MitoQ Prevents Human Breast Cancer Recurrence and Lung Metastasis in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. MitoQ Biodistribution in Mice
2.3. Cells and Cell Culture
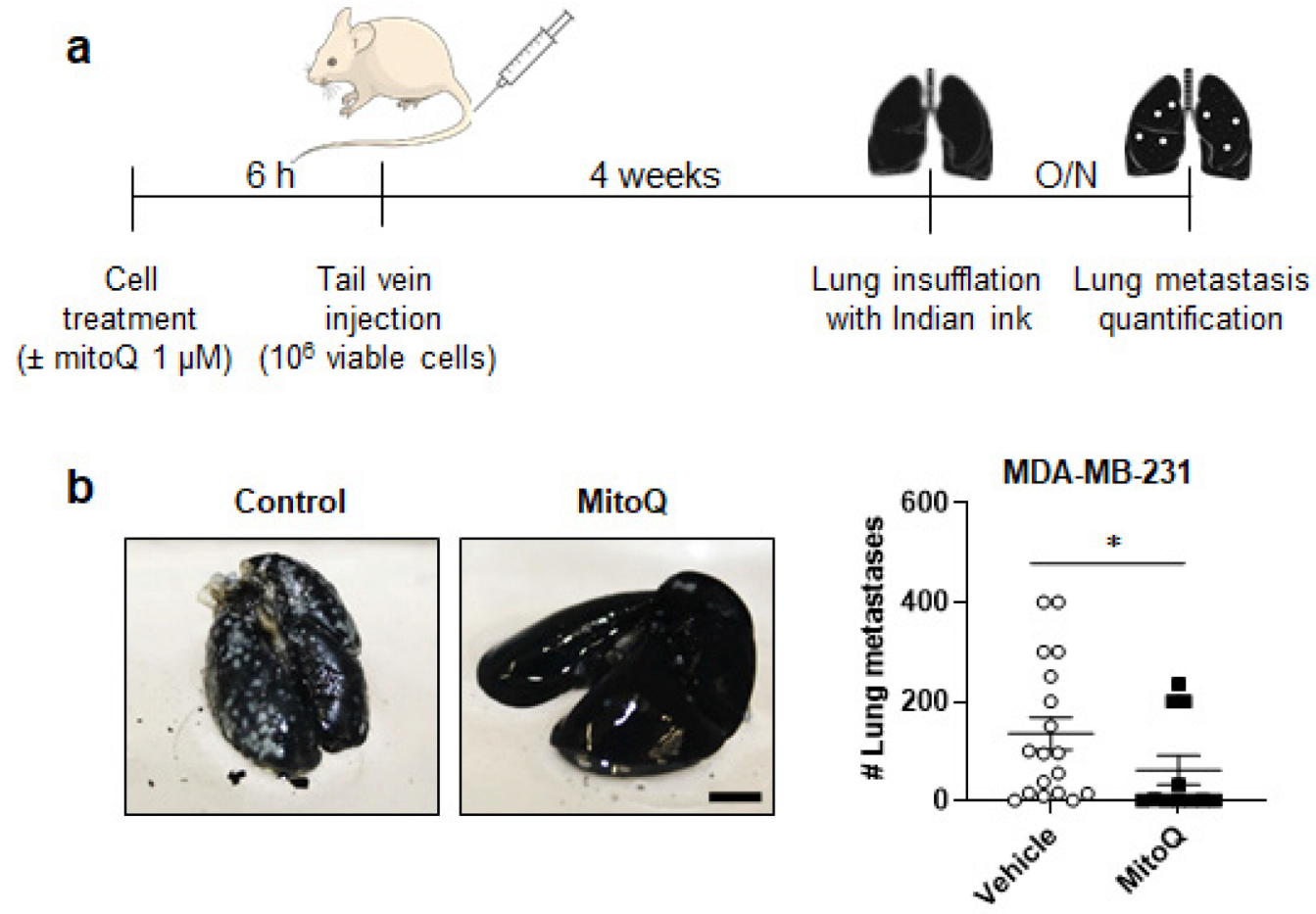
2.4. Experimental Metastasis Assay
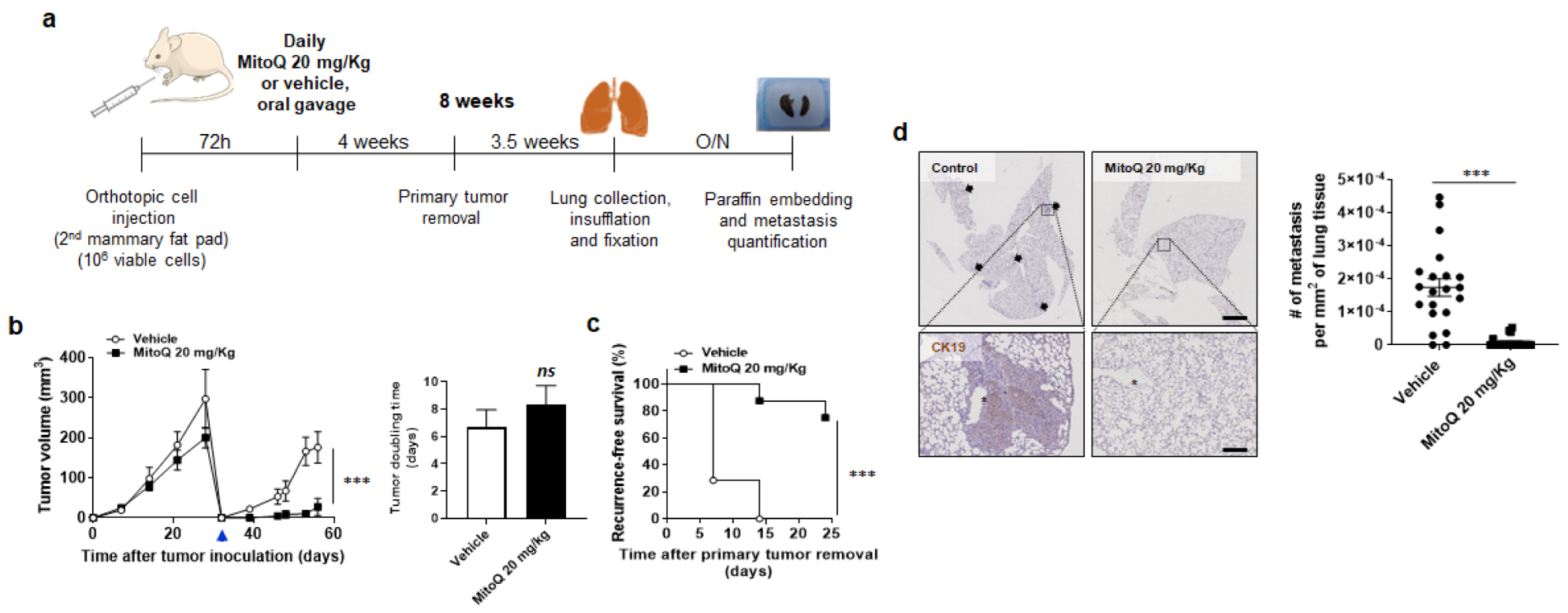
2.5. Orthotopic Metastasis Assay
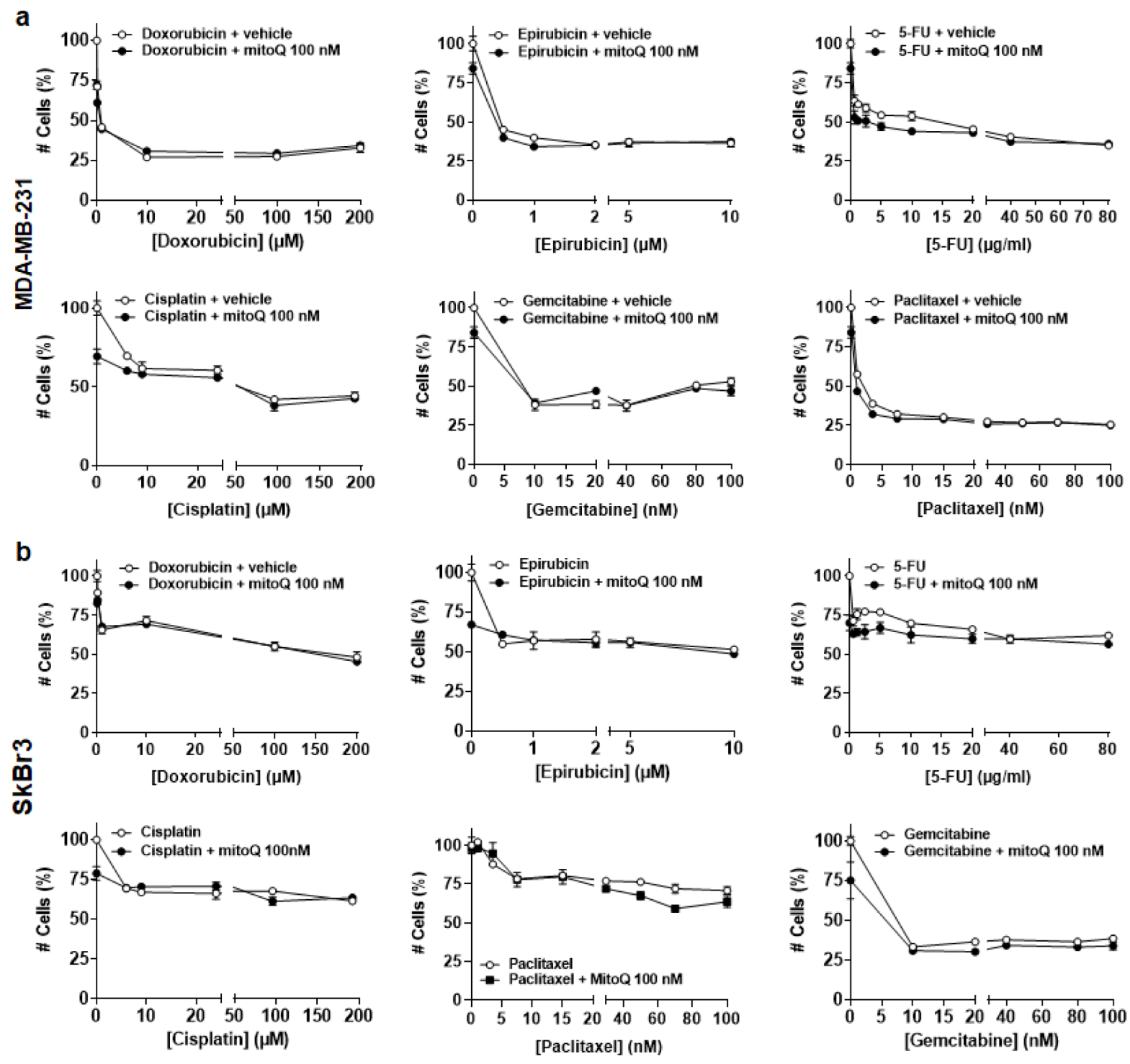
2.6. Cytotoxicity Assay
2.7. Spontaneous Tumor and Metastasis Model
2.8. Immunohistochemistry
2.9. Statistics
3. Results
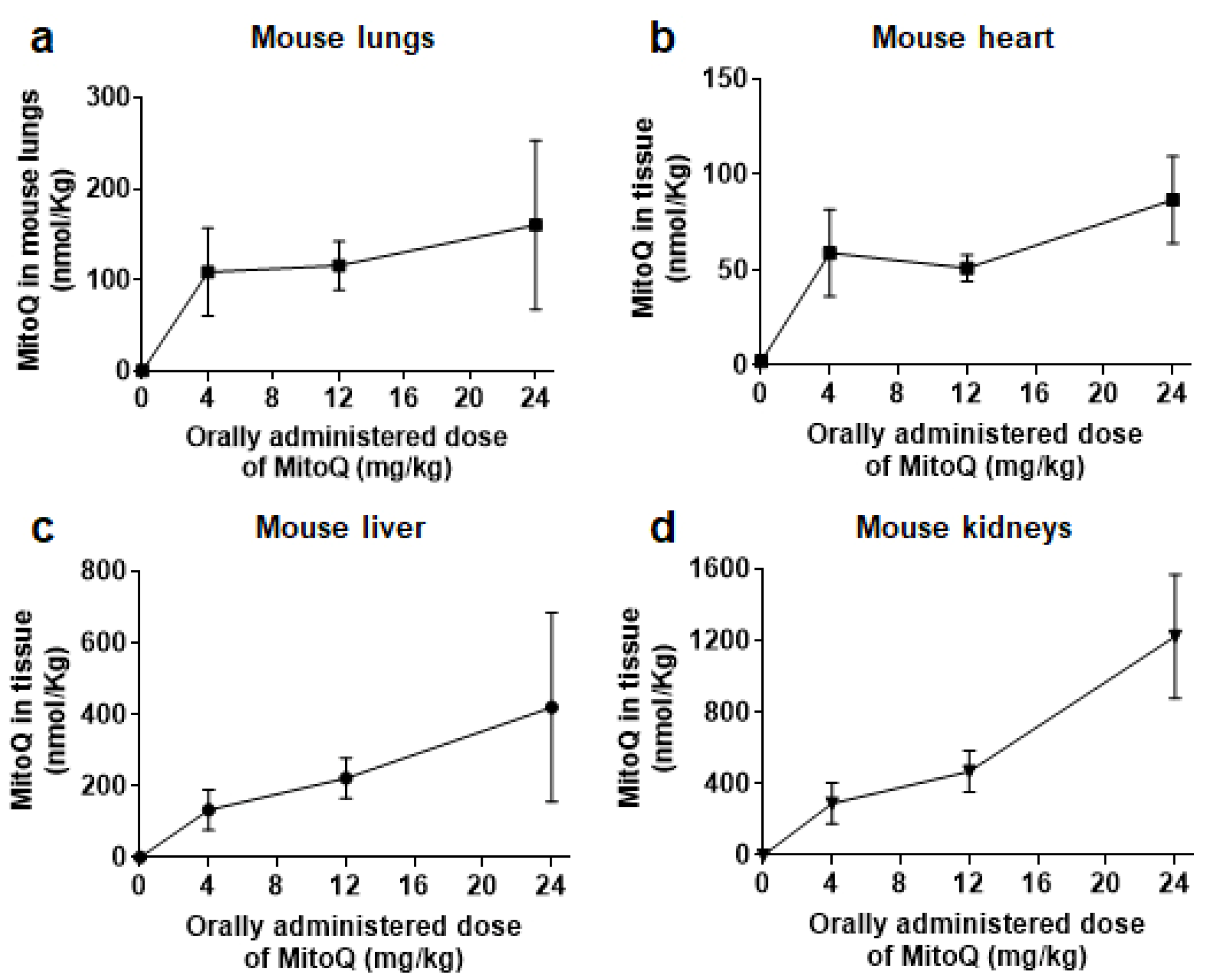
3.1. After Oral Delivery, MitoQ Accumulates in Mouse Organs
3.2. MitoQ Inhibits the Metastatic Take of Human Breast Cancer Cells in Mice
3.3. MitoQ Inhibits Primary Tumor Recurrence and the Spontaneous Metastasis of Orthotopic MDA-MB-231 Tumors in Mice
3.4. In Vitro, MitoQ Does Not Interfere with Chemotherapy-Induced Breast Cancer Cell Killing
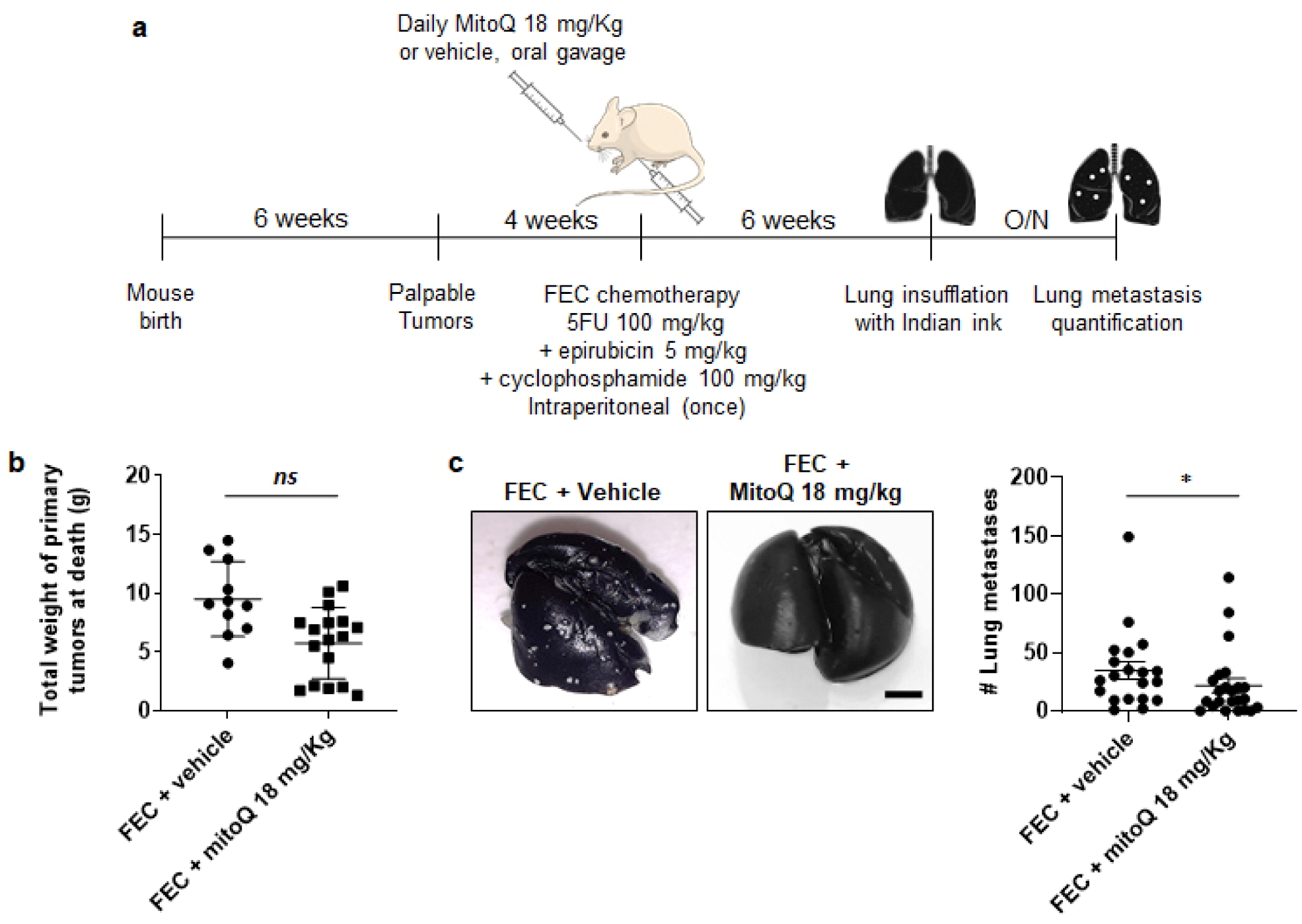
3.5. MitoQ Prevents the Metastatic Dissemination to the Lungs of Spontaneously Metastatic Breast Tumors in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Luo, M.; Brooks, M.; Wicha, M.S. Epithelial-mesenchymal plasticity of breast cancer stem cells: Implications for metastasis and therapeutic resistance. Curr. Pharm. Des. 2015, 21, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Heeke, A.L.; Tan, A.R. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev. 2021, 41, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Tesch, M.E.; Gelmon, K.A. Targeting HER2 in breast cancer: Latest developments on treatment sequencing and the introduction of biosimilars. Drugs 2020, 80, 1811–1830. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Stockler, M.; Puntoni, M.; Sormani, M.; Nanni, O.; Amadori, D.; Wilcken, N.; D’Amico, M.; DeCensi, A.; Bruzzi, P. Duration of chemotherapy for metastatic breast cancer: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Oncol. 2011, 29, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef]
- Lin, N.U.; Thomssen, C.; Cardoso, F.; Cameron, D.; Cufer, T.; Fallowfield, L.; Francis, P.A.; Kyriakides, S.; Pagani, O.; Senkus, E.; et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)-MBC Task Force: Surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast 2013, 22, 203–210. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; Perez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Payen, V.L.; Zampieri, L.X.; Porporato, P.E.; Sonveaux, P. Pro- and antitumor effects of mitochondrial reactive oxygen species. Cancer Metastasis Rev. 2019, 38, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Sonveaux, P. Paving the way for therapeutic prevention of tumor metastasis with agents targeting mitochondrial superoxide. Mol. Cell. Oncol. 2015, 2, e968043. [Google Scholar] [CrossRef]
- Korshunova, G.A.; Shishkina, A.V.; Skulachev, M.V. Design, synthesis, and some aspects of the biological activity of mitochondria-targeted antioxidants. Biochemistry 2017, 82, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Nazarewicz, R.R.; Dikalova, A.; Bikineyeva, A.; Ivanov, S.; Kirilyuk, I.A.; Grigor’ev, I.A.; Dikalov, S.I. Does scavenging of mitochondrial superoxide attenuate cancer prosurvival signaling pathways? Antioxid. Redox. Signal. 2013, 19, 344–349. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study, G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef]
- Gane, E.J.; Weilert, F.; Orr, D.W.; Keogh, G.F.; Gibson, M.; Lockhart, M.M.; Frampton, C.M.; Taylor, K.M.; Smith, R.A.; Murphy, M.P. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010, 30, 1019–1026. [Google Scholar] [CrossRef]
- Smith, R.A.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Capeloa, T.; Krzystyniak, J.; d’Hose, D.; Canas Rodriguez, A.; Payen, V.L.; Zampieri, L.X.; Van de Velde, J.A.; Benyahia, Z.; Pranzini, E.; Vazeille, T.; et al. MitoQ inhibits human breast cancer cell migration, invasion and clonogenicity. Cancers, Submitted as a companion paper to the present paper.
- Liu, X.; Murphy, M.P.; Xing, W.; Wu, H.; Zhang, R.; Sun, H. Mitochondria-targeted antioxidant MitoQ reduced renal damage caused by ischemia-reperfusion injury in rodent kidneys: Longitudinal observations of T2 -weighted imaging and dynamic contrast-enhanced MRI. Magn. Reson. Med. 2018, 79, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cuenca, S.; Cocheme, H.M.; Logan, A.; Abakumova, I.; Prime, T.A.; Rose, C.; Vidal-Puig, A.; Smith, A.C.; Rubinsztein, D.C.; Fearnley, I.M.; et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic. Biol. Med. 2010, 48, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Cailleau, R.; Young, R.; Olive, M.; Reeves, W.J., Jr. Breast tumor cell lines from pleural effusions. J. Natl. Cancer Inst. 1974, 53, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Fogh, J.; Trempe, G. New Human tumor cell lines. In Human Tumor Cells In Vitro; Fogh, J., Ed.; Plenum Publishing Corp.: New York, NY, USA, 1975; pp. 115–159. [Google Scholar]
- Cailleau, R.; Olive, M.; Cruciger, Q.V. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro 1978, 14, 911–915. [Google Scholar] [CrossRef]
- Plum, S.M.; Hanson, A.D.; Volker, K.M.; Vu, H.A.; Sim, B.K.; Fogler, W.E.; Fortier, A.H. Synergistic activity of recombinant human endostatin in combination with adriamycin: Analysis of in vitro activity on endothelial cells and in vivo tumor progression in an orthotopic murine mammary carcinoma model. Clin. Cancer Res. 2003, 9, 4619–4626. [Google Scholar]
- Zhao, Y.; Jing, Z.; Li, Y.; Mao, W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol. Rep. 2016, 36, 567–572. [Google Scholar] [CrossRef]
- Stanley, A.; Ashrafi, G.H.; Seddon, A.M.; Modjtahedi, H. Synergistic effects of various Her inhibitors in combination with IGF-1R, C-MET and Src targeting agents in breast cancer cell lines. Sci. Rep. 2017, 7, 3964. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhao, Z.F.; Chen, M.H.; Yuan, Q.H.; Li, Y.L.; Jiang, C.L. Epirubicin inhibits growth and alters the malignant phenotype of the U87 glioma cell line. Mol. Med. Rep. 2015, 12, 5917–5923. [Google Scholar] [CrossRef]
- Wu, Y.; Qian, Y.; Zhou, G.; Lv, J.; Yan, Q.; Dong, X. Effect of GEN1 interference on the chemosensitivity of the breast cancer MCF-7 and SKBR3 cell lines. Oncol. Lett. 2016, 11, 3597–3604. [Google Scholar] [CrossRef]
- Hernandez-Vargas, H.; Rodriguez-Pinilla, S.M.; Julian-Tendero, M.; Sanchez-Rovira, P.; Cuevas, C.; Anton, A.; Rios, M.J.; Palacios, J.; Moreno-Bueno, G. Gene expression profiling of breast cancer cells in response to gemcitabine: NF-kappaB pathway activation as a potential mechanism of resistance. Breast Cancer Res. Treat. 2007, 102, 157–172. [Google Scholar] [CrossRef]
- Guy, C.T.; Cardiff, R.D.; Muller, W.J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol. Cell. Biol. 1992, 12, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.; Fumoleau, P.; Spielmann, M.; Canon, J.L.; Delozier, T.; Serin, D.; Symann, M.; Kerbrat, P.; Soulie, P.; Eichler, F.; et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J. Clin. Oncol. 2006, 24, 5664–5671. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Erler, J.T. Quantification of lung metastases from in vivo mouse models. Adv. Exp. Med. Biol. 2016, 899, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Porteous, C.M.; Logan, A.; Evans, C.; Ledgerwood, E.C.; Menon, D.K.; Aigbirhio, F.; Smith, R.A.; Murphy, M.P. Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: Implications for mitochondria-specific therapies and probes. Biochim. Biophys. Acta 2010, 1800, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Sharpley, M.S.; Manas, A.R.; Frerman, F.E.; Hirst, J.; Smith, R.A.; Murphy, M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007, 282, 14708–14718. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer. Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Collins, K.P.; Aradi, A.E.; Conger, K.A.; Gustafson, D.L. Kinetics of cyclophosphamide metabolism in humans, dogs, cats, and mice and relationship to cytotoxic activity and pharmacokinetics. Drug Metab. Dispos. 2019, 47, 257–268. [Google Scholar] [CrossRef]
- Zimmer, A.S.; Steeg, P.S. Meaningful prevention of breast cancer metastasis: Candidate therapeutics, preclinical validation, and clinical trial concerns. J. Mol. Med. 2015, 93, 13–29. [Google Scholar] [CrossRef]
- James, A.M.; Cocheme, H.M.; Smith, R.A.; Murphy, M.P. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J. Biol. Chem. 2005, 280, 21295–21312. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Wang, X. The metabolic mechanisms of breast cancer metastasis. Front. Oncol. 2020, 10, 602416. [Google Scholar] [CrossRef]
- Ippolito, L.; Giannoni, E.; Chiarugi, P.; Parri, M. Mitochondrial redox hubs as promising targets for anticancer therapy. Front. Oncol. 2020, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Dong, Q.Z. Advance in metabolism and target therapy in breast cancer stem cells. World J. Stem Cells 2020, 12, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywinski, K.L.; Biel, T.G.; Kryndushkin, D.; Rao, V.A. Therapeutic Targeting of the mitochondria initiates excessive superoxide production and mitochondrial depolarization causing decreased mtDNA integrity. PLoS ONE 2016, 11, e0168283. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-targeted drugs. Curr. Mol. Pharmacol. 2019, 12, 202–214. [Google Scholar] [CrossRef]
- Kenny, T.C.; Craig, A.J.; Villanueva, A.; Germain, D. Mitohormesis primes tumor invasion and metastasis. Cell Rep. 2019, 27, 2292–2303.e6. [Google Scholar] [CrossRef]
- Payandeh, Z.; Pirpour Tazehkand, A.; Barati, G.; Pouremamali, F.; Kahroba, H.; Baradaran, B.; Samadi, N. Role of Nrf2 and mitochondria in cancer stem cells; in carcinogenesis, tumor progression, and chemoresistance. Biochimie 2020, 179, 32–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capeloa, T.; Krzystyniak, J.; Rodriguez, A.C.; Payen, V.L.; Zampieri, L.X.; Pranzini, E.; Derouane, F.; Vazeille, T.; Bouzin, C.; Duhoux, F.P.; et al. MitoQ Prevents Human Breast Cancer Recurrence and Lung Metastasis in Mice. Cancers 2022, 14, 1488. https://doi.org/10.3390/cancers14061488
Capeloa T, Krzystyniak J, Rodriguez AC, Payen VL, Zampieri LX, Pranzini E, Derouane F, Vazeille T, Bouzin C, Duhoux FP, et al. MitoQ Prevents Human Breast Cancer Recurrence and Lung Metastasis in Mice. Cancers. 2022; 14(6):1488. https://doi.org/10.3390/cancers14061488
Chicago/Turabian StyleCapeloa, Tania, Joanna Krzystyniak, Amanda Canas Rodriguez, Valéry L. Payen, Luca X. Zampieri, Erica Pranzini, Françoise Derouane, Thibaut Vazeille, Caroline Bouzin, François P. Duhoux, and et al. 2022. "MitoQ Prevents Human Breast Cancer Recurrence and Lung Metastasis in Mice" Cancers 14, no. 6: 1488. https://doi.org/10.3390/cancers14061488
APA StyleCapeloa, T., Krzystyniak, J., Rodriguez, A. C., Payen, V. L., Zampieri, L. X., Pranzini, E., Derouane, F., Vazeille, T., Bouzin, C., Duhoux, F. P., Murphy, M. P., Porporato, P. E., & Sonveaux, P. (2022). MitoQ Prevents Human Breast Cancer Recurrence and Lung Metastasis in Mice. Cancers, 14(6), 1488. https://doi.org/10.3390/cancers14061488








