Prevalence of Sleep Disorders, Risk Factors and Sleep Treatment Needs of Adolescents and Young Adult Childhood Cancer Patients in Follow-Up after Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Procedures
2.3. Measures
2.3.1. Holland Sleep Disorder Questionnaire (HSDQ)
2.3.2. Sociodemographic, Cancer-Related Factors and the Presence of Comorbid Health Conditions
2.3.3. Sleep Treatment Needs
2.4. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Prevalence of Sleep Disorders
3.3. Risk Factors
3.3.1. Sex
3.3.2. Age Group: Adolescents Versus Young Adults
3.3.3. Co-Morbid Health Conditions
3.3.4. Childhood Cancer-Related Risk Factors
3.4. Sleep Medication Use
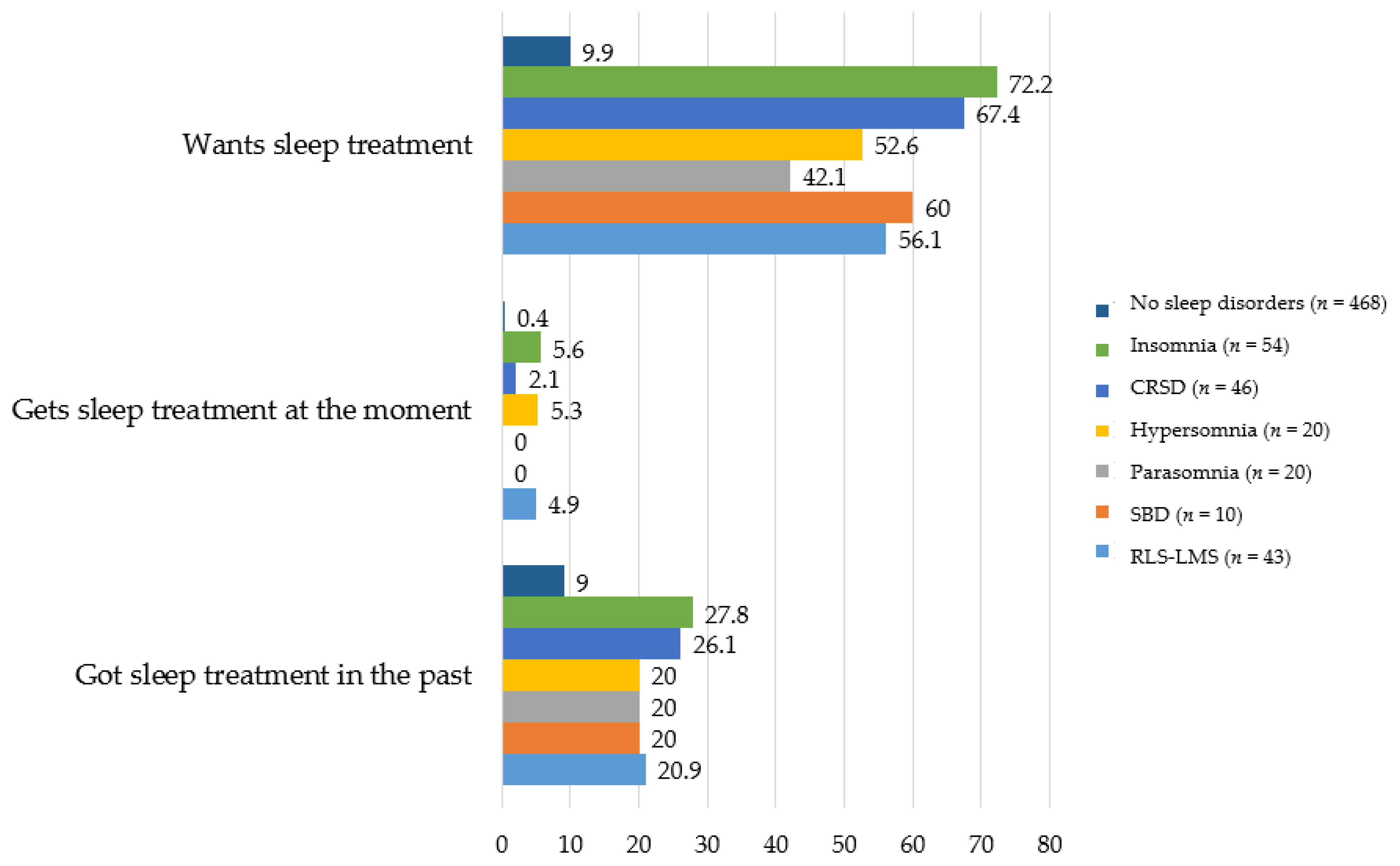
3.5. Treatment Needs
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combs, D.; Goodwin, J.L.; Quan, S.F.; Morgan, W.J.; Shetty, S.; Parthasarathy, S. Insomnia, health-related quality of life and health outcomes in children: A seven year longitudinal cohort. Sci. Rep. 2016, 6, 27921. [Google Scholar] [CrossRef]
- Svertsen, B.; Lallukka, T.; Salo, P.; Pallesen, S.; Hysing, M.; Krokstad, S.; Øverland, S. Insomnia as a risk factor for ill health: Results from the large population-based prospective HUNT Study in N orway. J. Sleep Res. 2014, 23, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H. Insomnia as a health risk factor. Behav. Sleep Med. 2003, 1, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Mallory, L.J.; Lichstein, K.L.; Durrence, H.H.; Riedel, B.W.; Bush, A.J. Comorbidity of chronic insomnia with medical problems. Sleep 2007, 30, 213–218. [Google Scholar] [CrossRef]
- Uhlig, B.L.; Sand, T.; Ødegård, S.S.; Hagen, K. Prevalence and associated factors of DSM-V insomnia in Norway: The Nord-Trøndelag Health Study (HUNT 3). Sleep Med. 2014, 15, 708–713. [Google Scholar] [CrossRef] [PubMed]
- McArdle, N.; Ward, S.V.; Bucks, R.S.; Maddison, K.; Smith, A.; Huang, R.-C.; Pennell, C.E.; Hillman, D.R.; Eastwood, P.R. The prevalence of common sleep disorders in young adults: A descriptive population-based study. Sleep 2020, 43, zsaa072. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.; Group, A.S.W. Insufficient sleep in adolescents and young adults: An update on causes and consequences. Pediatrics 2014, 134, e921–e932. [Google Scholar] [CrossRef] [PubMed]
- Colrain, I.M.; Baker, F.C. Changes in sleep as a function of adolescent development. Neuropsychol. Rev. 2011, 21, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.J.; Wolfson, A.R.; Tarokh, L.; Carskadon, M.A. An update on adolescent sleep: New evidence informing the perfect storm model. J. Adolesc. 2018, 67, 55–65. [Google Scholar] [CrossRef]
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A. Sleep is essential to health: An American Academy of Sleep Medicine position statement. J. Clin. Sleep Med. 2021, 17, 2115–2119. [Google Scholar] [CrossRef]
- Meltzer, L.J.; Johnson, C.; Crosette, J.; Ramos, M.; Mindell, J.A. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics 2010, 125, e1410–e1418. [Google Scholar] [CrossRef]
- Abad, V.C.; Guilleminault, C. Diagnosis and treatment of sleep disorders: A brief review for clinicians. Dialogues Clin. Neurosci. 2003, 5, 371. [Google Scholar] [PubMed]
- Daniel, L.C.; van Litsenburg, R.R.; Rogers, V.E.; Zhou, E.S.; Ellis, S.J.; Wakefield, C.E.; Stremler, R.; Walter, L.; Crabtree, V.M.; International Psycho-Oncology Society Pediatrics Special Interest Group. A call to action for expanded sleep research in pediatric oncology: A position paper on behalf of the International Psycho-Oncology Society Pediatrics Special Interest Group. Psycho-Oncology 2020, 29, 465–474. [Google Scholar] [CrossRef]
- Brown, A.L.; Raghubar, K.P.; Taylor, O.A.; Bernhardt, M.B.; Kahalley, L.S.; Pan, W.; Lupo, P.J.; Hockenberry, M.J.; Scheurer, M.E. Prospective patient-reported symptom profiles associated with pediatric acute lymphoblastic leukemia relapse. Supportive Care Cancer 2020, 29, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- van Gorp, M.; van Erp, L.M.E.; Maas, A.; Kremer, L.C.M.; van Dulmen-den Broeder, E.; Tissing, W.J.E.; Loonen, J.J.; van der Pal, H.J.H.; de Vries, A.C.H.; van den Heuvel-Eibrink, M.M.; et al. Increased health-related quality of life impairments of male and female survivors of childhood cancer: DCCSS LATER 2 psycho-oncology study. Cancer 2021. [Google Scholar] [CrossRef]
- Fidler, M.M.; Ziff, O.J.; Wang, S.; Cave, J.; Janardhanan, P.; Winter, D.L.; Kelly, J.; Mehta, S.; Jenkinson, H.; Frobisher, C. Aspects of mental health dysfunction among survivors of childhood cancer. Br. J. Cancer 2015, 113, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.H.; van der Graaf, W.T.; van der Meer, D.J.; Manten-Horst, E.; Husson, O. Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview. Cancers 2021, 13, 4847. [Google Scholar] [CrossRef] [PubMed]
- van Erp, L.; Maurice-Stam, H.; Kremer, L.; Tissing, W.; van der Pal, H.; de Vries, A.; van den Heuvel-Eibrink, M.; Versluys, B.; Huizinga, G.; Grootenhuis, M. A vulnerable age group: The impact of cancer on the psychosocial well-being of young adult childhood cancer survivors. Supportive Care Cancer 2021, 29, 4751–4761. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders; Diagnostic & Coding Manual; American Academy of Sleep Medicine: Darien, IL, USA, 2005; pp. 51–55. [Google Scholar]
- Rogers, V.E.; Zhu, S.; Ancoli-Israel, S.; Hinds, P.S. Impairment in circadian activity rhythms occurs during dexamethasone therapy in children with leukemia. Pediatric Blood Cancer 2014, 61, 1986–1991. [Google Scholar] [CrossRef]
- Steur, L.M.; Kaspers, G.J.; Van Someren, E.J.; Van Eijkelenburg, N.K.; Van der Sluis, I.M.; Dors, N.; Van den Bos, C.; Tissing, W.J.; Grootenhuis, M.A.; Van Litsenburg, R.R. Sleep–wake rhythm disruption is associated with cancer-related fatigue in pediatric acute lymphoblastic leukemia. Sleep 2020, 43, zsz320. [Google Scholar] [CrossRef]
- Green, D.M.; Cox, C.L.; Zhu, L.; Krull, K.R.; Srivastava, D.K.; Stovall, M.; Nolan, V.G.; Ness, K.K.; Donaldson, S.S.; Oeffinger, K.C. Risk factors for obesity in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2012, 30, 246. [Google Scholar] [CrossRef]
- Gami, A.S.; Caples, S.M.; Somers, V.K. Obesity and obstructive sleep apnea. Endocrinol. Metab. Clin. 2003, 32, 869–894. [Google Scholar] [CrossRef]
- Mandrell, B.N.; Wise, M.; Schoumacher, R.A.; Pritchard, M.; West, N.; Ness, K.K.; Crabtree, V.M.; Merchant, T.E.; Morris, B. Excessive daytime sleepiness and sleep-disordered breathing disturbances in survivors of childhood central nervous system tumors. Pediatric Blood Cancer 2012, 58, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Manley, P.E.; McKendrick, K.; McGillicudy, M.; Chi, S.N.; Kieran, M.W.; Cohen, L.E.; Kothare, S.; Scott, R.M.; Goumnerova, L.C.; Sun, P. Sleep dysfunction in long term survivors of craniopharyngioma. J. Neuro-Oncol. 2012, 108, 543–549. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, J.; Pillen, S.; van Litsenburg, R.; Vandenbussche, N.; de Bont, J.; Schouten-van Meeteren, A.; van Santen, H. The importance of specialized sleep investigations in children with a suprasellar tumor. Pituitary 2020, 23, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Garland, S.N.; Mao, J.J.; Applebaum, A.J.; Li, Q.S.; Gehrman, P.R.; DuHamel, K.N.; Verrico, Z. Hyperarousal and Insomnia in Survivors of Cancer. Int. J. Behav. Med. 2021, 28, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Roberts, C.R.; Duong, H.T. Chronic insomnia and its negative consequences for health and functioning of adolescents: A 12-month prospective study. J. Adolesc. Health 2008, 42, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.L.; Carpenter, J.S.; Manchanda, S.; Rand, K.L.; Skaar, T.C.; Weaver, M.; Chernyak, Y.; Zhong, X.; Igega, C.; Landis, C. Systematic review of sleep disorders in cancer patients: Can the prevalence of sleep disorders be ascertained? Cancer Med. 2015, 4, 183–200. [Google Scholar] [CrossRef]
- Daniel, L.C.; Wang, M.; Mulrooney, D.A.; Srivastava, D.K.; Schwartz, L.A.; Edelstein, K.; Brinkman, T.M.; Zhou, E.S.; Howell, R.M.; Gibson, T.M. Sleep, emotional distress, and physical health in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Psycho-Oncology 2019, 28, 903–912. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Ness, K.K.; Neglia, J.P.; Whitton, J.A.; Green, D.M.; Zeltzer, L.K.; Robison, L.L.; Mertens, A.C. Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the childhood cancer survivor study (CCSS). Sleep 2008, 31, 271–281. [Google Scholar] [CrossRef]
- Steur, L.M.; Grootenhuis, M.A.; Van Someren, E.J.; Van Eijkelenburg, N.K.; Van der Sluis, I.M.; Dors, N.; Van den Bos, C.; Tissing, W.J.; Kaspers, G.J.; Van Litsenburg, R.R. High prevalence of parent-reported sleep problems in pediatric patients with acute lymphoblastic leukemia after induction therapy. Pediatric Blood Cancer 2020, 67, e28165. [Google Scholar] [CrossRef]
- Zupanec, S.; Jones, H.; Stremler, R. Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. J. Pediatric Oncol. Nurs. 2010, 27, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Steur, L.M.; Kolk, R.H.; Mooij, F.; de Vries, R.; Grootenhuis, M.A.; Kaspers, G.J.; Van Litsenburg, R.R. The prevalence and risk factors of sleep problems in pediatric oncology: Its effect on quality of life during and after cancer treatment. Expert Rev. Qual. Life Cancer Care 2016, 1, 153–171. [Google Scholar] [CrossRef]
- Rosen, G.; Brand, S.R. Sleep in children with cancer: Case review of 70 children evaluated in a comprehensive pediatric sleep center. Supportive Care Cancer 2011, 19, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Altena, E.; Bjorvatn, B.; Blom, K.; Bothelius, K.; Devoto, A.; Espie, C.A.; Frase, L.; Gavriloff, D.; Tuuliki, H. The European Academy for Cognitive Behavioural Therapy for Insomnia: An initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J. Sleep Res. 2020, 29, e12967. [Google Scholar] [CrossRef] [PubMed]
- Peersmann, S.H.; van Straten, A.; Kaspers, G.J.; Thano, A.; van den Bergh, E.; Grootenhuis, M.A.; van Litsenburg, R.R. Does the guided online cognitive behavioral therapy for insomnia “i-Sleep youth” improve sleep of adolescents and young adults with insomnia after childhood cancer?(MICADO-study): Study protocol of a randomized controlled trial. Trials 2021, 22, 307. [Google Scholar] [CrossRef]
- Kerkhof, G.A.; Geuke, M.E.; Brouwer, A.; Rijsman, R.M.; Schimsheimer, R.J.; Van Kasteel, V. Holland Sleep Disorders Questionnaire: A new sleep disorders questionnaire based on the International Classification of Sleep Disorders-2. J. Sleep Res. 2013, 22, 104–107. [Google Scholar] [CrossRef]
- de Bruin, E.J.; Bögels, S.M.; Oort, F.J.; Meijer, A.M. Efficacy of cognitive behavioral therapy for insomnia in adolescents: A randomized controlled trial with internet therapy, group therapy and a waiting list condition. Sleep 2015, 38, 1913–1926. [Google Scholar] [CrossRef]
- de Bruin, E.J.; Bögels, S.M.; Oort, F.J.; Meijer, A.M. Improvements of adolescent psychopathology after insomnia treatment: Results from a randomized controlled trial over 1 year. J. Child Psychol. Psychiatry 2018, 59, 509–522. [Google Scholar] [CrossRef]
- de Bruin, E.J.; Meijer, A.M. The impact of online therapeutic feedback on outcome measures in Internet-CBTI for adolescents with insomnia. Sleep Med. 2017, 29, 68–75. [Google Scholar] [CrossRef]
- de Bruin, E.J.; Oort, F.J.; Bögels, S.M.; Meijer, A.M. Efficacy of internet and group-administered cognitive behavioral therapy for insomnia in adolescents: A pilot study. Behav. Sleep Med. 2014, 12, 235–254. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, E.J.; van Steensel, F.J.; Meijer, A.M. Cost-effectiveness of group and internet cognitive behavioral therapy for insomnia in adolescents: Results from a randomized controlled trial. Sleep 2016, 39, 1571–1581. [Google Scholar] [CrossRef]
- Dewald-Kaufmann, J.F.; Bruin, E.J.; Smits, M.; Zijlstra, B.J.; Oort, F.J.; Meijer, A.M. Chronic sleep reduction in adolescents—clinical cut-off scores for the Chronic Sleep Reduction Questionnaire (CSRQ). J. Sleep Res. 2018, 27, e12653. [Google Scholar] [CrossRef]
- Dewald-Kaufmann, J.F.; Oort, F.; Meijer, A. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: A randomized controlled trial. J. Child Psychol. Psychiatry 2014, 55, 273–283. [Google Scholar] [CrossRef]
- Meppelink, R.; de Bruin, E.I.; Bögels, S.M. Meditation or Medication? Mindfulness training versus medication in the treatment of childhood ADHD: A randomized controlled trial. BMC Psychiatry 2016, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- van Maanen, A.; Dewald-Kaufmann, J.F.; Oort, F.J.; de Bruin, E.J.; Smits, M.G.; Short, M.A.; Gradisar, M.; Kerkhof, G.A.; Meijer, A.M. Screening for sleep reduction in adolescents through self-report: Development and validation of the sleep reduction screening questionnaire (SRSQ). In Proceedings of the Child & Youth Care Forum; Springer: New York, NY, USA, 2014; pp. 607–619. [Google Scholar]
- Sen, T.; Spruyt, K. Pediatric sleep tools: An updated literature review. Front. Psychiatry 2020, 317. [Google Scholar] [CrossRef] [PubMed]
- Van Meter, A.R.; Anderson, E.A. Evidence base update on assessing sleep in youth. J. Clin. Child Adolesc. Psychol. 2020, 49, 701–736. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, G.A. Epidemiology of sleep and sleep disorders in The Netherlands. Sleep Med. 2017, 30, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Onderwijsindeling, S. Standard Educational Classification; Centraal Bureau voor de Statistiek [Statistics Netherlands]: Den Haag/Heerlen, The Netherlands, 2016. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Morin, C.M.; Jarrin, D.C. Epidemiology of insomnia: Prevalence, course, risk factors, and public health burden. Sleep Med. Clin. 2013, 8, 281–297. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Chung, K.F.; Kan, K.K.K.; Yeung, W.F. Insomnia in adolescents: Prevalence, help-seeking behaviors, and types of interventions. Child Adolesc. Ment. Health 2014, 19, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Roth, T.; Schultz, L.; Breslau, N. Epidemiology of DSM-IV insomnia in adolescence: Lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics 2006, 117, e247–e256. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Roberts, R.E.; Zulley, J.; Smirne, S.; Priest, R.G. Prevalence and patterns of problematic sleep among older adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef]
- Danielsson, K.; Markström, A.; Broman, J.-E.; von Knorring, L.; Jansson-Fröjmark, M. Delayed sleep phase disorder in a Swedish cohort of adolescents and young adults: Prevalence and associated factors. Chronobiol. Int. 2016, 33, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Lovato, N.; Gradisar, M.; Short, M.; Dohnt, H.; Micic, G. Delayed sleep phase disorder in an Australian school-based sample of adolescents. J. Clin. Sleep Med. 2013, 9, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Christen, S.; Roser, K.; Mulder, R.L.; Ilic, A.; Lie, H.C.; Loonen, J.J.; Mellblom, A.V.; Kremer, L.C.; Hudson, M.M.; Constine, L.S. Recommendations for the surveillance of cancer-related fatigue in childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. J. Cancer Surviv. 2020, 14, 923–938. [Google Scholar] [CrossRef]
- Steur, L.; Kaspers, G.; Van Someren, E.; Van Eijkelenburg, N.; Van der Sluis, I.; Dors, N.; van den Bos, C.; Tissing, W.; Grootenhuis, M.; Van Litsenburg, R. The impact of maintenance therapy on sleep-wake rhythms and cancer-related fatigue in pediatric acute lymphoblastic leukemia. Supportive Care Cancer 2020, 28, 5983–5993. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.C.; Meltzer, L.J.; Gross, J.Y.; Flannery, J.L.; Forrest, C.B.; Barakat, L.P. Sleep practices in pediatric cancer patients: Indirect effects on sleep disturbances and symptom burden. Psycho-Oncology 2021, 30, 910–918. [Google Scholar] [CrossRef]
- Bjorvatn, B.; Leissner, L.; Ulfberg, J.; Gyring, J.; Karlsborg, M.; Regeur, L.; Skeidsvoll, H.; Nordhus, I.H.; Pallesen, S. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005, 6, 307–312. [Google Scholar] [CrossRef]
- Picchietti, D.; Allen, R.P.; Walters, A.S.; Davidson, J.E.; Myers, A.; Ferini-Strambi, L. Restless legs syndrome: Prevalence and impact in children and adolescents—The Peds REST study. Pediatrics 2007, 120, 253–266. [Google Scholar] [CrossRef]
- Sánchez-Armengol, A.; Fuentes-Pradera, M.A.; Capote-Gil, F.; García-Díaz, E.; Cano-Gómez, S.; Carmona-Bernal, C.; Castillo-Gómez, J. Sleep-related breathing disorders in adolescents aged 12 to 16 years: Clinical and polygraphic findings. Chest 2001, 119, 1393–1400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fiorentino, L.; Rissling, M.; Liu, L.; Ancoli-Israel, S. The symptom cluster of sleep, fatigue and depressive symptoms in breast cancer patients: Severity of the problem and treatment options. Drug Discov. Today Dis. Models 2011, 8, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Brimeyer, C.; Adams, L.; Zhu, L.; Srivastava, D.K.; Wise, M.; Hudson, M.M.; Crabtree, V.M. Sleep complaints in survivors of pediatric brain tumors. Supportive Care Cancer 2016, 24, 23–31. [Google Scholar] [CrossRef]
- van Kooten, J.A.; Maurice-Stam, H.; Schouten, A.Y.; van Vuurden, D.G.; Granzen, B.; Gidding, C.; de Ruiter, M.A.; van Litsenburg, R.R.; Grootenhuis, M.A. High occurrence of sleep problems in survivors of a childhood brain tumor with neurocognitive complaints: The association with psychosocial and behavioral executive functioning. Pediatric Blood Cancer 2019, 66, e27947. [Google Scholar] [CrossRef] [PubMed]
- Verberne, L.M.; MAURICE-STAM, H.; Grootenhuis, M.A.; Van Santen, H.M.; Schouten-Van Meeteren, A.Y. Sleep disorders in children after treatment for a CNS tumour. J. Sleep Res. 2012, 21, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Jacola, L.M.; Conklin, H.M.; Scoggins, M.A.; Ashford, J.M.; Merchant, T.E.; Mandrell, B.N.; Ogg, R.J.; Curtis, E.; Wise, M.S.; Indelicato, D.J. Investigating the role of hypothalamic tumor involvement in sleep and cognitive outcomes among children treated for craniopharyngioma. J. Pediatric Psychol. 2016, 41, 610–622. [Google Scholar] [CrossRef][Green Version]
- Müller, H.L. Increased daytime sleepiness in patients with childhood craniopharyngioma and hypothalamic tumor involvement: Review of the literature and perspectives. Int. J. Endocrinol. 2010, 2010, 519607. [Google Scholar] [CrossRef]
- Koopman-Verhoeff, M.E.; van den Dries, M.A.; van Seters, J.J.; Luijk, M.P.; Tiemeier, H.; Luik, A.I. Association of sleep problems and melatonin use in school-aged children. JAMA Pediatrics 2019, 173, 883–885. [Google Scholar] [CrossRef]
- Melatonine. Farmacotherapeutisch Kompas. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/m/melatonine#indicaties (accessed on 25 November 2021).
- Keijzer, H.; Smits, M.G.; Duffy, J.F.; Curfs, L.M. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med. Rev. 2014, 18, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lea, S.; Martins, A.; Fern, L.A.; Bassett, M.; Cable, M.; Doig, G.; Morgan, S.; Soanes, L.; Whelan, M.; Taylor, R.M. The support and information needs of adolescents and young adults with cancer when active treatment ends. BMC Cancer 2020, 20, 697. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, M.J.; Harju, E.; Michel, G. The unmet needs of childhood cancer survivors in long-term follow-up care: A qualitative study. Psycho-Oncol. 2021, 30, 485–492. [Google Scholar] [CrossRef]
- Garland, S.N. A Step in the Right Direction: Making Cognitive-Behavioral Therapy for Insomnia More Accessible to People Diagnosed with Cancer; Oxford University Press US: New York, NY, USA, 2021. [Google Scholar]
- van Litsenburg, R.R.; Huisman, J.; Hoogerbrugge, P.M.; Egeler, R.M.; Kaspers, G.J.; Gemke, R.J. Impaired sleep affects quality of life in children during maintenance treatment for acute lymphoblastic leukemia: An exploratory study. Health Qual. Life Outcomes 2011, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Marloes van Gorp, M.; Maurice-Stam, H.; Teunissen, L.C.; Kilsdonk, E.; van Dijk, J.; Sulkers, M.; Tissing, W.J.E.; van Litsenburg, R.R.L.; Grootenhuis, M.A. Psychosocial function of Dutch children with cancer and their caregivers during different phases of the COVID-19 pandemic. Pediatr. Blood Cancer 2022, e29535. [Google Scholar] [CrossRef]
- Kocevska, D.; Blanken, T.F.; Van Someren, E.J.; Rösler, L. Sleep quality during the COVID-19 pandemic: Not one size fits all. Sleep Med. 2020, 76, 86–88. [Google Scholar] [CrossRef]
- Lin, Y.N.; Liu, Z.R.; Li, S.Q.; Li, C.X.; Zhang, L.; Li, N.; Sun, X.W.; Li, H.P.; Zhou, J.P.; Li, Q.Y. Burden of sleep disturbance during COVID-19 pandemic: A systematic review. Nat. Sci. Sleep 2021, 13, 933. [Google Scholar] [CrossRef]
| Characteristics | Responders (n = 576) | Non-responders (n = 456) | p-Value |
|---|---|---|---|
| Sociodemographic | |||
| Age at study invitation (y), mean (SD) | 17.00 (2.91) | 17.59 (2.91) | 0.18 |
| Age group, n (%) | |||
| Adolescents (12–17 y) | 328 (56.9) | 260 (57.0) | 0.98 |
| Young adult (18–26 y) | 248 (43.1) | 196 (43.0) | |
| Sex, n (%) | |||
| Female | 285 (49.5) | 184 (40.4) | <0.01 |
| Current educational level, n (%) | |||
| Low | 139 (24.1) | NA | |
| Middle | 326 (57.1) | ||
| High | 104 (18.1) | ||
| Country of birth, n (%) | |||
| The Netherlands | 548 (95.1) | NA | |
| Other | 20 (3.5) | ||
| Childhood cancer-specific | |||
| Age at diagnosis (y), mean (SD) | 12.83 (3.19) | 13.52 (2.62) | 0.89 |
| Time since diagnosis in years, mean (SD) | 3.95 (2.35) | 3.81 (1.98) | 0.28 |
| Diagnosis groups, n (%) | |||
| Hemato-oncology | 256 (44.4) | 212 (46.6) | |
| Neuro-oncology | 136 (23.6) | 95 (20.9) | 0.52 |
| Solid | 184 (31.9) | 148 (32.5) | |
| Time since end of treatment in years, mean (SD) | 3.21 (2.23) | NA | |
| Type of oncological treatment, n (%) | |||
| No treatment | 8 (1.4) | NA | |
| Chemotherapy | 423 (73.4) | ||
| Radiation | 147 (25.5) | ||
| Surgery | 312 (54.2) | ||
| Stem cell transplantation | 36 (6.3) | ||
| Mixed/Other | 29 (5.0) | ||
| Comorbid health problems, n (%) | |||
| No | 462 (80.2) | NA | |
| Yes | 102 (17.7) | ||
| Medical | 68 (11.8) | ||
| Psychological | 34 (5.9) |
| Sleep Disorders | Childhood Cancer Cohort | Published Data 1 | ||||
|---|---|---|---|---|---|---|
| Total Group N (%) n = 565 | Males, n (%) n = 282 | Females, n (%) n = 282 | Adolescents 12–17 y, n (%) n = 323 | Young Adults 18–26 y, n (%) n = 242 | Adults 18–70 y from the General Population 1, n (%) n = 2089 | |
| Insomnia | 54 (9.6) | 16 (5.7) | 38 (13.4) | 24 (7.4) | 30 (12.4) | 171 (8.2) |
| CRSD | 46 (8.1) | 18 (6.4) | 28 (9.9) | 18 (5.6) | 28 (11.6) | 110 (5.3) |
| RLS/LMS | 43 (7.6) | 15 (5.3) | 28 (9.9) | 21 (6.5) | 22 (9.1) | 261 (12.5) |
| Parasomnia | 20 (3.5) | 3 (1.1) | 17 (6.0) | 9 (2.8) | 11 (4.5) | 128 (6.1) |
| Hypersomnia | 20 (3.5) | 4 (1.4) | 16 (5.7) | 6 (1.9) | 14 (5.8) | 124 (5.9) |
| SBD | 10 (1.8) | 2 (0.7) | 8 (2.8) | 3 (0.9) | 7 (2.9) | 148 (7.1) |
| Risk Factors | Insomnia (n = 54) | CRSD (n = 46) | RLS/LMS (n = 43) | |||
|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable | |
| Female | 2.59 (1.41–4.76) ** | 2.33 (1.25–4.35) ** | 1.62 (0.87–3.00) | - | 1.96 (1.02–3.76) * | 1.89 (0.98–3.66) |
| Age group: young adults (ref: adolescents) | 1.76 (1.00–3.10) * | 1.55 (0.86–2.80) | 2.22 (1.20–4.11) * | 2.15 (1.13–4.07) * | 1.44 (0.77–2.68) | - |
| Having a comorbid health condition | 3.00 (1.62–5.53) *** | 2.89 (1.55–5.40) ** | 3.14 (1.64–6.02) ** | 2.98 (1.55–5.74) ** | 3.04 (1.57–5.91) ** | 3.02 (1.55–5.89) ** |
| Age at diagnosis in years | 1.04 (0.95–1.14) | - | 1.04 (0.94–1.16) | - | 1.08 (0.97–1.21) | - |
| Cancer diagnosis: (ref: CNS) | ||||||
| Hemato | 0.94 (0.46–1.92) | - | 2.15 (0.86–5.43) | - | 1.02 (0.46–2.25) | - |
| Solid | 1.01 (0.48–2.13) | - | 2.17 (0.83–5.66) | - | 1.02 (0.44–2.37) | - |
| Type of oncological treatment 1: (ref: Radiation and/or SCT) | ||||||
| Surgery only | 1.07 (0.74–1.54) | - | 0.84 (0.56–1.26) | - | 0.89 (0.59–1.36) | - |
| Chemotherapy with or without surgery | 0.92 (0.64–1.32) | - | 1.18 (0.78–1.77) | - | 1.09 (0.72–1.66) | - |
| Time since end of treatment (in years) | 1.05 (0.93–1.18) | - | 1.07 (0.94–1.21) | - | 1.03 (0.90–1.18) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peersmann, S.H.M.; Grootenhuis, M.A.; van Straten, A.; Kerkhof, G.A.; Tissing, W.J.E.; Abbink, F.; de Vries, A.C.H.; Loonen, J.; Kremer, L.C.M.; Kaspers, G.J.L.; et al. Prevalence of Sleep Disorders, Risk Factors and Sleep Treatment Needs of Adolescents and Young Adult Childhood Cancer Patients in Follow-Up after Treatment. Cancers 2022, 14, 926. https://doi.org/10.3390/cancers14040926
Peersmann SHM, Grootenhuis MA, van Straten A, Kerkhof GA, Tissing WJE, Abbink F, de Vries ACH, Loonen J, Kremer LCM, Kaspers GJL, et al. Prevalence of Sleep Disorders, Risk Factors and Sleep Treatment Needs of Adolescents and Young Adult Childhood Cancer Patients in Follow-Up after Treatment. Cancers. 2022; 14(4):926. https://doi.org/10.3390/cancers14040926
Chicago/Turabian StylePeersmann, Shosha H. M., Martha A. Grootenhuis, Annemieke van Straten, Gerard A. Kerkhof, Wim J. E. Tissing, Floor Abbink, Andrica C. H. de Vries, Jacqueline Loonen, Leontien C. M. Kremer, Gertjan J. L. Kaspers, and et al. 2022. "Prevalence of Sleep Disorders, Risk Factors and Sleep Treatment Needs of Adolescents and Young Adult Childhood Cancer Patients in Follow-Up after Treatment" Cancers 14, no. 4: 926. https://doi.org/10.3390/cancers14040926
APA StylePeersmann, S. H. M., Grootenhuis, M. A., van Straten, A., Kerkhof, G. A., Tissing, W. J. E., Abbink, F., de Vries, A. C. H., Loonen, J., Kremer, L. C. M., Kaspers, G. J. L., & van Litsenburg, R. R. L. (2022). Prevalence of Sleep Disorders, Risk Factors and Sleep Treatment Needs of Adolescents and Young Adult Childhood Cancer Patients in Follow-Up after Treatment. Cancers, 14(4), 926. https://doi.org/10.3390/cancers14040926






