Non-Invasive Biomarkers in the Diagnosis of Upper Urinary Tract Urothelial Carcinoma—A Systematic Review
Abstract
:Simple Summary
Abstract
1. Background
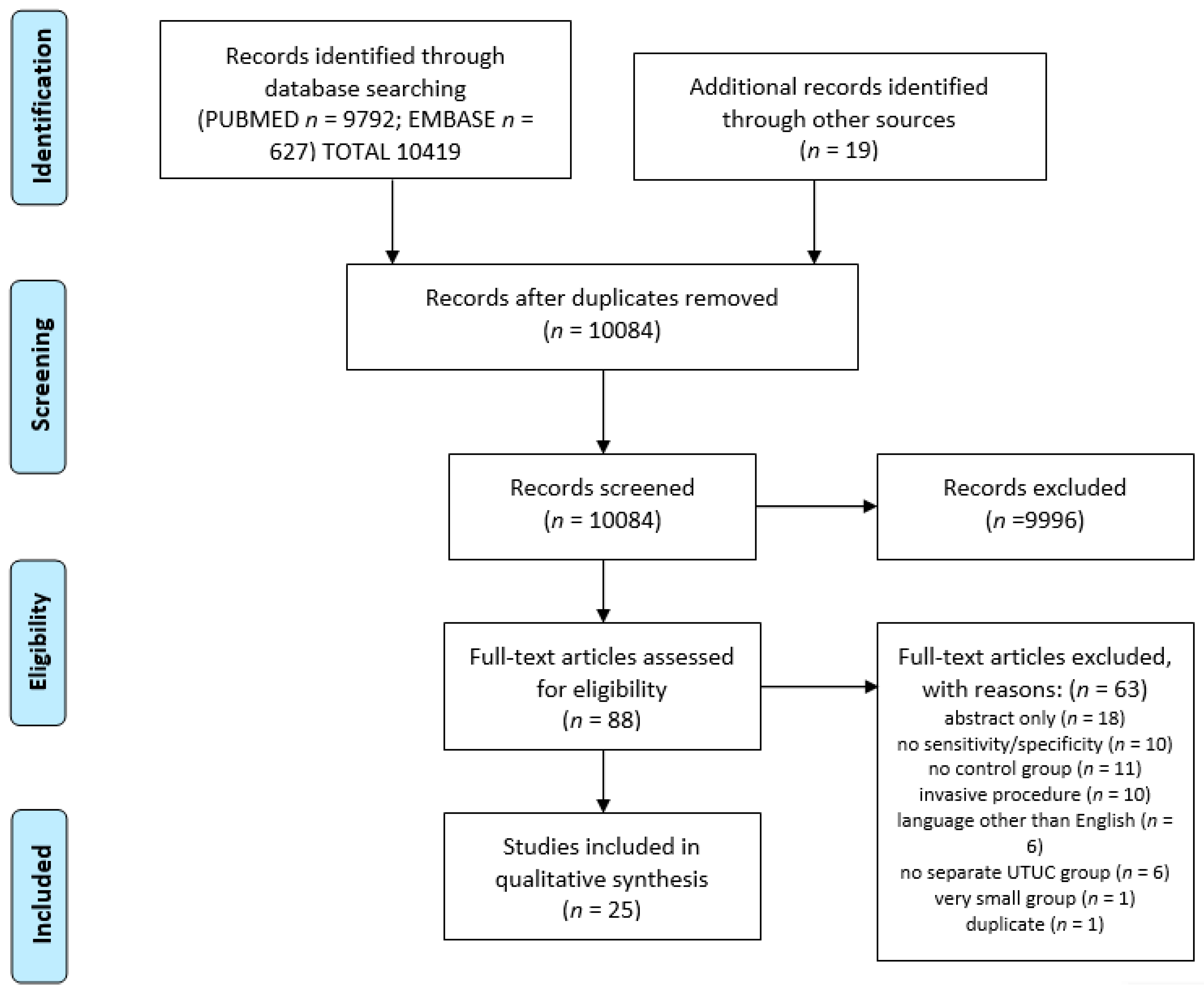
2. Material and Methods
3. Evidence Synthesis
4. Voided Urinary Cytology
5. Fluorescence In Situ Hybridization in Diagnostic Cytology
6. Other Urinary Tests
7. Blood-Based Tests
| TEST | Source | Author | UTUC pts Number | Country | M:F | Tumor Stage | Tumor Grade | Control pts Number | M:F | Control Group Characterization | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| diagnostic N-glycan score | serum | Tanaka [33] | 55 | Japan | n/a | n/a | n/a | 435 | 218:217 | 122 healthy; 96 PCa | 77.10 | 97.20 | 79.70 | 96.80 | |
| miRNA | serum | Kriebel [31] | 44 | Germany | 23:11 | Ta–18; T1–7; T2–3; T3–15; T4–1 | G1–6; G2–28; G3–10 | 34 | 23:11 | BPH, urethral stricture, incontinence, stones | 29.50–84.10 | 29.40–91.20 | n/a | n/a | 0.541–0.726 |
| miRNA | serum | Tao [32] | 58 | China | 41:17 | T1–41; T2–14; T3–20; T4–3 | LG 18; HG–40 | 42 | 30:12 | hematuria | 58.70–97.80 | 70–100 | n/a | n/a | 0.642–0.998 |
| phosphoprotein-1 | plasma | Li [34] | 105 | China | 45:60 | Ta–7; T1–41; T2–23; T3–34 | LG–51; HG–54 | 71 | n/a | 10 healthy; 2 enteritidis; 6 BPH; 27 stones; 15 hydronephrosis; 6 renal cyst; 5 adrenal adenoma | n/a | n/a | n/a | n/a | 0.838 |
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanguedolce, F.; Cormio, L. The complex relationship between upper urinary tract and bladder cancer: Clinical and predictive issues. Transl. Androl. Urol. 2018, 7 (Suppl. S2), S248–S251. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yu, W.; Liu, H.; Ding, F.; Zhang, Y.; Zhang, Y.; Wang, W.; Lou, F.; Cao, S.; Wang, H.; et al. Comparison of Genomic Characterization in Upper Tract Urothelial Carcinoma and Urothelial Carcinoma of the Bladder. Oncologist 2021, 26, e1395–e1405. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Módos, O.; Horváth, A.; Nyirády, P. Why are upper tract urothelial carcinoma two different diseases? Transl. Androl. Urol. 2016, 5, 636–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, J.; Mills, A.M.; Mahadevan, M.S.; Fan, J.; Culp, S.H.; Thomas, M.H.; Cathro, H.P. Universal Lynch Syndrome Screening Should be Performed in All Upper Tract Urothelial Carcinomas. Am. J. Surg. Pathol. 2018, 42, 1549–1555. [Google Scholar] [CrossRef]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.-M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernandez, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef]
- Raman, J.D.; Messer, J.; Sielatycki, J.A.; Hollenbeak, C.S. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011, 107, 1059–1064. [Google Scholar] [CrossRef]
- Raman, J.D.; Shariat, S.F.; Karakiewicz, P.I.; Lotan, Y.; Sagalowsky, A.I.; Roscigno, M.; Montorsi, F.; Bolenz, C.; Weizer, A.Z.; Wheat, J.C.; et al. Does preoperative symptom classification impact prognosis in patients with clinically localized upper-tract urothelial carcinoma managed by radical nephroureterectomy? Urol. Oncol. Semin. Orig. Investig. 2011, 29, 716–723. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Walsh, I.K.; Keane, P.F.; Ishak, L.M.; Flessland, K.A. The BTA stat test: A tumor marker for the detection of upper tract transitional cell carcinoma. Urology 2001, 58, 532–535. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Xia, M.; Yang, S.; Fei, L.; Zhang, M.; Ma, H.; Wang, L.; Chen, S.; Hanyu, M. The combined application of urinary liquid-based cytology with fluorescence in situ hybridization and p16/Ki-67 dual immunostaining is valuable for improving the early diagnosis of upper tract urothelial carcinomas. Diagn. Cytopathol. 2017, 45, 895–902. [Google Scholar] [CrossRef]
- Lodde, M.; Mian, C.; Wiener, H.; Haitel, A.; Pycha, A.; Marberger, M. Detection of upper urinary tract transitional cell carcinoma with ImmunoCyt: A preliminary report. Urology 2001, 58, 362–366. [Google Scholar] [CrossRef]
- Hayashi, Y.; Fujita, K.; Matsuzaki, K.; Matsushita, M.; Kawamura, N.; Koh, Y.; Nakano, K.; Wang, C.; Ishizuya, Y.; Yamamoto, Y.; et al. Diagnostic potential of TERT promoter and FGFR 3 mutations in urinary cell-free DNA in upper tract urothelial carcinoma. Cancer Sci. 2019, 110, 1771–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanovic, M.; Soldatovic, I.; Janjic, A.; Vuksanovic, A.; Džamic, Z.; Acimovic, M.; Hadzi-Djokic, J. Diagnostic Value of the Nuclear Matrix Protein 22 Test and Urine Cytology in Upper Tract Urothelial Tumors. Urol. Int. 2011, 87, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Wu, P.; Zheng, S.; Tan, W.; Zhou, H.; Zuo, Y.; Qi, H.; Zhang, P.; Peng, H.; Wang, Y. Evaluation of upper urinary tract tumors by FISH in Chinese patients. Cancer Genet. Cytogenet. 2010, 203, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Gomella, L.G.; Mann, M.J.; Cleary, R.C.; Hubosky, S.G.; Bagley, D.H.; Thumar, A.B.; McCue, P.A.; Lallas, C.D.; Trabulsi, E.J. Fluorescence in situ hybridization (FISH) in the diagnosis of bladder and upper tract urothelial carcinoma: The largest single-institution experience to date. Can. J. Urol. 2017, 24, 8620–8626. [Google Scholar] [PubMed]
- Akkad, T.; Brunner, A.; Pallwein, L.; Gozzi, C.; Bartsch, G.; Mikuz, G.; Steiner, H.; Verdorfer, I. Fluorescence In Situ Hybridization for Detecting Upper Urinary Tract Tumors—A Preliminary Report. Urology 2007, 70, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, G.; Nakata, W.; Tani, M.; Tsujimura, G.; Tsujimoto, Y.; Nin, M.; Mimura, A.; Miwa, H.; Tsujihata, M. High diagnostic efficacy of 5-aminolevulinic acid-induced fluorescent urine cytology for urothelial carcinoma. Int. J. Clin. Oncol. 2019, 24, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Todenhöfer, T.; Hennenlotter, J.; Tews, V.; Gakis, G.; Aufderklamm, S.; Kuehs, U.; Stenzl, A.; Schwentner, C. Impact of different grades of microscopic hematuria on the performance of urine-based markers for the detection of urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1148–1154. [Google Scholar] [CrossRef]
- Chen, A.A.; Grasso, M. Is There a Role for FISH in the Management and Surveillance of Patients with Upper Tract Transitional-Cell Carcinoma? J. Endourol. 2008, 22, 1371–1374. [Google Scholar] [CrossRef]
- Yu, Q.; Li, Y.; Li, G.; Li, T.; Zeng, H.; Yang, Z.; Wang, D. Prospective evaluation of FISH for detecting upper tract urothelial carcinoma in voided urine specimens. Oncol. Lett. 2016, 12, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Liu, Z.; Liu, L.; Yang, L.; Han, P.; Zhang, P.; Wei, Q. Prospective evaluation of fluorescence in situ hybridization for diagnosing urothelial carcinoma. Oncol. Lett. 2017, 13, 3928–3934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Zeng, Q.; Hou, J.; Gao, L.; Zhang, Z.; Xu, W.; Yang, B.; Sun, Y. Utility of a Modality Combining FISH and Cytology in Upper Tract Urothelial Carcinoma Detection in Voided Urine Samples of Chinese Patients. Urology 2011, 77, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiong, H.; Wei, C.; Cui, Z.; Jin, X.; Zhang, J. Utility of fluorescence in situ hybridization analysis for detecting upper urinary tract-urothelial carcinoma. J. Cancer Res. Ther. 2017, 13, 647. [Google Scholar] [PubMed]
- Marín-Aguilera, M.; Mengual, L.; Ribal, M.J.; Musquera, M.; Ars, E.; Villavicencio, H.; Algaba, F.; Alcaraz, A. Utility of Fluorescence In Situ Hybridization as a Non-invasive Technique in the Diagnosis of Upper Urinary Tract Urothelial Carcinoma. Eur. Urol. 2007, 51, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, W.; Deng, C.-H.; Zheng, F.-F.; Sun, X.-Z.; Wang, D.-H.; Dai, Y.-P. Utility of fluorescence in situ hybridization in the diagnosis of upper urinary tract urothelial carcinoma. Cancer Genet. Cytogenet. 2009, 189, 93–97. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Peng, L.; Tu, P.; Li, W.; Liu, L.; Cheng, W.; Wang, X.; Zhou, S.; Shi, S.; et al. Distinguishing Urothelial Carcinoma in the Upper Urinary Tract from Benign Diseases with Hematuria Using, FISH. Acta Cytol. 2012, 56, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Li, L.Y.; Pang, J.; Ruan, X.X.; Sun, Q.P.; Yang, W.J.; Gao, X. Fluorescence in situ hybridization assay detects upper urinary tract transitional cell carcinoma in patients with asymptomatic hematuria and negative urine cytology. Neoplasma 2012, 59, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Monteiro-Reis, S.; Leça, L.; Almeida, M.; Antunes, L.; Monteiro, P.; Dias, P.C.; Morais, A.; Oliveira, J.; Henrique, R.; Jerónimo, C. Accurate detection of upper tract urothelial carcinoma in tissue and urine by means of quantitative GDF15, TMEFF2 and VIM promoter methylation. Eur. J. Cancer 2014, 50, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.L.; Henrique, R.; Danielsen, S.A.; Duarte-Pereira, S.; Eknaes, M.; Skotheim, R.I.; Rodrigues, Â.; Magalhães, J.S.; Oliveira, J.; Lothe, R.A.; et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 5842–5851. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, M.; Hadzi-Djokic, J.; Dzamic, Z.; Acimovic, M. Evaluation of the bard BTA-test in the diagnosis of upper urinary tract tumours. Acta Chir. Iugosl. 2007, 54, 19–24. [Google Scholar] [CrossRef]
- Kriebel, S.; Schmidt, D.; Holdenrieder, S.; Goltz, D.; Kristiansen, G.; Moritz, R.; Fisang, C.; Müller, S.C.; Ellinger, J. Analysis of Tissue and Serum MicroRNA Expression in Patients with Upper Urinary Tract Urothelial Cancer. PLoS ONE 2015, 10, e0117284. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yang, X.; Li, P.; Wei, J.; Deng, X.; Cheng, Y.; Qin, C.; Ju, X.; Meng, X.; Li, J.; et al. Identification of circulating microRNA signatures for upper tract urothelial carcinoma detection. Mol. Med. Rep. 2015, 12, 6752–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Yoneyama, T.; Noro, D.; Imanishi, K.; Kojima, Y.; Hatakeyama, S.; Tobisawa, Y.; Mori, K.; Yamamoto, H.; Imai, A.; et al. Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas. Int. J. Mol. Sci. 2017, 18, 2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; He, S.; He, A.; Guan, B.; Ge, G.; Zhan, Y.; Wu, Y.; Gong, Y.; Peng, D.; Bao, Z.; et al. Identification of plasma secreted phosphoprotein 1 as a novel biomarker for upper tract urothelial carcinomas. Biomed. Pharm. 2019, 113, 108744. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Janisch, F.; Shariat, S.F.; Baltzer, P.; Fajkovic, H.; Kimura, S.; Iwata, T.; Korn, P.; Yang, L.; Glybochko, P.V.; Rink, M.; et al. Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): A systematic review and meta-analysis. World J. Urol. 2020, 38, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Gallioli, A.; Territo, A.; Mercadé, A.; Fontana, M.; Boissier, R.; Gaya, J.M.; Emiliani, E.; Sánchez-Puy, A.; Martínez, M.J.; Palou, J.; et al. The Impact of Ureteroscopy following Computerized Tomography Urography in the Management of Upper Tract Urothelial Carcinoma. J. Urol. 2021, 205, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Glockner, J.F.; Hartman, R.P.; King, B.F.; Leibovich, B.C.; Stanley, D.W.; Fitz-Gibbon, P.D.; Kawashima, A. Gadolinium enhanced magnetic resonance urography for upper urinary tract malignancy. J. Urol. 2010, 183, 1330–1365. [Google Scholar] [CrossRef]
- Ruvolo, C.C.; Nocera, L.; Stolzenbach, L.F.; Wenzel, M.; Califano, G.; Tian, Z.; Verze, P.; Shariat, S.F.; Saad, F.; Briganti, A.; et al. Tumor Size Predicts Muscle-invasive and Non-organ-confined Disease in Upper Tract Urothelial Carcinoma at Radical Nephroureterectomy. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]
- Voskuilen, C.S.; Schweitzer, D.; Jensen, J.B.; Nielsen, A.M.; Joniau, S.; Muilwijk, T.; Necchi, A.; Azizi, M.; Spiess, P.E.; Briganti, A.; et al. Diagnostic Value of 18F-fluorodeoxyglucose Positron Emission Tomography with Computed Tomography for Lymph Node Staging in Patients with Upper Tract Urothelial Carcinoma. Eur. Urol. Oncol. 2020, 3, 73–79. [Google Scholar] [CrossRef]
- Raica, M.; Mederle, O.; Ioiart, I. Upper urinary tract washing in the diagnosis of transitional cell carcinoma of the renal pelvis and calices. A comparison with voided urine. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 1995, 41, 101–105. [Google Scholar]
- Gelwan, E.; Zhang, M.L.; Allison, D.B.; Cowan, M.L.; DeLuca, J.; Fite, J.J.; Wangsiricharoen, S.; Williamson, B.; Zhou, A.; VandenBussche, C.J. Variability among observers utilizing the CellSolutions BestCyte Cell Sorter imaging system for the assessment of urinary tract cytology specimens. J. Am. Soc. Cytopathol. 2019, 8, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Nojima, S.; Terayama, K.; Shimoura, S.; Hijiki, S.; Nonomura, N.; Morii, E.; Okuno, Y.; Fujita, K. A deep learning system to diagnose the malignant potential of urothelial carcinoma cells in cytology specimens. Cancer Cytopathol. 2021, 129, 984–995. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Weiß, C.; Hoffmann, U.; Borggrefe, M.; Akin, I.; Behnes, M.; Kim, C.W.S.-H. Advantages and Limitations of Current Biomarker Research: From Experimental Research to Clinical Application. Curr. Pharm. Biotechnol. 2017, 18, 445–455. [Google Scholar] [CrossRef] [PubMed]
| Author | UTUC pts Number | Country | M:F | Tumor Stage | Tumor Grade | Control pts Number | M:F | Control Group Characterization | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Walsh [9] | 27 | US | 20:7 | Ta-16-(59.3); T1–7 (25.9); T2–3 (11.1); T3–1 (3.7) | G1–2 (7.4); G2–19 (70.4); G3–6 (22.2) | 54 | 41:13 | 26 stones; 28 hematuria | 11 | 54 | 11 | 55 |
| Sun [10] | 61 | China | 32:29 | n/a | LG-15; HG-46 | 18 | n/a | healthy | 59 | 94.4 | n/a | n/a |
| Lodde [11] | 16 | Austria | n/a | Ta-6; T1–2; T2 > −6 | G1–3; G2–6; G3–7 | 21 | n/a | misc. | 50 | 100 | n/a | n/a |
| Hayashi [12] | 56 | Japan | 46:10 | Ta or Tis-17; T1–11; T2 ± 28 | LG-7; HG-47; unknown-2 | n/a | n/a | hematuria, benign urological disorders, negative UC surveillance | 44.6 | 96 | 92.6 | 60.8 |
| Jovanovic [13] | 34 | Serbia | 22:12 | Ta-8; T1–8; T2–4; T3–13; T4–1 | G1–7; G2–15; G3–12 | 25 | 08:14 | stones | 58.8 | 96 | 95.2 | 63.1 |
| Shan [14] | 50 | China | 32:18 | Ta-6; Tis–3; T1–9; T2–9; T3–22; T4–1 | G1–10; G2–13; G3–27 | 25 | 16:9 | healthy | 40 | 96 | n/a | n/a |
| Gomella [15] | 147 | US | n/a | n/a | n/a | 92 | n/a | n/a | 42.2 | 90.2 | 87.3 | 49.4 |
| Akkad [16] | 9 | Austria | 5:4 | Ta-2; Tis–1; T1–1; T2–1; T3–4 | G2–5; G3–4 | 7 | 3:4 | suspected for UTUC but T0 | 60 | 80 | 75 | 66 |
| Yamamichi [17] | 76 | Japan | n/a | n/a | n/a | 158 | n/a | 117 BPH; 18 stones; 17 infection; 6 other | 71.1 | 95.6 | 88.5 | 87.3 |
| Todenhoefer [18] | 62 | Germany | n/a | n/a | n/a | n/a | n/a | hematuria | 67.7 | 82.8 | n/a | n/a |
| Chen [19] | 23 | US | n/a | n/a | LG-15; HG-8 | 5 | n/a | n/a | 13 | 80 | n/a | n/a |
| Yu [20] | 19 | China | 15:4 | Ta-6; Tis–3; T1–5; T2–5; | G1–7; G2–8; G3–4 | 98 | n/a | suspected for UTUC but T0 | 42.1 | 94.9 | 100 | 90.3 |
| Lin [21] | 19 | China | n/a | n/a | n/a | 46 | n/a | suspected for UTUC but T0 | 31.6 | 100 | n/a | n/a |
| Xu [22] | 71 | China | 54:17 | Ta-34; T1–12; T2–17; T3–6; T4–2 | LG-39; HG-32 | 45 | n/a | 15 UTI; 20 stones; 10 healthy | 45.1 | 100 | n/a | n/a |
| Yu [23] | 44 | China | 25:19 | Ta-1; T1-6; T2–24; T3–7; T4–5 | LG-14; HG-30 | 26 | n/a | stones or BPH | 27.3 | 100 | n/a | n/a |
| Marin-Aguilera [24] | 30 | Spain | 25:5 | Ta-5; Tis–1; T1–5; T2–5; T3–10; Tx-4 | LG-5; HG-25 | 19 | n/a | healthy | 36 | 100 | n/a | n/a |
| Luo [25] | 21 | China | 12:9 | Ta-3; T1–2; T2–7; T3–7; T4–2 | LG-8; HG-13 | 10 | n/a | healthy | 23.8 | 100 | n/a | n/a |
| Author | UTUC pts Number | Country | M:F | Tumor Stage | Tumor Grade | Control pts Number | M:F | Control Group Characterization | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun [10] | 61 | China | 32:29 | n/a | LG-15; HG-46 | 10 | n/a | healthy | 67.2 | 90 | n/a | n/a |
| Wang [26] | 34 | China | 26:8 | Ta-1; T1–15; T2–6; T3-8; T4-4 | LG-14; HG-20 | 33 | 21:12 | hematuria | 73.5 | 93.9 | 92.6 | 77.5 |
| Shan [14] | 50 | China | 32:18 | Ta–6; Tis–3; T1–9; T2–9; T3–22; T4–1 | G1-10; G2-13; G3-27 | 25 | 16:9 | healthy | 84 | 96 | n/a | n/a |
| Gomella [15] | 52 | US | n/a | n/a | n/a | 28 | n/a | n/a | 51.9 | 89.3 | 90 | 50 |
| Huang [27] | 9 | China | 8:1 | T1–4; T2–4; T3–1 | G1-1; G2-2; G3-6 | 276 | 140:136 | asymptomatic hematuria and negative VUC | 100 | 99.3 | 81.8 | 100 |
| Akkad [16] | 9 | Austria | 5:4 | Ta–2; Tis–1; T1–1; T2–1; T3–4 | G2-5; G3-4 | 7 | 3:4 | suspected for UTUC but T0 | 87.5 | 80 | 87.5 | 80 |
| Todenhoefer [18] | 26 | Germany | n/a | n/a | n/a | n/a | n/a | hematuria | 61.5 | 80.1 | n/a | n/a |
| Chen [19] | 20 | US | n/a | n/a | LG-14; HG-6 | 5 | n/a | n/a | 35 | 80 | n/a | n/a |
| Yu [20] | 19 | China | 15:4 | Ta–6; Tis–3; T1–5; T2–5; | G1-7; G2-8; G3-4 | 98 | n/a | suspected for UTUC but T0 | 84.2 | 89.8 | 80 | 97.8 |
| Lin [21] | 19 | China | n/a | n/a | n/a | 46 | n/a | suspected for UTUC but T0 | 73.7 | 89.1 | n/a | n/a |
| Xu [22] | 71 | China | 54:17 | Ta–34; T1–12; T2–17; T3–6; T4–2 | LG-39; HG-32 | 45 | n/a | 15 UTI; 20 stones; 10 healthy | 78.9 | 97.8 | n/a | n/a |
| Yu [23] | 44 | China | 25:19 | Ta–1; T1–6; T2–24; T3–7; T4–5 | LG-14; HG-30 | 26 | n/a | stones or BPH | 79.5 | 96.2 | n/a | n/a |
| Marin-Aguilera [24] | 30 | Spain | 25:5 | Ta–5; Tis–1; T1–5; T2–5; T3–10; Tx–4 | LG-5; HG-25 | 19 | n/a | healthy | 76.7 | 94.7 | n/a | n/a |
| Luo [25] | 21 | China | 12:9 | Ta–3; T1–2; T2–7; T3–7; T4–2 | LG-8; HG-13 | 10 | n/a | healthy | 85.7 | 100 | n/a | n/a |
| TEST | Author | UTUC pts Number | Country | M:F | Tumor Stage | Tumor Grade | Control pts Number | M:F | Control Group Characterization | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BTA stat | Walsh [9] | 27 | US | 20:07 | Ta–16- (59.3); T1–7 (25.9); T2–3 (11.1); T3–1 (3.7) | G1–2 (7.4); G2–19 (70.4); G3-6 (22.2) | 54 | 41:13:00 | 26 stones; 28 hematuria | 82 | 89 | 79 | 91 |
| NMP22 | Jovanovic [13] | 34 | Serbia | 22:12 | Ta–8; T1–8; T2–4; T3–13; T4–1 | G1–7; G2–15; G3–12 | 25 | 08:14 | stones | 70.5 | 92 | 92.3 | 69.6 |
| NMP22 | Todenhoefer [18] | 20 | Germany | n/a | n/a | n/a | n/a | n/a | hematuria | 70 | 43.2 | n/a | n/a |
| uCyt | Lodde [11] | 16 | Austria | n/a | Ta–6; T1–2; T2 > −6 | G1–3; G2–6; G3–7 | 21 | n/a | misc. | 75 | 95 | n/a | n/a |
| uCyt | Todenhoefer [18] | 9 | Germany | n/a | n/a | n/a | n/a | n/a | hematuria | 55.6 | 79.2 | n/a | n/a |
| VIM/GDF15/TMEFF2 methylation panel | Monteiro-Reis [28] | 22 | Portugal | 12:10 | Ta–15; T1 ± 7 | LG–3; HG–19 | 20 | 11:09 | 3 healthy; 10 RCC; 7 PCa | 91 | 100 | 100 | 91 |
| p16/Ki-67 dual immunolabelling | Sun [10] | 32 | China | 16:16 | n/a | LG–8; HG–24 | 9 | n/a | healthy | 53.1 | 100 | n/a | n/a |
| FGFR3 mutation | Hayashi [12] | 56 | Japan | 46:10:00 | Ta or Tis–17; T1–11; T2 ± 28 | LG–7; HG–47; unknown-2 | n/a | n/a | hematuria, benign urological disorders, negative UC surveillance | 16.1 | 100 | 100 | 51.5 |
| TERT promoter + FGFR3 | Hayashi [12] | 56 | Japan | 46:10:00 | Ta or Tis–17; T1–11; T2 ± 28 | LG–7; HG–47; unknown-2 | n/a | n/a | hematuria, benign urological disorders, negative UC surveillance | 55.4 | 100 | 100 | 66.7 |
| TERT promoter mutation | Hayashi [12] | 56 | Japan | 46:10:00 | Ta or Tis–17; T1–11; T2 ± 28 | LG–7; HG–47; unknown-2 | n/a | n/a | hematuria, benign urological disorders, negative UC surveillance | 46.4 | 100 | 100 | 62.5 |
| VUC + TERT + FGFR3 | Hayashi [12] | 56 | Japan | 46:11:00 | Ta or Tis–17; T1–11; T2 ± 29 | LG–7; HG–47; unknown-2 | n/a | n/a | hematuria, benign urological disorders, negative UC surveillance | 78.6 | 96 | 95.7 | 80 |
| BTA bard + VUC | Jovanovic [30] | 35 | Serbia | 22:13 | n/a | n/a | 35 | n/a | stones | 81.3 | 73.3 | 76.5 | 83.3 |
| 5-ALA VUC | Yamamichi [17] | 76 | Japan | n/a | n/a | n/a | 158 | n/a | 117-BPH; 18-stones; 17-infection; 6-other | 90.8 | 96.2 | 92 | 95.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białek, Ł.; Bilski, K.; Dobruch, J.; Krajewski, W.; Szydełko, T.; Kryst, P.; Poletajew, S. Non-Invasive Biomarkers in the Diagnosis of Upper Urinary Tract Urothelial Carcinoma—A Systematic Review. Cancers 2022, 14, 1520. https://doi.org/10.3390/cancers14061520
Białek Ł, Bilski K, Dobruch J, Krajewski W, Szydełko T, Kryst P, Poletajew S. Non-Invasive Biomarkers in the Diagnosis of Upper Urinary Tract Urothelial Carcinoma—A Systematic Review. Cancers. 2022; 14(6):1520. https://doi.org/10.3390/cancers14061520
Chicago/Turabian StyleBiałek, Łukasz, Konrad Bilski, Jakub Dobruch, Wojciech Krajewski, Tomasz Szydełko, Piotr Kryst, and Sławomir Poletajew. 2022. "Non-Invasive Biomarkers in the Diagnosis of Upper Urinary Tract Urothelial Carcinoma—A Systematic Review" Cancers 14, no. 6: 1520. https://doi.org/10.3390/cancers14061520
APA StyleBiałek, Ł., Bilski, K., Dobruch, J., Krajewski, W., Szydełko, T., Kryst, P., & Poletajew, S. (2022). Non-Invasive Biomarkers in the Diagnosis of Upper Urinary Tract Urothelial Carcinoma—A Systematic Review. Cancers, 14(6), 1520. https://doi.org/10.3390/cancers14061520







