Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgery and Chemotherapy
2.3. Radiation Treatment Planning
2.4. Hematological Data
2.5. Data Analysis and Statistics
3. Results
3.1. Patient Characteristics
3.2. Surgery and Chemotherapy
3.3. Proton Therapy
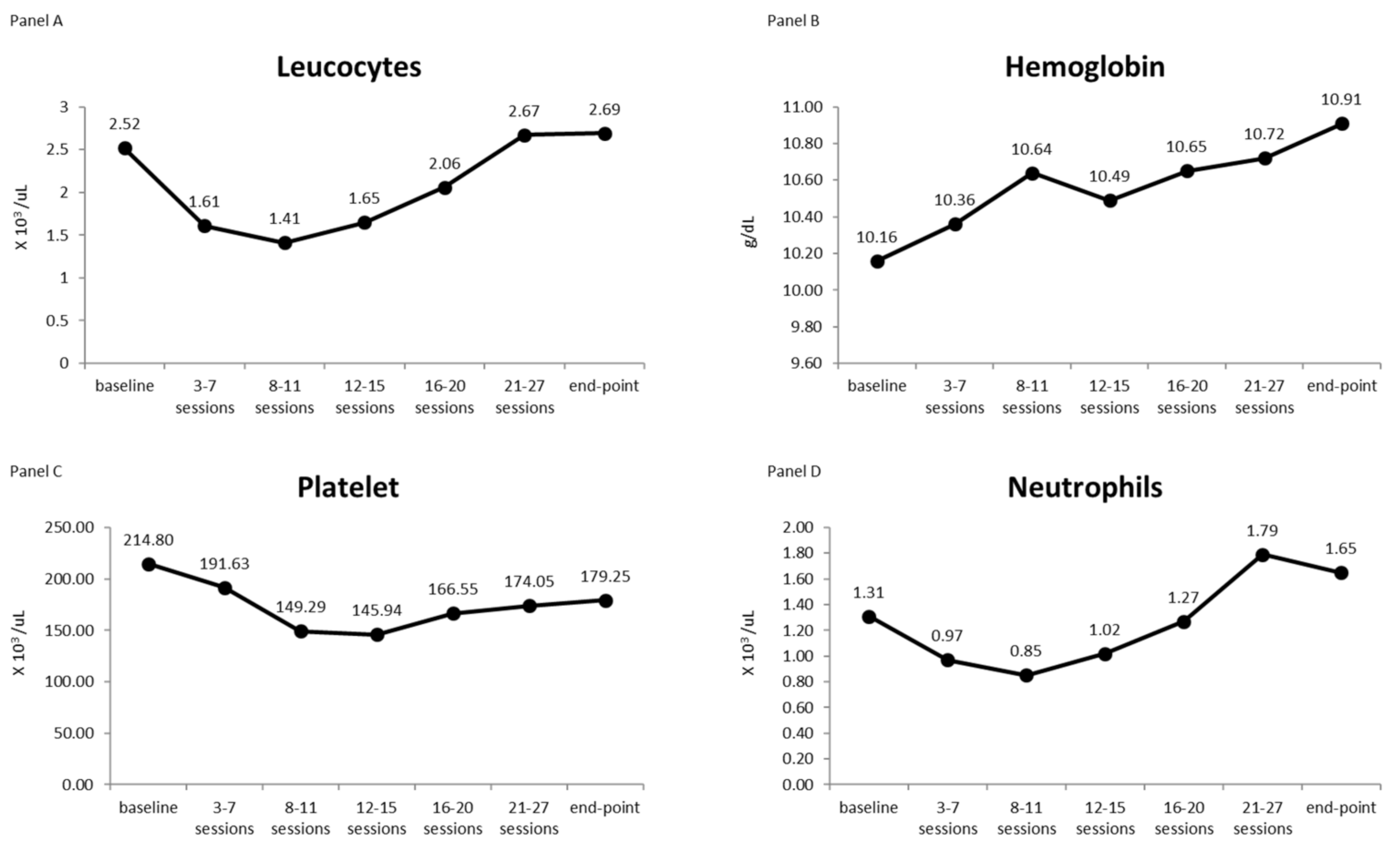
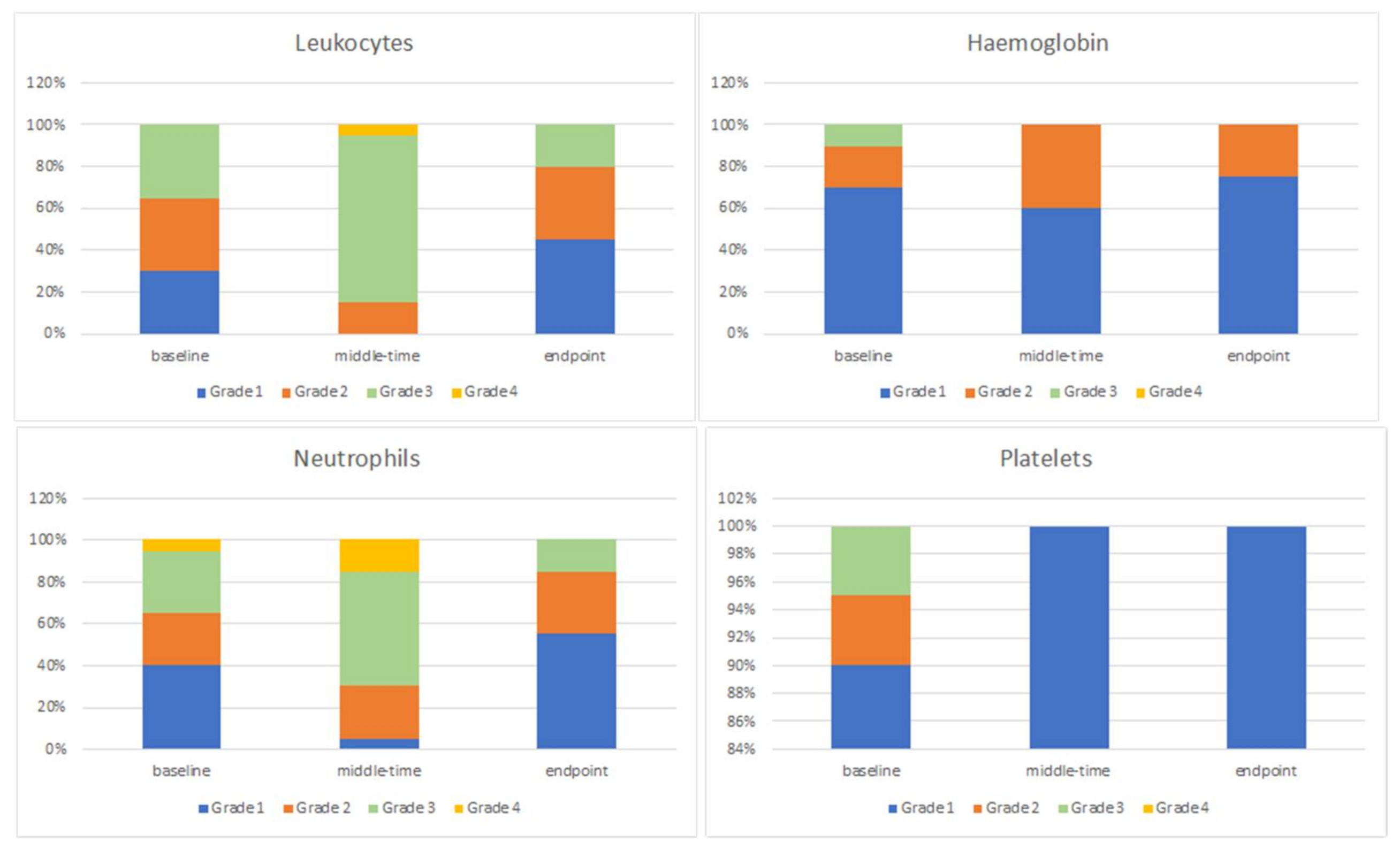
3.4. Blood Count
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smoll, N.R.; Drummond, K.J. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J. Clin. Neurosci. 2012, 19, 1541–1544. [Google Scholar] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.S.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-Oncology 2016, 18 (Suppl. S5), v1–v75. [Google Scholar] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [PubMed] [Green Version]
- Jakacki, R.I.; Burger, P.C.; Kocak, M.; Boyett, J.M.; Goldwein, J.; Mehta, M.; Packer, R.J.; Tarbell, N.J.; Pollack, I.J. Outcome and prognostic factors for children with supratentorial primitive neuroectodermal tumors treated with carboplatin during radiotherapy: A report from the Children’s Oncology Group: Outcome for Children with Supratentorial PNET. Pediatr. Blood Cancer 2015, 62, 776–783. [Google Scholar]
- Pizer, B.L.; Weston, C.L.; Robinson, K.J.; Ellison, D.W.; Ironside, J.; Saran, F.; Lashford, L.S.; Tait, D.; Lucraft, H.; Walkeret, D.A.; et al. Analysis of patients with supratentorial primitive neuro-ectodermal tumours entered into the SIOP/UKCCSG PNET 3 study. Eur. J. Cancer 2006, 42, 1120–1128. [Google Scholar]
- Massimino, M.; Gandola, L.; Biassoni, V.; Spreafico, F.; Schiavello, E.; Poggi, G.; Pecori, E.; Vajna De Pava, M.; Modena, P.; Antonelli, M.; et al. Evolving of therapeutic strategies for CNS-PNET: Childhood CNS-PNET. Pediatr. Blood Cancer 2013, 60, 2031–2035. [Google Scholar]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar]
- Deutsch, M. Medulloblastoma: Staging and treatment outcome. Int. J. Radiat Oncol. Biol. Phys. 1988, 14, 1103–1107. [Google Scholar]
- Mizumoto, M.; Oshiro, Y.; Yamamoto, T.; Kohzuki, H.; Sakurai, H. Proton Beam Therapy for Pediatric Brain Tumor. Neurol. Med. Chir. 2017, 57, 343–355. [Google Scholar]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [PubMed]
- Jefferies, S.; Rajan, B.; Ashley, S.; Traish, D.; Brada, M. Haematological toxicity of cranio-spinal irradiation. Radiother. Oncol. 1998, 48, 23–27. [Google Scholar] [PubMed]
- Chang, E.L.; Allen, P.; Wu, C.; Ater, J.; Kuttesch, J.; Maor, M.H. Acute toxicity and treatment interruption related to electron and photon craniospinal irradiation in pediatric patients treated at the University of Texas M. D. Anderson Cancer Cent. Int. J. Radiat Oncol. Biol. Phys. 2002, 52, 1008–1016. [Google Scholar]
- Kumar, N.; Miriyala, R.; Thakur, P.; Madan, R.; Salunke, P.; Yadav, B.; Gupta, A. Impact of acute hematological toxicity on treatment interruptions during cranio-spinal irradiation in medulloblastoma: A tertiary care institute experience. J. Neurooncol. 2017, 134, 309–315. [Google Scholar] [PubMed]
- Song, S.; Park, H.J.; Yoon, J.H.; Kim, D.W.; Park, J.; Shin, D.; Shin, S.H.; Kang, H.J.; Kim, S.-K.; Phi, J.; et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol. 2014, 53, 1158–1164. [Google Scholar] [PubMed]
- Prabhu, R.S.; Cassidy, R.J.; Landry, J.C. Radiation therapy and neutropenia. Curr. Probl. Cancer 2015, 39, 292–296. [Google Scholar] [PubMed]
- Baliga, S.; Yock, T.I. Proton beam therapy in pediatric oncology. Curr. Opin. Pediatr. 2019, 31, 28–34. [Google Scholar]
- Liu, K.X.; Ioakeim-Ioannidou, M.; Susko, M.S.; Rao, A.D.; Yeap, B.Y.; Snijders, A.M.; Ladra, M.M.; Vogel, J.; Zaslowe-Dude, C.; Marcus, K.J.; et al. A Multi-institutional Comparative Analysis of Proton and Photon Therapy-Induced Hematologic Toxicity in Patients with Medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 726–735. [Google Scholar]
- Breen, W.G.; Paulino, A.C.; Hartsell, W.F.; Mangona, V.S.; Perkins, S.M.; Indelicato, D.J.; Harmsen, W.S.; Tranby, B.N.; Bajaj, B.V.M.; Gallotto, S.L.; et al. Factors Associated with Acute Toxicity in Pediatric Patients Treated with Proton Radiation Therapy: A Report From the Pediatric Proton Consortium Registry. Pract. Radiat. Oncol. 2021, 12, S1879850021003441. [Google Scholar]
- Gandola, L.; Massimino, M.; Cefalo, G.; Solero, C.; Spreafico, F.; Pecori, E.; Riva, D.; Collini, P.; Pignoli, E.; Giangaspero, F.; et al. Hyperfractionated Accelerated Radiotherapy in the Milan Strategy for Metastatic Medulloblastoma. JCO 2009, 27, 566–571. [Google Scholar]
- Farace, P.; Bizzocchi, N.; Righetto, R.; Fellin, F.; Fracchiolla, F.; Lorentini, S.; Widesott, L.; Algranati, C.; Rombi, B.; Vennarini, S.; et al. Supine craniospinal irradiation in pediatric patients by proton pencil beam scanning. Radiother. Oncol. 2017, 123, 112–118. [Google Scholar] [PubMed]
- Particle Radiotherapy. Available online: https://link.springer.com/book/10.1007/978-81-322-2622-2 (accessed on 26 January 2022).
- Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf (accessed on 2 February 2022).
- Rubin, P.; Constine, L.S.; Marks, L.B. ALERT—Adverse Late Effects of Cancer Treatment; Chapter 24; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, pp. 624–650. [Google Scholar]
- De Naurois, J.; Novitzky-Basso, I.; Gill, M.J.; Marti Marti, F.; Cullen, M.H.; Roila, F. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2010, 21, v252–v256. [Google Scholar] [PubMed]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. JCO 2015, 33, 3199–3212. [Google Scholar]
- Brown, A.P.; Barney, A.P.; Grosshans, D.R.; McAleer, M.F.; De Groot, J.F.; Puduvalli, V.K.; Tucker, S.L.; Crawford, C.N.; Khan, M.; Khatua, S.; et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int. J. Radiant. Oncol. Biol. Phys. 2013, 86, 277–284. [Google Scholar]
- Mac Manus, M.P.; McCormick, D.; Trimble, A.; Abram, W.P. Value of granulocyte colony stimulating factor in radiotherapy induced neutropenia: Clinical and laboratory studies. Eur. J. Cancer 1995, 31, 302–307. [Google Scholar]
- Probert, J.C.; Parker, B.R. The Effects of Radiation Therapy on Bone Growth. Radiology 1975, 114, 8. [Google Scholar]
- Hoeben, B.A.; Carrie, C.; Timmermann, B.; Mandeville, H.C.; Gandola, L.; Dieckmann, K.; Albiac, M.R.; Magelssen, H.; Lassen-Ramshad, Y.; Ondrováet, B.; et al. Management of vertebral radiotherapy dose in paediatric patients with cancer: Consensus recommendations from the SIOPE radiotherapy working group. Lancet Oncol. 2019, 20, e155–e166. [Google Scholar]
- Mauch, P.; Constine, L.; Greenberger, J.; Knospe, W.; Sullivan, J.; Liesveld, J.L.; Deeget, H.J. Hemopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat Oncol. Biol. Phys. 1995, 31, 1319–1339. [Google Scholar]
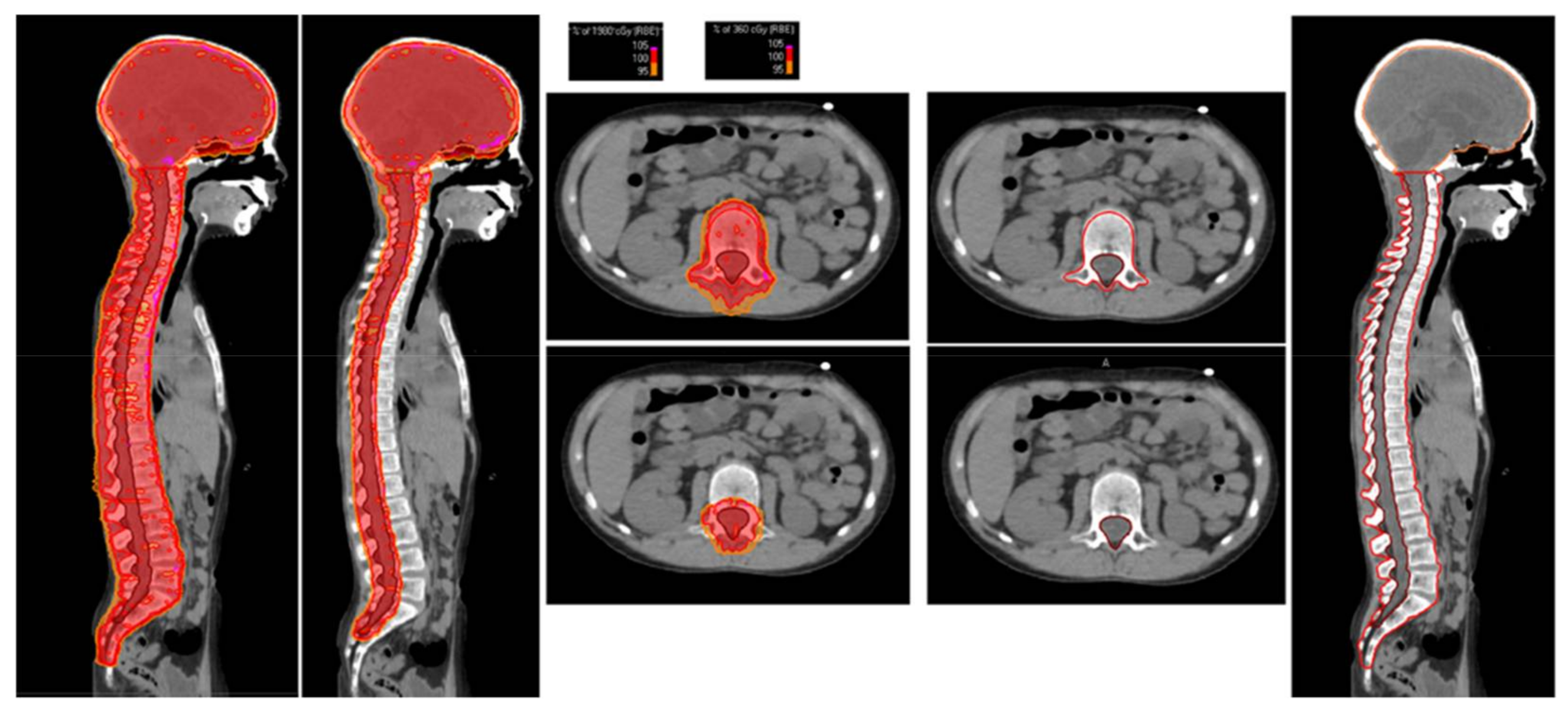
| Age at Diagnosis | Median | Range |
|---|---|---|
| 6.95 years | 3.2–18.43 years | |
| n | % | |
| Sex | ||
| Male | 13 | 65 |
| Female | 7 | 35 |
| Histology | ||
| Medulloblastoma | 17 | 85 |
| Pineoblastoma | 2 | 10 |
| CNS Neuroblastoma | 1 | 5 |
| Metastatic disease | ||
| Yes | 10 | 50 |
| No | 10 | 50 |
| Surgery | ||
| Total resection | 14 | 70 |
| Subtotal resection | 4 | 20 |
| Biopsy | 2 | 10 |
| Chemotherapy | ||
| Standard dose | 20 | 100 |
| High-dose chemotherapy and HSCT | 3 | 15 |
| Doses, Gy-RBE | ||
| Craniospinal dose (19.8 + 16.2) | 36 | 36 (all patients) |
| Total dose (CSI + boost primary site) | 54 | 54 (all patients) |
| Boost Metastasis site (only 3 patients) | 45 | 18–50.4 |
| Time | Mean | SD | 95% C.I. for Mean | Min | Max | Median | ||
|---|---|---|---|---|---|---|---|---|
| Leucocytes | Baseline | 2.52 | 0.97 | 2.07 | 2.97 | 1.40 | 5.10 | 2.35 |
| 3rd–7th sessions | 1.61 | 0.41 | 1.41 | 1.80 | 0.80 | 2.70 | 1.50 | |
| 8th–11th sessions | 1.41 | 0.44 | 1.18 | 1.64 | 0.70 | 2.70 | 1.40 | |
| 12th–15th sessions | 1.65 | 0.61 | 1.35 | 1.95 | 0.60 | 2.60 | 1.65 | |
| 16th–20th sessions | 2.06 | 0.51 | 1.82 | 2.30 | 1.00 | 3.00 | 2.05 | |
| 21st–27th sessions | 2.67 | 0.95 | 2.23 | 3.11 | 1.50 | 5.50 | 2.35 | |
| Endpoint | 2.69 | 0.91 | 2.26 | 3.11 | 1.10 | 4.50 | 2.50 | |
| Hemoglobin | Baseline | 10.16 | 1.07 | 9.66 | 10.66 | 7.80 | 12.10 | 10.35 |
| 3rd–7th sessions | 10.36 | 0.78 | 9.98 | 10.74 | 9.00 | 12.30 | 10.40 | |
| 8th–11th sessions | 10.64 | 0.67 | 10.29 | 10.98 | 9.20 | 12.00 | 10.70 | |
| 12th–15th sessions | 10.49 | 0.86 | 10.06 | 10.92 | 8.60 | 11.70 | 10.70 | |
| 16th–20th sessions | 10.65 | 1.25 | 10.06 | 11.23 | 8.00 | 13.20 | 10.65 | |
| 21st–27th sessions | 10.72 | 1.01 | 10.25 | 11.19 | 8.90 | 12.80 | 10.65 | |
| Endpoint | 10.91 | 1.09 | 10.40 | 11.41 | 8.90 | 13.30 | 11.15 | |
| Platelets | Baseline | 214.80 | 106.73 | 164.85 | 264.75 | 36.00 | 409.00 | 231.5 |
| 3rd–7th sessions | 191.63 | 78.12 | 153.98 | 229.28 | 74.00 | 370.00 | 184.0 | |
| 8th–11th sessions | 149.29 | 40.47 | 128.49 | 170.10 | 74.00 | 241.00 | 150.0 | |
| 12th–15th sessions | 145.94 | 49.76 | 121.20 | 170.69 | 79.00 | 264.00 | 140.0 | |
| 16th–20th sessions | 166.55 | 55.76 | 140.45 | 192.65 | 86.00 | 272.00 | 164.5 | |
| 21st–27th sessions | 174.05 | 57.51 | 147.14 | 200.96 | 70.00 | 290.00 | 161.0 | |
| Endpoint | 179.25 | 53.32 | 154.29 | 204.21 | 78.00 | 304.00 | 166.5 | |
| Neutrophils | Baseline | 1.31 | 0.66 | 1.00 | 1.62 | 0.30 | 2.60 | 1.15 |
| 3rd–7th sessions | 0.97 | 0.35 | 0.80 | 1.14 | 0.40 | 1.90 | 1.00 | |
| 8th–11th sessions | 0.85 | 0.36 | 0.66 | 1.03 | 0.30 | 1.90 | 0.80 | |
| 12th–15th sessions | 1.02 | 0.52 | 0.76 | 1.27 | 0.10 | 1.80 | 1.00 | |
| 16th–20th sessions | 1.27 | 0.49 | 1.03 | 1.50 | 0.10 | 2.30 | 1.25 | |
| 21st–27th sessions | 1.79 | 0.74 | 1.44 | 2.13 | 0.90 | 3.80 | 1.60 | |
| Endpoint | 1.65 | 0.60 | 1.36 | 1.94 | 0.70 | 2.80 | 1.60 | |
| Time | p-Value of Wilcoxon Signed-Rank Sum Test | |
|---|---|---|
| Leucocytes | Baseline vs. Midpoint | <0.0001 |
| Midpoint vs. Endpoint | <0.0001 | |
| Baseline vs. Endpoint | 0.6408 | |
| Hemoglobin | Baseline vs. Midpoint | 0.1896 |
| Midpoint vs. Endpoint | 0.3958 | |
| Baseline vs. Endpoint | 0.0100 | |
| Platelets | Baseline vs. Midpoint | 0.0046 |
| Midpoint vs. Endpoint | 0.0008 | |
| Baseline vs. Endpoint | 0.0954 | |
| Neutrophils | Baseline vs. Midpoint | 0.0005 |
| Midpoint vs. Endpoint | <0.0001 | |
| Baseline vs. Endpoint | 0.2502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vennarini, S.; Del Baldo, G.; Lorentini, S.; Pertile, R.; Fabozzi, F.; Merli, P.; Megaro, G.; Scartoni, D.; Carai, A.; Tornesello, A.; et al. Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors. Cancers 2022, 14, 1653. https://doi.org/10.3390/cancers14071653
Vennarini S, Del Baldo G, Lorentini S, Pertile R, Fabozzi F, Merli P, Megaro G, Scartoni D, Carai A, Tornesello A, et al. Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors. Cancers. 2022; 14(7):1653. https://doi.org/10.3390/cancers14071653
Chicago/Turabian StyleVennarini, Sabina, Giada Del Baldo, Stefano Lorentini, Riccardo Pertile, Francesco Fabozzi, Pietro Merli, Giacomina Megaro, Daniele Scartoni, Andrea Carai, Assunta Tornesello, and et al. 2022. "Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors" Cancers 14, no. 7: 1653. https://doi.org/10.3390/cancers14071653
APA StyleVennarini, S., Del Baldo, G., Lorentini, S., Pertile, R., Fabozzi, F., Merli, P., Megaro, G., Scartoni, D., Carai, A., Tornesello, A., Colafati, G. S., Cacchione, A., & Mastronuzzi, A. (2022). Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors. Cancers, 14(7), 1653. https://doi.org/10.3390/cancers14071653








