-ABL1 + CML: Potential Diagnostic Value and Functional Implications. Exp. Hematol. 2017, 51, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kitamura, H.; Hijikata, A.; Tomizawa-Murasawa, M.; Tanaka, S.; Takagi, S.; Uchida, N.; Suzuki, N.; Sone, A.; Najima, Y.; et al. Identification of Therapeutic Targets for Quiescent, Chemotherapy-Resistant Human Leukemia Stem Cells. Sci. Transl. Med. 2010, 2, 17ra9. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Cerny-Reiterer, S.; Gleixner, K.V.; Blatt, K.; Herndlhofer, S.; Rabitsch, W.; Jager, E.; Mitterbauer-Hohendanner, G.; Streubel, B.; Selzer, E.; et al. CD34+/CD38- Stem Cells in Chronic Myeloid Leukemia Express Siglec-3 (CD33) and Are Responsive to the CD33-Targeting Drug Gemtuzumab/Ozogamicin. Haematologica 2012, 97, 219–226. [Google Scholar] [CrossRef]
- Herrmann, H.; Sadovnik, I.; Eisenwort, G.; Rülicke, T.; Blatt, K.; Herndlhofer, S.; Willmann, M.; Stefanzl, G.; Baumgartner, S.; Greiner, G.; et al. Delineation of Target Expression Profiles in CD34+/CD38− and CD34+/CD38+ Stem and Progenitor Cells in AML and CML. Blood Adv. 2020, 4, 5118–5132. [Google Scholar] [CrossRef]
- Jilani, I.; Estey, E.; Huh, Y.; Joe, Y.; Manshouri, T.; Yared, M.; Giles, F.; Kantarjian, H.; Cortes, J.; Thomas, D.; et al. Differences in CD33 Intensity Between Various Myeloid Neoplasms. Am. J. Clin. Pathol. 2002, 118, 560–566. [Google Scholar] [CrossRef]
- Kersten, B.; Valkering, M.; Wouters, R.; van Amerongen, R.; Hanekamp, D.; Kwidama, Z.; Valk, P.; Ossenkoppele, G.; Zeijlemaker, W.; Kaspers, G.; et al. CD45RA, a Specific Marker for Leukaemia Stem Cell Sub-Populations in Acute Myeloid Leukaemia. Br. J. Haematol. 2016, 173, 219–235. [Google Scholar] [CrossRef]
- Wisniewski, D.; Affer, M.; Willshire, J.; Clarkson, B. Further Phenotypic Characterization of the Primitive Lineage− CD34+CD38−CD90+CD45RA− Hematopoietic Stem Cell/Progenitor Cell Sub-Population Isolated from Cord Blood, Mobilized Peripheral Blood and Patients with Chronic Myelogenous Leukemia. Blood Cancer J. 2011, 1, e36. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C.; Galvan, M.D.; Bohlson, S.S. CD93: Recent Advances and Implications in Disease. Curr. Drug Targets 2012, 13, 411–420. [Google Scholar] [CrossRef]
- Inoue, A.; Kobayashi, C.I.; Shinohara, H.; Miyamoto, K.; Yamauchi, N.; Yuda, J.; Akao, Y.; Minami, Y. Chronic Myeloid Leukemia Stem Cells and Molecular Target Therapies for Overcoming Resistance and Disease Persistence. Int. J. Hematol. 2018, 108, 365–370. [Google Scholar] [CrossRef]
- Kinstrie, R.; Horne, G.A.; Morrison, H.; Irvine, D.; Munje, C.; Castañeda, E.G.; Moka, H.A.; Dunn, K.; Cassels, J.E.; Parry, N.; et al. CD93 Is Expressed on Chronic Myeloid Leukemia Stem Cells and Identifies a Quiescent Population Which Persists after Tyrosine Kinase Inhibitor Therapy. Leukemia 2020, 34, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Manié, S.; Billard, M.; Ashman, L.; Gerlier, D.; Boucheix, C.; Rubinstein, E. Multiple Levels of Interactions within the Tetraspanin Web. Biochem. Biophys. Res. Commun. 2003, 304, 107–112. [Google Scholar] [CrossRef]
- Touzet, L.; Dumezy, F.; Roumier, C.; Berthon, C.; Bories, C.; Quesnel, B.; Preudhomme, C.; Boyer, T. CD9 in Acute Myeloid Leukemia: Prognostic Role and Usefulness to Target Leukemic Stem Cells. Cancer Med. 2019, 8, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- van Rhenen, A.; van Dongen, G.A.M.S.; Kelder, A.; Rombouts, E.J.; Feller, N.; Moshaver, B.; Walsum, M.S.; Zweegman, S.; Ossenkoppele, G.J.; Jan Schuurhuis, G. The Novel AML Stem Cell–Associated Antigen CLL-1 Aids in Discrimination between Normal and Leukemic Stem Cells. Blood 2007, 110, 2659–2666. [Google Scholar] [CrossRef]
- Askmyr, M.; Ågerstam, H.; Hansen, N.; Gordon, S.; Arvanitakis, A.; Rissler, M.; Juliusson, G.; Richter, J.; Järås, M.; Fioretos, T. Selective Killing of Candidate AML Stem Cells by Antibody Targeting of IL1RAP. Blood 2013, 121, 3709–3713. [Google Scholar] [CrossRef]
- Jaras, M.; Johnels, P.; Hansen, N.; Agerstam, H.; Tsapogas, P.; Rissler, M.; Lassen, C.; Olofsson, T.; Bjerrum, O.W.; Richter, J.; et al. Isolation and Killing of Candidate Chronic Myeloid Leukemia Stem Cells by Antibody Targeting of IL-1 Receptor Accessory Protein. Proc. Natl. Acad. Sci. USA 2010, 107, 16280–16285. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Chen, Y.; Yeh, Y.; Huang, H. CD69 Partially Inhibits Apoptosis and Erythroid Differentiation via CD24, and Their Knockdown Increase Imatinib Sensitivity in BCR-ABL-positive Cells. J. Cell. Physiol. 2018, 233, 7467–7479. [Google Scholar] [CrossRef]
- Landberg, N.; von Palffy, S.; Askmyr, M.; Lilljebjörn, H.; Sandén, C.; Rissler, M.; Mustjoki, S.; Hjorth-Hansen, H.; Richter, J.; Ågerstam, H.; et al. CD36 Defines Primitive Chronic Myeloid Leukemia Cells Less Responsive to Imatinib but Vulnerable to Antibody-Based Therapeutic Targeting. Haematologica 2018, 103, 447–455. [Google Scholar] [CrossRef]
- Sachs, K.; Sarver, A.L.; Noble-Orcutt, K.E.; LaRue, R.S.; Antony, M.L.; Chang, D.; Lee, Y.; Navis, C.M.; Hillesheim, A.L.; Nykaza, I.R.; et al. Single-Cell Gene Expression Analyses Reveal Distinct Self-Renewing and Proliferating Subsets in the Leukemia Stem Cell Compartment in Acute Myeloid Leukemia. Cancer Res. 2020, 80, 458–470. [Google Scholar] [CrossRef]
- O’Reilly, E.; Zeinabad, H.A.; Szegezdi, E. Hematopoietic versus Leukemic Stem Cell Quiescence: Challenges and Therapeutic Opportunities. Blood Rev. 2021, 50, 100850. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L. Dissecting the Bone Marrow HSC Niches. Cell Res. 2016, 26, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Crane, G.M.; Jeffery, E.; Morrison, S.J. Adult Haematopoietic Stem Cell Niches. Nat. Rev. Immunol. 2017, 17, 573–590. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar Niches Maintain Haematopoietic Stem Cell Quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Acar, M.; Kocherlakota, K.S.; Murphy, M.M.; Peyer, J.G.; Oguro, H.; Inra, C.N.; Jaiyeola, C.; Zhao, Z.; Luby-Phelps, K.; Morrison, S.J. Deep Imaging of Bone Marrow Shows Non-Dividing Stem Cells Are Mainly Perisinusoidal. Nature 2015, 526, 126–130. [Google Scholar] [CrossRef]
- Poulos, M.G.; Guo, P.; Kofler, N.M.; Pinho, S.; Gutkin, M.C.; Tikhonova, A.; Aifantis, I.; Frenette, P.S.; Kitajewski, J.; Rafii, S.; et al. Endothelial Jagged-1 Is Necessary for Homeostatic and Regenerative Hematopoiesis. Cell Rep. 2013, 4, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Barbier, V.; Nowlan, B.; Jacobsen, R.N.; Forristal, C.E.; Patton, J.T.; Magnani, J.L.; Lévesque, J.-P. Vascular Niche E-Selectin Regulates Hematopoietic Stem Cell Dormancy, Self Renewal and Chemoresistance. Nat. Med. 2012, 18, 1651–1657. [Google Scholar] [CrossRef]
- Galán-Díez, M.; Kousteni, S. The Osteoblastic Niche in Hematopoiesis and Hematological Myeloid Malignancies. Curr. Mol. Biol. Rep. 2017, 3, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Itkin, T.; Ludin, A.; Gradus, B.; Gur-Cohen, S.; Kalinkovich, A.; Schajnovitz, A.; Ovadya, Y.; Kollet, O.; Canaani, J.; Shezen, E.; et al. FGF-2 Expands Murine Hematopoietic Stem and Progenitor Cells via Proliferation of Stromal Cells, c-Kit Activation, and CXCL12 down-Regulation. Blood 2012, 120, 1843–1855. [Google Scholar] [CrossRef]
- Takam Kamga, P.; Bazzoni, R.; Dal Collo, G.; Cassaro, A.; Tanasi, I.; Russignan, A.; Tecchio, C.; Krampera, M. The Role of Notch and Wnt Signaling in MSC Communication in Normal and Leukemic Bone Marrow Niche. Front. Cell Dev. Biol. 2021, 8, 599276. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ema, H.; Karlsson, G.; Yamaguchi, T.; Miyoshi, H.; Shioda, S.; Taketo, M.M.; Karlsson, S.; Iwama, A.; Nakauchi, H. Nonmyelinating Schwann Cells Maintain Hematopoietic Stem Cell Hibernation in the Bone Marrow Niche. Cell 2011, 147, 1146–1158. [Google Scholar] [CrossRef]
- Arai, F.; Hosokawa, K.; Toyama, H.; Matsumoto, Y.; Suda, T. Role of N-Cadherin in the Regulation of Hematopoietic Stem Cells in the Bone Marrow Niche: Function of N-Cadherin in the Regulation of HSPCs. Ann. N. Y. Acad. Sci. 2012, 1266, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhou, H.; Nemes, K.; Yen, D.; Zhao, W.; Bramlett, C.; Wang, B.; Lu, R.; Shen, K. Membrane-Bound SCF and VCAM-1 Synergistically Regulate the Morphology of Hematopoietic Stem Cells. J. Cell Biol. 2021, 220, e202010118. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; DiPersio, J.F. Mobilization of Hematopoietic Stem and Leukemia Cells. J. Leukoc. Biol. 2012, 91, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Coulombel, L.; Auffray, I.; Gaugler, M.-H.; Rosemblatt, M. Expression and Function of Integrins on Hematopoietic Progenitor Cells. Acta Haematol. 1997, 97, 13–21. [Google Scholar] [CrossRef]
- Lee-Thedieck, C.; Spatz, J.P. Biophysical Regulation of Hematopoietic Stem Cells. Biomater. Sci. 2014, 2, 1548–1561. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Alonso, J.L.; Goldmann, W.H. Cellular mechanotransduction. AIMS Biophys. 2016, 3, 50–62. [Google Scholar] [CrossRef]
- Riether, C.; Schürch, C.M.; Ochsenbein, A.F. Regulation of Hematopoietic and Leukemic Stem Cells by the Immune System. Cell Death Differ. 2015, 22, 187–198. [Google Scholar] [CrossRef]
- Schepers, K.; Pietras, E.M.; Reynaud, D.; Flach, J.; Binnewies, M.; Garg, T.; Wagers, A.J.; Hsiao, E.C.; Passegué, E. Myeloproliferative Neoplasia Remodels the Endosteal Bone Marrow Niche into a Self-Reinforcing Leukemic Niche. Cell Stem Cell 2013, 13, 285–299. [Google Scholar] [CrossRef]
- Sipkins, D.A.; Wei, X.; Wu, J.W.; Runnels, J.M.; Côté, D.; Means, T.K.; Luster, A.D.; Scadden, D.T.; Lin, C.P. In Vivo Imaging of Specialized Bone Marrow Endothelial Microdomains for Tumour Engraftment. Nature 2005, 435, 969–973. [Google Scholar] [CrossRef]
- Brenner, A.K.; Nepstad, I.; Bruserud, Ø. Mesenchymal Stem Cells Support Survival and Proliferation of Primary Human Acute Myeloid Leukemia Cells through Heterogeneous Molecular Mechanisms. Front. Immunol. 2017, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Jacamo, R.; Chen, Y.; Wang, Z.; Ma, W.; Zhang, M.; Spaeth, E.L.; Wang, Y.; Battula, V.L.; Mak, P.Y.; Schallmoser, K.; et al. Reciprocal Leukemia-Stroma VCAM-1/VLA-4-Dependent Activation of NF-KB Mediates Chemoresistance. Blood 2014, 123, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Klein, T.; Born, G.; Hilpert, M.; Scherberich, A.; Lengerke, C.; Skoda, R.C.; Bourgine, P.E.; Martin, I. Culturing Patient-Derived Malignant Hematopoietic Stem Cells in Engineered and Fully Humanized 3D Niches. Proc. Natl. Acad. Sci. USA 2021, 118, e2114227118. [Google Scholar] [CrossRef]
- Ito, S.; Barrett, A.J.; Dutra, A.; Pak, E.; Miner, S.; Keyvanfar, K.; Hensel, N.F.; Rezvani, K.; Muranski, P.; Liu, P.; et al. Long Term Maintenance of Myeloid Leukemic Stem Cells Cultured with Unrelated Human Mesenchymal Stromal Cells. Stem Cell Res. 2015, 14, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ema, H. Mechanisms of Self-Renewal in Hematopoietic Stem Cells. Int. J. Hematol. 2016, 103, 498–509. [Google Scholar] [CrossRef]
- Gothot, A.; van der Loo, J.C.M.; Clapp, D.W.; Srour, E.F. Cell Cycle-Related Changes in Repopulating Capacity of Human Mobilized Peripheral Blood CD34+ Cells in Non-Obese Diabetic/Severe Combined Immune-Deficient Mice. Blood 1998, 92, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.C.; Dörr, K.; Niebel, A.; Daria, D.; Schrezenmeier, H.; Rojewski, M.; Filippi, M.-D.; Hasenberg, A.; Gunzer, M.; Scharffetter-Kochanek, K.; et al. Cdc42 Activity Regulates Hematopoietic Stem Cell Aging and Rejuvenation. Cell Stem Cell 2012, 10, 520–530. [Google Scholar] [CrossRef]
- Ito, K.; Turcotte, R.; Cui, J.; Zimmerman, S.E.; Pinho, S.; Mizoguchi, T.; Arai, F.; Runnels, J.M.; Alt, C.; Teruya-Feldstein, J.; et al. Self-Renewal of a Purified Tie2+ Hematopoietic Stem Cell Population Relies on Mitochondrial Clearance. Science 2016, 354, 1156–1160. [Google Scholar] [CrossRef]
- Loeffler, D.; Wehling, A.; Schneiter, F.; Zhang, Y.; Müller-Bötticher, N.; Hoppe, P.S.; Hilsenbeck, O.; Kokkaliaris, K.D.; Endele, M.; Schroeder, T. Asymmetric Lysosome Inheritance Predicts Activation of Haematopoietic Stem Cells. Nature 2019, 573, 426–429. [Google Scholar] [CrossRef]
- Ting, S.B.; Deneault, E.; Hope, K.; Cellot, S.; Chagraoui, J.; Mayotte, N.; Dorn, J.F.; Laverdure, J.-P.; Harvey, M.; Hawkins, E.D.; et al. Asymmetric Segregation and Self-Renewal of Hematopoietic Stem and Progenitor Cells with Endocytic Ap2a2. Blood 2012, 119, 2510–2522. [Google Scholar] [CrossRef]
- Nebenfuehr, S.; Kollmann, K.; Sexl, V. The Role of CDK6 in Cancer. Int. J. Cancer 2020, 147, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Rollins, B.J. Cyclin C/Cdk3 Promotes Rb-Dependent G0 Exit. Cell 2004, 117, 239–251. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK Inhibitors: Cell Cycle Regulators and Beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yoshihara, H.; Hosokawa, K.; Tai, I.; Shinmyozu, K.; Tsukahara, F.; Maru, Y.; Nakayama, K.; Nakayama, K.I.; Suda, T. P57Kip2 and P27Kip1 Cooperate to Maintain Hematopoietic Stem Cell Quiescence through Interactions with Hsc70. Cell Stem Cell 2011, 9, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.A.; Hwa, J.; Nolta, J.A. Molecular Mechanism of Transforming Growth Factor Beta-mediated Cell-cycle Modulation in Primary Human CD34(+) Progenitors. Blood 2002, 99, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, P.; Shen, H.; Yu, H.; Song, X.; Zhang, L.; Zhang, P.; Cheng, H.; Xie, Z.; Hao, S.; et al. Small-Molecule Inhibitors Targeting INK4 Protein P18INK4C Enhance Ex Vivo Expansion of Haematopoietic Stem Cells. Nat. Commun. 2015, 6, 6328. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, H.; Franklin, D.S.; Scadden, D.T.; Cheng, T. In Vivo Self-Renewing Divisions of Haematopoietic Stem Cells Are Increased in the Absence of the Early G1-Phase Inhibitor, P18INK4C. Nat. Cell Biol. 2004, 6, 436–442. [Google Scholar] [CrossRef]
- Hilpert, M.; Legrand, C.; Bluteau, D.; Balayn, N.; Betems, A.; Bluteau, O.; Villeval, J.-L.; Louache, F.; Gonin, P.; Debili, N.; et al. P19INK4d Controls Hematopoietic Stem Cells in a Cell-Autonomous Manner during Genotoxic Stress and through the Microenvironment during Aging. Stem Cell Rep. 2014, 3, 1085–1102. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular Regulation of Stem Cell Quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Hao, S.; Chen, C.; Cheng, T. Cell Cycle Regulation of Hematopoietic Stem or Progenitor Cells. Int. J. Hematol. 2016, 103, 487–497. [Google Scholar] [CrossRef]
- Bowie, M.B.; Kent, D.G.; Copley, M.R.; Eaves, C.J. Steel Factor Responsiveness Regulates the High Self-Renewal Phenotype of Fetal Hematopoietic Stem Cells. Blood 2007, 109, 5043–5048. [Google Scholar] [CrossRef]
- Fox, N.; Priestley, G.; Papayannopoulou, T.; Kaushansky, K. Thrombopoietin Expands Hematopoietic Stem Cells after Transplantation. J. Clin. Investig. 2002, 110, 389–394. [Google Scholar] [CrossRef]
- Kunisato, A.; Chiba, S.; Nakagami-Yamaguchi, E.; Kumano, K.; Saito, T.; Masuda, S.; Yamaguchi, T.; Osawa, M.; Kageyama, R.; Nakauchi, H.; et al. HES-1 Preserves Purified Hematopoietic Stem Cells Ex Vivo and Accumulates Side Population Cells in Vivo. Blood 2003, 101, 1777–1783. [Google Scholar] [CrossRef]
- Stier, S.; Cheng, T.; Dombkowski, D.; Carlesso, N.; Scadden, D.T. Notch1 Activation Increases Hematopoietic Stem Cell Self-Renewal in Vivo and Favors Lymphoid over Myeloid Lineage Outcome. Blood 2002, 99, 2369–2378. [Google Scholar] [CrossRef]
- Arai, F.; Hirao, A.; Ohmura, M.; Sato, H.; Matsuoka, S.; Takubo, K.; Ito, K.; Koh, G.Y.; Suda, T. Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell 2004, 118, 149–161. [Google Scholar] [CrossRef]
- Nie, Y.; Han, Y.-C.; Zou, Y.-R. CXCR4 Is Required for the Quiescence of Primitive Hematopoietic Cells. J. Exp. Med. 2008, 205, 777–783. [Google Scholar] [CrossRef]
- Yamazaki, S.; Iwama, A.; Takayanagi, S.; Eto, K.; Ema, H.; Nakauchi, H. TGF-β as a Candidate Bone Marrow Niche Signal to Induce Hematopoietic Stem Cell Hibernation. Blood 2009, 113, 1250–1256. [Google Scholar] [CrossRef]
- Buza-Vidas, N.; Antonchuk, J.; Qian, H.; Månsson, R.; Luc, S.; Zandi, S.; Anderson, K.; Takaki, S.; Nygren, J.M.; Jensen, C.T.; et al. Cytokines Regulate Postnatal Hematopoietic Stem Cell Expansion: Opposing Roles of Thrombopoietin and LNK. Genes Dev. 2006, 20, 2018–2023. [Google Scholar] [CrossRef]
- Geddis, A.E.; Fox, N.E.; Kaushansky, K. Phosphatidylinositol 3-Kinase Is Necessary but Not Sufficient for Thrombopoietin-Induced Proliferation in Engineered Mpl-Bearing Cell Lines as Well as in Primary Megakaryocytic Progenitors. J. Biol. Chem. 2001, 276, 34473–34479. [Google Scholar] [CrossRef]
- Kimura, S.; Roberts, A.W.; Metcalf, D.; Alexander, W.S. Hematopoietic Stem Cell Deficiencies in Mice Lacking C-Mpl, the Receptor for Thrombopoietin. Proc. Natl. Acad. Sci. USA 1998, 95, 1195–1200. [Google Scholar] [CrossRef]
- Kollmann, S.; Grausenburger, R.; Klampfl, T.; Prchal-Murphy, M.; Bastl, K.; Pisa, H.; Knab, V.M.; Brandstoetter, T.; Doma, E.; Sperr, W.R.; et al. A STAT5B–CD9 Axis Determines Self-Renewal in Hematopoietic and Leukemic Stem Cells. Blood 2021, 138, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Rojnuckarin, P.; Drachman, J.G.; Kaushansky, K. Thrombopoietin-Induced Activation of the Mitogen-Activated Protein Kinase (MAPK) Pathway in Normal Megakaryocytes: Role in Endomitosis. Blood 1999, 94, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Seita, J.; Weissman, I.L. Hematopoietic Stem Cell: Self-Renewal versus Differentiation: Hematopoietic Stem Cell. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, H.; Arai, F.; Hosokawa, K.; Hagiwara, T.; Takubo, K.; Nakamura, Y.; Gomei, Y.; Iwasaki, H.; Matsuoka, S.; Miyamoto, K.; et al. Thrombopoietin/MPL Signaling Regulates Hematopoietic Stem Cell Quiescence and Interaction with the Osteoblastic Niche. Cell Stem Cell 2007, 1, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Lodish, H.F. Cytokines Regulating Hematopoietic Stem Cell Function. Curr. Opin. Hematol. 2008, 15, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Antonchuk, J.; Sauvageau, G.; Humphries, R.K. HOXB4 Overexpression Mediates Very Rapid Stem Cell Regeneration and Competitive Hematopoietic Repopulation. Exp. Hematol. 2001, 29, 1125–1134. [Google Scholar] [CrossRef]
- Qian, H.; Buza-Vidas, N.; Hyland, C.D.; Jensen, C.T.; Antonchuk, J.; Månsson, R.; Thoren, L.A.; Ekblom, M.; Alexander, W.S.; Jacobsen, S.E.W. Critical Role of Thrombopoietin in Maintaining Adult Quiescent Hematopoietic Stem Cells. Cell Stem Cell 2007, 1, 671–684. [Google Scholar] [CrossRef]
- Maurer, B.; Kollmann, S.; Pickem, J.; Hoelbl-Kovacic, A.; Sexl, V. STAT5A and STAT5B—Twins with Different Personalities in Hematopoiesis and Leukemia. Cancers 2019, 11, 1726. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Tse, W.; Bunting, K.D. Conditional Deletion of STAT5 in Adult Mouse Hematopoietic Stem Cells Causes Loss of Quiescence and Permits Efficient Nonablative Stem Cell Replacement. Blood 2009, 113, 4856–4865. [Google Scholar] [CrossRef]
- Fatrai, S.; Wierenga, A.T.J.; Daenen, S.M.G.J.; Vellenga, E.; Schuringa, J.J. Identification of HIF2α as an Important STAT5 Target Gene in Human Hematopoietic Stem Cells. Blood 2011, 117, 3320–3330. [Google Scholar] [CrossRef]
- Lampreia, F.P.; Carmelo, J.G.; Anjos-Afonso, F. Notch Signaling in the Regulation of Hematopoietic Stem Cell. Curr. Stem Cell Rep. 2017, 3, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, C.; Gramantieri, L.; Minguzzi, M.; Fornari, F.; Chieco, P.; Grazi, G.L.; Bolondi, L. CDKN1C/P57 Is Regulated by the Notch Target Gene Hes1 and Induces Senescence in Human Hepatocellular Carcinoma. Am. J. Pathol. 2012, 181, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Guiu, J.; Shimizu, R.; D’Altri, T.; Fraser, S.T.; Hatakeyama, J.; Bresnick, E.H.; Kageyama, R.; Dzierzak, E.; Yamamoto, M.; Espinosa, L.; et al. Hes Repressors Are Essential Regulators of Hematopoietic Stem Cell Development Downstream of Notch Signaling. J. Exp. Med. 2013, 210, 71–84. [Google Scholar] [CrossRef]
- Palomero, T.; Lim, W.K.; Odom, D.T.; Sulis, M.L.; Real, P.J.; Margolin, A.; Barnes, K.C.; O’Neil, J.; Neuberg, D.; Weng, A.P.; et al. NOTCH1 Directly Regulates C-MYC and Activates a Feed-Forward-Loop Transcriptional Network Promoting Leukemic Cell Growth. Proc. Natl. Acad. Sci. USA 2006, 103, 18261–18266. [Google Scholar] [CrossRef]
- de Pooter, R.F.; Schmitt, T.M.; de la Pompa, J.L.; Fujiwara, Y.; Orkin, S.H.; Zúñiga-Pflücker, J.C. Notch Signaling Requires GATA-2 to Inhibit Myelopoiesis from Embryonic Stem Cells and Primary Hemopoietic Progenitors. J. Immunol. 2006, 176, 5267–5275. [Google Scholar] [CrossRef]
- Perez-Roger, I. Cyclins D1 and D2 Mediate Myc-Induced Proliferation via Sequestration of P27Kip1 and P21Cip1. EMBO J. 1999, 18, 5310–5320. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Wu, Y.; Shi, K.; Bing, L.; Hao, J. Wnt/β-Catenin Signaling Pathway Upregulates c-Myc Expression to Promote Cell Proliferation of P19 Teratocarcinoma Cells. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 2104–2113. [Google Scholar] [CrossRef]
- Fleming, H.E.; Janzen, V.; Lo Celso, C.; Guo, J.; Leahy, K.M.; Kronenberg, H.M.; Scadden, D.T. Wnt Signaling in the Niche Enforces Hematopoietic Stem Cell Quiescence and Is Necessary to Preserve Self-Renewal In Vivo. Cell Stem Cell 2008, 2, 274–283. [Google Scholar] [CrossRef]
- Luis, T.C.; Naber, B.A.E.; Roozen, P.P.C.; Brugman, M.H.; de Haas, E.F.E.; Ghazvini, M.; Fibbe, W.E.; van Dongen, J.J.M.; Fodde, R.; Staal, F.J.T. Canonical Wnt Signaling Regulates Hematopoiesis in a Dosage-Dependent Fashion. Cell Stem Cell 2011, 9, 345–356. [Google Scholar] [CrossRef]
- Zhang, Y.; Dépond, M.; He, L.; Foudi, A.; Kwarteng, E.O.; Lauret, E.; Plo, I.; Desterke, C.; Dessen, P.; Fujii, N.; et al. CXCR4/CXCL12 Axis Counteracts Hematopoietic Stem Cell Exhaustion through Selective Protection against Oxidative Stress. Sci. Rep. 2016, 6, 37827. [Google Scholar] [CrossRef]
- Blank, U.; Karlsson, S. TGF-b Signaling in the Control of Hematopoietic Stem Cells. Blood 2015, 125, 3542–3550. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.A.; Taylor, N.; Nolta, J.A. Reduction in Levels of the Cyclin-Dependent Kinase Inhibitor P27kip-1 Coupled with Transforming Growth Factor Neutralization Induces Cell-Cycle Entry and Increases Retroviral Transduction of Primitive Human Hematopoietic Cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13006–13011. [Google Scholar] [CrossRef] [PubMed]
- Ducos, K.; Panterne, B.; Fortunel, N.; Hatzfeld, A.; Monier, M.-N.; Hatzfeld, J. p21(cip1) mRNA is Controlled by Endogenous Transforming Growth Factor-Beta1 in Quiescent Human Hematopoietic Stem/Progenitor Cells. J. Cell. Physiol. 2000, 184, 80–85. [Google Scholar] [CrossRef]
- Vaidya, A.; Kale, V. Hematopoietic Stem Cells, Their Niche, and the Concept of Co-Culture Systems: A Critical Review. J. Stem Cells 2015, 19, 13–31. [Google Scholar]
- Wang, X.; Dong, F.; Zhang, S.; Yang, W.; Yu, W.; Wang, Z.; Zhang, S.; Wang, J.; Ma, S.; Wu, P.; et al. TGF-Β1 Negatively Regulates the Number and Function of Hematopoietic Stem Cells. Stem Cell Rep. 2018, 11, 274–287. [Google Scholar] [CrossRef]
- Asai, T.; Liu, Y.; Di Giandomenico, S.; Bae, N.; Ndiaye-Lobry, D.; Deblasio, A.; Menendez, S.; Antipin, Y.; Reva, B.; Wevrick, R.; et al. Necdin, a P53 Target Gene, Regulates the Quiescence and Response to Genotoxic Stress of Hematopoietic Stem/Progenitor Cells. Blood 2012, 120, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Elf, S.E.; Miyata, Y.; Sashida, G.; Liu, Y.; Huang, G.; Di Giandomenico, S.; Lee, J.M.; Deblasio, A.; Menendez, S.; et al. P53 Regulates Hematopoietic Stem Cell Quiescence. Cell Stem Cell 2009, 4, 37–48. [Google Scholar] [CrossRef]
- Iavarone, A.; Garg, P.; Lasorella, A.; Hsu, J.; Israel, M.A. The Helix-Loop-Helix Rotein Id-2 Enhances Cell ProliferPion and Binds to the Retinobl-Asto a Protein. Genes Dev. 1994, 16, 1270–1284. [Google Scholar] [CrossRef]
- Mori, S. Lactation Defect in Mice Lacking the Helix-Loop-Helix Inhibitor Id2. EMBO J. 2000, 19, 5772–5781. [Google Scholar] [CrossRef]
- Taniura, H.; Matsumoto, K.; Yoshikawa, K. Physical and Functional Interactions of Neuronal Growth Suppressor Necdin with P53. J. Biol. Chem. 1999, 274, 16242–16248. [Google Scholar] [CrossRef]
- Lacorazza, H.D.; Yamada, T.; Liu, Y.; Miyata, Y.; Sivina, M.; Nunes, J.; Nimer, S.D. The Transcription Factor MEF/ELF4 Regulates the Quiescence of Primitive Hematopoietic Cells. Cancer Cell 2006, 9, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ficara, F.; Murphy, M.J.; Lin, M.; Cleary, M.L. Pbx1 Regulates Self-Renewal of Long-Term Hematopoietic Stem Cells by Maintaining Their Quiescence. Cell Stem Cell 2008, 2, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Goyama, S.; Nitta, E.; Yoshino, T.; Kako, S.; Watanabe-Okochi, N.; Shimabe, M.; Imai, Y.; Takahashi, K.; Kurokawa, M. EVI-1 Interacts with Histone Methyltransferases SUV39H1 and G9a for Transcriptional Repression and Bone Marrow Immortalization. Leukemia 2010, 24, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Frelin, C.; Xie, S.; Ferrari, R.; Dunant, C.F.; Zandi, S.; Neumann, A.; Plumb, I.; Doulatov, S.; Chen, J.; et al. CDK6 Levels Regulate Quiescence Exit in Human Hematopoietic Stem Cells. Cell Stem Cell 2015, 16, 302–313. [Google Scholar] [CrossRef]
- Scheicher, R.; Hoelbl-Kovacic, A.; Bellutti, F.; Tigan, A.-S.; Prchal-Murphy, M.; Heller, G.; Schneckenleithner, C.; Salazar-Roa, M.; Zöchbauer-Müller, S.; Zuber, J.; et al. CDK6 as a Key Regulator of Hematopoietic and Leukemic Stem Cell Activation. Blood 2015, 125, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Laurenti, E.; Trumpp, A. Balancing Dormant and Self-Renewing Hematopoietic Stem Cells. Curr. Opin. Genet. Dev. 2009, 19, 461–468. [Google Scholar] [CrossRef]
- Takizawa, H.; Regoes, R.R.; Boddupalli, C.S.; Bonhoeffer, S.; Manz, M.G. Dynamic Variation in Cycling of Hematopoietic Stem Cells in Steady State and Inflammation. J. Exp. Med. 2011, 208, 273–284. [Google Scholar] [CrossRef]
- Trumpp, A.; Essers, M.; Wilson, A. Awakening Dormant Haematopoietic Stem Cells. Nat. Rev. Immunol. 2010, 10, 201–209. [Google Scholar] [CrossRef]
- Cortes, J. Natural History and Staging of Chronic Myelogenous Leukemia. Hematol. Oncol. Clin. N. Am. 2004, 18, 569–584. [Google Scholar] [CrossRef]
- Corces, M.R.; Buenrostro, J.D.; Wu, B.; Greenside, P.G.; Chan, S.M.; Koenig, J.L.; Snyder, M.P.; Pritchard, J.K.; Kundaje, A.; Greenleaf, W.J.; et al. Lineage-Specific and Single-Cell Chromatin Accessibility Charts Human Hematopoiesis and Leukemia Evolution. Nat. Genet. 2016, 48, 1193–1203. [Google Scholar] [CrossRef]
- Jan, M.; Snyder, T.M.; Corces-Zimmerman, M.R.; Vyas, P.; Weissman, I.L.; Quake, S.R.; Majeti, R. Clonal Evolution of Preleukemic Hematopoietic Stem Cells Precedes Human Acute Myeloid Leukemia. Sci. Transl. Med. 2012, 4, 149ra118. [Google Scholar] [CrossRef]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohle, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A.; et al. IDH Mutation Impairs Histone Demethylation and Results in a Block to Cell Differentiation. Nature 2012, 483, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, D.G.; Griffin, J.D. The Roles of FLT3 in Hematopoiesis and Leukemia. Blood 2002, 100, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Small, D. Mutant FLT3 Signaling Contributes to a Block in Myeloid Differentiation. Leuk. Lymphoma 2005, 46, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.N.; Kalleda, N.; Stavropoulou, V.; Schwaller, J. The Impact of the Cellular Origin in Acute Myeloid Leukemia: Learning from Mouse Models. HemaSphere 2019, 3, e152. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-Gene Stemness Score for Rapid Determination of Risk in Acute Leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef]
- Duployez, N.; Marceau-Renaut, A.; Villenet, C.; Petit, A.; Rousseau, A.; Ng, S.W.K.; Paquet, A.; Gonzales, F.; Barthélémy, A.; Leprêtre, F.; et al. The Stem Cell-Associated Gene Expression Signature Allows Risk Stratification in Pediatric Acute Myeloid Leukemia. Leukemia 2019, 33, 348–357. [Google Scholar] [CrossRef]
- Li, S.; Garrett-Bakelman, F.E.; Chung, S.S.; Sanders, M.A.; Hricik, T.; Rapaport, F.; Patel, J.; Dillon, R.; Vijay, P.; Brown, A.L.; et al. Distinct Evolution and Dynamics of Epigenetic and Genetic Heterogeneity in Acute Myeloid Leukemia. Nat. Med. 2016, 22, 792–799. [Google Scholar] [CrossRef]
- Simsek, T.; Kocabas, F.; Zheng, J.; DeBerardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The Distinct Metabolic Profile of Hematopoietic Stem Cells Reflects Their Location in a Hypoxic Niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef]
- Kuntz, E.M.; Baquero, P.; Michie, A.M.; Dunn, K.; Tardito, S.; Holyoake, T.L.; Helgason, G.V.; Gottlieb, E. Targeting Mitochondrial Oxidative Phosphorylation Eradicates Therapy-Resistant Chronic Myeloid Leukemia Stem Cells. Nat. Med. 2017, 23, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Nieborowska-Skorska, M.; Kopinski, P.K.; Ray, R.; Hoser, G.; Ngaba, D.; Flis, S.; Cramer, K.; Reddy, M.M.; Koptyra, M.; Penserga, T.; et al. Rac2-MRC-CIII–Generated ROS Cause Genomic Instability in Chronic Myeloid Leukemia Stem Cells and Primitive Progenitors. Blood 2012, 119, 4253–4263. [Google Scholar] [CrossRef] [PubMed]
- Sallmyr, A.; Fan, J.; Datta, K.; Kim, K.-T.; Grosu, D.; Shapiro, P.; Small, D.; Rassool, F. Internal Tandem Duplication of FLT3 (FLT3/ITD) Induces Increased ROS Production, DNA Damage, and Misrepair: Implications for Poor Prognosis in AML. Blood 2008, 111, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Adane, B.; Ye, H.; Khan, N.; Pei, S.; Minhajuddin, M.; Stevens, B.M.; Jones, C.L.; D’Alessandro, A.; Reisz, J.A.; Zaberezhnyy, V.; et al. The Hematopoietic Oxidase NOX2 Regulates Self-Renewal of Leukemic Stem Cells. Cell Rep. 2019, 27, 238–254.e6. [Google Scholar] [CrossRef]
- Grossmann, V.; Kohlmann, A.; Zenger, M.; Schindela, S.; Eder, C.; Weissmann, S.; Schnittger, S.; Kern, W.; Müller, M.C.; Hochhaus, A.; et al. A Deep-Sequencing Study of Chronic Myeloid Leukemia Patients in Blast Crisis (BC-CML) Detects Mutations in 76.9% of Cases. Leukemia 2011, 25, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef]
- Rebechi, M.T.; Pratz, K.W. Genomic Instability Is a Principle Pathologic Feature of FLT3 ITD Kinase Activity in Acute Myeloid Leukemia Leading to Clonal Evolution and Disease Progression. Leuk. Lymphoma 2017, 58, 2040–2050. [Google Scholar] [CrossRef]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef]
- Hao, X.; Gu, H.; Chen, C.; Huang, D.; Zhao, Y.; Xie, L.; Zou, Y.; Shu, H.S.; Zhang, Y.; He, X.; et al. Metabolic Imaging Reveals a Unique Preference of Symmetric Cell Division and Homing of Leukemia-Initiating Cells in an Endosteal Niche. Cell Metab. 2019, 29, 950–965.e6. [Google Scholar] [CrossRef]
- Pei, S.; Minhajuddin, M.; Adane, B.; Khan, N.; Stevens, B.M.; Mack, S.C.; Lai, S.; Rich, J.N.; Inguva, A.; Shannon, K.M.; et al. AMPK/FIS1-Mediated Mitophagy Is Required for Self-Renewal of Human AML Stem Cells. Cell Stem Cell 2018, 23, 86–100.e6. [Google Scholar] [CrossRef]
- Hattori, A.; Tsunoda, M.; Konuma, T.; Kobayashi, M.; Nagy, T.; Glushka, J.; Tayyari, F.; McSkimming, D.; Kannan, N.; Tojo, A.; et al. Cancer Progression by Reprogrammed BCAA Metabolism in Myeloid Leukaemia. Nature 2017, 545, 500–504. [Google Scholar] [CrossRef]
- Bazinet, A.; Popradi, G. A General Practitioner’s Guide to Hematopoietic Stem-Cell Transplantation. Curr. Oncol. 2019, 26, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Jenq, R.R.; van den Brink, M.R.M. Allogeneic Haematopoietic Stem Cell Transplantation: Individualized Stem Cell and Immune Therapy of Cancer. Nat. Rev. Cancer 2010, 10, 213–221. [Google Scholar] [CrossRef] [PubMed]
- van Galen, P.; Kreso, A.; Mbong, N.; Kent, D.G.; Fitzmaurice, T.; Chambers, J.E.; Xie, S.; Laurenti, E.; Hermans, K.; Eppert, K.; et al. The Unfolded Protein Response Governs Integrity of the Haematopoietic Stem-Cell Pool during Stress. Nature 2014, 510, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Karigane, D.; Kobayashi, H.; Morikawa, T.; Ootomo, Y.; Sakai, M.; Nagamatsu, G.; Kubota, Y.; Goda, N.; Matsumoto, M.; Nishimura, E.K.; et al. P38α Activates Purine Metabolism to Initiate Hematopoietic Stem/Progenitor Cell Cycling in Response to Stress. Cell Stem Cell 2016, 19, 192–204. [Google Scholar] [CrossRef]
- Koide, S.; Sigurdsson, V.; Radulovic, V.; Saito, K.; Zheng, Z.; Lang, S.; Soneji, S.; Iwama, A.; Miharada, K. CD244 Expression Represents Functional Decline of Murine Hematopoietic Stem Cells after in Vitro Culture. iScience 2022, 25, 103603. [Google Scholar] [CrossRef]
- Kruta, M.; Sunshine, M.J.; Chua, B.A.; Fu, Y.; Chawla, A.; Dillingham, C.H.; Hidalgo San Jose, L.; De Jong, B.; Zhou, F.J.; Signer, R.A.J. Hsf1 Promotes Hematopoietic Stem Cell Fitness and Proteostasis in Response to Ex Vivo Culture Stress and Aging. Cell Stem Cell 2021, 28, 1950–1965.e6. [Google Scholar] [CrossRef]
- Ludin, A.; Itkin, T.; Gur-Cohen, S.; Mildner, A.; Shezen, E.; Golan, K.; Kollet, O.; Kalinkovich, A.; Porat, Z.; D’Uva, G.; et al. Monocytes-Macrophages That Express α-Smooth Muscle Actin Preserve Primitive Hematopoietic Cells in the Bone Marrow. Nat. Immunol. 2012, 13, 1072–1082. [Google Scholar] [CrossRef]
- Miharada, K.; Sigurdsson, V.; Karlsson, S. Dppa5 Improves Hematopoietic Stem Cell Activity by Reducing Endoplasmic Reticulum Stress. Cell Rep. 2014, 7, 1381–1392. [Google Scholar] [CrossRef]
- Walter, D.; Lier, A.; Geiselhart, A.; Thalheimer, F.B.; Huntscha, S.; Sobotta, M.C.; Moehrle, B.; Brocks, D.; Bayindir, I.; Kaschutnig, P.; et al. Exit from Dormancy Provokes DNA-Damage-Induced Attrition in Haematopoietic Stem Cells. Nature 2015, 520, 549–552. [Google Scholar] [CrossRef]
- Xiao, N.; Jani, K.; Morgan, K.; Okabe, R.; Cullen, D.E.; Jesneck, J.L.; Raffel, G.D. Hematopoietic Stem Cells Lacking Ott1 Display Aspects Associated with Aging and Are Unable to Maintain Quiescence during Proliferative Stress. Blood 2012, 119, 4898–4907. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Spencer, J.A.; Yeh, S.-C.A.; Turcotte, R.; Kokkaliaris, K.D.; Panero, R.; Ramos, A.; Guo, G.; Seyedhassantehrani, N.; Esipova, T.V.; et al. Live-Animal Imaging of Native Haematopoietic Stem and Progenitor Cells. Nature 2020, 578, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Takubo, K.; Suda, T. Bone Marrow Long Label-Retaining Cells Reside in the Sinusoidal Hypoxic Niche. Biochem. Biophys. Res. Commun. 2008, 366, 335–339. [Google Scholar] [CrossRef]
- Parmar, K.; Mauch, P.; Vergilio, J.-A.; Sackstein, R.; Down, J.D. Distribution of Hematopoietic Stem Cells in the Bone Marrow According to Regional Hypoxia. Proc. Natl. Acad. Sci. USA 2007, 104, 5431–5436. [Google Scholar] [CrossRef]
- Winkler, I.G.; Barbier, V.; Wadley, R.; Zannettino, A.C.W.; Williams, S.; Lévesque, J.-P. Positioning of Bone Marrow Hematopoietic and Stromal Cells Relative to Blood Flow in Vivo: Serially Reconstituting Hematopoietic Stem Cells Reside in Distinct Nonperfused Niches. Blood 2010, 116, 375–385. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; O’Leary, H.A.; Huang, X.; Mantel, C. The Importance of Hypoxia and Extra Physiologic Oxygen Shock/Stress for Collection and Processing of Stem and Progenitor Cells to Understand True Physiology/Pathology of These Cells Ex Vivo. Curr. Opin. Hematol. 2015, 22, 273–278. [Google Scholar] [CrossRef]
- Cipolleschi, M.; Dello Sbarba, P.; Olivotto, M. The Role of Hypoxia in the Maintenance of Hematopoietic Stem Cells. Blood 1993, 82, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Danet, G.H.; Pan, Y.; Luongo, J.L.; Bonnet, D.A.; Simon, M.C. Expansion of Human SCID-Repopulating Cells under Hypoxic Conditions. J. Clin. Investig. 2003, 112, 126–135. [Google Scholar] [CrossRef]
- Ivanović, Z.; Sbarba, P.D.; Trimoreau, F.; Faucher, J.-L.; Praloran, V. Primitive Human HPCs Are Better Maintained and Expanded in Vitro at 1 Percent Oxygen than at 20 Percent. Transfusion 2000, 40, 1482–1488. [Google Scholar] [CrossRef]
- Kobayashi, H.; Morikawa, T.; Okinaga, A.; Hamano, F.; Hashidate-Yoshida, T.; Watanuki, S.; Hishikawa, D.; Shindou, H.; Arai, F.; Kabe, Y.; et al. Environmental Optimization Enables Maintenance of Quiescent Hematopoietic Stem Cells Ex Vivo. Cell Rep. 2019, 28, 145–158.e9. [Google Scholar] [CrossRef]
- Pernes, G.; Flynn, M.C.; Lancaster, G.I.; Murphy, A.J. Fat for Fuel: Lipid Metabolism in Haematopoiesis. Clin. Transl. Immunol. 2019, 8, e1098. [Google Scholar] [CrossRef]
- Purton, L.E. Roles of Retinoids and Retinoic Acid Receptors in the Regulation of Hematopoietic Stem Cell Self-Renewal and Differentiation. PPAR Res. 2007, 2007, 087934. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-H.; Gudas, L.J. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Cabezas-Wallscheid, N.; Buettner, F.; Sommerkamp, P.; Klimmeck, D.; Ladel, L.; Thalheimer, F.B.; Pastor-Flores, D.; Roma, L.P.; Renders, S.; Zeisberger, P.; et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 2017, 169, 807–823.e19. [Google Scholar] [CrossRef] [PubMed]
- Oedekoven, C.A.; Belmonte, M.; Bode, D.; Hamey, F.K.; Shepherd, M.S.; Che, J.L.C.; Boyd, G.; McDonald, C.; Belluschi, S.; Diamanti, E.; et al. Hematopoietic Stem Cells Retain Functional Potential and Molecular Identity in Hibernation Cultures. Stem Cell Rep. 2021, 16, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.C.; Ishida, R.; Kikuchi, M.; Sudo, K.; Morita, M.; Crisostomo, R.V.; Yamamoto, R.; Loh, K.M.; Nakamura, Y.; Watanabe, M.; et al. Long-Term Ex Vivo Haematopoietic-Stem-Cell Expansion Allows Nonconditioned Transplantation. Nature 2019, 571, 117–121. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Ishida, R.; Nakauchi, H.; Yamazaki, S. Long-Term Ex Vivo Expansion of Mouse Hematopoietic Stem Cells. Nat. Protoc. 2020, 15, 628–648. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Ku, H.; Lyman, S.D.; Ogawa, M. In Vitro Expansion of Hematopoietic Progenitors and Maintenance of Stem Cells: Comparison Between FLT3/FLK-2 Ligand and KIT Ligand. Blood 1997, 89, 1915–1921. [Google Scholar] [CrossRef]
- Zhang, S.; Morita, M.; Wang, Z.; Ooehara, J.; Zhang, S.; Xie, M.; Bai, H.; Yu, W.; Wang, X.; Dong, F.; et al. Interleukin-12 Supports in Vitro Self-Renewal of Long-Term Hematopoietic Stem Cells. Blood Sci. 2019, 1, 92–101. [Google Scholar] [CrossRef]
- Huynh, H.; Iizuka, S.; Kaba, M.; Kirak, O.; Zheng, J.; Lodish, H.F.; Zhang, C.C. Insulin-Like Growth Factor-Binding Protein 2 Secreted by a Tumorigenic Cell Line Supports Ex Vivo Expansion of Mouse Hematopoietic Stem Cells. Stem Cells 2008, 26, 1628–1635. [Google Scholar] [CrossRef]
- Ieyasu, A.; Ishida, R.; Kimura, T.; Morita, M.; Wilkinson, A.C.; Sudo, K.; Nishimura, T.; Ohehara, J.; Tajima, Y.; Lai, C.-Y.; et al. An All-Recombinant Protein-Based Culture System Specifically Identifies Hematopoietic Stem Cell Maintenance Factors. Stem Cell Rep. 2017, 8, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; Eaves, C.J. Expansion in Vitro of Adult Murine Hematopoietic Stem Cells with Transplantable Lympho-Myeloid Reconstituting Ability. Proc. Natl. Acad. Sci. USA 1997, 94, 13648–13653. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Ku, H.; Hirayama, F.; Souza, L.M.; Ogawa, M. Interleukin 3 or Interleukin 1 Abrogates the Reconstituting Ability of Hematopoietic Stem Cells. Proc. Natl. Acad. Sci. USA 1996, 93, 4040–4044. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Yonemura, Y.; Ku, H. In Vitro Expansion of Hematopoietic Stem Cells. Stem Cells 1997, 15, 7–12. [Google Scholar] [CrossRef]
- Yagi, M.; Ritchie, K.A.; Sitnicka, E.; Storey, C.; Roth, G.J.; Bartelmez, S. Sustained Ex Vivo Expansion of Hematopoietic Stem Cells Mediated by Thrombopoietin. Proc. Natl. Acad. Sci. USA 1999, 96, 8126–8131. [Google Scholar] [CrossRef]
- Du, J.; He, H.; Li, Z.; He, J.; Bai, Z.; Liu, B.; Lan, Y. Integrative Transcriptomic Analysis of Developing Hematopoietic Stem Cells in Human and Mouse at Single-Cell Resolution. Biochem. Biophys. Res. Commun. 2021, 558, 161–167. [Google Scholar] [CrossRef]
- Eaves, C.J. Hematopoietic Stem Cells: Concepts, Definitions, and the New Reality. Blood 2015, 125, 2605–2613. [Google Scholar] [CrossRef]
- Göttgens, B. Regulatory Network Control of Blood Stem Cells. Blood 2015, 125, 2614–2620. [Google Scholar] [CrossRef]
- Radtke, F.; Wilson, A.; Stark, G.; Bauer, M.; van Meerwijk, J.; MacDonald, H.R.; Aguet, M. Deficient T Cell Fate Specification in Mice with an Induced Inactivation of Notch1. Immunity 1999, 10, 547–558. [Google Scholar] [CrossRef]
- Dallas, M.H.; Varnum-Finney, B.; Delaney, C.; Kato, K.; Bernstein, I.D. Density of the Notch Ligand Delta1 Determines Generation of B and T Cell Precursors from Hematopoietic Stem Cells. J. Exp. Med. 2005, 201, 1361–1366. [Google Scholar] [CrossRef]
- Varnum-Finney, B.; Brashem-Stein, C.; Bernstein, I.D. Combined Effects of Notch Signaling and Cytokines Induce a Multiple Log Increase in Precursors with Lymphoid and Myeloid Reconstituting Ability. Blood 2003, 101, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.H.; Varnum-Finney, B.; Martin, P.J.; Bernstein, I.D. Enhanced T-Cell Reconstitution by Hematopoietic Progenitors Expanded Ex Vivo Using the Notch Ligand Delta. Blood 2007, 109, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- Tolosano, E.; Altruda, F. Hemopexin: Structure, Function, and Regulation. DNA Cell Biol. 2002, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhshian, E.; Verstegen, M.M.; Visser, T.P.; Kheradmandkia, S.; Geerts, D.; Arshad, S.; Riaz, N.; Grosveld, F.; van Til, N.P.; Meijerink, J.P.P. Angiopoietin-Like Protein 3 Promotes Preservation of Stemness during Ex Vivo Expansion of Murine Hematopoietic Stem Cells. PLoS ONE 2014, 9, e105642. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in Mutant IDH2 Relapsed or Refractory Acute Myeloid Leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef]
- Kragh-Hansen, U. Molecular Aspects of Ligand Binding to Serum Albumin. Pharmacol. Rev. 1981, 33, 17–53. [Google Scholar]
- Nishimura, T.; Hsu, I.; Martinez-Krams, D.C.; Nakauchi, Y.; Majeti, R.; Yamazaki, S.; Nakauchi, H.; Wilkinson, A.C. Use of Polyvinyl Alcohol for Chimeric Antigen Receptor T-Cell Expansion. Exp. Hematol. 2019, 80, 16–20. [Google Scholar] [CrossRef]
- Zhang, C.C.; Lodish, H.F. Insulin-like Growth Factor 2 Expressed in a Novel Fetal Liver Cell Population Is a Growth Factor for Hematopoietic Stem Cells. Blood 2004, 103, 2513–2521. [Google Scholar] [CrossRef]
- Fan, X.; Gay, F.P.; Lim, F.W.; Ang, J.M.; Chu, P.P.; Bari, S.; Hwang, W.Y. Low-Dose Insulin-like Growth Factor Binding Proteins 1 and 2 and Angiopoietin-like Protein 3 Coordinately Stimulate Ex Vivo Expansion of Human Umbilical Cord Blood Hematopoietic Stem Cells as Assayed in NOD/SCID Gamma Null Mice. Stem Cell Res. Ther. 2014, 5, 71. [Google Scholar] [CrossRef]
- Ribeiro-Filho, A.C.; Levy, D.; Ruiz, J.L.M.; da Cunha Mantovani, M.; Bydlowski, S.P. Traditional and Advanced Cell Cultures in Hematopoietic Stem Cell Studies. Cells 2019, 8, 1628. [Google Scholar] [CrossRef]
- Zhang, C.C.; Kaba, M.; Iizuka, S.; Huynh, H.; Lodish, H.F. Angiopoietin-like 5 and IGFBP2 Stimulate Ex Vivo Expansion of Human Cord Blood Hematopoietic Stem Cells as Assayed by NOD/SCID Transplantation. Blood 2008, 111, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Niazi, V.; Taheri, M.; Basiri, A. Effect of Small Molecule on Ex Vivo Expansion of Cord Blood Hematopoietic Stem Cells: A Concise Review. Front. Cell Dev. Biol. 2021, 9, 649115. [Google Scholar] [CrossRef] [PubMed]
- Boitano, A.E.; Wang, J.; Romeo, R.; Bouchez, L.C.; Parker, A.E.; Sutton, S.E.; Walker, J.R.; Flaveny, C.A.; Perdew, G.H.; Denison, M.S.; et al. Aryl Hydrocarbon Receptor Antagonists Promote the Expansion of Human Hematopoietic Stem Cells. Science 2010, 329, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Shpall, E.J.; Rezvani, K. Cord Blood Expansion Has Arrived. Blood 2021, 138, 1381–1382. [Google Scholar] [CrossRef]
- Thordardottir, S.; Hangalapura, B.N.; Hutten, T.; Cossu, M.; Spanholtz, J.; Schaap, N.; Radstake, T.R.D.J.; van der Voort, R.; Dolstra, H. The Aryl Hydrocarbon Receptor Antagonist StemRegenin 1 Promotes Human Plasmacytoid and Myeloid Dendritic Cell Development from CD34+ Hematopoietic Progenitor Cells. Stem Cells Dev. 2014, 23, 955–967. [Google Scholar] [CrossRef]
- Singh, K.P.; Casado, F.L.; Opanashuk, L.A.; Gasiewicz, T.A. The Aryl Hydrocarbon Receptor Has a Normal Function in the Regulation of Hematopoietic and Other Stem/Progenitor Cell Populations. Biochem. Pharmacol. 2009, 77, 577–587. [Google Scholar] [CrossRef]
- Liu, H.; Ramachandran, I.; Gabrilovich, D.I. Regulation of Plasmacytoid Dendritic Cell Development in Mice by Aryl Hydrocarbon Receptor. Immunol. Cell Biol. 2014, 92, 200–203. [Google Scholar] [CrossRef]
- Wagner, J.E.; Brunstein, C.G.; Boitano, A.E.; DeFor, T.E.; McKenna, D.; Sumstad, D.; Blazar, B.R.; Tolar, J.; Le, C.; Jones, J.; et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell 2016, 18, 144–155. [Google Scholar] [CrossRef]
- Casper, R.F.; Quesne, M.; Rogers, I.M.; Shirota, T.; Jolivet, A.; Milgrom, E.; Savouret, J.-F. Resveratrol Has Antagonist Activity on the Aryl Hydrocarbon Receptor: Implications for Prevention of Dioxin Toxicity. Mol. Pharmacol. 1999, 56, 784–790. [Google Scholar]
- Lin, H.-Y.; Lansing, L.; Merillon, J.-M.; Davis, F.B.; Tang, H.-Y.; Shih, A.; Vitrac, X.; Krisa, S.; Keating, T.; Cao, H.J.; et al. Integrin AVβ3 Contains a Receptor Site for Resveratrol. FASEB J. 2006, 20, 1742–1744. [Google Scholar] [CrossRef]
- Chagraoui, J.; Girard, S.; Spinella, J.-F.; Simon, L.; Bonneil, E.; Mayotte, N.; MacRae, T.; Coulombe-Huntington, J.; Bertomeu, T.; Moison, C.; et al. UM171 Preserves Epigenetic Marks That Are Reduced in Ex Vivo Culture of Human HSCs via Potentiation of the CLR3-KBTBD4 Complex. Cell Stem Cell 2021, 28, 48–62.e6. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Roy, J.; Lachance, S.; Delisle, J.-S.; Marinier, A.; Busque, L.; Roy, D.-C.; Barabé, F.; Ahmad, I.; Bambace, N.; et al. Hematopoietic Stem Cell Transplantation Using Single UM171-Expanded Cord Blood: A Single-Arm, Phase 1–2 Safety and Feasibility Study. Lancet Haematol. 2020, 7, e134–e145. [Google Scholar] [CrossRef]
- Fares, I.; Chagraoui, J.; Gareau, Y.; Gingras, S.; Ruel, R.; Mayotte, N.; Csaszar, E.; Knapp, D.J.H.F.; Miller, P.; Ngom, M.; et al. Pyrimidoindole Derivatives Are Agonists of Human Hematopoietic Stem Cell Self-Renewal. Science 2014, 345, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Peled, T.; Shoham, H.; Aschengrau, D.; Yackoubov, D.; Frei, G.; Rosenheimer, G.N.; Lerrer, B.; Cohen, H.Y.; Nagler, A.; Fibach, E.; et al. Nicotinamide, a SIRT1 Inhibitor, Inhibits Differentiation and Facilitates Expansion of Hematopoietic Progenitor Cells with Enhanced Bone Marrow Homing and Engraftment. Exp. Hematol. 2012, 40, 342–355.e1. [Google Scholar] [CrossRef]
- Peck, B.; Chen, C.-Y.; Ho, K.-K.; Di Fruscia, P.; Myatt, S.S.; Coombes, R.C.; Fuchter, M.J.; Hsiao, C.-D.; Lam, E.W.-F. SIRT Inhibitors Induce Cell Death and P53 Acetylation through Targeting Both SIRT1 and SIRT2. Mol. Cancer Ther. 2010, 9, 844–855. [Google Scholar] [CrossRef]
- Horwitz, M.E.; Chao, N.J.; Rizzieri, D.A.; Long, G.D.; Sullivan, K.M.; Gasparetto, C.; Chute, J.P.; Morris, A.; McDonald, C.; Waters-Pick, B.; et al. Umbilical Cord Blood Expansion with Nicotinamide Provides Long-Term Multilineage Engraftment. J. Clin. Investig. 2014, 124, 3121–3128. [Google Scholar] [CrossRef]
- Papa, L.; Zimran, E.; Djedaini, M.; Ge, Y.; Ozbek, U.; Sebra, R.; Sealfon, S.C.; Hoffman, R. Ex Vivo Human HSC Expansion Requires Coordination of Cellular Reprogramming with Mitochondrial Remodeling and P53 Activation. Blood Adv. 2018, 2, 2766–2779. [Google Scholar] [CrossRef]
- Zimran, E.; Papa, L.; Djedaini, M.; Patel, A.; Iancu-Rubin, C.; Hoffman, R. Expansion and Preservation of the Functional Activity of Adult Hematopoietic Stem Cells Cultured Ex Vivo with a Histone Deacetylase Inhibitor. Stem Cells Transl. Med. 2020, 9, 531–542. [Google Scholar] [CrossRef]
- Rocha, V.; Gluckman, E. Clinical Use of Umbilical Cord Blood Hematopoietic Stem Cells. Biol. Blood Marrow Transplant. 2006, 12, 34–41. [Google Scholar] [CrossRef]
- Delaney, C.; Heimfeld, S.; Brashem-Stein, C.; Voorhies, H.; Manger, R.L.; Bernstein, I.D. Notch-Mediated Expansion of Human Cord Blood Progenitor Cells Capable of Rapid Myeloid Reconstitution. Nat. Med. 2010, 16, 232–236. [Google Scholar] [CrossRef]
- Papa, L.; Djedaini, M.; Hoffman, R. Ex Vivo HSC Expansion Challenges the Paradigm of Unidirectional Human Hematopoiesis. Ann. N. Y. Acad. Sci. 2020, 1466, 39–50. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, M.L.; Weisdorf, D.J.; Brunstein, C.G.; Cao, Q.; DeFor, T.E.; Verneris, M.R.; Blazar, B.R.; Wagner, J.E. Acute Graft-versus-Host Disease after Unrelated Donor Umbilical Cord Blood Transplantation: Analysis of Risk Factors. Blood 2009, 113, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- Kasai, F.; Hirayama, N.; Ozawa, M.; Iemura, M.; Kohara, A. Changes of Heterogeneous Cell Populations in the Ishikawa Cell Line during Long-Term Culture: Proposal for an in Vitro Clonal Evolution Model of Tumor Cells. Genomics 2016, 107, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Dexter, T.M.; Moore, M.A.; Sheridan, A.P. Maintenance of Hemopoietic Stem Cells and Production of Differentiated Progeny in Allogeneic and Semiallogeneic Bone Marrow Chimeras in Vitro. J. Exp. Med. 1977, 145, 1612–1616. [Google Scholar] [CrossRef]
- Klco, J.M.; Spencer, D.H.; Miller, C.A.; Griffith, M.; Lamprecht, T.L.; O’Laughlin, M.; Fronick, C.; Magrini, V.; Demeter, R.T.; Fulton, R.S.; et al. Functional Heterogeneity of Genetically Defined Subclones in Acute Myeloid Leukemia. Cancer Cell 2014, 25, 379–392. [Google Scholar] [CrossRef]
- Miles, L.A.; Bowman, R.L.; Merlinsky, T.R.; Csete, I.S.; Ooi, A.T.; Durruthy-Durruthy, R.; Bowman, M.; Famulare, C.; Patel, M.A.; Mendez, P.; et al. Single-Cell Mutation Analysis of Clonal Evolution in Myeloid Malignancies. Nature 2020, 587, 477–482. [Google Scholar] [CrossRef]
- Zeijlemaker, W.; Gratama, J.W.; Schuurhuis, G.J. Tumor Heterogeneity Makes AML a “Moving Target” for Detection of Residual Disease: Phenotype Instability and MRD in AML. Cytom. B Clin. Cytom. 2014, 86, 3–14. [Google Scholar] [CrossRef]
- Brenner, A.K.; Aasebø, E.; Hernandez-Valladares, M.; Selheim, F.; Berven, F.; Grønningsæter, I.-S.; Bartaula-Brevik, S.; Bruserud, Ø. The Capacity of Long-Term in Vitro Proliferation of Acute Myeloid Leukemia Cells Supported Only by Exogenous Cytokines Is Associated with a Patient Subset with Adverse Outcome. Cancers 2019, 11, 73. [Google Scholar] [CrossRef]
- Lin, H.; Damen, J.E.; Walasek, M.A.; Szilvassy, S.J.; Turhan, A.G.; Louis, S.A.; Eaves, A.C.; Wognum, A.W. Feeder-Free and Serum-Free in Vitro Assay for Measuring the Effect of Drugs on Acute and Chronic Myeloid Leukemia Stem/Progenitor Cells. Exp. Hematol. 2020, 90, 52–64.e11. [Google Scholar] [CrossRef]
- Pabst, C.; Krosl, J.; Fares, I.; Boucher, G.; Ruel, R.; Marinier, A.; Lemieux, S.; Hébert, J.; Sauvageau, G. Identification of Small Molecules That Support Human Leukemia Stem Cell Activity Ex Vivo. Nat. Methods 2014, 11, 436–442. [Google Scholar] [CrossRef]
- Huang, J.; Nguyen-McCarty, M.; Hexner, E.O.; Danet-Desnoyers, G.; Klein, P.S. Maintenance of Hematopoietic Stem Cells through Regulation of Wnt and MTOR Pathways. Nat. Med. 2012, 18, 1778–1785. [Google Scholar] [CrossRef]
- Bhavanasi, D.; Wen, K.W.; Liu, X.; Vergez, F.; Danet-Desnoyers, G.; Carroll, M.; Huang, J.; Klein, P.S. Signaling Mechanisms That Regulate Ex Vivo Survival of Human Acute Myeloid Leukemia Initiating Cells. Blood Cancer J. 2017, 7, 636. [Google Scholar] [CrossRef][Green Version]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic Stem Cell Self-Renewal in Vivo and Ex Vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef]
- Breems, D.A.; Blokland, E.A.W.; Siebel, K.E.; Mayen, A.E.M.; Engels, L.J.A.; Ploemacher, R.E. Stroma-Contact Prevents Loss of Hematopoietic Stem Cell Quality During Ex Vivo Expansion of CD34+ Mobilized Peripheral Blood Stem Cells. Blood 1998, 91, 111–117. [Google Scholar] [CrossRef]
- Robinson, S.N.; Ng, J.; Niu, T.; Yang, H.; McMannis, J.D.; Karandish, S.; Kaur, I.; Fu, P.; Del Angel, M.; Messinger, R.; et al. Superior Ex Vivo Cord Blood Expansion Following Co-Culture with Bone Marrow-Derived Mesenchymal Stem Cells. Bone Marrow Transplant. 2006, 37, 359–366. [Google Scholar] [CrossRef]
- de Lima, M.; McNiece, I.; Robinson, S.N.; Munsell, M.; Eapen, M.; Horowitz, M.; Alousi, A.; Saliba, R.; McMannis, J.D.; Kaur, I.; et al. Cord-Blood Engraftment with Ex Vivo Mesenchymal-Cell Coculture. N. Engl. J. Med. 2012, 367, 2305–2315. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’Ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Nakahara, F.; Borger, D.K.; Wei, Q.; Pinho, S.; Maryanovich, M.; Zahalka, A.H.; Suzuki, M.; Cruz, C.D.; Wang, Z.; Xu, C.; et al. Engineering a Haematopoietic Stem Cell Niche by Revitalizing Mesenchymal Stromal Cells. Nat. Cell Biol. 2019, 21, 560–567. [Google Scholar] [CrossRef]
- Butler, J.M.; Nolan, D.J.; Vertes, E.L.; Varnum-Finney, B.; Kobayashi, H.; Hooper, A.T.; Seandel, M.; Shido, K.; White, I.A.; Kobayashi, M.; et al. Endothelial Cells Are Essential for the Self-Renewal and Repopulation of Notch-Dependent Hematopoietic Stem Cells. Cell Stem Cell 2010, 6, 251–264. [Google Scholar] [CrossRef]
- Seandel, M.; Butler, J.M.; Kobayashi, H.; Hooper, A.T.; White, I.A.; Zhang, F.; Vertes, E.L.; Kobayashi, M.; Zhang, Y.; Shmelkov, S.V.; et al. Generation of a Functional and Durable Vascular Niche by the Adenoviral E4ORF1 Gene. Proc. Natl. Acad. Sci. USA 2008, 105, 19288–19293. [Google Scholar] [CrossRef]
- Li, H.; Pei, H.; Xie, X.; Wang, S.; Jia, Y.; Zhang, B.; Fan, Z.; Liu, Y.; Bai, Y.; Han, Y.; et al. Liver Sinusoidal Endothelial Cells Promote the Expansion of Human Cord Blood Hematopoietic Stem and Progenitor Cells. Int. J. Mol. Sci. 2019, 20, 1985. [Google Scholar] [CrossRef]
- Azadniv, M.; Myers, J.R.; McMurray, H.R.; Guo, N.; Rock, P.; Coppage, M.L.; Ashton, J.; Becker, M.W.; Calvi, L.M.; Liesveld, J.L. Bone Marrow Mesenchymal Stromal Cells from Acute Myelogenous Leukemia Patients Demonstrate Adipogenic Differentiation Propensity with Implications for Leukemia Cell Support. Leukemia 2020, 34, 391–403. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Gjertsen, B.T.; von Volkman, H.L. In Vitro Culture of Human Acute Myelogenous Leukemia (AML) Cells in Serum-Free Media: Studies of Native AML Blasts and AML Cell Lines. J. Hematother. Stem Cell Res. 2000, 9, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Garrido, S.M.; Appelbaum, F.R.; Willman, C.L.; Banker, D.E. Acute Myeloid Leukemia Cells Are Protected from Spontaneous and Drug-Induced Apoptosis by Direct Contact with a Human Bone Marrow Stromal Cell Line (HS-5). Exp. Hematol. 2001, 29, 448–457. [Google Scholar] [CrossRef]
- Mayani, H.; Flores-Figueroa, E.; Chávez-González, A. In Vitro Biology of Human Myeloid Leukemia. Leuk. Res. 2009, 33, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, D.G.J.; Groen, R.W.J.; Janssen, J.J.W.M.; Cloos, J. Ex Vivo Cultures and Drug Testing of Primary Acute Myeloid Leukemia Samples: Current Techniques and Implications for Experimental Design and Outcome. Drug Resist. Updat. 2020, 53, 100730. [Google Scholar] [CrossRef]
- van Gosliga, D.; Schepers, H.; Rizo, A. Establishing Long-Term Cultures with Self-Renewing Acute Myeloid Leukemia Stem/Progenitor Cells. Exp. Hematol. 2007, 35, 1538–1549. [Google Scholar] [CrossRef]
- Griessinger, E.; Anjos-Afonso, F.; Pizzitola, I.; Rouault-Pierre, K.; Vargaftig, J.; Taussig, D.; Gribben, J.; Lassailly, F.; Bonnet, D. A Niche-Like Culture System Allowing the Maintenance of Primary Human Acute Myeloid Leukemia-Initiating Cells: A New Tool to Decipher Their Chemoresistance and Self-Renewal Mechanisms. Stem Cells Transl. Med. 2014, 3, 520–529. [Google Scholar] [CrossRef]
- Rombouts, W.; Broyl, A.; Martens, A.; Slater, R.; Ploemacher, R. Human Acute Myeloid Leukemia Cells with Internal Tandem Duplications in the Flt3 Gene Show Reduced Proliferative Ability in Stroma Supported Long-Term Cultures. Leukemia 1999, 13, 1071–1078. [Google Scholar] [CrossRef]
- Zhou, B.; Tsaknakis, G.; Coldwell, K.E.; Khoo, C.P.; Roubelakis, M.G.; Chang, C.-H.; Pepperell, E.; Watt, S.M. A Novel Function for the Haemopoietic Supportive Murine Bone Marrow MS-5 Mesenchymal Stromal Cell Line in Promoting Human Vasculogenesis and Angiogenesis. Br. J. Haematol. 2012, 157, 299–311. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Malek, S.N.; Zheng, P.; Liu, Y. Targeting HIF1α Eliminates Cancer Stem Cells in Hematological Malignancies. Cell Stem Cell 2011, 8, 399–411. [Google Scholar] [CrossRef]
- Chandran, P.; Le, Y.; Li, Y.; Sabloff, M.; Mehic, J.; Rosu-Myles, M.; Allan, D.S. Mesenchymal Stromal Cells from Patients with Acute Myeloid Leukemia Have Altered Capacity to Expand Differentiated Hematopoietic Progenitors. Leuk. Res. 2015, 39, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Geyh, S.; Rodríguez-Paredes, M.; Jäger, P.; Khandanpour, C.; Cadeddu, R.-P.; Gutekunst, J.; Wilk, C.M.; Fenk, R.; Zilkens, C.; Hermsen, D.; et al. Functional Inhibition of Mesenchymal Stromal Cells in Acute Myeloid Leukemia. Leukemia 2016, 30, 683–691. [Google Scholar] [CrossRef]
- Battula, V.L.; Le, P.M.; Sun, J.C.; Nguyen, K.; Yuan, B.; Zhou, X.; Sonnylal, S.; McQueen, T.; Ruvolo, V.; Michel, K.A.; et al. AML-Induced Osteogenic Differentiation in Mesenchymal Stromal Cells Supports Leukemia Growth. JCI Insight 2017, 2, e90036. [Google Scholar] [CrossRef] [PubMed]
- Binato, R.; de Almeida Oliveira, N.C.; Du Rocher, B.; Abdelhay, E. The Molecular Signature of AML Mesenchymal Stromal Cells Reveals Candidate Genes Related to the Leukemogenic Process. Cancer Lett. 2015, 369, 134–143. [Google Scholar] [CrossRef]
- Desbourdes, L.; Javary, J.; Charbonnier, T.; Ishac, N.; Bourgeais, J.; Iltis, A.; Chomel, J.-C.; Turhan, A.; Guilloton, F.; Tarte, K.; et al. Alteration Analysis of Bone Marrow Mesenchymal Stromal Cells from De Novo Acute Myeloid Leukemia Patients at Diagnosis. Stem Cells Dev. 2017, 26, 709–722. [Google Scholar] [CrossRef]
- Le, Y.; Fraineau, S.; Chandran, P.; Sabloff, M.; Brand, M.; Lavoie, J.R.; Gagne, R.; Rosu-Myles, M.; Yauk, C.L.; Richardson, R.B.; et al. Adipogenic Mesenchymal Stromal Cells from Bone Marrow and Their Hematopoietic Supportive Role: Towards Understanding the Permissive Marrow Microenvironment in Acute Myeloid Leukemia. Stem Cell Rev. Rep. 2016, 12, 235–244. [Google Scholar] [CrossRef]
- Chang-Liu, C.-M.; Woloschak, G.E. Effect of Passagenumber on Cellular Response to DNA-Damaging Agents: Cell Survival and Gene Expression. Cancer Lett. 1995, 10, 77–86. [Google Scholar] [CrossRef]
- Raic, A.; Naolou, T.; Mohra, A.; Chatterjee, C.; Lee-Thedieck, C. 3D Models of the Bone Marrow in Health and Disease: Yesterday, Today, and Tomorrow. MRS Commun. 2019, 9, 37–52. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Maglio, M.; Fini, M. A Systematic Review on in Vitro 3D Bone Metastases Models: A New Horizon to Recapitulate the Native Clinical Scenario? Oncotarget 2016, 7, 44803–44820. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.M.; Futrega, K.; Osiecki, M.; Kabiri, M.; Kul, B.; Rice, A.; Atkinson, K.; Brooke, G.; Doran, M. Micromarrows—Three-Dimensional Coculture of Hematopoietic Stem Cells and Mesenchymal Stromal Cells. Tissue Eng. Part C Methods 2012, 18, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures-Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Ham, S.L.; Joshi, R.; Thakuri, P.S.; Tavana, H. Liquid-Based Three-Dimensional Tumor Models for Cancer Research and Drug Discovery. Exp. Biol. Med. 2016, 241, 939–954. [Google Scholar] [CrossRef]
- Lewis, E.E.L.; Wheadon, H.; Lewis, N.; Yang, J.; Mullin, M.; Hursthouse, A.; Stirling, D.; Dalby, M.J.; Berry, C.C. A Quiescent, Regeneration-Responsive Tissue Engineered Mesenchymal Stem Cell Bone Marrow Niche Model via Magnetic Levitation. ACS Nano 2016, 10, 8346–8354. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef]
- Lee-Thedieck, C.; Spatz, J.P. Artificial Niches: Biomimetic Materials for Hematopoietic Stem Cell Culture. Macromol. Rapid Commun. 2012, 33, 1432–1438. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels 2022, 8, 46. [Google Scholar] [CrossRef]
- Bai, T.; Li, J.; Sinclair, A.; Imren, S.; Merriam, F.; Sun, F.; O’Kelly, M.B.; Nourigat, C.; Jain, P.; Delrow, J.J.; et al. Expansion of Primitive Human Hematopoietic Stem Cells by Culture in a Zwitterionic Hydrogel. Nat. Med. 2019, 25, 1566–1575. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Cryogels for Biomedical Applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Raic, A.; Rödling, L.; Kalbacher, H.; Lee-Thedieck, C. Biomimetic Macroporous PEG Hydrogels as 3D Scaffolds for the Multiplication of Human Hematopoietic Stem and Progenitor Cells. Biomaterials 2014, 35, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Ventura Ferreira, M.S.; Jahnen-Dechent, W.; Labude, N.; Bovi, M.; Hieronymus, T.; Zenke, M.; Schneider, R.K.; Neurs, S. Cord Blood-Hematopoietic Stem Cell Expansion in 3D Fibrin Scaffolds with Stromal Support. Biomaterials 2012, 33, 6987–6997. [Google Scholar] [CrossRef] [PubMed]
- Franc, S.; Rousseau, J.C.; Garrone, R.; van der Rest, M.; Moradi-Améli, M. Microfibrillar Composition of Umbilical Cord Matrix: Characterization of Fibrillin, Collagen VI and Intact Collagen, V. Placenta 1998, 19, 95–104. [Google Scholar] [CrossRef]
- Jadalannagari, S.; Converse, G.; McFall, C.; Buse, E.; Filla, M.; Villar, M.T.; Artigues, A.; Mellot, A.J.; Wang, J.; Detamore, M.S.; et al. Decellularized Wharton’s Jelly from Human Umbilical Cord as a Novel 3D Scaffolding Material for Tissue Engineering Applications. PLoS ONE 2017, 12, e0172098. [Google Scholar] [CrossRef]
- Rödling, L.; Schwedhelm, I.; Kraus, S.; Bieback, K.; Hansmann, J.; Lee-Thedieck, C. 3D Models of the Hematopoietic Stem Cell Niche under Steady-State and Active Conditions. Sci. Rep. 2017, 7, 4625. [Google Scholar] [CrossRef]
- Bessy, T.; Itkin, T.; Passaro, D. Bioengineering the Bone Marrow Vascular Niche. Front. Cell Dev. Biol. 2021, 9, 645496. [Google Scholar] [CrossRef]
- Kotha, S.S.; Hayes, B.J.; Phong, K.T.; Redd, M.A.; Bomsztyk, K.; Ramakrishnan, A.; Torok-Storb, B.; Zheng, Y. Engineering a Multicellular Vascular Niche to Model Hematopoietic Cell Trafficking. Stem Cell Res. Ther. 2018, 9, 77. [Google Scholar] [CrossRef]
- Reinisch, A.; Hernandez, D.C.; Schallmoser, K.; Majeti, R. Generation and Use of a Humanized Bone-Marrow-Ossicle Niche for Hematopoietic Xenotransplantation into Mice. Nat. Protoc. 2017, 12, 2169–2188. [Google Scholar] [CrossRef]
- Torisawa, Y.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone Marrow–on–a–Chip Replicates Hematopoietic Niche Physiology in Vitro. Nat. Methods 2014, 11, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Aljitawi, O.S.; Li, D.; Xiao, Y.; Zhang, D.; Ramachandran, K.; Stehno-Bittel, L.; Van Veldhuizen, P.; Lin, T.L.; Kambhampati, S.; Garimella, R. A Novel Three-Dimensional Stromal-Based Model for in Vitro Chemotherapy Sensitivity Testing of Leukemia Cells. Leuk. Lymphoma 2014, 55, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Blanco, T.M.; Mantalaris, A.; Bismarck, A.; Panoskaltsis, N. The Development of a Three-Dimensional Scaffold for Ex Vivo Biomimicry of Human Acute Myeloid Leukaemia. Biomaterials 2010, 31, 2243–2251. [Google Scholar] [CrossRef]
- Bray, L.J.; Binner, M.; Körner, Y.; von Bonin, M.; Bornhäuser, M.; Werner, C. A Three-Dimensional Ex Vivo Tri-Culture Model Mimics Cell-Cell Interactions between Acute Myeloid Leukemia and the Vascular Niche. Haematologica 2017, 102, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Leisten, I.; Kramann, R.; Ventura Ferreira, M.S.; Bovi, M.; Neuss, S.; Ziegler, P.; Wagner, W.; Knüchel, R.; Schneider, R.K. 3D Co-Culture of Hematopoietic Stem and Progenitor Cells and Mesenchymal Stem Cells in Collagen Scaffolds as a Model of the Hematopoietic Niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef]
- Tun, T.; Miyoshi, H.; Aung, T.; Takahashi, S.; Shimizu, R.; Kuroha, T.; Yamamoto, M.; Ohshima, N. Effect of Growth Factors on Ex Vivo Bone Marrow Cell Expansion Using Three-Dimensional Matrix Support. Artif. Organs 2002, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Velliou, E.G.; Dos Santos, S.B.; Papathanasiou, M.M.; Fuentes-Gari, M.; Misener, R.; Panoskaltsis, N.; Pistikopoulos, E.N.; Mantalaris, A. Towards Unravelling the Kinetics of an Acute Myeloid Leukaemia Model System under Oxidative and Starvation Stress: A Comparison between Two- and Three-Dimensional Cultures. Bioprocess Biosyst. Eng. 2015, 38, 1589–1600. [Google Scholar] [CrossRef]
- Argiropoulos, B.; Humphries, R.K. Hox Genes in Hematopoiesis and Leukemogenesis. Oncogene 2007, 26, 6766–6776. [Google Scholar] [CrossRef]
- Doma, E.; Mayer, I.M.; Brandstoetter, T.; Maurer, B.; Grausenburger, R.; Menzl, I.; Zojer, M.; Hoelbl-Kovacic, A.; Carlsson, L.; Heller, G.; et al. A Robust Approach for the Generation of Functional Hematopoietic Progenitor Cell Lines to Model Leukemic Transformation. Blood Adv. 2021, 5, 39–53. [Google Scholar] [CrossRef]
- Geoffroy, M.-C.; Esnault, C.; de Thé, H. Retinoids in Hematology: A Timely Revival? Blood 2021, 137, 2429–2437. [Google Scholar] [CrossRef]
- Sauvageau, G. Overexpression of HOXB4 in Hematopoietic Cells Causes the Selective Expansion of more Primitive Populations in Vitro and Vivo. Genes Dev. 1995, 14, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Redecke, V.; Wu, R.; Zhou, J.; Finkelstein, D.; Chaturvedi, V.; High, A.A.; Häcker, H. Hematopoietic Progenitor Cell Lines with Myeloid and Lymphoid Potential. Nat. Methods 2013, 10, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Sitnicka, E.; Collins, S. Lymphohematopoietic Progenitors Immortalized by a Retroviral Vector Harboring a Dominant-Negative Retinoic Acid Receptor Can Recapitulate Lymphoid, Myelold, and Erythrold Development. Genes Dev. 1994, 12, 2831–2841. [Google Scholar] [CrossRef]
- Lappin, T.R.; Grier, D.G.; Thompson, A.; Halliday, H.L. Hox Genes: Seductive Science, Mysterious Mechanisms. Ulst. Med. J. 2006, 9, 23–31. [Google Scholar]
- Abuhantash, M.; Collins, E.M.; Thompson, A. Role of the HOXA Cluster in HSC Emergence and Blood Cancer. Biochem. Soc. Trans. 2021, 49, 1817–1827. [Google Scholar] [CrossRef]
- Yu, H.; Neale, G.; Zhang, H.; Lee, H.M.; Ma, Z.; Zhou, S.; Forget, B.G.; Sorrentino, B.P. Downregulation of Prdm16 MRNA Is a Specific Antileukemic Mechanism during HOXB4-Mediated HSC Expansion in Vivo. Blood 2014, 124, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Morita, K.; Hill, M.C.; Jiang, Y.; Kitano, A.; Saito, Y.; Wang, F.; Mao, X.; Hoegenauer, K.A.; Morishita, K.; et al. PRDM16s Transforms Megakaryocyte-Erythroid Progenitors into Myeloid Leukemia–Initiating Cells. Blood 2019, 134, 614–625. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, Z.; Cheng, T. New Paradigms on Hematopoietic Stem Cell Differentiation. Protein Cell 2020, 11, 34–44. [Google Scholar] [CrossRef]
- Antonchuk, J.; Sauvageau, G.; Humphries, R.K. HOXB4-Induced Expansion of Adult Hematopoietic Stem Cells Ex Vivo. Cell 2002, 109, 39–45. [Google Scholar] [CrossRef]
- Krosl, J.; Sauvageau, G. AP-1 Complex Is Effector of Hox-Induced Cellular Proliferation and Transformation. Oncogene 2000, 8, 5134–5141. [Google Scholar] [CrossRef]
- Ohta, H.; Sekulovic, S.; Bakovic, S.; Eaves, C.J.; Pineault, N.; Gasparetto, M.; Smith, C.; Sauvageau, G.; Humphries, R.K. Near-Maximal Expansions of Hematopoietic Stem Cells in Culture Using NUP98-HOX Fusions. Exp. Hematol. 2007, 35, 817–830. [Google Scholar] [CrossRef]
- Beslu, N.; Krosl, J.; Laurin, M.; Mayotte, N.; Humphries, K.R.; Sauvageau, G. Molecular Interactions Involved in HOXB4-Induced Activation of HSC Self-Renewal. Blood 2004, 104, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Cellot, S.; Krosl, J.; Chagraoui, J.; Meloche, S.; Humphries, R.K.; Sauvageau, G. Sustained in Vitro Trigger of Self-Renewal Divisions in Hoxb4hiPbx1lo Hematopoietic Stem Cells. Exp. Hematol. 2007, 35, 802.e1–802.e19. [Google Scholar] [CrossRef]
- Pineault, N.; Abramovich, C.; Ohta, H.; Humphries, R.K. Differential and Common Leukemogenic Potentials of Multiple NUP98-Hox Fusion Proteins Alone or with Meis1. Mol. Cell. Biol. 2004, 24, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Broxmeyer, H.E.; Lee, M.R. Generating Autologous Hematopoietic Cells from Human-Induced Pluripotent Stem Cells through Ectopic Expression of Transcription Factors. Curr. Opin. Hematol. 2017, 24, 283–288. [Google Scholar] [CrossRef]
- Izawa, K.; Yamazaki, S.; Becker, H.J.; Bhadury, J.; Kakegawa, T.; Sakaguchi, M.; Tojo, A. Activated HoxB4-Induced Hematopoietic Stem Cells from Murine Pluripotent Stem Cells via Long-Term Programming. Exp. Hematol. 2020, 89, 68–79.e7. [Google Scholar] [CrossRef] [PubMed]
- Weston, W.; Zayas, J.; Perez, R.; George, J.; Jurecic, R. Dynamic Equilibrium of Heterogeneous and Interconvertible Multipotent Hematopoietic Cell Subsets. Sci. Rep. 2015, 4, 5199. [Google Scholar] [CrossRef]
- Dai, B.; Wang, P. In Vitro Differentiation of Adult Bone Marrow Progenitors into Antigen-Specific CD4 Helper T Cells Using Engineered Stromal Cells Expressing a Notch Ligand and a Major Histocompatibility Complex Class II Protein. Stem Cells Dev. 2009, 18, 235–245. [Google Scholar] [CrossRef]
- Kutlesa, S.; Zayas, J.; Valle, A.; Levy, R.B.; Jurecic, R. T-Cell Differentiation of Multipotent Hematopoietic Cell Line EML in the OP9-DL1 Coculture System. Exp. Hematol. 2009, 37, 909–923. [Google Scholar] [CrossRef][Green Version]
- Pina, C.; Fugazza, C.; Tipping, A.J.; Brown, J.; Soneji, S.; Teles, J.; Peterson, C.; Enver, T. Inferring Rules of Lineage Commitment in Haematopoiesis. Nat. Cell Biol. 2012, 14, 287–294. [Google Scholar] [CrossRef]
- Ye, Z.-J.; Kluger, Y.; Lian, Z.; Weissman, S.M. Two Types of Precursor Cells in a Multipotential Hematopoietic Cell Line. Proc. Natl. Acad. Sci. USA 2005, 102, 18461–18466. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Zhang, H.; Schulz, V.; Tuck, D.P.; Forget, B.G. Downstream Targets of HOXB4 in a Cell Line Model of Primitive Hematopoietic Progenitor Cells. Blood 2010, 116, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Pinto do O, P.; Kolterud, Å.; Carlsson, L. Expression of the LIM-Homeobox Gene LH2 Generates Immortalized Steel Factor-Dependent Multipotent Hematopoietic Precursors. EMBO J. 1998, 17, 5744–5756. [Google Scholar] [CrossRef] [PubMed]
- do Pinto, Ó.P.; Richter, K.; Carlsson, L. Hematopoietic Progenitor/Stem Cells Immortalized ByLhx2 Generate Functional Hematopoietic Cells in Vivo. Blood 2002, 99, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- Hobert, O.; Westphal, H. Functions of LIM-Homeobox Genes. Trends Genet. 2000, 16, 75–83. [Google Scholar] [CrossRef]
- Porter, F.D.; Drago, J.; Xu, Y.; Cheema, S.S.; Wassif, C.; Huang, S.-P.; Lee, E.; Grinberg, A.; Massalas, J.S.; Bodine, D.; et al. Lhx2, a LIM Homeobox Gene, Is Required for Eye, Forebrain, and Definitive Erythrocyte Development. Development 1997, 124, 2935–2944. [Google Scholar] [CrossRef]
- Kitajima, K.; Kawaguchi, M.; Iacovino, M.; Kyba, M.; Hara, T. Molecular Functions of the LIM-Homeobox Transcription Factor Lhx2 in Hematopoietic Progenitor Cells Derived from Mouse Embryonic Stem Cells. Stem Cells 2013, 31, 2680–2689. [Google Scholar] [CrossRef]
- Adnan-Awad, S.; Kim, D.; Hohtari, H.; Javarappa, K.K.; Brandstoetter, T.; Mayer, I.; Potdar, S.; Heckman, C.A.; Kytölä, S.; Porkka, K.; et al. Characterization of P190-Bcr-Abl Chronic Myeloid Leukemia Reveals Specific Signaling Pathways and Therapeutic Targets. Leukemia 2021, 35, 1964–1975. [Google Scholar] [CrossRef]
- Schmalzbauer, B.S.; Thondanpallil, T.; Heller, G.; Schirripa, A.; Sperl, C.M.; Mayer, I.M.; Knab, V.M.; Nebenfuehr, S.; Zojer, M.; Mueller, A.C.; et al. CDK6 Degradation Is Counteracted by p16INK4A and p18INK4C in AML. Cancers 2022, 14, 1554. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).