Protein Degradation by E3 Ubiquitin Ligases in Cancer Stem Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Cancer Stem Cells (CSCs)
3. Ubiquitination Process
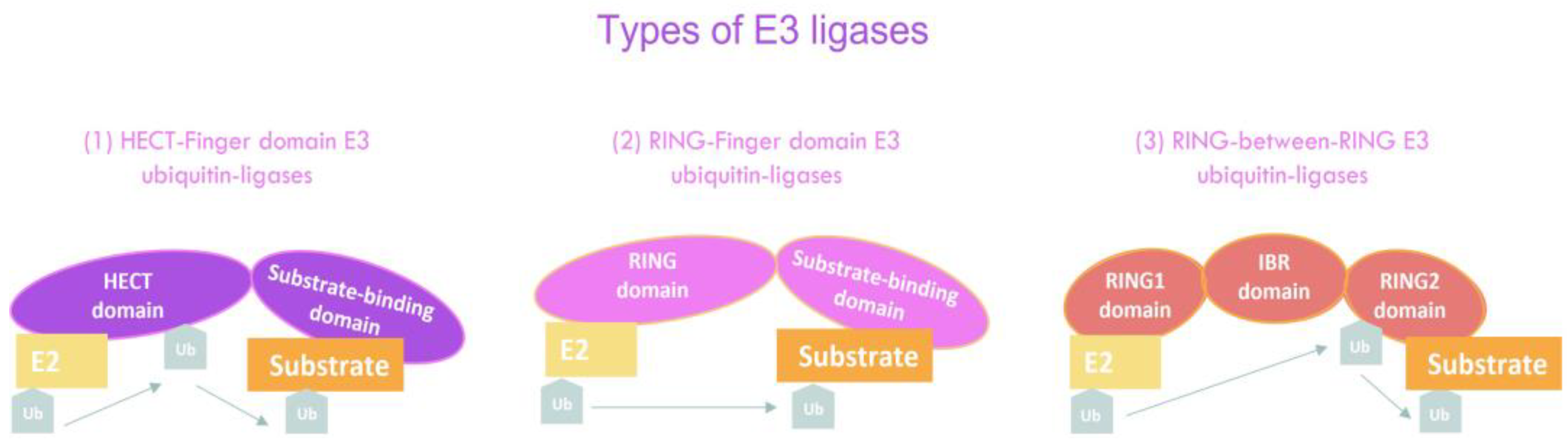
4. E3 Ubiquitin Ligases in Cancer Stem Cells
4.1. RING-Finger Domain E3 Ubiquitin Ligases
4.1.1. CBL Proteins
4.1.2. SCF Family: F-Box Proteins
4.1.3. Ring-Finger Proteins (RNF)
4.1.4. SIAH
4.1.5. MDM2
4.1.6. TRIM
4.1.7. MARCH
4.2. HECT-Domain E3 Ubiquitin Ligases
4.2.1. Nedd4 Family
4.2.2. Other HECT-Domain E3 Ubiquitin Ligases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in Cancer: Cancer Stem Cells versus Clonal Evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; de Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef]
- Avgustinova, A.; Benitah, S.A. The epigenetics of tumour initiation: Cancer stem cells and their chromatin. Curr. Opin. Genet. Dev. 2016, 36, 8–15. [Google Scholar] [CrossRef]
- Chua, B.A.; van der Werf, I.; Jamieson, C.; Signer, R.A. Post-Transcriptional Regulation of Homeostatic, Stressed, and Malignant Stem Cells. Cell Stem Cell 2020, 26, 138–159. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef]
- Weissman, A.M.; Shabek, N.; Ciechanover, A. The predator becomes the prey: Regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol. 2011, 12, 605–620. [Google Scholar] [CrossRef]
- Pattabiraman, D.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef]
- Quintana, E.; Shackleton, M.; Foster, H.R.; Fullen, D.R.; Sabel, M.S.; Johnson, T.M.; Morrison, S.J. Phenotypic Heterogeneity among Tumorigenic Melanoma Cells from Patients that Is Reversible and Not Hierarchically Organized. Cancer Cell 2010, 18, 510–523. [Google Scholar] [CrossRef]
- van den Hoogen, C.; van der Horst, G.; Cheung, H.; Buijs, J.T.; Lippitt, J.M.; Guzmán-Ramírez, N.; Hamdy, F.C.; Eaton, C.L.; Thalmann, G.N.; Cecchini, M.G.; et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010, 70, 5163–5173. [Google Scholar] [CrossRef]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E.; et al. Glycine Decarboxylase Activity Drives Non-Small Cell Lung Cancer Tumor-Initiating Cells and Tumorigenesis. Cell 2012, 148, 259–272. [Google Scholar] [CrossRef]
- Dobbin, Z.C.; Landen, C.N. Isolation and Characterization of Potential Cancer Stem Cells from Solid Human Tumors—Potential Applications. Curr. Protoc. Pharmacol. 2013, 63, 14–28. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Yu, Y.; Nautiyal, J.; Patel, B.B.; Majumdar, A.P. The Wnt/β-catenin pathway regulates growth and maintenance of colonospheres. Mol. Cancer 2010, 9, 212. [Google Scholar] [CrossRef]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Wang, J.; Wakeman, T.P.; Lathia, J.D.; Hjelmeland, A.B.; Wang, X.F.; White, R.R.; Rich, J.N.; Sullenger, B.A. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010, 28, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sarrió, D.; Franklin, C.K.; Mackay, A.; Reis-Filho, J.S.; Isacke, C.M. Epithelial and Mesenchymal Subpopulations within Normal Basal Breast Cell Lines Exhibit Distinct Stem Cell/Progenitor Properties. Stem Cells 2012, 30, 292–303. [Google Scholar] [CrossRef] [PubMed]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Hadjimichael, C.; Chanoumidou, K.; Papadopoulou, N.; Arampatzi, P.; Papamatheakis, J.; Kretsovali, A. Common stemness regulators of embryonic and cancer stem cells. World J. Stem Cells 2015, 7, 1150–1184. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Pádua, D.; Figueira, P.; Ribeiro, I.; Almeida, R.; Mesquita, P. The Relevance of Transcription Factors in Gastric and Colorectal Cancer Stem Cells Identification and Eradication. Front. Cell Dev. Biol. 2020, 8, 442. [Google Scholar] [CrossRef]
- Kreso, A.; O’Brien, C.A.; van Galen, P.; Gan, O.I.; Notta, F.; Brown, A.M.K.; Ng, K.; Ma, J.; Wienholds, E.; Dunant, C.; et al. Variable Clonal Repopulation Dynamics Influence Chemotherapy Response in Colorectal Cancer. Science 2013, 339, 543–548. [Google Scholar] [CrossRef]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting Cancer Stemness in the Clinic: From Hype to Hope. Cell Stem Cell 2019, 24, 25–40. [Google Scholar] [CrossRef]
- Berdasco, M.; Esteller, M. DNA methylation in stem cell renewal and multipotency. Stem Cell Res. Ther. 2011, 2, 42. [Google Scholar] [CrossRef]
- Muñoz, P.; Iliou, M.S.; Esteller, M. Epigenetic alterations involved in cancer stem cell reprogramming. Mol. Oncol. 2012, 6, 620–636. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A.; Varshavsky, A. Basic Medical Research Award. The ubiquitin system. Nat. Med. 2000, 6, 1073–1081. [Google Scholar] [CrossRef]
- Popovic, D.; Vucic, D.; Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014, 20, 1242–1253. [Google Scholar] [CrossRef]
- Rape, M. Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 59–70. [Google Scholar] [CrossRef]
- Galisson, F.; Mahrouche, L.; Courcelles, M.; Bonneil, E.; Meloche, S.; Chelbi-Alix, M.K.; Thibault, P. A Novel Proteomics Approach to Identify SUMOylated Proteins and Their Modification Sites in Human Cells. Mol. Cell. Proteom. 2011, 10, S1–S15. [Google Scholar] [CrossRef]
- Ohh, M.; Kim, W.Y.; Moslehi, J.J.; Chen, Y.; Chau, V.; Read, M.A.; Kaelin, W.G. An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Rep. 2002, 3, 177–182. [Google Scholar] [CrossRef]
- Swaney, D.L.; Beltrao, P.; Starita, L.; Guo, A.; Rush, J.; Fields, S.; Krogan, N.J.; Villén, J. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 2013, 10, 676–682. [Google Scholar] [CrossRef]
- Buetow, L.; Huang, D.T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2016, 17, 626–642. [Google Scholar] [CrossRef]
- Kang, B.; Sun, X.-H. Regulation of cancer stem cells by RING finger ubiquitin ligases. Stem Cell Investig. 2014, 1, 5. [Google Scholar]
- Cooper, J.A.; Kaneko, T.; Li, S.S.C. Cell Regulation by Phosphotyrosine-Targeted Ubiquitin Ligases. Mol. Cell. Biol. 2015, 35, 1886–1897. [Google Scholar] [CrossRef]
- Berndsen, C.; Wolberger, C. New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 2014, 21, 301–307. [Google Scholar] [CrossRef]
- Scheffner, M.; Kumar, S. Mammalian HECT ubiquitin-protein ligases: Biological and pathophysiological aspects. Biochim. Biophys. Acta 2014, 1843, 61–74. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, A.; Casas-Pais, A.; Roca-Lema, D.; Graña, B.; Romay, G.; Figueroa, A. Regulation of Epithelial–Mesenchymal Plasticity by the E3 Ubiquitin-Ligases in Cancer. Cancers 2020, 12, 3093. [Google Scholar] [CrossRef] [PubMed]
- Uchida, C.; Kitagawa, M. RING-, HECT-, and RBR-type E3 Ubiquitin Ligases: Involvement in Human Cancer. Curr. Cancer Drug Targets 2016, 16, 157–174. [Google Scholar] [CrossRef]
- Tian, M.; Zeng, T.; Liu, M.; Han, S.; Lin, H.; Lin, Q.; Li, L.; Jiang, T.; Li, G.; Lin, H.; et al. A cell-based high-throughput screening method based on a ubiquitin-reference technique for identifying modulators of E3 ligases. J. Biol. Chem. 2019, 294, 2880–2891. [Google Scholar] [CrossRef]
- Appel, A. Drugs: More shots on target. Nature 2011, 480, S40–S42. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dixit, V.M. Drugging the undruggables: Exploring the ubiquitin system for drug development. Cell Res. 2016, 26, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.; Calabrese, M.F.; Bullock, A.N.; Crews, C.M. Targeted protein degradation: Expanding the toolbox. Nat. Rev. Drug Discov. 2019, 18, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.M.; Aranda-Orgilles, B.; Strikoudis, A.; Apostolou, E.; Loizou, E.; Moran-Crusio, K.; Farnsworth, C.L.; Koller, A.A.; Dasgupta, R.; Silva, J.C.; et al. Regulation of Pluripotency and Cellular Reprogramming by the Ubiquitin-Proteasome System. Cell Stem Cell 2012, 11, 783–798. [Google Scholar] [CrossRef]
- Baharvand, H.; Hajheidari, M.; Ashtiani, S.K.; Salekdeh, G.H. Proteomic signature of human embryonic stem cells. Proteomics 2006, 6, 3544–3549. [Google Scholar] [CrossRef]
- Vilchez, D.; Boyer, L.; Morantte, I.; Lutz, M.; Merkwirth, C.; Joyce, D.; Spencer, B.; Page, L.; Masliah, E.; Berggren, W.T.; et al. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature 2012, 489, 304–308. [Google Scholar] [CrossRef]
- Cai, N.; Li, M.; Qu, J.; Liu, G.-H.; Belmonte, J.C.I. Post-translational modulation of pluripotency. J. Mol. Cell Biol. 2012, 4, 262–265. [Google Scholar] [CrossRef]
- Suresh, B.; Lee, J.; Kim, K.-S.; Ramakrishna, S. The Importance of Ubiquitination and Deubiquitination in Cellular Reprogramming. Stem Cells Int. 2016, 2016, 6705927. [Google Scholar] [CrossRef]
- Strikoudis, A.; Guillamot, M.; Aifantis, I. Regulation of stem cell function by protein ubiquitylation. EMBO Rep. 2014, 15, 365–382. [Google Scholar] [CrossRef]
- Lv, K.; Jiang, J.; Donaghy, R.; Riling, C.R.; Cheng, Y.; Chandra, V.; Rozenova, K.; An, W.; Mohapatra, B.C.; Goetz, B.T.; et al. CBL family E3 ubiquitin ligases control JAK2 ubiquitination and stability in hematopoietic stem cells and myeloid malignancies. Genes Dev. 2017, 31, 1007–1023. [Google Scholar] [CrossRef]
- An, W.; Nadeau, S.A.; Mohapatra, B.; Feng, D.; Zutshi, N.; Storck, M.; Arya, P.; Talmadge, J.E.; Meza, J.L.; Band, V.; et al. Loss of Cbl and Cbl-b ubiquitin ligases abrogates hematopoietic stem cell quiescence and sensitizes leukemic disease to chemotherapy. Oncotarget 2015, 6, 10498–10509. [Google Scholar] [CrossRef]
- Yin, Y.; Xie, C.-M.; Li, H.; Tan, M.; Chen, G.; Schiff, R.; Xiong, X.; Sun, Y. The FBXW2–MSX2–SOX2 axis regulates stem cell property and drug resistance of cancer cells. Proc. Natl. Acad. Sci. USA 2019, 116, 20528–20538. [Google Scholar] [CrossRef]
- Gallo, L.H.; Ko, J.; Donoghue, D.J. The importance of regulatory ubiquitination in cancer and metastasis. Cell Cycle 2017, 16, 634–648. [Google Scholar] [CrossRef]
- Wang, Z.; Inuzuka, H.; Fukushima, H.; Wan, L.; Gao, D.; Shaik, S.; Sarkar, F.H.; Wei, W. Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep. 2011, 13, 36–43. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Bellon, M.; Nicot, C. FBXW7: A critical tumor suppressor of human cancers. Mol. Cancer 2018, 17, 115. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, M.O.; Cho, Y.-Y.; Yao, K.; Kim, D.J.; Jeong, C.-H.; Yu, D.H.; Bae, K.B.; Cho, E.J.; Jung, S.K.; et al. ERK1 phosphorylates Nanog to regulate protein stability and stem cell self-renewal. Stem Cell Res. 2014, 13, 1–11. [Google Scholar] [CrossRef]
- Xu, P.; Scott, D.C.; Xu, B.; Yao, Y.; Feng, R.; Cheng, L.; Mayberry, K.; Wang, Y.-D.; Bi, W.; Palmer, L.E.; et al. FBXO11-mediated proteolysis of BAHD1 relieves PRC2-dependent transcriptional repression in erythropoiesis. Blood 2021, 137, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.-X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.; Van De Wetering, M.; Van Es, J.H.; Mohammed, S.; Heck, A.; Maurice, M.; et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012, 488, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Lebensohn, A.M.; Rohatgi, R. R-spondins can potentiate WNT signaling without LGRs. Elife 2018, 7, e33126. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Zhou, Z.; She, Y.-M.; Chun, A.; Cyr, T.D.; Yang, X. Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability. Proc. Natl. Acad. Sci. USA 2011, 108, 4870–4875. [Google Scholar] [CrossRef]
- Yeung, B.; Ho, K.-C.; Yang, X. WWP1 E3 Ligase Targets LATS1 for Ubiquitin-Mediated Degradation in Breast Cancer Cells. PLoS ONE 2013, 8, e61027. [Google Scholar] [CrossRef]
- Yang, F.; Xing, Y.; Li, Y.; Chen, X.; Jiang, J.; Ai, Z.; Wei, Y. Monoubiquitination of Cancer Stem Cell Marker CD133 at Lysine 848 Regulates Its Secretion and Promotes Cell Migration. Mol. Cell. Biol. 2018, 38, e00024-18. [Google Scholar] [CrossRef]
- Novellasdemunt, L.; Kucharska, A.; Jamieson, C.; Prange-Barczynska, M.; Baulies, A.; Antas, P.; van der Vaart, J.; Gehart, H.; Maurice, M.M.; Li, V.S. NEDD4 and NEDD4L regulate Wnt signalling and intestinal stem cell priming by degrading LGR5 receptor. EMBO J. 2020, 39, e102771. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, B.; Jiang, X.; Charlat, O.; Chen, A.; Mickanin, C.; Bauer, A.; Xu, W.; Yan, X.; Cong, F. The SIAH E3 ubiquitin ligases promote Wnt/β-catenin signaling through mediating Wnt-induced Axin degradation. Genes Dev. 2017, 31, 904–915. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wong, C.C.; Zhang, J.; Dong, Y.; Li, X.; Kang, W.; Chan, F.K.; Sung, J.J.Y.; Yu, J. RNF6 Promotes Colorectal Cancer by Activating the Wnt/β-Catenin Pathway via Ubiquitination of TLE3. Cancer Res. 2018, 78, 1958–1971. [Google Scholar] [CrossRef]
- Thomas, J.J.; Abed, M.; Heuberger, J.; Novak, R.; Zohar, Y.; Lopez, A.P.B.; Trausch-Azar, J.S.; Ilagan, M.X.; Benhamou, D.; Dittmar, G.; et al. RNF4-Dependent Oncogene Activation by Protein Stabilization. Cell Rep. 2016, 16, 3388–3400. [Google Scholar] [CrossRef]
- Liu, L.; Wong, C.C.; Gong, B.; Yu, J. Functional significance and therapeutic implication of ring-type E3 ligases in colorectal cancer. Oncogene 2018, 37, 148–159. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, F.; Chen, W.; Gao, C.; Wang, J. RNF144A suppresses ovarian cancer stem cell properties and tumor progression through regulation of LIN28B degradation via the ubiquitin-proteasome pathway. Cell Biol. Toxicol. 2021, 1–16. [Google Scholar] [CrossRef]
- Chen, W.; Patel, D.; Jia, Y.; Yu, Z.; Liu, X.; Shi, H.; Liu, H. MARCH8 Suppresses Tumor Metastasis and Mediates Degradation of STAT3 and CD44 in Breast Cancer Cells. Cancers 2021, 13, 2550. [Google Scholar] [CrossRef]
- Chène, P. Inhibiting the p53–MDM2 interaction: An important target for cancer therapy. Nat. Cancer 2003, 3, 102–109. [Google Scholar] [CrossRef]
- Andrews, A.; Warner, K.; Rodriguez-Ramirez, C.; Pearson, A.T.; Nör, F.; Zhang, Z.; Kerk, S.; Kulkarni, A.S.; Helman, J.I.; Brenner, J.C.; et al. Ablation of Cancer Stem Cells by Therapeutic Inhibition of the MDM2–p53 Interaction in Mucoepidermoid Carcinoma. Clin. Cancer Res. 2019, 25, 1588–1600. [Google Scholar] [CrossRef]
- Kao, S.-H.; Cheng, W.-C.; Wang, Y.-T.; Wu, H.-T.; Yeh, H.-Y.; Chen, Y.-J.; Tsai, M.-H.; Wu, K.-J. Regulation of miRNA biogenesis and histone modification by K63-polyubiquitinated DDX17 controls cancer stem-like features. Cancer Res. 2019, 79, 2549–2563. [Google Scholar] [CrossRef]
- Ci, Y.; Li, X.; Chen, M.; Zhong, J.; North, B.J.; Inuzuka, H.; He, X.; Li, Y.; Guo, J.; Dai, X. SCFβ-TRCP E3 ubiquitin ligase targets the tumor suppressor ZNRF3 for ubiquitination and degradation. Protein Cell 2018, 9, 879–889. [Google Scholar] [CrossRef]
- Gu, J.; Mao, W.; Ren, W.; Xu, F.; Zhu, Q.; Lu, C.; Lin, Z.; Zhang, Z.; Chu, Y.; Liu, R.; et al. Ubiquitin-protein ligase E3C maintains non-small-cell lung cancer stemness by targeting AHNAK-p53 complex. Cancer Lett. 2019, 443, 125–134. [Google Scholar] [CrossRef]
- Sato, T.; Okumura, F.; Ariga, T.; Hatakeyama, S. TRIM6 interacts with c-Myc and maintains pluripotency of mouse embryonal stem cells. J. Cell Sci. 2012, 125, 1544–1555. [Google Scholar] [CrossRef]
- Jaworska, A.M.; Wlodarczyk, N.A.; Mackiewicz, A.; Czerwinska, P. The role of TRIM family proteins in the regulation of cancer stem cell self-renewal. Stem Cells 2019, 38, 165–173. [Google Scholar] [CrossRef]
- Ito, K.; Bernardi, R.; Morotti, A.; Matsuoka, S.; Saglio, G.; Ikeda, Y.; Rosenblatt, J.; Avigan, D.E.; Teruya-Feldstein, J.; Pandolfi, P.P. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 2008, 453, 1072–1078. [Google Scholar] [CrossRef]
- Zhou, W.; Bao, S. PML-mediated signaling and its role in cancer stem cells. Oncogene 2014, 33, 1475–1484. [Google Scholar] [CrossRef]
- Ito, K.; Carracedo, A.; Weiss, D.; Arai, F.; Ala, U.; Avigan, D.E.; Schafer, Z.T.; Evans, R.M.; Suda, T.; Lee, C.-H.; et al. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat. Med. 2012, 18, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Y.-J.; Fakih, M.; Wiatrek, R.L.; Duldulao, M.; Chen, Z.; Chu, P.; Garcia-Aguilar, J.; Chen, Y. Role of SUMO activating enzyme in cancer stem cell maintenance and self-renewal. Nat. Commun. 2016, 7, 12326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; Yin, Y.-H.; Chen, H.-Z.; Feng, S.-Y.; Liu, J.-L.; Chen, L.; Fu, W.-L.; Sun, G.-C.; Yu, X.-G.; Xu, D.-G. TRIM24 promotes stemness and invasiveness of glioblastoma cells via activating Sox2 expression. Neuro-Oncology 2020, 22, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.A.; Gooding, A.J.; Valadkhan, S.; Schiemann, W.P. lncRNA BORG:TRIM28 Complexes Drive Metastatic Progression by Inducing α6 Integrin/CD49f Expression in Breast Cancer Stem Cells. Mol. Cancer Res. 2021, 19, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Kaneko, Y. Trim32 Facilitates Degradation of MYCN on Spindle Poles and Induces Asymmetric Cell Division in Human Neuroblastoma Cells. Cancer Res. 2014, 74, 5620–5630. [Google Scholar] [CrossRef]
- Izumi, H.; Kaneko, Y. Symmetry breaking in human neuroblastoma cells. Mol. Cell. Oncol. 2014, 1, e968510. [Google Scholar] [CrossRef][Green Version]
- Liyasova, M.S.; Ma, K.; Lipkowitz, S. Molecular Pathways: Cbl Proteins in Tumorigenesis and Antitumor Immunity—Opportunities for Cancer Treatment. Clin. Cancer Res. 2015, 21, 1789–1794. [Google Scholar] [CrossRef]
- Yang, H.; Lu, X.; Liu, Z.; Chen, L.; Xu, Y.; Wang, Y.; Wei, G.; Chen, Y. FBXW7 suppresses epithelial-mesenchymal transition, stemness and metastatic potential of cholangiocarcinoma cells. Oncotarget 2015, 6, 6310–6325. [Google Scholar] [CrossRef]
- Ranganathan, P.; Weaver, K.L.; Capobianco, A.J. Notch signalling in solid tumours: A little bit of everything but not all the time. Nat. Rev. Cancer 2011, 11, 338–351. [Google Scholar] [CrossRef]
- Harrison, H.; Farnie, G.; Brennan, K.R.; Clarke, R. Breast Cancer Stem Cells: Something Out of Notching? Cancer Res. 2010, 70, 8973–8976. [Google Scholar] [CrossRef]
- McGowan, P.M.; Simedrea, C.; Ribot, E.J.; Foster, P.J.; Palmieri, D.; Steeg, P.S.; Allan, A.L.; Chambers, A.F. Notch1 Inhibition Alters the CD44hi/CD24lo Population and Reduces the Formation of Brain Metastases from Breast Cancer. Mol. Cancer Res. 2011, 9, 834–844. [Google Scholar] [CrossRef]
- Nishina, S.-I.; Shiraha, H.; Nakanishi, Y.; Tanaka, S.; Matsubara, M.; Takaoka, N.; Uemura, M.; Horiguchi, S.; Kataoka, J.; Iwamuro, M.; et al. Restored expression of the tumor suppressor gene RUNX3 reduces cancer stem cells in hepatocellular carcinoma by suppressing Jagged1-Notch signaling. Oncol. Rep. 2011, 26, 523–531. [Google Scholar] [CrossRef]
- Alcalay, M.; Meani, N.; Gelmetti, V.; Fantozzi, A.; Fagioli, M.; Orleth, A.; Riganelli, D.; Sebastiani, C.; Cappelli, E.; Casciari, C.; et al. Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J. Clin. Investig. 2003, 112, 1751–1761. [Google Scholar] [CrossRef]
- Matsuoka, S.; Oike, Y.; Onoyama, I.; Iwama, A.; Arai, F.; Takubo, K.; Mashimo, Y.; Oguro, H.; Nitta, E.; Ito, K.; et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 2008, 22, 986–991. [Google Scholar] [CrossRef]
- Qiu, F.; Jin, Y.; Pu, J.; Huang, Y.; Hou, J.; Zhao, X.; Lu, Y. Aberrant FBXW7-mediated ubiquitination and degradation of ZMYND8 enhances tumor progression and stemness in bladder cancer. Exp. Cell Res. 2021, 407, 112807. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.-H.; Tseng, S.-H. Regulation of ZMYND8 to Treat Cancer. Molecules 2021, 26, 1083. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, L.; Su, P.; Lei, X.; Liu, X.; Wang, H.; Lu, L.; Bai, Y.; Xiong, T.; Li, D.; et al. MSX2 mediates entry of human pluripotent stem cells into mesendoderm by simultaneously suppressing SOX2 and activating NODAL signaling. Cell Res. 2015, 25, 1314–1332. [Google Scholar] [CrossRef]
- D’Annibale, S.; Kim, J.; Magliozzi, R.; Low, T.Y.; Mohammed, S.; Heck, A.; Guardavaccaro, D. Proteasome-dependent Degradation of Transcription Factor Activating Enhancer-binding Protein 4 (TFAP4) Controls Mitotic Division. J. Biol. Chem. 2014, 289, 7730–7737. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Vanderbilt, D.B.; Lin, C.-C.; Martin, K.H.; Brundage, K.M.; Ruppert, J.M. SOX9 inhibits β-TrCP-mediated protein degradation to promote nuclear GLI1 expression and cancer stem cell properties. J. Cell Sci. 2015, 128, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Plechanovová, A.; Jaffray, E.G.; McMahon, S.A.; Johnson, K.A.; Navrátilová, I.; Naismith, J.H.; Hay, R.T. Mechanism of ubiquitylation by dimeric RING ligase RNF4. Nat. Struct. Mol. Biol. 2011, 18, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.C.; Kleinman, J.I.; Brittain, S.M.; Lee, P.S.; Chung, C.Y.S.; Kim, K.; Petri, Y.; Thomas, J.R.; Tallarico, J.A.; McKenna, J.M.; et al. Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications. ACS Chem. Biol. 2019, 14, 2430–2440. [Google Scholar] [CrossRef]
- Holzer, P.; Masuya, K.; Furet, P.; Kallen, J.; Valat-Stachyra, T.; Ferretti, S.; Berghausen, J.; Bouisset-Leonard, M.; Buschmann, N.; Pissot-Soldermann, C.; et al. Discovery of a Dihydroisoquinolinone Derivative (NVP-CGM097): A Highly Potent and Selective MDM2 Inhibitor Undergoing Phase 1 Clinical Trials in p53wt Tumors. J. Med. Chem. 2015, 58, 6348–6358. [Google Scholar] [CrossRef]
- Wang, X.; Herr, R.A.; Hansen, T. Viral and cellular MARCH ubiquitin ligases and cancer. Semin. Cancer Biol. 2008, 18, 441–450. [Google Scholar] [CrossRef]
- Eaton, D.C.; Malik, B.; Bao, H.-F.; Yu, L.; Jain, L. Regulation of Epithelial Sodium Channel Trafficking by Ubiquitination. Proc. Am. Thorac. Soc. 2010, 7, 54–64. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Chen, X.; Li, J.; Yao, W.; Huang, S.; Gu, A.; Lei, Q.-Y.; Mao, Y.; Wen, W. A multi-lock inhibitory mechanism for fine-tuning enzyme activities of the HECT family E3 ligases. Nat. Commun. 2019, 10, 3162. [Google Scholar] [CrossRef]
- Jeon, S.-A.; Kim, D.W.; Lee, D.-B.; Cho, J.-Y. NEDD4 Plays Roles in the Maintenance of Breast Cancer Stem Cell Characteristics. Front. Oncol. 2020, 10, 1680. [Google Scholar] [CrossRef]
- Lee, H.-J.; Li, C.-F.; Ruan, D.; He, J.; Montal, E.D.; Lorenz, S.; Girnun, G.D.; Chan, C.-H. Non-proteolytic ubiquitination of Hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat. Commun. 2019, 10, 2625. [Google Scholar] [CrossRef]
- Shibata, M.; Hoque, M.O. Targeting Cancer Stem Cells: A Strategy for Effective Eradication of Cancer. Cancers 2019, 11, 732. [Google Scholar] [CrossRef]

| Protein | Substrates | Functional Roles | References |
|---|---|---|---|
| CBL | JAK2 | Intervenes in the development of hematopoietic stem cells (HSCs). | [54,55] |
| FBXW2 | MSX2 | Involved in the pluripotency and maintenance of the properties of stem cells, through the degradation of MSX2, a repressor of SOX2. | [56] |
| FBXW7 | Notch1, ZMYND8 | Controls proteasome-mediated degradation of Notch and ZMYND8 impacting on CSCs in different types of cancers. Plays a critical role regulating the balance between self-renewal and dormancy of stem cells. | [57,58,59] |
| FBXW8 | Nanog | Prevents the maintenance of the characteristic properties of stem cells, losing the capacity for pluripotency and self-renewal. | [60] |
| FBXO11 | BAHD1 | Targets BAHD1 influencing on the transcriptional repression mediated by PRC2 during erythropoiesis. | [61] |
| RNF43, ZNRF3 | Frizzel and LRP6 | Negative regulators of Wnt signaling by targeting its coreceptors to degradation, influencing stemness. | [62,63,64] |
| WWP1, ITCH | LATS1 | Promote the Hippo pathway main regulator LATS1 degradation impairing stem cell differentiation and self-renewal. | [65,66] |
| NEDD4 | LGR5, DVL2 | Plays an important role for ISC self-renewal by regulating Wnt/β-catenin signaling pathway. Degrades LGR5 and DVL2, downregulating stemness and cell migration. | [67,68] |
| SIAH1/2 | Axin | Promotes axin degradation leading to an excessive accumulation of β-catenin that favors the excessive expression of genes related to the stem process. | [69] |
| RNF6 | TLE3 | Enhances β-catenin activity by suppressing its inhibitor (TLE3). Participates in the regulation of cell proliferation and differentiation. | [70] |
| RNF4 | β-catenin, Myc, c-Jun, Notch | Stabilizes short-lived oncogenic transcription factors. Positively regulates Wnt and Notch signaling pathways, important for pluripotency, cell proliferation and stem cell differentiation. | [71,72] |
| RNF144A | LIN28B | Prevents epithelial ovarian cancer (EOC) cells from acquiring stem cell properties by inducing LIN28B degradation. | [73] |
| MARCH8 | CD44, STAT3 | Degrades STAT3 and CD44 thereby impairing the phenotypic functions regulated by cancer stem cells. | [74] |
| MDM2 | p53 | Degrades one of the most important tumor suppressors (p53). Acts in multiple cellular processes, such as cell cycle regulation, DNA repair and cell differentiation. | [75,76] |
| HECTH9 | DDX17 | Promotes DDX17 poly-ubiquitination by K63 under hypoxia conditions that induces the transcription of genes related to cancer stemness properties. | [77] |
| β-TrCP | ZNRF3, β-catenin | Negatively regulates Wnt signaling by targeting β-catenin and positively regulates it by targeting ZNRF3. | [78] |
| UBE3C | AHNAK | Promotes AHNAK degradation. AHNAK is a p53 cofactor that inhibits stemness-related gene transcription. Therefore, UBEC3 acts as a key post-translational mechanism involved in maintaining the CSC properties of non-small cell lung cancer (NSCLC). | [79] |
| TRIM6 | c-Myc | Promotes the differentiation of embryonic stem cells by enhancing the activity of central transcription factors and the induction of specific signaling pathways. | [80] |
| TRIM16 | Gli-1 | Suppresses the properties of CSCs by degrading Gli-1, the effector of the Hh signaling pathway. | [81] |
| TRIM19 | Unknown | TRIM19 (or PML) positively regulates CSCs division and maintenance. In leukemia-initiating cells, TRIM19-null shows remarkable reduction in survival, indicating the positive role of leukemia-initiating maintenance. | [82,83,84] |
| TRIM21 | Oct-1 | Ubiquitinates Oct-1 and consequently reduces its stability, leading to a loss of self-renewal of CSCs. Oct-1 is a transcription factor that positively regulates ALDH1A1, important for the maintenance of CSC properties. | [85] |
| TRIM24 | Sox2 | Promotes stemness and invasiveness of the glioblastoma stem cells by activating the pluripotency transcription factor Sox2. | [86] |
| TRIM28 | Unknown | Interacts with BORG and its association promotes the expression of Nanog, Aldh1a3 and Itga6 enhancing the stem cell phenotype in triple negative breast cancer. | [87] |
| TRIM32 | c-Myc, MYCN | Promotes a RING-mediated ubiquitination and proteasomal degradation of c-Myc, inducing cell differentiation. It also induces asymmetric cell division and suppresses sphere formation in neuroblastoma initiating cells by promoting MYCN degradation. | [88,89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga, M.; Rodríguez-Alonso, A.; Alfonsín, G.; Rodríguez, J.J.E.; Breijo, S.M.; Chantada, V.; Figueroa, A. Protein Degradation by E3 Ubiquitin Ligases in Cancer Stem Cells. Cancers 2022, 14, 990. https://doi.org/10.3390/cancers14040990
Quiroga M, Rodríguez-Alonso A, Alfonsín G, Rodríguez JJE, Breijo SM, Chantada V, Figueroa A. Protein Degradation by E3 Ubiquitin Ligases in Cancer Stem Cells. Cancers. 2022; 14(4):990. https://doi.org/10.3390/cancers14040990
Chicago/Turabian StyleQuiroga, Macarena, Andrea Rodríguez-Alonso, Gloria Alfonsín, Juan José Escuder Rodríguez, Sara M. Breijo, Venancio Chantada, and Angélica Figueroa. 2022. "Protein Degradation by E3 Ubiquitin Ligases in Cancer Stem Cells" Cancers 14, no. 4: 990. https://doi.org/10.3390/cancers14040990
APA StyleQuiroga, M., Rodríguez-Alonso, A., Alfonsín, G., Rodríguez, J. J. E., Breijo, S. M., Chantada, V., & Figueroa, A. (2022). Protein Degradation by E3 Ubiquitin Ligases in Cancer Stem Cells. Cancers, 14(4), 990. https://doi.org/10.3390/cancers14040990







