Decisional Conflict after Deciding on Potential Participation in Early Phase Clinical Cancer Trials: Dependent on Global Health Status, Satisfaction with Communication, and Timing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
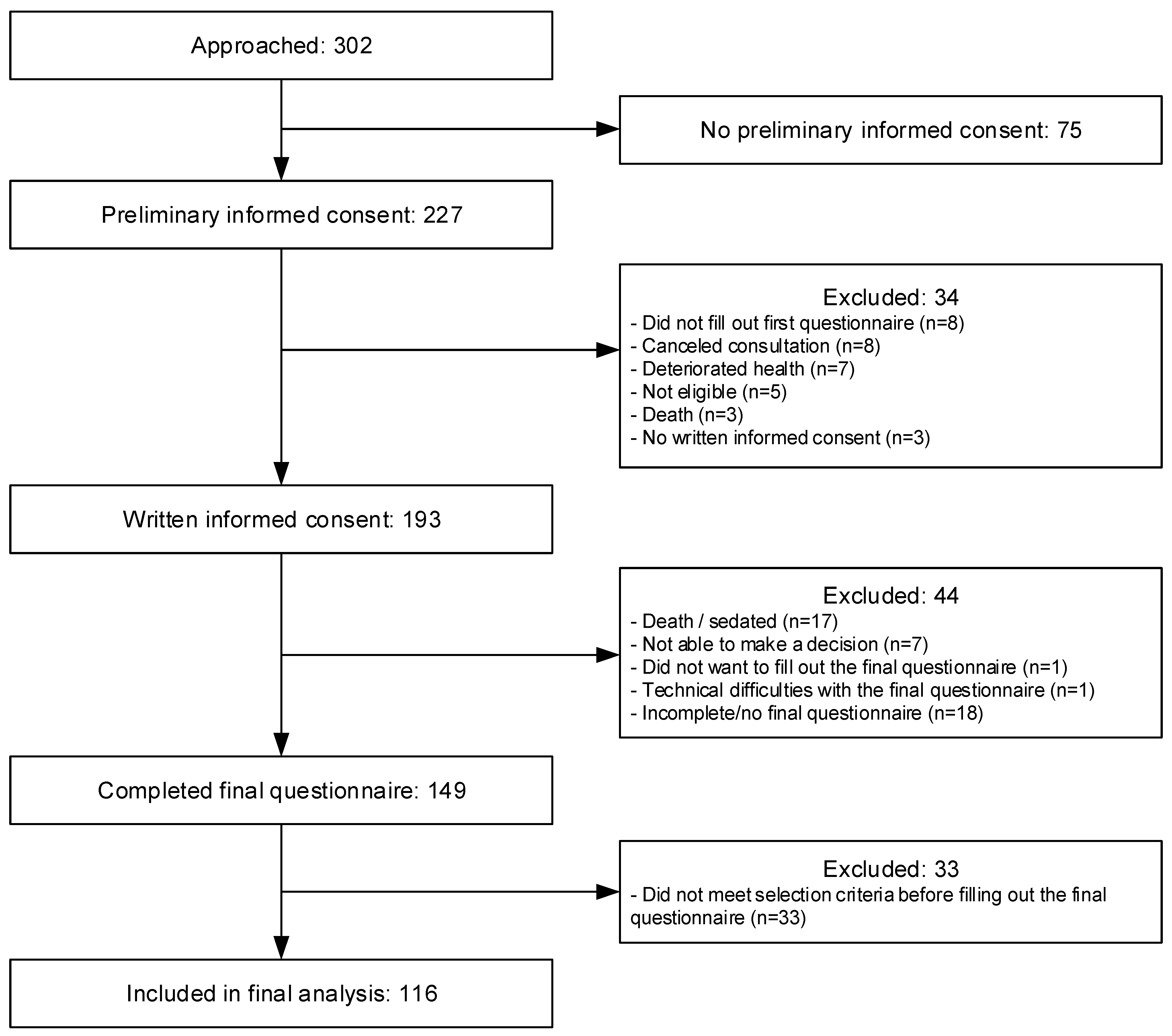
2.2. Participants
2.3. Measurements
2.4. Procedure
2.5. Statistical Analysis
3. Results
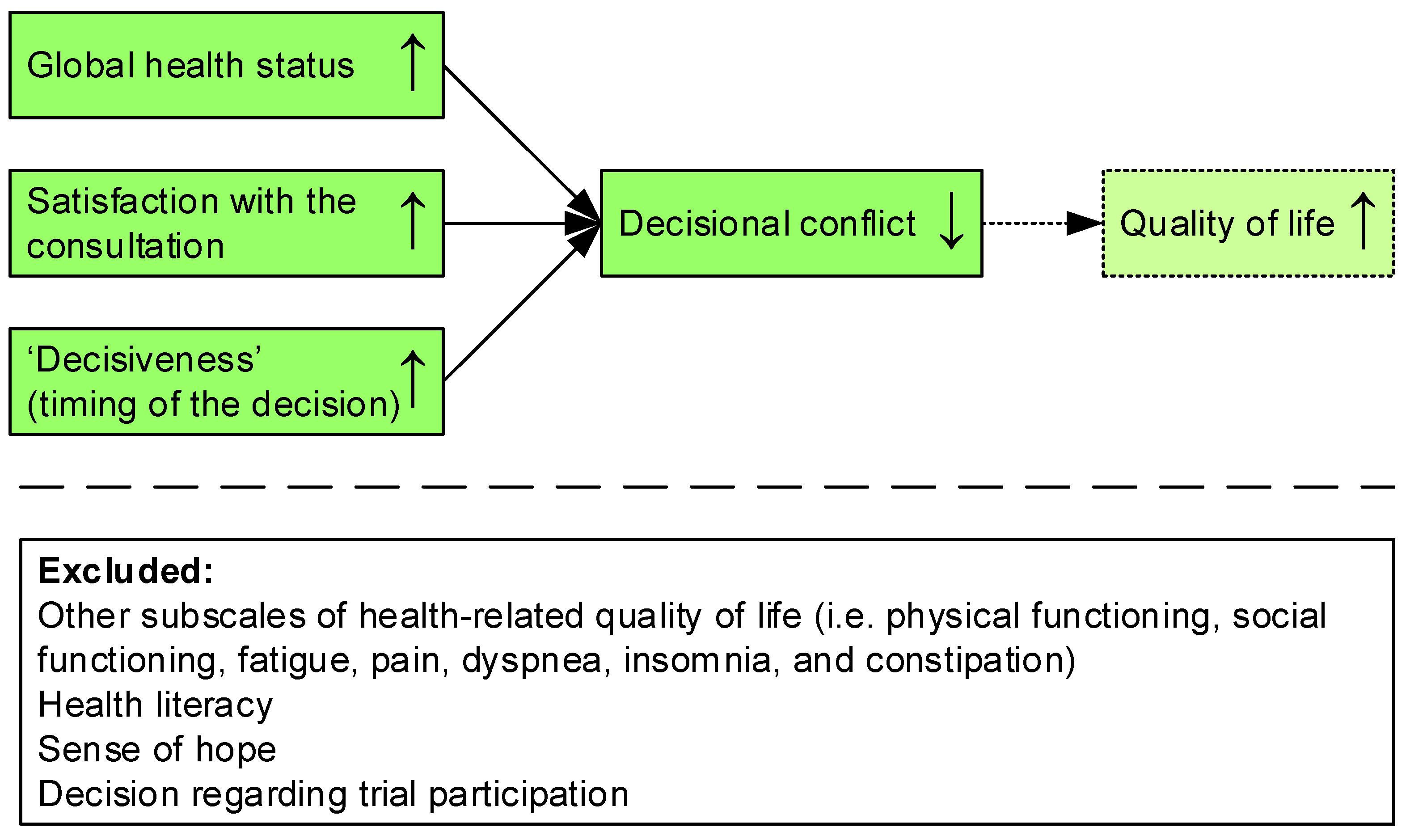
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Lent, L.G.G.; Jabbarian, L.J.; van Gurp, J.; Hasselaar, J.; Lolkema, M.P.; van Weert, J.C.M.; van der Rijt, C.C.D.; de Jonge, M.J.A. Identifying patient values impacting the decision whether to participate in early phase clinical cancer trials: A systematic review. Cancer Treat. Rev. 2021, 98, 102217. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.; Kaplan, S.; Ware, J.E. Expanding patient involvement in care: Effects on patient outcomes. Ann. Intern. Med. 1985, 102, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.; Kaplan, S.H.; Ware, J.E.; Yano, E.M.; Frank, H.J.L. Patients’ participation in medical care. J. Gen. Intern. Med. 1988, 3, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Sepucha, K.R.; Borkhoff, C.M.; Lally, J.; Levin, C.A.; Matlock, D.D.; Ng, C.J.; Ropka, M.E.; Stacey, D.; Joseph-Williams, N.; Wills, C.E.; et al. Establishing the effectiveness of patient decision aids: Key constructs and measurement instruments. BMC Med. Inform. Decis. Mak. 2013, 13, S12. [Google Scholar] [CrossRef] [PubMed]
- Garvelink, M.M.; Boland, L.; Klein, K.; Nguyen, D.V.; Menear, M.; Bekker, H.L.; Eden, K.B.; LeBlanc, A.; O’Connor, A.M.; Stacey, D. Decisional conflict scale findings among patients and surrogates making health decisions: Part II of an anniversary review. Med. Decis. Mak. 2019, 39, 316–327. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.M. Validation of a decisional conflict scale. Med. Decis. Mak. 1995, 15, 25–30. [Google Scholar] [CrossRef]
- O’Connor, A.M. User Manual: Decisional Conflict Scale. Available online: https://decisionaid.ohri.ca/eval_dcs.html (accessed on 29 March 2021).
- Garvelink, M.M.; Boland, L.; Klein, K.; Nguyen, D.V.; Menear, M.; Bekker, H.L.; Eden, K.B.; LeBlanc, A.; O’Connor, A.M.; Stacey, D. Decisional Conflict Scale use over 20 years: The anniversary review. Med. Decis. Mak. 2019, 39, 301–314. [Google Scholar] [CrossRef]
- Flynn, K.E.; Weinfurt, K.P.; Seils, D.M.; Lin, L.; Burnett, C.B.; Schulman, K.A.; Meropol, N.J. Decisional conflict among patients who accept or decline participation in phase I oncology studies. J. Empir. Res. Hum. Res. Ethics 2008, 3, 69–77. [Google Scholar] [CrossRef]
- Kates, J.M. Treatment-related Decisional Conflict, Quality of Life, and Comorbidity in Older Adults with Cancer. Asia-Pac. J. Oncol. Nurs. 2018, 5, 421. [Google Scholar] [CrossRef]
- Woudstra, A.J.; Smets, E.M.A.; Verdam, M.G.E.; Fransen, M.P. The Role of Health Literacy in Explaining the Relation between Educational Level and Decision Making about Colorectal Cancer Screening. Int. J. Environ. Res. Public Health 2019, 16, 4644. [Google Scholar] [CrossRef]
- Catt, S.; Langridge, C.; Fallowfield, L.; Talbot, D.C.; Jenkins, V. Reasons given by patients for participating, or not, in Phase 1 cancer trials. Eur. J. Cancer 2011, 47, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Nurgat, Z.A.; Craig, W.; Campbell, N.C.; Bissett, J.D.; Cassidy, J.; Nicolson, M.C. Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br. J. Cancer 2005, 92, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Lis, C.G.; Rodeghier, M.; Gupta, D. Distribution and determinants of patient satisfaction in oncology: A review of the literature. Patient Prefer. Adherence 2009, 3, 287. [Google Scholar] [PubMed]
- van Lent, L.G.G.; Stoel, N.K.; van Weert, J.C.M.; van Gurp, J.; de Jonge, M.J.A.; Lolkema, M.P.; Gort, E.H.; Pulleman, S.M.; Oomen-de Hoop, E.; Hasselaar, J. Realizing better doctor-patient dialogue about choices in palliative care and early phase clinical trial participation: Towards an online value clarification tool (OnVaCT). BMC Palliat. Care 2019, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Koedoot, N.; Molenaar, S.; Oosterveld, P.; Bakker, P.; de Graeff, A.; Nooy, M.; Varekamp, I.; de Haes, H. The decisional conflict scale: Further validation in two samples of Dutch oncology patients. Patient Educ. Couns. 2001, 45, 187–193. [Google Scholar] [CrossRef]
- Bjordal, K.; De Graeff, A.; Fayers, P.M.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.J.; Meyza, J.W.; Bredart, A. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Chew, L.D.; Bradley, K.A.; Boyko, E.J. Brief questions to identify patients with inadequate health literacy. Health 2004, 11, 12. [Google Scholar]
- Fransen, M.P.; Van Schaik, T.M.; Twickler, T.B.; Essink-Bot, M.L. Applicability of internationally available health literacy measures in the Netherlands. J. Health Commun. 2011, 16, 134–149. [Google Scholar] [CrossRef]
- Schlatmann, F.W.M.; Hofmeester, I.; van Balken, M.R. Met “Ik geef u onze folder mee” heeft een op de tien nóg geen idee. Tijdschr. Voor Urol. 2016, 6, 94–96. [Google Scholar] [CrossRef][Green Version]
- Herth, K. Abbreviated instrument to measure hope: Development and psychometric evaluation. J. Adv. Nurs. 1992, 17, 1251–1259. [Google Scholar] [CrossRef]
- Van Gestel-Timmermans, H.; Van Den Bogaard, J.; Brouwers, E.; Herth, K.; Van Nieuwenhuizen, C. Hope as a determinant of mental health recovery: A psychometric evaluation of the Herth Hope Index-Dutch version. Scand. J. Caring Sci. 2010, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zandbelt, L.C.; Smets, E.M.A.; Oort, F.J.; Godfried, M.H.; de Haes, H.C.J.M. Determinants of physicians’ patient-centred behaviour in the medical specialist encounter. Soc. Sci. Med. 2006, 63, 899–910. [Google Scholar] [CrossRef] [PubMed]
- van der Biessen, D.A.; van der Helm, P.G.; Klein, D.; van der Burg, S.; Mathijssen, R.H.; Lolkema, M.P.; de Jonge, M.J. Understanding how coping strategies and quality of life maintain hope in patients deliberating phase I trial participation. Psycho-Oncology 2018, 27, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Mead, N.; Bower, P. Patient-centred consultations and outcomes in primary care: A review of the literature. Patient Educ. Couns. 2002, 48, 51–61. [Google Scholar] [CrossRef]
- Janssen, S.M.; Lagro-Janssen, A.L.M. Physician’s gender, communication style, patient preferences and patient satisfaction in gynecology and obstetrics: A systematic review. Patient Educ. Couns. 2012, 89, 221–226. [Google Scholar] [CrossRef]
- Trice, E.D.; Prigerson, H.G. Communication in end-stage cancer: Review of the literature and future research. J. Health Commun. 2009, 14, 95–108. [Google Scholar] [CrossRef]
- Thorne, S.; Hislop, T.G.; Kim-Sing, C.; Oglov, V.; Oliffe, J.L.; Stajduhar, K.I. Changing communication needs and preferences across the cancer care trajectory: Insights from the patient perspective. Support. Care Cancer 2014, 22, 1009–1015. [Google Scholar] [CrossRef]
- Jenkins, V.; Solis-Trapala, I.; Langridge, C.; Catt, S.; Talbot, D.C.; Fallowfield, L.J. What oncologists believe they said and what patients believe they heard: An analysis of phase I trial discussions. J. Clin. Oncol. 2010, 29, 61–68. [Google Scholar] [CrossRef]
- Brown, R.; Bylund, C.L.; Siminoff, L.A.; Slovin, S.F. Seeking informed consent to phase I cancer clinical trials: Identifying oncologists’ communication strategies. Psycho-Oncology 2011, 20, 361–368. [Google Scholar] [CrossRef]
- Scott, N.W.; Fayers, P.; Aaronson, N.K.; Bottomley, A.; de Graeff, A.; Groenvold, M.; Gundy, C.; Koller, M.; Petersen, M.A.; Sprangers, M.A.G. EORTC QLQ-C30 Reference Values Manual. Available online: https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf (accessed on 24 August 2021).
- Heijmans, M.; Brabers, A.E.M.; Rademakers, J. Health Literacy in the Netherlands (Dutch Title: Health literacy in Nederland). Available online: https://www.nivel.nl/nl/publicatie/health-literacy-nederland (accessed on 24 August 2021).
- CBS. How Many People with a Migration Background Do There Live in the Netherlands? (Dutch Title: Hoeveel Mensen Met een Migratieachtergrond Wonen in Nederland?). Available online: https://www.cbs.nl/nl-nl/dossier/dossier-asiel-migratie-en-integratie/hoeveel-mensen-met-een-migratieachtergrond-wonen-in-nederland-#:~:text=Van%20de%20totale%20Nederlandse%20bevolking,daarmee%20tot%20de%20tweede%20generatie (accessed on 24 August 2021).
- Diviani, N.; van den Putte, B.; Giani, S.; van Weert, J.C.M. Low health literacy and evaluation of online health information: A systematic review of the literature. J. Med. Internet Res. 2015, 17, e112. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, S.H. Simplification improves understanding of informed consent information in clinical trials regardless of health literacy level. Clin. Trials 2015, 12, 232–236. [Google Scholar] [CrossRef] [PubMed]
- McClement, S.E.; Chochinov, H.M. Hope in advanced cancer patients. Eur. J. Cancer 2008, 44, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
| Outcome | Instrument |
|---|---|
| Primary outcome (final questionnaire) | |
| Decisional conflict | The validated Dutch version [16] of the Decisional Conflict Scale (DCS) [6,7] was used. The DCS consists of 16 items on a 5-point Likert scale (0 = strongly agree, 4 = strongly disagree) within the informed (3 items), values clarity (3 items), support (3 items), uncertainty (3 items), and effective decision (4 items) subscales. In line with the user manual [7], all items were summed, divided by 16, and multiplied by 25 to get a total score for decisional conflict on a 0–100 scale. Scores below 25 are associated with being able to implement a decision and those above 37.5 with decision delay. |
| Baseline measurements (baseline questionnaire) | |
| Quality of life | Quality of life was assessed with the QLQ-C30 version 3.0 [17] in Dutch of the European Organization for Research and Treatment of Cancer (EORTC). The QLQ-C30 consists of subscales/items for global health status (2 items), physical functioning (5 items), role functioning (2 items), emotional functioning (4 items), cognitive functioning (2 items), social functioning (2 items), fatigue (3 items), nausea and vomiting (2 items), pain (2 items), dyspnea (1 item), insomnia (1 item), appetite loss (1 item), constipation (1 item), diarrhea (1 item), and financial difficulties (1 item). Global health status was assessed on a 7-point Likert scale (1–7), whereas all functional and symptom scales/items were assessed on a 4-point Likert scale (1–4). In line with the EORTC manual [18], linear transformation was applied to obtain a score between 0–100 for all scales and items. |
| Health literacy | Patient ability to perform the basic reading and numerical tasks required to function in the health care environment was assessed using the 3-item Set of Brief Screening Questions (SBSQ) [19] on a 5-point Likert scale (1–5). We used the previously translated Dutch version (SBSQ-D) [20,21]. Based on previous literature, health literacy was considered low if the mean of the three items was ≤2, and adequate for >2. |
| Sense of hope | The Herth Hope Index (HHI) [22] was used to measure a global non-time-oriented sense of hope. The 12 items have a 4-point Likert scale (1–4) and were previously translated and authorized in Dutch [23]. Since it was advised for the Dutch version to use the scale as a whole rather than using subscales, the items were summed to a total score within a range between 12–48. |
| Other measurements (final questionnaire) | |
| Satisfaction with the communication | Satisfaction with the consultation was measured with one question (“How satisfied were you with the initial consultation?”) that could be assessed on a 7-point Likert-scale (1 = completely unsatisfied, 7 = completely satisfied). |
| Timing of the decision | Timing of the decision was measured with one question (“When did you approximately decide to participate or not in an early phase clinical trial?”) with 5 answer possibilities (1 = before initial consultation, 2 = within 1 week after the initial consultation, 3 = within 1–2 weeks after the initial consultation, 4 = more than 2 weeks after the initial consultation, 5 = not yet). For the analysis, the latter two possibilities were combined. |
| Patient Characteristics | M (SD) or n (%) |
|---|---|
| Gender, n (%) | |
| Female | 38 (32.8) |
| Male | 78 (67.2) |
| Age, M (SD) | 62.5 (8.8) |
| Education level, n (%) | |
| Low (no education to lowest level of secondary education) | 29 (25.0) |
| Middle (senior general secondary and pre-university education) | 46 (39.7) |
| High (higher vocational education and university) | 41 (35.3) |
| Nationality, n (%) | |
| Dutch | 115 (99.1) |
| Other | 1 (0.9) |
| Living situation, n (%) | |
| Alone | 12 (10.3) |
| With partner | 73 (62.9) |
| With partner and child(ren) | 27 (23.3) |
| With child(ren) or other relative(s) | 4 (3.4) |
| Working situation, n (%) | |
| Paid job | 32 (27.6) |
| No job (anymore) | 66 (56.9) |
| In health insurance act | 10 (8.6) |
| Other (e.g., voluntary work) | 8 (6.9) |
| Hospital, n (%) | |
| Erasmus MC | 84 (72.4) |
| Netherlands Cancer Institute | 23 (19.8) |
| UMC Utrecht | 9 (7.8) |
| WHO performance status at initial consultation, n (%) | |
| 0 | 27 (23.3) |
| 1 or 2 | 80 (69.0) |
| Missing/unknown | 9 (7.8) |
| Primary diagnosis, n (%) | |
| Colorectal/anal cancer | 35 (30.2) |
| Esophageal/stomach cancer | 11 (9.5) |
| Hepatobiliary/pancreatic cancer | 20 (17.2) |
| Gynecological cancer | 6 (5.2) |
| Lung cancer/mesothelioma | 8 (6.9) |
| Urinary tract cancer | 26 (22.4) |
| Breast cancer | 3 (2.6) |
| Melanoma/skin cancer | 3 (2.6) |
| Other | 4 (3.4) |
| Metastases, n (%) | |
| Yes | 110 (94.8) |
| No | 6 (5.2) |
| Number of previous lines of therapy, M (SD) | 2.6 (1.6) |
| Missing/unknown, n (%) | 6 (5.2) |
| Participated in another (phase II/III) clinical trial, n (%) | |
| Yes | 26 (22.4) |
| No | 90 (77.6) |
| Patient Measurements | M (SD) or n (%) | p-Value |
|---|---|---|
| Decisional conflict (DCS), M (SD) | ||
| Informed subscale | 31.5 (21.3) | N/A |
| Values clarity subscale | 36.6 (20.6) | N/A |
| Support subscale | 24.2 (18.1) | N/A |
| Uncertainty subscale | 36.6 (24.9) | N/A |
| Effective decision making subscale | 23.3 (20.4) | N/A |
| Total decisional conflict | 30.0 (16.9) | N/A |
| Quality of life (QLQ-C30), M (SD) | ||
| Global health status | ||
| Global health status | 67.4 (18.4) | <0.001 ‡ |
| Functional scales | ||
| Physical functioning | 80.1 (16.8) | 0.052 ‡ |
| Role functioning | 68.4 (24.6) | 0.105 ‡ |
| Emotional functioning | 76.3 (18.6) | 0.190 ‡ |
| Cognitive functioning | 87.2 (18.8) | 0.377 ‡ |
| Social functioning | 78.7 (23.8) | 0.031 ‡ |
| Symptom scales/items | ||
| Fatigue | 28.6 (20.8) | 0.025 ‡ |
| Nausea and vomiting | 8.2 (15.5) | 0.822 ‡ |
| Pain | 22.4 (24.5) | 0.038 ‡ |
| Dyspnea | 13.5 (21.1) | 0.077 ‡ |
| Insomnia | 19.3 (26.8) | 0.099‡ |
| Appetite loss | 17.8 (25.4) | 0.655 ‡ |
| Constipation | 12.9 (24.0) | 0.021 ‡ |
| Diarrhea | 11.5 (19.2) | 0.869 ‡ |
| Financial difficulties | 7.2 (16.9) | 0.334 ‡ |
| Health literacy, M (SD) | 4.5 (0.6) | 0.023 ‡ |
| Sense of Hope (HHI), M (SD) | 36.8 (4.9) | 0.003 ‡ |
| Satisfaction with the consultation, M (SD) | 6.0 (1.3) | <0.001 ‡ |
| Missing, n (%) | 1 (0.7) | |
| Decision regarding trial participation, n (%) | 0.036 ┴ | |
| Decided to further consider participation | 77 (66.4) | |
| Decided to not further consider participation (and not to participate) | 39 (33.6) | |
| Timing of the decision, n (%) | <0.001 † | |
| Before initial consultation | 38 (32.8) | |
| Within 1 week after the initial consultation | 47 (40.5) | |
| 1–2 weeks after the initial consultation | 9 (7.8) | |
| More than 2 weeks after the initial consultation | 22 (19.0) |
| DCS Score M (SD) | |
|---|---|
| Decision regarding trial participation | |
| Decided to further consider participation | 27.7 (17.1) |
| Decided to not further consider participation (and not to participate) | 34.6 (15.7) |
| Timing of the decision | |
| Before initial consultation | 22.4 (17.0) |
| Within 1 week after the initial consultation | 26.3 (14.1) |
| 1–2 weeks after the initial consultation | 43.1 (7.5) |
| More than 2 weeks after the initial consultation | 45.8 (11.0) |
| F | df | Adjusted R2 | p-Value | |
|---|---|---|---|---|
| Model statistics | 14.532 | (5, 110) | 0.370 | <0.001 |
| b | 95% CI | β | p-value | |
| Global health status | −0.170 | (−0.311, −0.030) | −0.185 | 0.018 |
| Satisfaction with the consultation | −3.153 | (−5.117, −1.189) | −0.246 | 0.002 |
| Timing of the decision * | ||||
| Before initial consultation | −19.445 | (−26.812, −12.079) | −0.543 | <0.001 |
| Within 1 week after the initial consultation | −14.644 | (−21.900, −7.388) | −0.427 | <0.001 |
| 1–2 weeks after the initial consultation | −2.047 | (−12.588, 8.494) | −0.033 | 0.701 |
| Constant | 72.798 | (58.826, 86.769) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Lent, L.G.G.; de Jonge, M.J.A.; van der Ham, M.; van Mil, M.; Gort, E.H.; Hasselaar, J.; Oomen-de Hoop, E.; van der Rijt, C.C.D.; van Weert, J.C.M.; Lolkema, M.P. Decisional Conflict after Deciding on Potential Participation in Early Phase Clinical Cancer Trials: Dependent on Global Health Status, Satisfaction with Communication, and Timing. Cancers 2022, 14, 1500. https://doi.org/10.3390/cancers14061500
van Lent LGG, de Jonge MJA, van der Ham M, van Mil M, Gort EH, Hasselaar J, Oomen-de Hoop E, van der Rijt CCD, van Weert JCM, Lolkema MP. Decisional Conflict after Deciding on Potential Participation in Early Phase Clinical Cancer Trials: Dependent on Global Health Status, Satisfaction with Communication, and Timing. Cancers. 2022; 14(6):1500. https://doi.org/10.3390/cancers14061500
Chicago/Turabian Stylevan Lent, Liza G. G., Maja J. A. de Jonge, Mirte van der Ham, Marjolein van Mil, Eelke H. Gort, Jeroen Hasselaar, Esther Oomen-de Hoop, Carin C. D. van der Rijt, Julia C. M. van Weert, and Martijn P. Lolkema. 2022. "Decisional Conflict after Deciding on Potential Participation in Early Phase Clinical Cancer Trials: Dependent on Global Health Status, Satisfaction with Communication, and Timing" Cancers 14, no. 6: 1500. https://doi.org/10.3390/cancers14061500
APA Stylevan Lent, L. G. G., de Jonge, M. J. A., van der Ham, M., van Mil, M., Gort, E. H., Hasselaar, J., Oomen-de Hoop, E., van der Rijt, C. C. D., van Weert, J. C. M., & Lolkema, M. P. (2022). Decisional Conflict after Deciding on Potential Participation in Early Phase Clinical Cancer Trials: Dependent on Global Health Status, Satisfaction with Communication, and Timing. Cancers, 14(6), 1500. https://doi.org/10.3390/cancers14061500







