Association of Low-Grade Glioma Diagnosis and Management Approach with Mental Health Disorders: A MarketScan Analysis 2005–2014
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
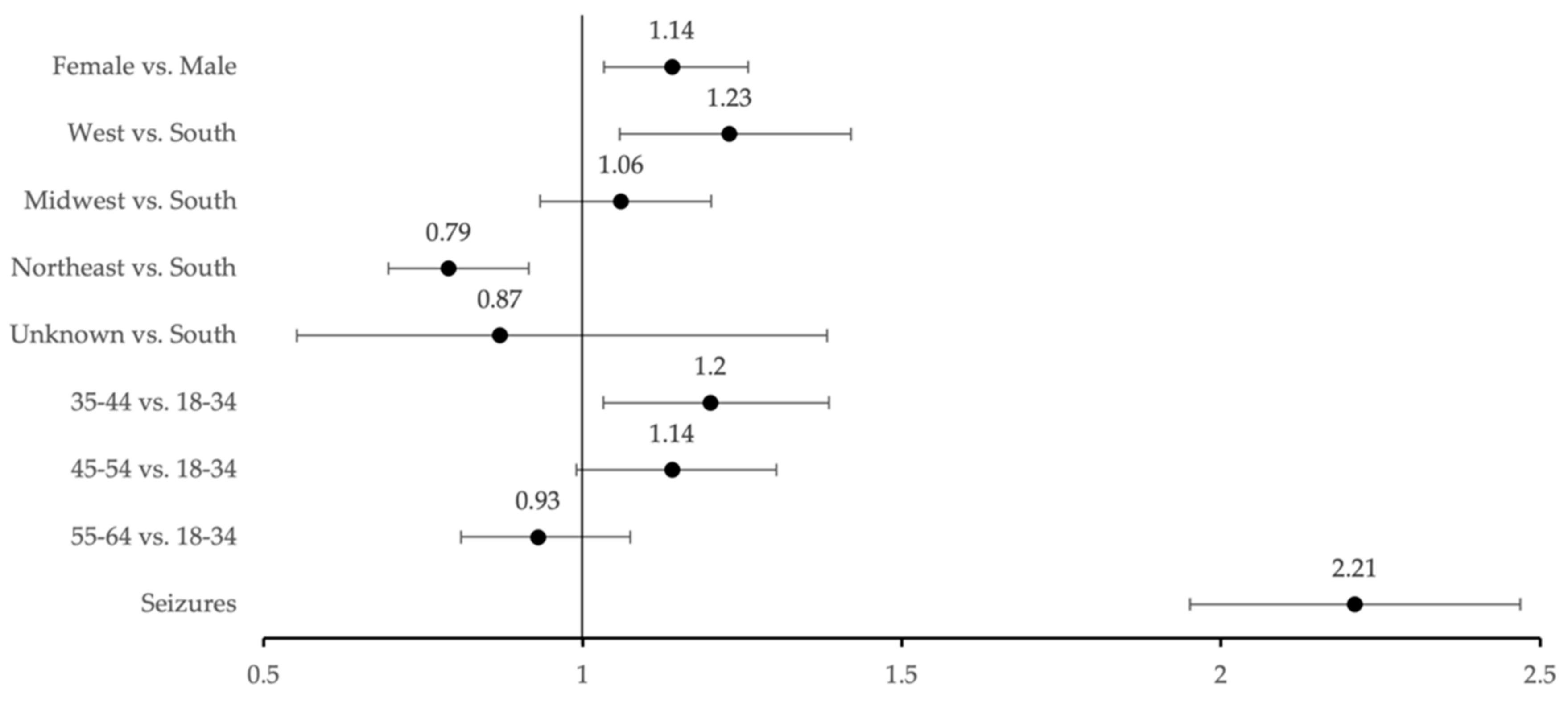
3.1. Prevalence of MHD in LGG Patients
3.2. New Onset of MHDs Post-LGG Diagnosis
3.3. Impact of Glioma-Related Seizures on MHD Prevalence
3.4. Impact of First-Line Treatment on MHD Prevalence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Krunchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomas, D.A.; Issa Laack, N.N.; Rao, R.D.; Meyer, F.B.; Shaw, E.G.; O’Neill, B.P.; Giannini, C.; Brown, P.D. Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro-Oncology 2009, 11, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Taillandier, L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. Neuro-Oncology 2015, 17, 332–342. [Google Scholar] [PubMed] [Green Version]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Golmard, J.L.; Bauchet, L.; Pallaud, J.; Peruzzi, P.; Baron, M.H.; Kajus, M.; Guyotat, J.; et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: A series of 1097 cases: Clinical article. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Duffau, H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: A consecutive series with 11-year follow-up. Acta Neurochir. 2016, 158, 51–58. [Google Scholar] [CrossRef]
- Xia, L.; Fang, C.; Chen, G.; Sun, C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: A systematic review and meta-analysis. BMC Cancer 2018, 18, 48. [Google Scholar] [CrossRef]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgard, G.; Solheim, O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Bunevicius, A.; Tamasauskas, S.; Deltuva, V.; Tamasauskas, A.; Radziunas, A.; Bunevicius, R. Predictors of health-related quality of life in neurosurgical brain tumor patients: Focus on patient-centered perspective. Acta Neurochir. 2014, 156, 367–374. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Steel, Z.; Marnane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wu, C.; Han, N.; Liu, N.; Han, S.; Liu, X.; Li, S.; Yan, C. Depressive and anxiety disorders worsen the prognosis of glioblastoma. Aging 2020, 12, 20095–20110. [Google Scholar] [CrossRef]
- Edelstein, K.; Coate, L.; Massey, C.; Jewitt, N.C.; Mason, W.P.; Devins, G.M. Illness intrusiveness and subjective well-being in patients with glioblastoma. J. Neurooncol. 2016, 126, 127–135. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, C.; Xia, J.; Zhao, D.; Tang, H.; Wu, H.; Chen, J. Association between depression and brain tumor: A systematic review and meta-analysis. Oncotarget 2017, 8, 94932–94943. [Google Scholar] [CrossRef]
- Ernst, J.; Friedrich, M.; Vehling, S.; Koch, U.; Mehnert-Theuerkauf, A. Cancer-Related Distress: How Often Does It Co-occur With a Mental Disorder?—Results of a Secondary Analysis. Front. Psychol. 2021, 12, 660588. [Google Scholar] [CrossRef]
- Satin, J.R.; Linden, W.; Phillips, J.M. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef]
- Rooney, A.G.; Carson, A.; Grant, R. Depression in cerebral glioma patients: A systematic review of observational studies. J. Natl. Cancer Inst. 2011, 103, 61–76. [Google Scholar] [CrossRef]
- Litofsky, N.S.; Farace, E.; Anderson, F., Jr.; Meyers, C.A.; Huang, W.; Laws, E.R., Jr. Glioma Outcomes Project Investigators. Depression in patients with high-grade glioma: Results of the Glioma Outcomes Project. Neurosurgery 2004, 54, 358–366, discussion 366–367. [Google Scholar]
- Díaz, J.L.; Barreto, P.; Gallego, J.M.; Barbero, J.; Bayés, R.; Barcia, J.A. Proper information during the surgical decision-making process lowers the anxiety of patients with high-grade gliomas. Acta Neurochir. 2009, 151, 357–362. [Google Scholar] [CrossRef]
- Pelletier, G.; Verhoef, M.J.; Khatri, N.; Hagen, N. Quality of life in brain tumor patients: The relative contributions of depression, fatigue, emotional distress, and existential issues. J. Neurooncol. 2002, 57, 41–49. [Google Scholar] [CrossRef]
- Pringle, A.M.; Taylor, R.; Whittle, I.R. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br. J. Neurosurg. 1999, 13, 46–51. [Google Scholar] [CrossRef]
- Mainio, A.; Hakko, H.; Niemelä, A.; Koivukangas, J.; Räsänen, P. Depression and functional outcome in patients with brain tumors: A population-based 1-year follow-up study. J. Neurosurg. 2005, 103, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Adamson, D.; Chang, S.; Hansen, L. Health Research Data for the Real World: The MarketScan Databases; Thomson Medstat: New York, NY, USA, 2006. [Google Scholar]
- Aizcorbe, A.; Liebman, E.; Pack, S.; Cutler, D.M.; Chernew, M.E.; Rosen, A.B. Measuring health care costs of individuals with employer-sponsored health insurance in the U.S.: A comparison of survey and claims data. Stat. J. IAOS 2012, 28, 43–51. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health; CBHSQ Methodology Report; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2021.
- Anderson, S.I.; Taylor, R.; Whittle, I.R. Mood disorders in patients after treatment for primary intracranial tumours. Br. J. Neurosurg. 1999, 13, 480–485. [Google Scholar] [CrossRef]
- Arnold, S.D.; Forman, L.M.; Brigidi, B.D.; Carter, K.E.; Schweitzer, H.A.; Quinn, H.E.; Guill, A.B.; Herndon, J.E., 2nd; Raynor, R.H. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro-Oncology 2008, 10, 171–181. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Taphoorn, M.J.; Klein, M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004, 3, 159–168. [Google Scholar] [CrossRef]
- Litofsky, N.S.; Resnick, A.G. The relationships between depression and brain tumors. J. Neurooncol. 2009, 94, 153–161. [Google Scholar] [CrossRef]
- Sparacia, G.; Parla, G.; Re, V.L.; Cannella, R.; Mamone, G.; Carollo, V.; Midiri, M.; Grasso, G. Resting-State Functional Connectome in Patients with Brain Tumors Before and After Surgical Resection. World Neurosurg. 2020, 141, e182–e194. [Google Scholar] [CrossRef]
- Drysdale, A.T.; Grosenick, L.; Downar, J.; Dunlop, K.; Mansouri, F.; Meng, Y.; Fetcho, R.N.; Zebley, B.; Oathes, D.J.; Etkin, A.; et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017, 23, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Briggs, R.G.; Conner, A.K.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Sali, G.; Battiste, J.D.; O’Donoghue, D.L.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 18: The Connectional Anatomy of Human Brain Networks. Oper. Neurosurg. 2018, 15, S470–S480. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Sugihara, G. Diagnostic ambiguity of subthreshold depression: Minor depression vs. adjustment disorder with depressive mood. Acta Psychiatr. Scand. 2006, 114, 144, Author Reply 145. [Google Scholar] [CrossRef] [PubMed]
- Valentine, A.D.; Passik, S.D.; Massie, M.J. Psychiatric and Psychosocial Issues. In Cancer in the Nervous System, 2nd ed.; Levin, V., Ed.; University Press: Oxford, UK, 2002; pp. 572–589. [Google Scholar]
- Hejrati, N.; Spieler, D.; Samuel, R.; Regli, L.; Weyerbrock, A.; Surbeck, W. Conscious Experience and Psychological Consequences of Awake Craniotomy. World Neurosurg. 2019, 129, e381–e386. [Google Scholar] [CrossRef] [PubMed]
- Boele, F.W.; Zant, M.; Heine, E.C.E.; Aaronson, N.K.; Taphoorn, M.J.B.; Reijneveld, J.C.; Postma, T.J.; Heimans, J.J.; Klein, M. The association between cognitive functioning and health-related quality of life in low-grade glioma patients. Neurooncol. Pract. 2014, 1, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Hingray, C.; McGonigal, A.; Kotwas, I.; Micouland-Franchi, J.A. The Relationship Between Epilepsy and Anxiety Disorders. Curr. Psychiatry Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Grassi, L.; Spiegel, D.; Riba, M. Advancing psychosocial care in cancer patients. F1000Res 2017, 6, 2083. [Google Scholar] [CrossRef] [Green Version]
- Singer, S.; Roick, J.; Danker, H.; Kortmann, R.D.; Papsdorf, K.; Taubenheim, S.; Renovanz, M.; Jahne, K.; Meixensberger, J. Psychiatric co-morbidity, distress, and use of psycho-social services in adult glioma patients-a prospective study. Acta Neurochir. 2018, 160, 1187–1194. [Google Scholar] [CrossRef]
- Ownsworth, T.; Chambers, S.; Damborg, E.; Casey, L.; Walker, D.G.; Shum, D.H.K. Evaluation of the making sense of brain tumor program: A randomized controlled trial of a home-based psychosocial intervention. Psychooncology 2015, 24, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Maurer, R.; Daggubati, L.; Ba, D.M.; Liu, G.; Leslie, D.; Goyal, N.; Zacharia, B.E. Mental health disorders in patients with untreated meningiomas: An observational cohort study using the nationwide MarketScan database. Neurooncol. Pract. 2020, 7, 507–513. [Google Scholar] [CrossRef]
- Maurer, R.; McNutt, S.; Daggubati, L.; Ba, D.M.; Liu, G.; Leslie, D.; Goyal, N.; Zacharia, B.E. Mental health disorders in newly diagnosed non-functional pitutary adenoma under initial observation: An observational cohort study using the nationwide MarketScan database. Pituitary 2021, 25, 86–91. [Google Scholar] [CrossRef]
- Lee, J.H.; Ba, D.; Liu, G.; Leslie, D.; Zacharia, B.E.; Goyal, N. Association of Head and Neck Cancer with Mental Health Disorders in a Large Insurance Claims Database. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 339–344. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wooding, S.; Grant, J. The answer is 17 years, what is the question: Understanding time lags in translational research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef]
- Haneef, Z.; Stern, J.; Dewar, S.; Engel, J., Jr. Referral pattern for epilepsy surgery after evidence-based recommendations: A retrospective study. Neurology 2010, 75, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.C.; Thompson, R.C.; Kalkanis, S.N.; Barker, F.G. Prophylactic antiepileptic drug administration following brain tumor resection: Results of a recent AANS/CNS Section on Tumors survey. J. Neurosurg. 2017, 126, 1772–1778. [Google Scholar] [CrossRef]

| Characteristic | No. (%) of Patients |
|---|---|
| Age, years | |
| 18–34 | 4297 (21.03) |
| 35–44 | 4191 (20.51) |
| 45–54 | 5995 (29.34) |
| 55–64 | 5949 (29.12) |
| Sex | |
| Male | 8105 (39.67) |
| Female | 12,327 (60.33) |
| US Region | |
| South | 8392 (41.07) |
| West | 2763 (13.52) |
| Midwest | 5020 (24.57) |
| Northeast | 4019 (19.67) |
| Unknown | 238 (1.16) |
| Mental Health Disorder | |
| Yes | 12,436 (60.9) |
| No | 7996 (39.1) |
| Study Group, No. (%) of Patients | ||
|---|---|---|
| No MHD Post-LGG Diagnosis | MHD Post-LGG Diagnosis | |
| Characteristic | 9543 (83.3) | 1915 (16.7) |
| Age, years | ||
| 18–34 | 2241 (23.48) | 425 (22.19) |
| 35–44 | 1918 (20.10) | 435 (22.72) |
| 45–54 | 2720 (28.50) | 574 (29.97) * |
| 55–64 | 2720 (28.50) | 481 (25.12) |
| Sex | ||
| Male | 4476 (46.90) | 836 (43.66) |
| Female | 5067 (53.10) | 1079 (56.32) * |
| US Region | ||
| South | 3926 (41.14) | 791 (41.31) * |
| West | 1223 (12.82) | 302 (15.77) |
| Midwest | 2166 (22.70) | 462 (24.13) |
| Northeast | 2103 (22.04) | 338 (17.65) |
| Unknown | 125 (1.31) | 22 (1.15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhanja, D.; Ba, D.; Tuohy, K.; Wilding, H.; Trifoi, M.; Padmanaban, V.; Liu, G.; Sughrue, M.; Zacharia, B.; Leslie, D.; et al. Association of Low-Grade Glioma Diagnosis and Management Approach with Mental Health Disorders: A MarketScan Analysis 2005–2014. Cancers 2022, 14, 1376. https://doi.org/10.3390/cancers14061376
Bhanja D, Ba D, Tuohy K, Wilding H, Trifoi M, Padmanaban V, Liu G, Sughrue M, Zacharia B, Leslie D, et al. Association of Low-Grade Glioma Diagnosis and Management Approach with Mental Health Disorders: A MarketScan Analysis 2005–2014. Cancers. 2022; 14(6):1376. https://doi.org/10.3390/cancers14061376
Chicago/Turabian StyleBhanja, Debarati, Djibril Ba, Kyle Tuohy, Hannah Wilding, Mara Trifoi, Varun Padmanaban, Guodong Liu, Michael Sughrue, Brad Zacharia, Douglas Leslie, and et al. 2022. "Association of Low-Grade Glioma Diagnosis and Management Approach with Mental Health Disorders: A MarketScan Analysis 2005–2014" Cancers 14, no. 6: 1376. https://doi.org/10.3390/cancers14061376
APA StyleBhanja, D., Ba, D., Tuohy, K., Wilding, H., Trifoi, M., Padmanaban, V., Liu, G., Sughrue, M., Zacharia, B., Leslie, D., & Mansouri, A. (2022). Association of Low-Grade Glioma Diagnosis and Management Approach with Mental Health Disorders: A MarketScan Analysis 2005–2014. Cancers, 14(6), 1376. https://doi.org/10.3390/cancers14061376









