Cost-Effectiveness of Molecularly Guided Treatment in Diffuse Large B-Cell Lymphoma (DLBCL) in Patients under 60
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
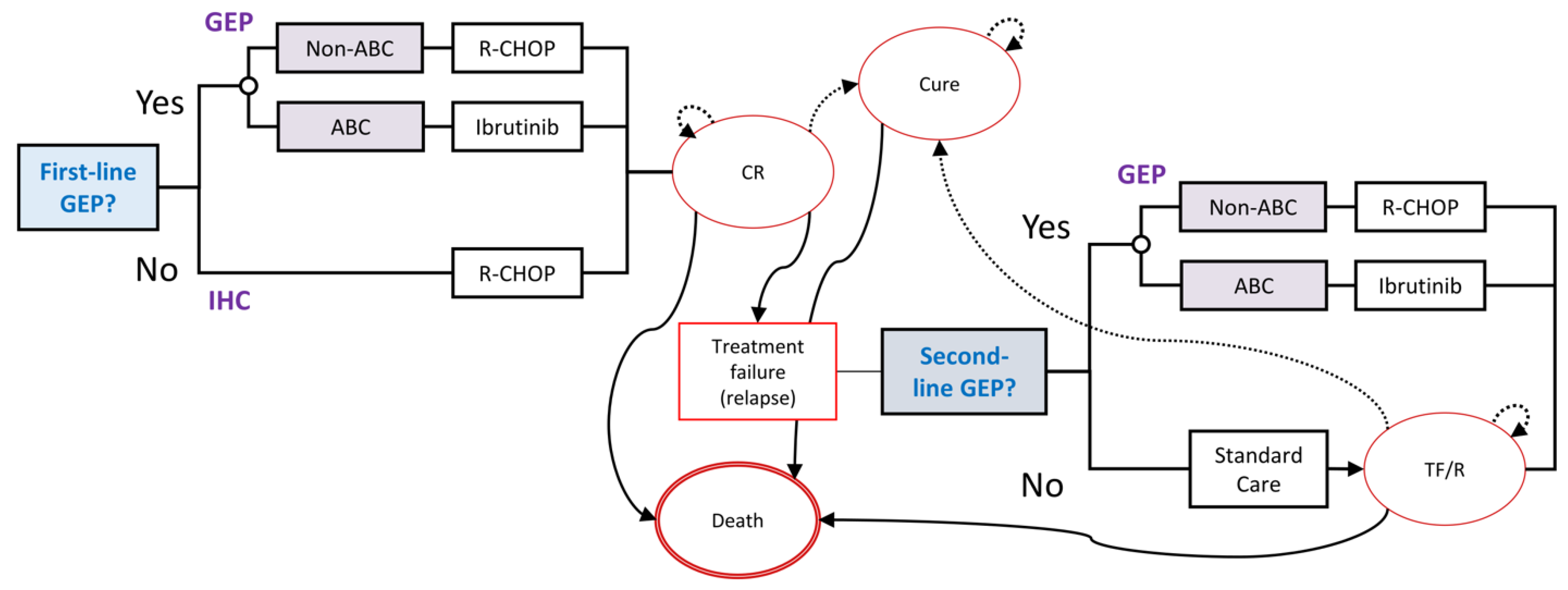
2.1. Model Structure
2.2. Model Parameters
2.2.1. Survival and Costs with Standard Care
2.2.2. Effectiveness and Cost of GEP-Informed Treatment
2.2.3. Health State Utilities
2.2.4. Cost-Effectiveness Analysis
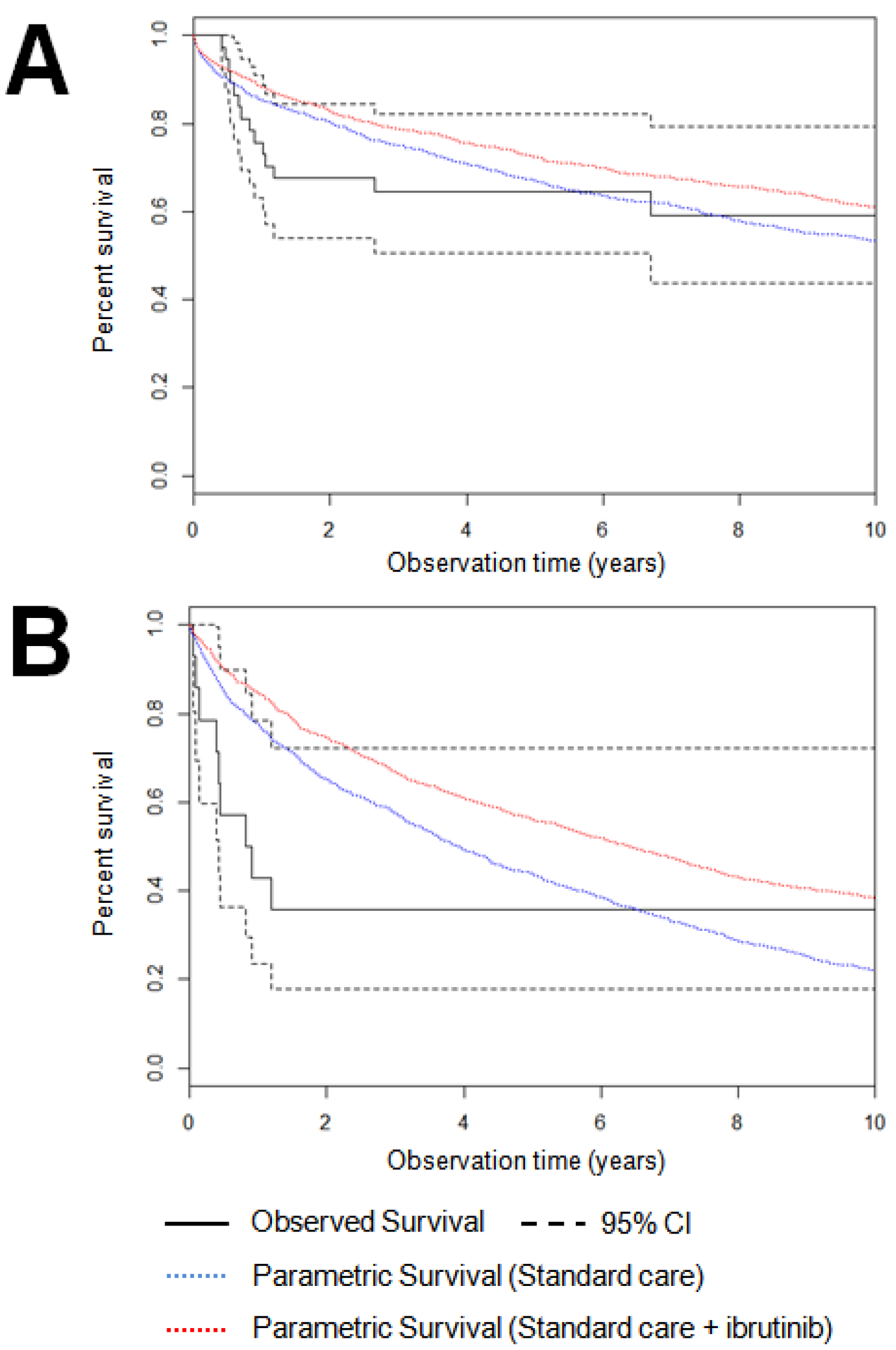
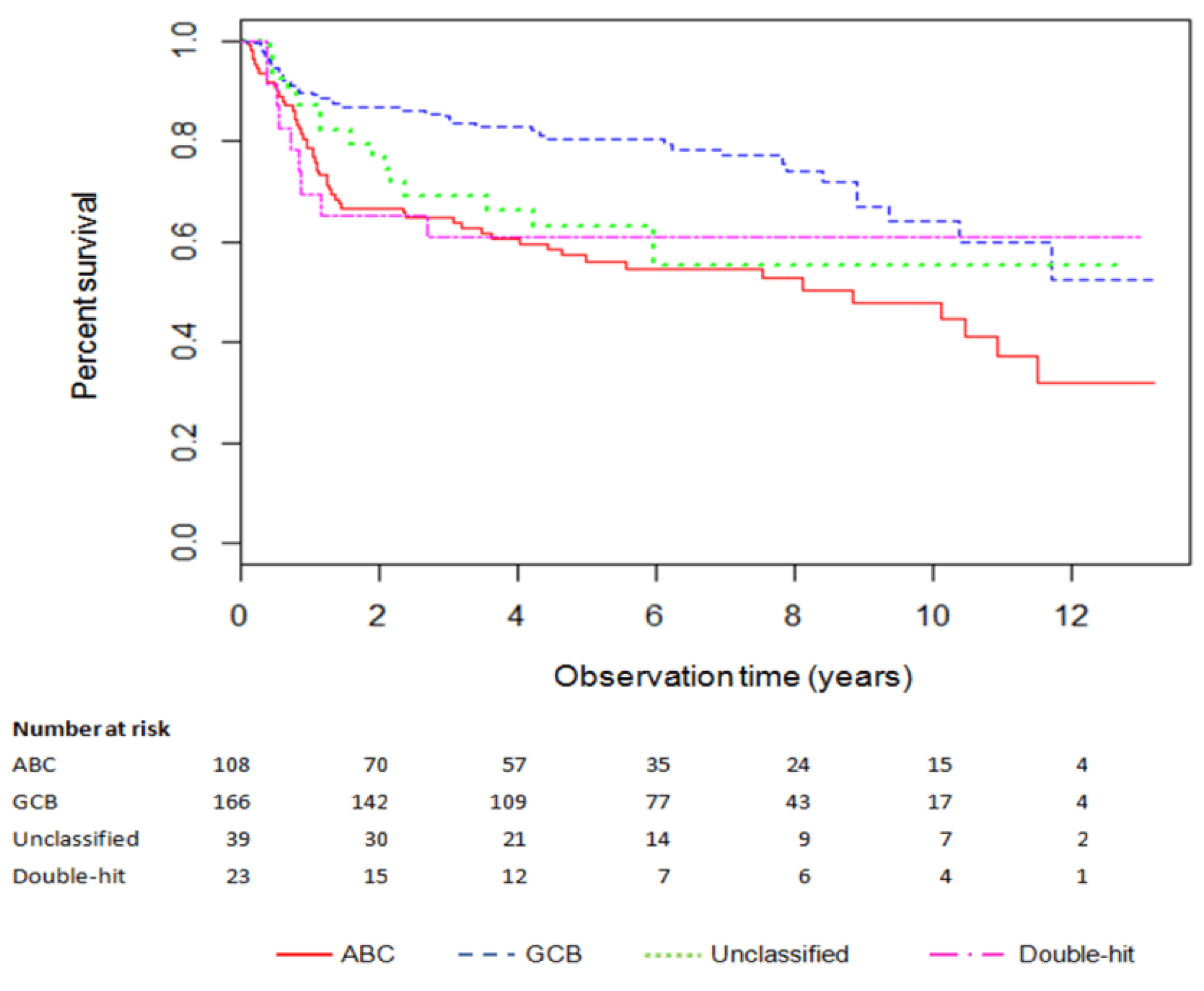
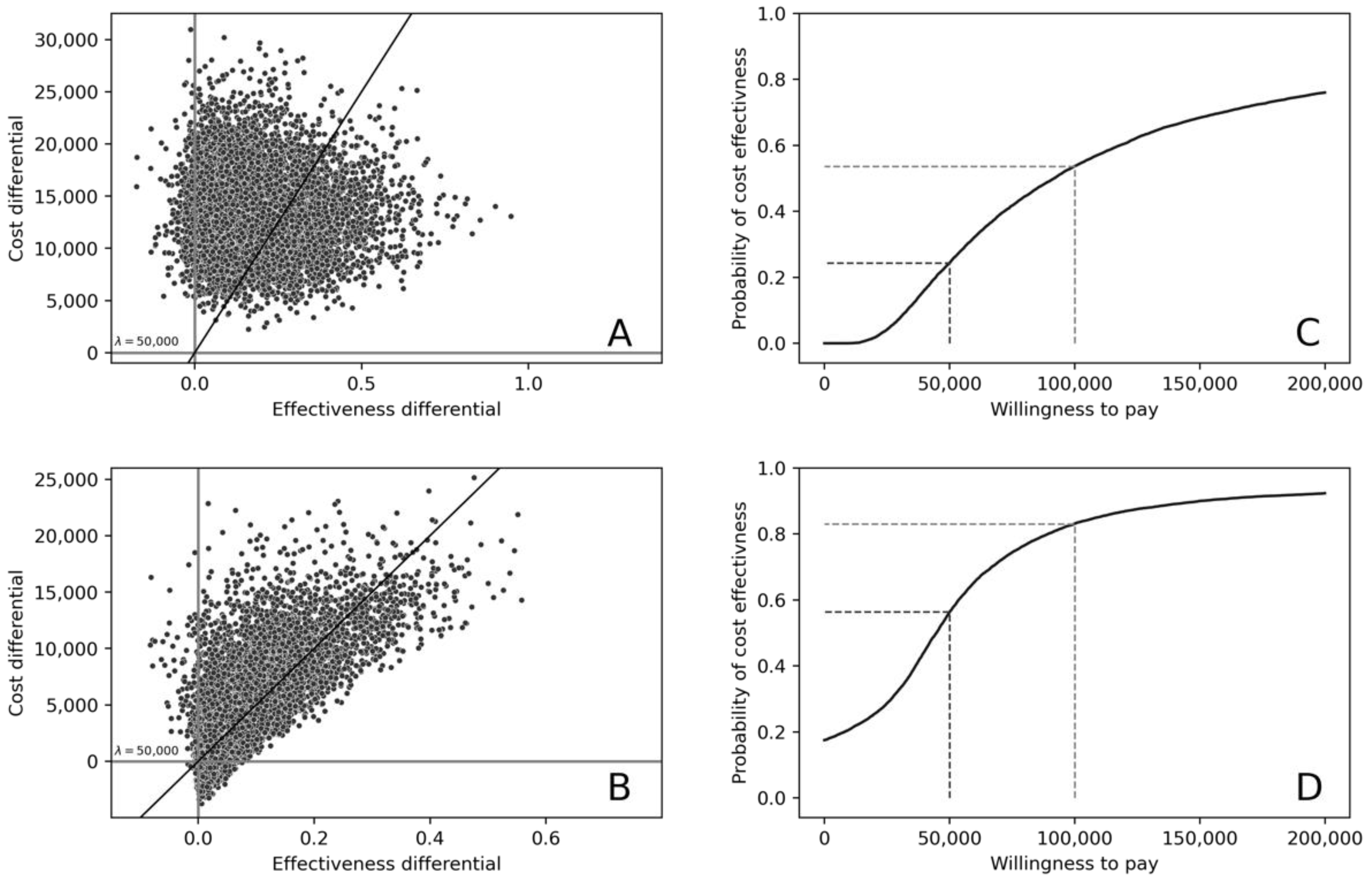
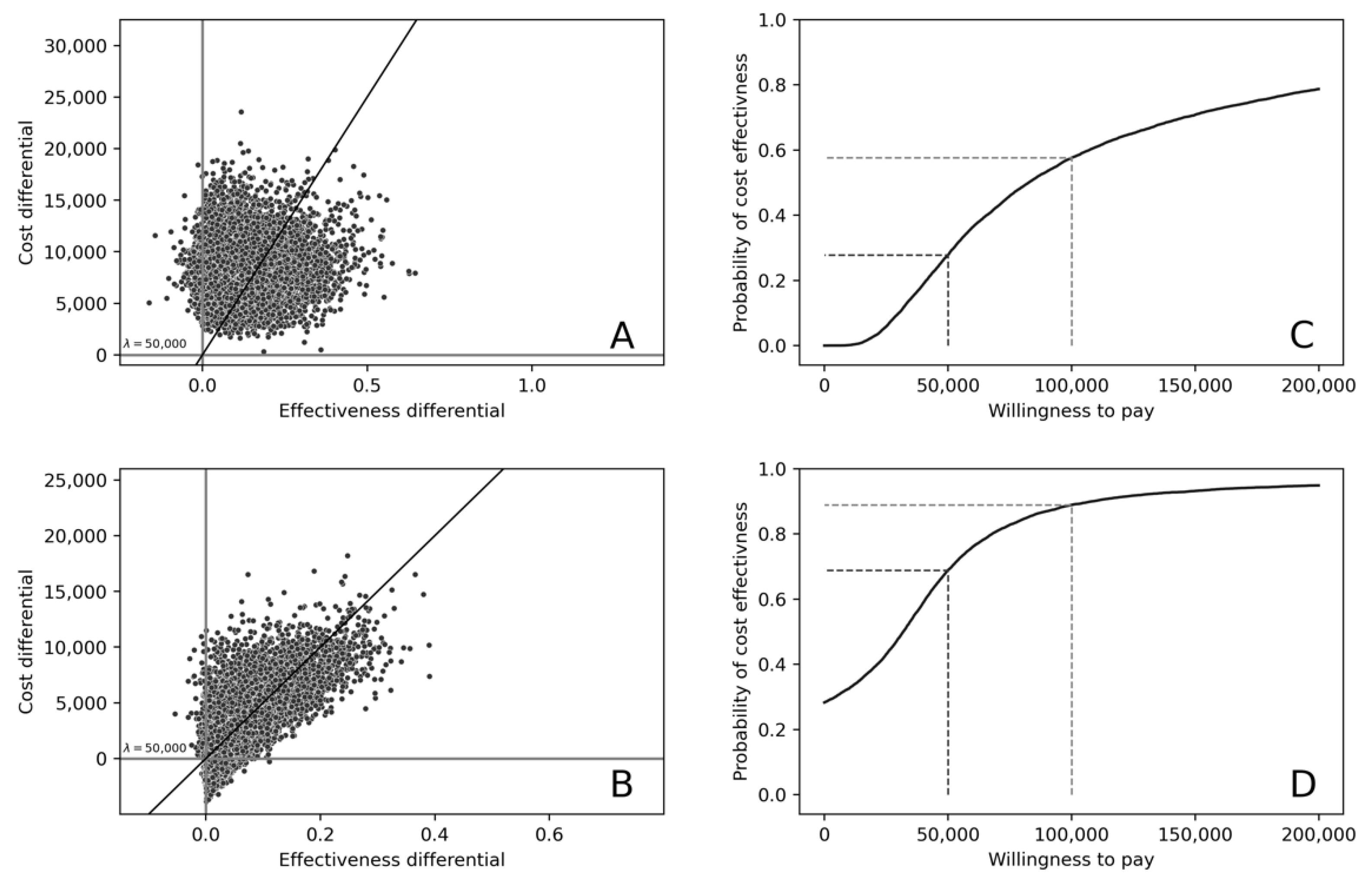
3. Results
Cost-Effectiveness of Treatment in Patients under 60 Years of Age
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Months Following Diagnosis | Cure, Complete Response | Relapsed Disease | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| 6 | $30,425 | $1543 | $19,308 | $2561 |
| 12 | $4590 | $250 | $10,417 | $10,530 |
| 18 | $2496 | $118 | $7441 | $10,382 |
| 24 | $1870 | $194 | $5703 | $6857 |
| 30 | $1593 | $161 | $4495 | $3904 |
| 36 | $1447 | $139 | $3592 | $2142 |
| 42 | $1363 | $124 | $2894 | $1267 |
| 48 | $1314 | $115 | $2344 | $935 |
| 54 | $1287 | $112 | $1904 | $745 |
| 60 | $1274 | $115 | $1551 | $661 |
| Months Following Diagnosis | Cure, Complete Response | Relapsed Disease | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| 6 | $78,540 | $1543 | $67,423 | $2561 |
| 12 | $52,705 | $250 | $58,532 | $10,530 |
| 18 | $50,611 | $118 | $55,556 | $10,382 |
| 24 | $49,985 | $194 | $53,818 | $6857 |
| 30 | $49,708 | $161 | $52,610 | $3904 |
| 36 | $49,562 | $139 | $51,707 | $2142 |
| 42 | $49,478 | $124 | $51,009 | $1267 |
| 48 | $49,429 | $115 | $50,459 | $935 |
| 54 | $49,402 | $112 | $50,019 | $745 |
| 60 | $49,389 | $115 | $49,666 | $661 |
Appendix B. Methodological and Computational Note: Generation of Transition Probabilities from Stochastic Survival Data
- Time from Complete Response to Treatment Failure/Relapse
- Time from Treatment Failure/Relapse to Death
- Time from Complete Response to Death
- Time from Cure to Death
| _t | Coef. | Std. Err. | z | P > |z| | [95% Conf. Interval] | |
|---|---|---|---|---|---|---|
| _cons | −4.861465 | 0.562269 | −8.65 | 0.000 | −5.963492 | −3.759438 |
| /ln_p | −0.5228583 | 0.1233935 | −4.24 | 0.000 | −0.7647051 | −0.2810115 |
| P 1/p | 0.5928236 1.686842 | 0.0731506 0.2081453 | 0.4654712 1.324469 | 0.7550196 2.148361 | ||
References
- Lymphoma Canada. DLBCL—Diffuse Large B Cell Lymphoma; Lymphoma Canada: Mississauga, ON, Canada, 2017; Available online: http://www.lymphoma.ca/DLBCL (accessed on 9 January 2021).
- Sehn, L.H.; Donaldson, J.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Klasa, R.; MacPherson, N.; O’Reilly, S.; Spinelli, J.J.; Sutherland, J.; et al. Introduction of Combined CHOP Plus Rituximab Therapy Dramatically Improved Outcome of Diffuse Large B-Cell Lymphoma in British Columbia. J. Clin. Oncol. 2005, 23, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M.; Link, B.K.; Grossbard, M.L.; Czuczman, M.; Grillo-Lopez, A.; Gilman, P.; Lowe, A.; Kunkel, L.A.; Fisher, R.I. Phase II Study of Rituximab in Combination with CHOP Chemotherapy in Patients with Previously Untreated, Aggressive Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2001, 19, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Gascoyne, R.D. Diffuse large B-cell lymphoma: Optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015, 125, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.; Beca, J.; Krahn, M.; Hodgson, D.; Lee, L.; Crump, M.; Bremner, K.E.; Luo, J.; Mamdani, M.; Bell, C.M.; et al. Real world costs and cost-effectiveness of Rituximab for diffuse large B-cell lymphoma patients: A population-based analysis. BMC Cancer 2014, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.M.; Marra, C.A.; Connors, J.M.; Najafzadeh, M.; Sehn, L.; Peacock, S.J. Cost-Effectiveness of the Addition of Rituximab to CHOP Chemotherapy in First-Line Treatment for Diffuse Large B-Cell Lymphoma in a Population-Based Observational Cohort in British Columbia, Canada. Value Health 2010, 13, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Wright, G.W.; Emre, N.C.T.; Kohlhammer, H.; Dave, S.S.; Davis, R.E.; Carty, S.; Lam, L.T.; Shaffer, A.L.; Xiao, W.; et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 13520–13525. [Google Scholar] [CrossRef]
- Scott, D.W.; Mottok, A.; Ennishi, D.; Wright, G.W.; Farinha, P.; Ben-Neriah, S.; Kridel, R.; Barry, G.S.; Hother, C.; Abrisqueta, P.; et al. Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J. Clin. Oncol. 2015, 33, 2848–2856. [Google Scholar] [CrossRef]
- Scott, D.W.; Wright, G.W.; Williams, P.M.; Lih, C.-J.; Walsh, W.; Jaffe, E.; Rosenwald, A.; Campo, E.; Chan, W.C.; Connors, J.M.; et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 2014, 123, 1214–1217. [Google Scholar] [CrossRef]
- Fu, K.; Weisenburger, D.D.; Choi, W.W.; Perry, K.D.; Smith, L.M.; Shi, X.; Hans, C.P.; Greiner, T.C.; Bierman, P.J.; Bociek, R.G.; et al. Addition of Rituximab to Standard Chemotherapy Improves the Survival of Both the Germinal Center B-Cell–Like and Non–Germinal Center B-Cell–Like Subtypes of Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2008, 26, 4587–4594. [Google Scholar] [CrossRef]
- De Jong, D.; Rosenwald, A.; Chhanabhai, M.; Gaulard, P.; Klapper, W.; Lee, A. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: Validation of tissue microarray as a prerequisite for broad clinical applications—A study from the Lunenburg Lymphoma Biomarker Consortium. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.; Roschewski, M.; Wilson, W.H. Precision Treatment of Distinct Molecular Subtypes of Diffuse Large B-cell Lymphoma: Ascribing Treatment Based on the Molecular Phenotype. Clin. Cancer Res. 2014, 20, 5182–5193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roschewski, M.; Staudt, L.M.; Wilson, W.H. Diffuse large B-cell lymphoma—treatment approaches in the molecular era. Nat. Rev. Clin. Oncol. 2014, 11, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.; Younes, A. Precision therapy for lymphoma—current state and future directions. Nat. Rev. Clin. Oncol. 2014, 11, 585–596. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Younes, A.; Sehn, L.H.; Johnson, P.; Zinzani, P.L.; Hong, X.; Zhu, J.; Patti, C.; Belada, D.; Samoilova, O.; Suh, C.; et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non–Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 1285–1295. [Google Scholar] [CrossRef]
- Wilson, W.H.; Wright, G.W.; Huang, D.W.; Hodkinson, B.; Balasubramanian, S.; Fan, Y.; Vermeulen, J.; Shreeve, M.; Staudt, L.M. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 2021, 39, 1643–1653.e3. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e14. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technology in Health (CADTH). Guidelines for the Economic Evaluation of Health Technologies; CADTH: Ottawa, ON, Canada, 2017. [Google Scholar]
- Briggs, A.; Claxton, K.; Sculpher, M. Decision Modelling for Health Economic Evaluation; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Costa, S.; Scott, D.W.; Steidl, C.; Peacock, S.J.; Regier, D.A. Real-World Costing Analysis for Diffuse Large B-Cell Lymphoma in British Columbia. Curr. Oncol. 2019, 26, 108–113. [Google Scholar] [CrossRef]
- Costa, S.; Regier, D.; Meissner, B.; Cromwell, I.; Ben-Neriah, S.; Chavez, E.; Hung, S.; Steidl, C.; Scott, D.; Marra, M.; et al. A Time-and-Motion Approach to Micro-Costing of High-Throughput Genomic Assays. Curr. Oncol. 2016, 23, 304–313. [Google Scholar] [CrossRef]
- CADTH pan-Canadian Oncology Drug Review Final Economic Guidance Report. Ibrutinib (Imbruvica) for Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. 2015. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr-ibrutinib-cll-sll-fn-egr.pdf (accessed on 3 January 2021).
- CADTH pan-Canadian Oncology Drug Review Final Economic Guidance Report. Brentuximab Vedotin (Adcetris) for Hodgkin Lymphoma. 2013. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr-adcetrishl-fn-egr.pdf (accessed on 3 January 2021).
- CADTH pan-Canadian Oncology Drug Review Final Economic Guidance Report. Lenalidomide (Revlimid) for Newly Diagnosed Multiple Myeloma. 2016. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_lenalidomide_revlimid_nd-mm_fn_egr.pdf (accessed on 3 January 2021).
- ClinicalTrials.gov. Efficacy and Safety Study of Lenalidomide Plus R-CHOP Chemotherapy Versus Placebo Plus R-CHOP Chemotherapy in Untreated ABC Type Diffuse Large B-Cell Lymphoma (ROBUST). 2017; ClinicalTrials.gov Identifier: NCT02285062. Available online: https://clinicaltrials.gov/ct2/show/NCT02285062 (accessed on 9 January 2021).
- ClinicalTrials.gov. A Study of the Bruton’s Tyrosine Kinase Inhibitor, PCI-32765 (Ibrutinib), in Combination with Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients with Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma. 2017; ClinicalTrials.gov Identifier: NCT01855750. Available online: https://clinicaltrials.gov/ct2/show/NCT01855750 (accessed on 9 January 2021).
- ClinicalTrials.gov. A Study of Brentuximab Vedotin in Relapsed or Refractory Non-Hodgkin Lymphoma. 2015; ClinicalTrials.gov Identifier: NCT01421667. Available online: https://clinicaltrials.gov/ct2/show/NCT01421667 (accessed on 9 January 2021).
- Knight, C.; Hind, D.; Brewer, N.; Abbott, V. Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: Systematic review and economic evaluation. Health Technol. Assess. 2004, 8, iii–ix. [Google Scholar] [CrossRef] [PubMed]
- Doxorubicin, Cyclophosphamide, Vincristine, Prednisone and Rituximab (CHOP-R). Chemotherapy protocols—Lymphoma & Myeloma 2014. Available online: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Lymphoma-Myeloma/LYCHOPR_Protocol_1Oct2014.pdf (accessed on 20 July 2016).
- Siebert, U.; Alagoz, O.; Bayoumi, A.M.; Jahn, B.; Owens, D.K.; Cohen, D.J.; Kuntz, K.M. State-Transition Modeling: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health 2012, 15, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Consumer Price Index, Health and Personal Care, by Province (British Columbia). 2017. Available online: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ161k-eng.htm (accessed on 9 January 2021).
- Staton, A.D.; Chen, Q.; Ayer, T.; Goldstein, D.; Koff, J.L.; Flowers, C.R. Cost-Effectiveness of Subtype-Based Treatment Strategies for Diffuse Large B-Cell Lymphoma Patients (DLBCL). Blood 2015, 126, 4476. [Google Scholar] [CrossRef]
- Chen, Q.; Staton, A.D.; Ayer, T.; Goldstein, D.; Koff, J.L.; Flowers, C.R. Exploring the potential cost-effectiveness of precision medicine treatment strategies for diffuse large B-cell lymphoma. Leuk. Lymphoma 2017, 59, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Weymann, D.; Pataky, R.; Regier, D.A. Economic Evaluations of Next-Generation Precision Oncology: A Critical Review. JCO Precis. Oncol. 2018, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Veenstra, D.L.; Basu, A.; Carlson, J.J. Demand for Precision Medicine: A Discrete-Choice Experiment and External Validation Study. PharmacoEconomics 2019, 38, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Staiger, A.; Ziepert, M.; Horn, H.; Scott, D.; Barth, T.; Bernd, H.-W. Clinical Impact of the Cell-of-Origin Classification and the MYC/BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. 2017, 35, 2515–2526. [Google Scholar] [CrossRef]
| Study Cohorts | |||
|---|---|---|---|
| Characteristics | Retrospective Cohort n = 751 | GEP-Classified Cohort n = 339 | GEP-Classified Cohort < 60 n = 128 |
| Age at diagnosis | |||
| Mean (range) | 65 (18–93) | 62 (16–92) | 48 (16–59) |
| Age group | |||
| 0–19 | <1% | <1% | 2% |
| 0–59 | 32% | 37% | 98% |
| 60–69 | 28% | 29% | NA |
| 70–79 | 27% | 26% | NA |
| 80+ | 13% | 7% | NA |
| Sex | |||
| Male | 58% | 63% | 68% |
| ECOG performance status at diagnosis | |||
| 0–1 | 62% | 68% | 69% |
| 2–4 | 38% | 31% | 31% |
| missing | <1% | 1% | 0% |
| Stage of disease at diagnosis ‡ | |||
| Limited | 29% | 30% | 30% |
| Advanced | 71% | 69% | 70% |
| Other | - | 1% | 0% |
| Molecular Profiling Costs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Mean | Standard Error | ||||||
| GEP (Lymph2Cx) assay | Micro-costing study [23] | $438 * | $68 | Gamma | ||||
| Hans IHC-based algorithm | BC Cancer Estimates | $366 * | $54 | |||||
| Treatment costs | ||||||||
| Mean | Standard error | |||||||
| GEP-informed treatment, first 6 cycles § | Published estimates [24] | $48,115 * | $10,585 | Gamma | ||||
| Treatment efficacy—Hazard ratio | ||||||||
| R-CHOP | Mean | Standard error | ||||||
| GEP-informed treatment (ABC subtype, <60 y) | Younes et al., 2019 [17] | −0.5465 * | 0.01159 | Normal | ||||
| Proportion of cell-of-origin subtype | ||||||||
| Mean | Standard error | Alpha | ||||||
| ABC | Published estimates [9] | 0.32 * | 0.00255 | 108 | Dirichlet | |||
| GCB | 0.5 * | 0.00205 | 166 | |||||
| Unclassified | 0.11 * | 0.00281 | 38 | |||||
| Double-hit | 0.07 * | 0.00294 | 23 | |||||
| Utilities | ||||||||
| Mean | Standard error | |||||||
| Complete response, cure states | Published estimates (Knight et al., 2004) [30] | 0.81 * | 0.04 | Beta | ||||
| Treatment failure/relapse state | 0.49 * | 0.013 | ||||||
| Transition probabilities—Weibull GLM regression coefficients | ||||||||
| From BC Cancer retrospective IHC/GEP-assigned cohort | ||||||||
| Log (Scale) Log (SE) | Shape (ρ) SE | |||||||
| Complete Response to Treatment Failure/Relapse | ABC | 4.888 * | −0.62 * | Normal | ||||
| 1.048 | 0.251 | |||||||
| GCB | 5.334 * | −0.703 | ||||||
| 0.992 | 0.252 | |||||||
| Unclassified | −4.87 * | −0.651 | ||||||
| 1.523 | 0.37 | |||||||
| Double-hit | 3.722 * | 0.963 * | ||||||
| 0.983 | 0.324 | |||||||
| Complete Response to Death | ABC | −5.354 * | −1.062 | Normal | ||||
| 1.811 | 0.664 | |||||||
| GCB | −12.206 | −0.039 | ||||||
| 7.21 | 0.875 | |||||||
| Unclassified | −9.629 * | −0.045 | ||||||
| 3.624 | 0.483 | |||||||
| Double-hit | −10.722 | −0.017 | ||||||
| 6.843 | 0.846 | |||||||
| Treatment Failure/Relapse to Death | ABC | −3.339 * | −0.802 * | Normal | ||||
| 0.99 | 0.3 | |||||||
| GCB | −3.183 * | −0.835 * | ||||||
| 0.924 | 0.29 | |||||||
| Unclassified | −3.635 | −0.519 | ||||||
| 1.385 | 0.344 | |||||||
| Double-hit | −9.487 | 0.7 | ||||||
| 2.784 | 0.846 | |||||||
| Cure to Death | ABC | −11.801 * | 0.309 | Normal | ||||
| 3.833 | 0.372 | |||||||
| GCB | −16.508 | 0.675 * | ||||||
| 4.066 | 0.274 | |||||||
| Unclassified | −7.699 * | 0.309 | ||||||
| 4.817 | 0.89 | |||||||
| Double-hit | −16.508 * | 0.675 * | ||||||
| 4.066 | 0.274 | |||||||
| Strategy | Cost | SD | ΔC | QALY | SD | ΔE | ICER |
|---|---|---|---|---|---|---|---|
| First Line | |||||||
| Standard Care | $48,768 | 5781 | 5.443 | 1.603 | |||
| GEP-informed | $61,993 | 6488 | $13,226 | 5.613 | 1.598 | 0.17 | $77,806 |
| Second Line | |||||||
| Standard Care | $48,768 | 5782 | 5.443 | 1.603 | |||
| GEP-informed | $53,038 | 7813 | $4270 | 5.522 | 1.593 | 0.079 | $53,909 |
| Strategy | Cost | SD | ΔC | QALY | SD | ΔE | ICER |
|---|---|---|---|---|---|---|---|
| First Line | |||||||
| Standard Care | 48,817.5 | 5797.82 | 8386.55 | 5.438 | 1.601 | 0.117 | 71,706.37 |
| GEP-informed | 57,204.05 | 5948.03 | 5.555 | 1.597 | |||
| Second Line | |||||||
| Standard Care | 48,817.5 | 5797.82 | 2253.57 | 5.438 | 1.601 | 0.056 | 40,531.74 |
| GEP-informed | 51,071.07 | 6945.73 | 5.493 | 1.594 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regier, D.A.; Chan, B.; Costa, S.; Scott, D.W.; Steidl, C.; Connors, J.M.; Karsan, A.; Marra, M.A.; Kridel, R.; Cromwell, I.; et al. Cost-Effectiveness of Molecularly Guided Treatment in Diffuse Large B-Cell Lymphoma (DLBCL) in Patients under 60. Cancers 2022, 14, 908. https://doi.org/10.3390/cancers14040908
Regier DA, Chan B, Costa S, Scott DW, Steidl C, Connors JM, Karsan A, Marra MA, Kridel R, Cromwell I, et al. Cost-Effectiveness of Molecularly Guided Treatment in Diffuse Large B-Cell Lymphoma (DLBCL) in Patients under 60. Cancers. 2022; 14(4):908. https://doi.org/10.3390/cancers14040908
Chicago/Turabian StyleRegier, Dean A., Brandon Chan, Sarah Costa, David W. Scott, Christian Steidl, Joseph M. Connors, Aly Karsan, Marco A. Marra, Robert Kridel, Ian Cromwell, and et al. 2022. "Cost-Effectiveness of Molecularly Guided Treatment in Diffuse Large B-Cell Lymphoma (DLBCL) in Patients under 60" Cancers 14, no. 4: 908. https://doi.org/10.3390/cancers14040908
APA StyleRegier, D. A., Chan, B., Costa, S., Scott, D. W., Steidl, C., Connors, J. M., Karsan, A., Marra, M. A., Kridel, R., Cromwell, I., & Pollard, S. (2022). Cost-Effectiveness of Molecularly Guided Treatment in Diffuse Large B-Cell Lymphoma (DLBCL) in Patients under 60. Cancers, 14(4), 908. https://doi.org/10.3390/cancers14040908






