Impact of Mogamulizumab in Real-Life Advanced Cutaneous T-Cell Lymphomas: A Multicentric Retrospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dictionnaire Médical de l’Académie de Médecine. Available online: https://dictionnaire.academie-medecine.fr/index.php?q=lymphome (accessed on 17 October 2021).
- Dobos, G.; Pohrt, A.; Ram-Wolff, C.; Lebbé, C.; Bouaziz, J.-D.; Battistella, M.; Bagot, M.; De Masson, A. Epidemiology of Cutaneous T-Cell Lymphomas: A Systematic Review and Meta-Analysis of 16,953 Patients. Cancers 2020, 12, 2921. [Google Scholar] [CrossRef]
- Saunes, M.; Nilsen, T.L.; Johannesen, T. Incidence of primary cutaneous T-cell lymphoma in Norway. Br. J. Dermatol. 2009, 160, 376–379. [Google Scholar] [CrossRef]
- Bradford, P.T.; Devesa, S.S.; Anderson, W.F.; Toro, J.R. Cutaneous lymphoma incidence patterns in the United States: A population-based study of 3884 cases. Blood 2009, 113, 5064–5073. [Google Scholar] [CrossRef]
- Wilson, L.D.; Hinds, G.A.; Yu, J. Age, Race, Sex, Stage, and Incidence of Cutaneous Lymphoma. Clin. Lymphoma Myeloma Leuk. 2012, 12, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Olsen, E.; Vonderheid, E.; Pimpinelli, N.; Willemze, R.; Kim, Y.; Knobler, R.; Zackheim, H.; Duvic, M.; Estrach, T.; Lamberg, S.; et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 1713–1722. [Google Scholar] [CrossRef] [Green Version]
- Demierre, M.-F.; Gan, S.; Jones, J.; Miller, D.R. Significant impact of cutaneous T-cell lymphoma on patients’ quality of life. Cancer 2006, 107, 2504–2511. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Hodak, E.; Bagot, M.; Stranzenbach, R.; Stadler, R.; Ortiz-Romero, P.L.; Papadavid, E.; Evison, F.; Knobler, R.; Quaglino, P.; et al. Blood classification and blood response criteria in mycosis fungoides and Sézary syndrome using flow cytometry: Recommendations from the EORTC cutaneous lymphoma task force. Eur. J. Cancer 2018, 93, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Jawed, S.I.; Myskowski, P.L.; Horwitz, S.; Moskowitz, A.; Querfeld, C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). J. Am. Acad. Dermatol. 2014, 70, 205.e1–205.e16. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2016, 37, 163–176. [Google Scholar] [CrossRef]
- Hurabielle, C.; Michel, L.; Ram-Wolff, C.; Battistella, M.; Jean-Louis, F.; Beylot-Barry, M.; D’Incan, M.; Bensussan, A.; Bagot, M. Expression of Sézary Biomarkers in the Blood of Patients with Erythrodermic Mycosis Fungoides. J. Investig. Dermatol. 2016, 136, 317–320. [Google Scholar] [CrossRef] [Green Version]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome–Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.C.; Elmes, J.B.; Shibu, P.A.; Larck, C.; Park, S.I. Mogamulizumab: An Anti-CC Chemokine Receptor 4 Antibody for T-Cell Lymphomas. Ann. Pharmacother. 2019, 54, 371–379. [Google Scholar] [CrossRef]
- Bonnet, P.; Battistella, M.; Roelens, M.; Ram-Wolff, C.; Herms, F.; Frumholtz, L.; Bouaziz, J.; Brice, P.; Moins-Teisserenc, H.; Bagot, M.; et al. Association of autoimmunity and long-term complete remission in patients with Sézary syndrome treated with mogamulizumab. Br. J. Dermatol. 2018, 180, 419–420. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Savarese, D.M.F. Common Terminology Criteria for Adverse Events (CTCAE) v5.0, Department of Health and Human Services. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 25 February 2022).
- Satake, A.; Konishi, A.; Azuma, Y.; Tsubokura, Y.; Yoshimura, H.; Hotta, M.; Nakanishi, T.; Fujita, S.; Nakaya, A.; Ito, T.; et al. Clinical efficacy of mogamulizumab for relapsed/refractory aggressive adult T-cell leukemia/lymphoma: A retrospective analysis. Eur. J. Haematol. 2020, 105, 704–711. [Google Scholar] [CrossRef]
- Schechter, G.; Sausville, E.; Fischmann, A.; Soehnlen, F.; Eddy, J.; Matthews, M.; Gazdar, A.; Guccion, J.; Munson, D.; Makuch, R. Evaluation of circulating malignant cells provides prognostic information in cutaneous T cell lymphoma. Blood 1987, 69, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Olsen, E.A.; Whittaker, S.; Willemze, R.; Pinter-Brown, L.; Foss, F.M.; Geskin, L.J.; Schwartz, L.H.; Horwitz, S.M.; Guitart, J.; Zic, J.; et al. Primary Cutaneous Lymphoma: Recommendations for Clinical Trial Design and Staging Update from the ISCL, USCLC, and EORTC. Blood 2021, 110, 1713–1722. [Google Scholar] [CrossRef] [Green Version]
- Afifi, S.; Mohamed, S.; Zhao, J.; Foss, F. A drug safety evaluation of mogamulizumab for the treatment of cutaneous T-Cell lymphoma. Expert Opin. Drug Saf. 2019, 18, 769–776. [Google Scholar] [CrossRef]
- Cowan, R.; Scarisbrick, J.; Zinzani, P.; Nicolay, J.; Sokol, L.; Pinter-Brown, L.; Quaglino, P.; Iversen, L.; Dummer, R.; Musiek, A.; et al. Efficacy and safety of mogamulizumab by patient baseline blood tumour burden: A post hoc analysis of the MAVORIC trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2225–2238. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, J.; Li, J.; Zhao, Y.; Zhang, T.; Yang, R.; Ma, X. Safety and efficacy profile of mogamulizumab (Poteligeo) in the treatment of cancers: An update evidence from 14 studies. BMC Cancer 2021, 21, 618. [Google Scholar] [CrossRef]
- Bagot, M.; Porcu, P.; Marie-Cardine, A.; Battistella, M.; William, B.M.; Vermeer, M.; Whittaker, S.; Rotolo, F.; Ram-Wolff, C.; Khodadoust, M.S.; et al. IPH4102, a first-in-class anti-KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T-cell lymphoma: An international, first-in-human, open-label, phase 1 trial. Lancet Oncol. 2019, 20, 1160–1170. [Google Scholar] [CrossRef]
- Khodadoust, M.S.; Rook, A.H.; Porcu, P.; Foss, F.; Moskowitz, A.J.; Shustov, A.; Shanbhag, S.; Sokol, L.; Fling, S.P.; Ramchurren, N.; et al. Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study. J. Clin. Oncol. 2020, 38, 20–28. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients with Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.D.S.; Banerjee, S.; Kruglov, O.; Viller, N.N.; Horwitz, S.M.; Lesokhin, A.; Zain, J.; Querfeld, C.; Chen, R.; Okada, C.; et al. Targeting CD47 in Sézary syndrome with SIRPαFc. Blood Adv. 2019, 3, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Silence, K.; Dreier, T.; Moshir, M.; Ulrichts, P.; E Gabriels, S.M.; Saunders, M.; Wajant, H.; Brouckaert, P.; Huyghe, L.; Van Hauwermeiren, T.; et al. ARGX-110, a highly potent antibody targeting CD70, eliminates tumors via both enhanced ADCC and immune checkpoint blockade. mAbs 2013, 6, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Weiner, D.M.; Durgin, J.S.; Wysocka, M.; Rook, A.H. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: Current and future approaches. J. Am. Acad. Dermatol. 2020, 84, 597–604. [Google Scholar] [CrossRef]
| Basic Features | Population (n = 21) | Responders (n = 13) | Non-Responders (n = 8) | p-Value |
|---|---|---|---|---|
| Sex (n, %) | 0.067 | |||
| Male | 12 (57.1) | 5 (38.5) | 7 (87.5) | - |
| Female | 9 (42.9) | 8 (61.5) | 1 (12.5) | - |
| Age (moy ± δ) | 68.0 ± 10.7 | 70.0 ± 11.9 | 64.8 ± 7.9 | 0.286 |
| Smoker (n, %) (n = 18 [12/6]) | 4 (22.2) | 2 (16.7) | 2 (33.3) | 0.569 |
| Alcohol consumption (n, %) (n = 18 [12/6]) | 4 (22.2) | 2 (16.7) | 2 (33.3) | 0.569 |
| Lymphoma type (n, %) | ||||
| Sézary | 16 (76.2) | 10 (76.9) | 6 (75.0) | 1.000 |
| Mycosis fungoïde | 5 (23.8) | 3 (23.1) | 2 (25.0) | |
| Delay from diagnosis to mogamulizumab introduction (in months) [median, Q1–Q3] | 32.0 [22.0–87.0] | 25.0 [15.0–87.0] | 44.5 [33.5–107.0] | 0.169 |
| T4-stage at mogamulizumab introduction (n, %) | 12 (57.1) | 7 (53.9) | 5 (62.5) | 1.000 |
| ECOG status at mogamulizumab introduction (n, %) | 0.194 | |||
| 0 | 10 (47.6) | 5 (38.5) | 5 (62.5) | 0.387 |
| 1 | 6 (28.6) | 4 (30.8) | 2 (25.0) | 1.000 |
| 2 | 4 (19.1) | 4 (30.8) | 0 (0.0) | 0.131 |
| 3 | 1 (4.8) | 0 (0.0) | 1 (12.5) | - |
| LDH level at mogamulizumab introduction (UI/L) [median, Q1–Q3] (n = 17 [10/7]) | 299.0 [211.0–365.0] | 260.0 [202.0–358.0] | 310.0 [211.0–379.0] | 0.591 |
| LDH level superior to 245 UI/L at mogamulizumab introduction (n, %) (n = 18 [10/8]) | 10 (55.6) | 5 (50.0) | 5 (62.5) | 0.664 |
| Number of Sézary cells at mogamulizumab introduction (g/L) [median, Q1–Q3] (n = 20 [12/8]) | 0.88 [0.02–6.66] | 2.85 [0.20–9.50] | 0.39 [0.003–3.15] | 0.296 |
| CD4/CD8 ratio before mogamulizumab introduction [median, Q1–Q3] (n = 17 [11/6]) | 9.6 [3.3–88.0] | 9.6 [3.0–89.0] | 8.4 [3.3–40.0] | 0.580 |
| CD4/CD8 ratio >10 before mogamulizumab introduction (n, %) (n = 17 [11/6]) | 7 (41.2) | 5 (45.5) | 2 (33.3) | 1.000 |
| Number of therapeutic lines [median, Q1–Q3] | 4.0 [3.0–5.0] | 3.0 [3.0–5.0] | 5.0 [4.5–6.0] | 0.070 |
| Patch type cutaneous before introduction of mogamulizumab (n, %) | 8 (38.1) | 6 (46.2) | 2 (25.0) | 0.400 |
| Plaque type cutaneous lesions (infiltrated) before introduction of mogamulizumab (n, %) | 7 (33.3) | 5 (38.5) | 2 (25.0) | 0.656 |
| Tumor type cutaneous lesions before introduction of mogamulizumab (n, %) | 2 (9.5) | 1 (7.7) | 1 (12.5) | 1.000 |
| Presence of any adenopathy before introduction of mogamulizumab (n, %) | 12 (57.1) | 7 (53.9) | 5 (62.5) | 1.000 |
| Cutaneous area affected before introduction of mogamulizumab (n, %) | 0.110 | |||
| 0% | 1 (4.8) | 0 (0.0) | 1 (12.5) | 0.381 |
| <10% | 2 (9.5) | 2 (15.4) | 0 (0.0) | 0.505 |
| 10–50% | 1 (4.8) | 0 (0.0) | 1 (12.5) | 0.381 |
| 5–80% | 4 (19.1) | 4 (30.8) | 0 (0.0) | 0.131 |
| >80% | 13 (61.9) | 7 (53.9) | 6 (75.0) | 0.400 |
| Basic Features | Population (n = 21) | Responders (n = 13) | Non-Responders (n = 8) | p-Value |
|---|---|---|---|---|
| Evolution (n, %) | ||||
| Full response | 4 (19.1) | 4 (30.8) | - | - |
| Partial response | 8 (38.1) | 8 (61.5) | - | - |
| Stability | 1 (4.8) | 1 (4.8) | - | - |
| Progression | 3 (14.3) | - | 3 (37.5) | - |
| Death | 5 (23.8) | - | 5 (62.5) | - |
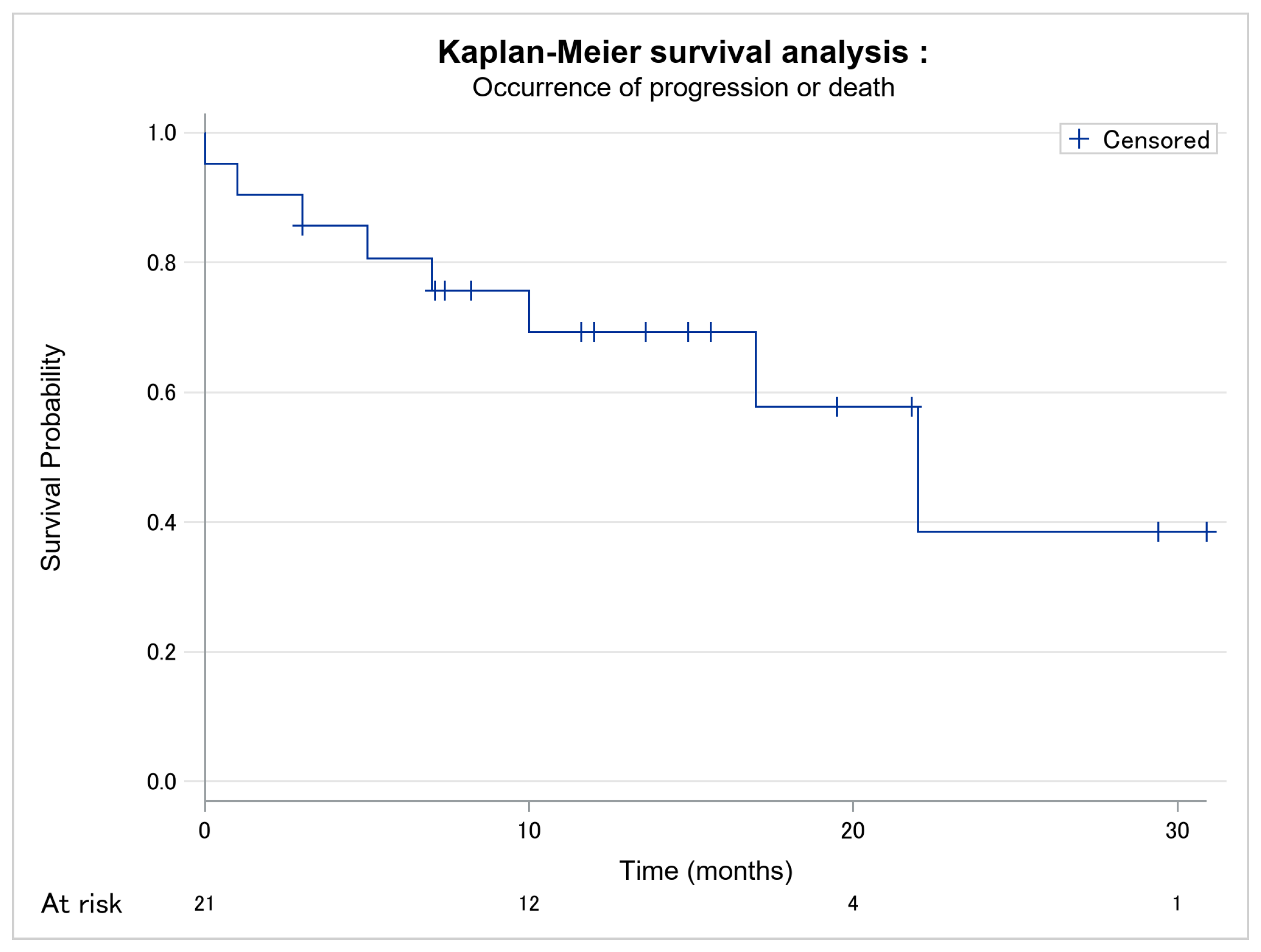
| Time of follow-up (in months) [median, Q1–Q3] | 11.6 [7.0–17.0] | 13.6 [8.2–19.5] | 6.0 [2.0–13.5] | - |
| Time of response to Moga before progression or death (in months) [median, Q1–Q3] (n = 8) | - | - | 6.0 [2.0–13.5] | - |
| Before progression (n = 3) | - | - | 17.0 [3.0–22.0] | - |
| Before death (n = 5) | - | - | 5.0 [1.0–7.0] | |
| Responder’s time of follow-up in months) [median, Q1–Q3] (n = 13) | - | 13.6 [8.2–19.5] | - | - |
| Full response (n = 4) | - | 22.5 [15.3–30.2] | - | - |
| Partial response (n = 8) | - | 9.9 [7.3–16.6] | - | - |
| Stability (n = 1) | - | 12.0 [12.0–12.0] | - | - |
| Visceral progression (n, %) | 1 (4.8) | - | 1 (12.5) | - |
| Presence of one or several adenopathy (n, %) | 5 (23.8) | - | 5 (62.5) | - |
| LDH-level at endpoint (UI/L) [median, Q1–Q3] (n = 14 [9/5]) | 268.0 [201.0–363.0] | 287.0 [221.0–323.0] | 249.0 [201.0–549.0] | 0.790 |
| LDH-level superior 245 UI/L at end-point (n, %) (n = 14 [9/5]) | 8 (57.1) | 5 (55.6) | 3 (60.0) | 1.000 |
| Number of Sézary cells (progression or endpoint, g/L) [median, Q1–Q3] (n = 20 [13/7]) | 0.00 [0.00–0.80] | 0.00 [0.00–0.65] | 0.55 [0.00–3.13] | 0.165 |
| CD4/CD8 ratio at endpoint [median, Q1–Q3] n = 12 [8/4]) | 3.8 [1.1–7.2] | 4.2 [1.1–7.2] | 3.3 [1.1–31.6] | 1.000 |
| CD4/CD8 ratio >10 at end-point (n, %) (n = 13 [9/4]) | 2 (15.4) | 1 (11.1) | 1 (25.0) | 1.000 |
| Total number of lymphocytes (progression or endpoint, g/L) [median, Q1–Q3] | 0.99 [0.70–2.14] | 0.99 [0.80–2.08] | 1.20 [0.65–3.67] | 0.800 |
| Clinical presence of skin lesions (progression or end-point (n, %) | 18 (85.7) | 10 (76.9) | 8 (100.0) | 0.257 |
| Basic Features | Population (n = 21) | Responders (n = 13) | Non-Responders (n = 8) | p-Value |
|---|---|---|---|---|
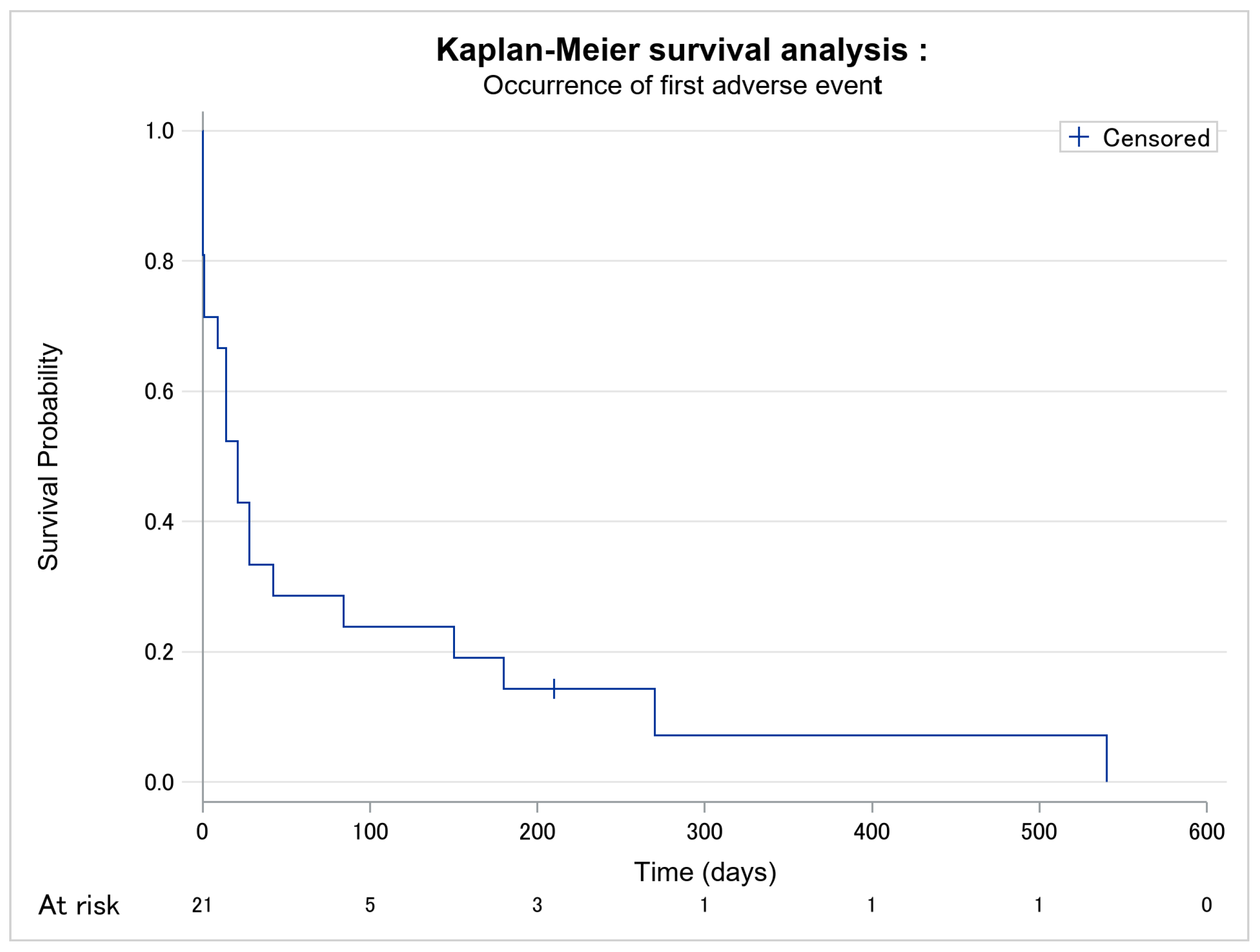
| Adverse event occurrence (n, %) | 20 (95.2) | 13 (100.0) | 7 (87.5) | 0.381 |
| Delay between mogamulizumab introduction and first adverse event (days) [median, Q1–Q3] | 21.0 [1.0–84.0] | 21.0 [14.0–84.0] | 18.5 [0.5–126.0] | 0.913 |
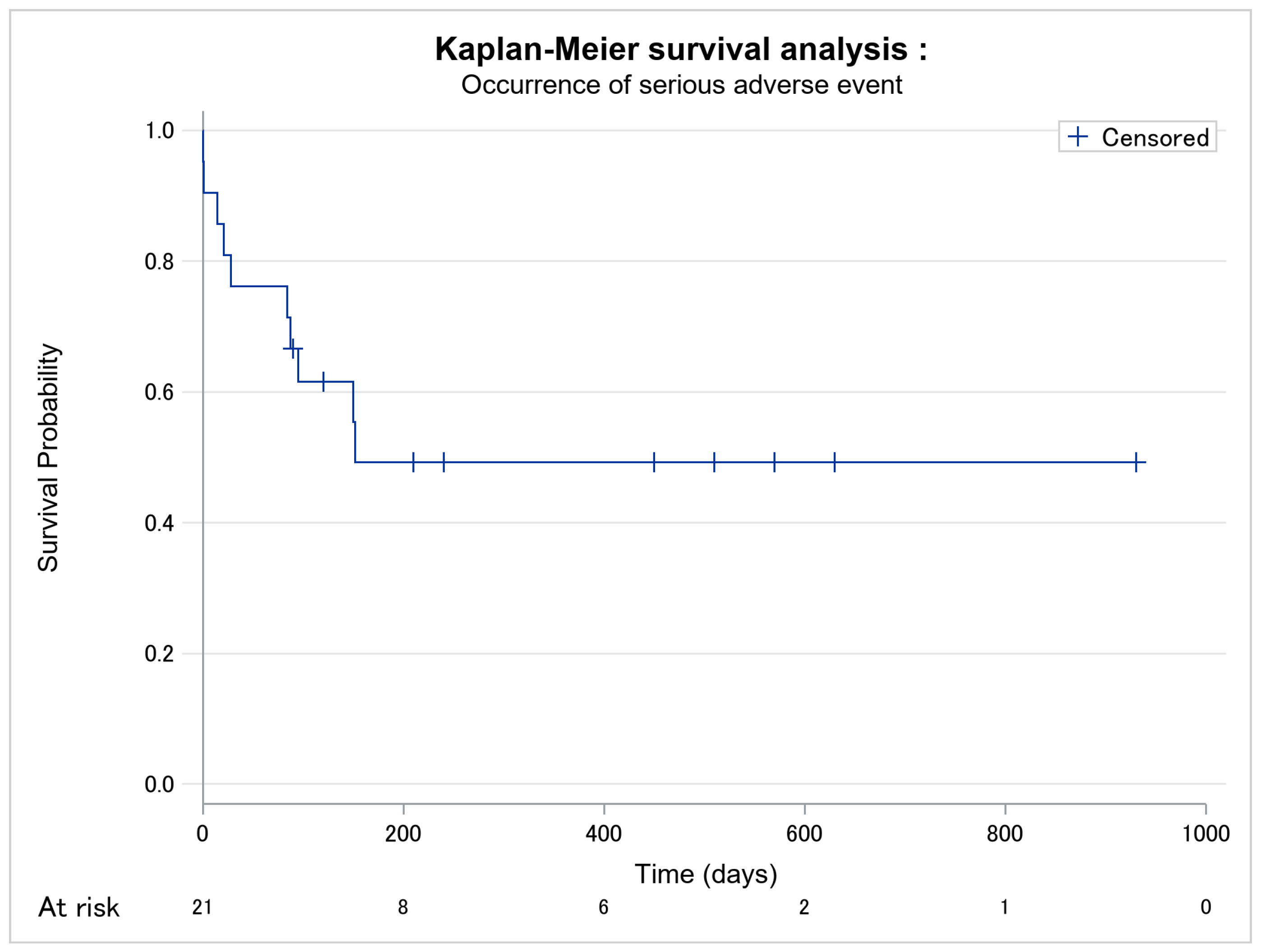
| Occurrence of severe adverse event (n, %) | 10 (47.6) | 5 (38.5) | 5 (62.5) | 0.387 |
| Delay between mogamulizumab introduction and first severe adverse event (days) [median, Q1–Q3] | 120.0 [84.0–450.0] | 152.0 [120.0–450.0] | 88.5 [14.5–152.5] | 0.089 |
| Number of adverse events per patient since mogamulizumab [median, Q1–Q3] | 2.0 [1.0–3.0] | 2.0 [1.0–3.0] | 1.5 [1.0–2.5] | 0.515 |
| Population (n = 21) | Responders (n = 13) | Non-Responders (n = 8) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| before Moga | with Moga | p-Value | before Moga | with Moga | p-Value | before Moga | with Moga | p-Value | |
| LDH-level (UI/L) [median, Q1–Q3] | 299.0 [211.0–365.0] | 268.0 [201.0–363.0] | 0.671 | 260.0 [202.0–358.0] | 287.0 [221.0–323.0] | 1.000 | 310.0 [211.0–379.0] | 249.0 [201.0–549.0] | 0.813 |
| LDH-level superior to 245 UI/L (n, %) | 10 (55.6) | 8 (57.1) | 1.000 | 5 (50.0) | 5 (55.6) | 1.000 | 5 (62.5) | 3 (60.0) | 1.000 |
| Number of Sézary cells(g/L) [median, Q1–Q3] | 0.88 [0.02–6.66] | 0.00 [0.00–0.80] | 0.004 | 2.85 [0.20–9.50] | 0.00 [0.00–0.65] | 0.006 | 0.39 [0.003–3.15] | 0.55 [0.00–3.13] | 0.438 |
| CD4/CD8 ratio [median, Q1–Q3] | 9.6 [3.3–88.0] | 3.8 [1.1–7.2] | 0.001 | 9.6 [3.0–89.0] | 4.2 [1.1–7.2] | 0.016 | 8.4 [3.3–40.0] | 3.3 [1.1–31.6] | 0.125 |
| CD4/CD8 >10 (n, %) | 7 (41.2) | 2 (15.4) | 0.455 | 5 (45.5) | 1 (11.1) | 1.000 | 2 (33.3) | 1 (25.0) | 1.000 |
| Presence of adenopathy (n, %) | 12 (57.1) | 8 (38.1) | 0.367 | 7 (53.9) | 3 (23.1) | 1.000 | 5 (62.5) | 5 (62.5) | 0.464 |
| Affected cutaneous area (n, %) | 20 (95.2) | 18 (85.7) | 1.000 | 13 (100.0) | 10 (76.9) | - | 7 (87.5) | 8 (100.0) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouandet, M.; Nakouri, I.; Nadin, L.; Kieny, A.; Samimi, M.; Adamski, H.; Quéreux, G.; Chaby, G.; Dompmartin, A.; L’Orphelin, J.-M. Impact of Mogamulizumab in Real-Life Advanced Cutaneous T-Cell Lymphomas: A Multicentric Retrospective Cohort Study. Cancers 2022, 14, 1659. https://doi.org/10.3390/cancers14071659
Jouandet M, Nakouri I, Nadin L, Kieny A, Samimi M, Adamski H, Quéreux G, Chaby G, Dompmartin A, L’Orphelin J-M. Impact of Mogamulizumab in Real-Life Advanced Cutaneous T-Cell Lymphomas: A Multicentric Retrospective Cohort Study. Cancers. 2022; 14(7):1659. https://doi.org/10.3390/cancers14071659
Chicago/Turabian StyleJouandet, Marie, Inès Nakouri, Lawrence Nadin, Alice Kieny, Mahtab Samimi, Henri Adamski, Gaëlle Quéreux, Guillaume Chaby, Anne Dompmartin, and Jean-Matthieu L’Orphelin. 2022. "Impact of Mogamulizumab in Real-Life Advanced Cutaneous T-Cell Lymphomas: A Multicentric Retrospective Cohort Study" Cancers 14, no. 7: 1659. https://doi.org/10.3390/cancers14071659
APA StyleJouandet, M., Nakouri, I., Nadin, L., Kieny, A., Samimi, M., Adamski, H., Quéreux, G., Chaby, G., Dompmartin, A., & L’Orphelin, J.-M. (2022). Impact of Mogamulizumab in Real-Life Advanced Cutaneous T-Cell Lymphomas: A Multicentric Retrospective Cohort Study. Cancers, 14(7), 1659. https://doi.org/10.3390/cancers14071659





