Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results from a Pilot Study in Patients with Metastatic Non-Small-Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection
2.2. Nivolumab Treatment and Timing
2.3. Assessments
2.4. Statistical Considerations
3. Results
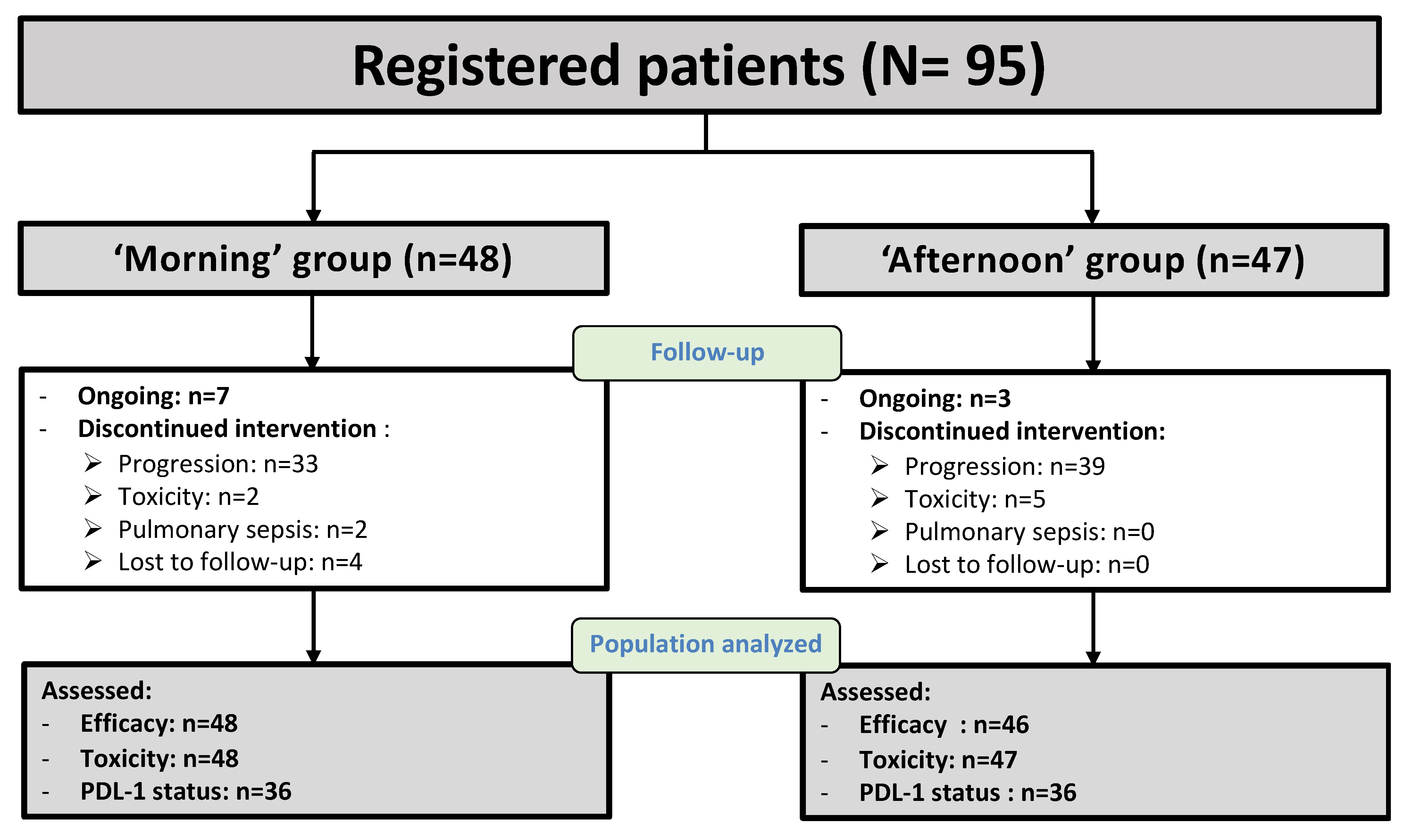
3.1. Patient Characteristics
3.2. Treatment Safety
3.3. Tumor Responses
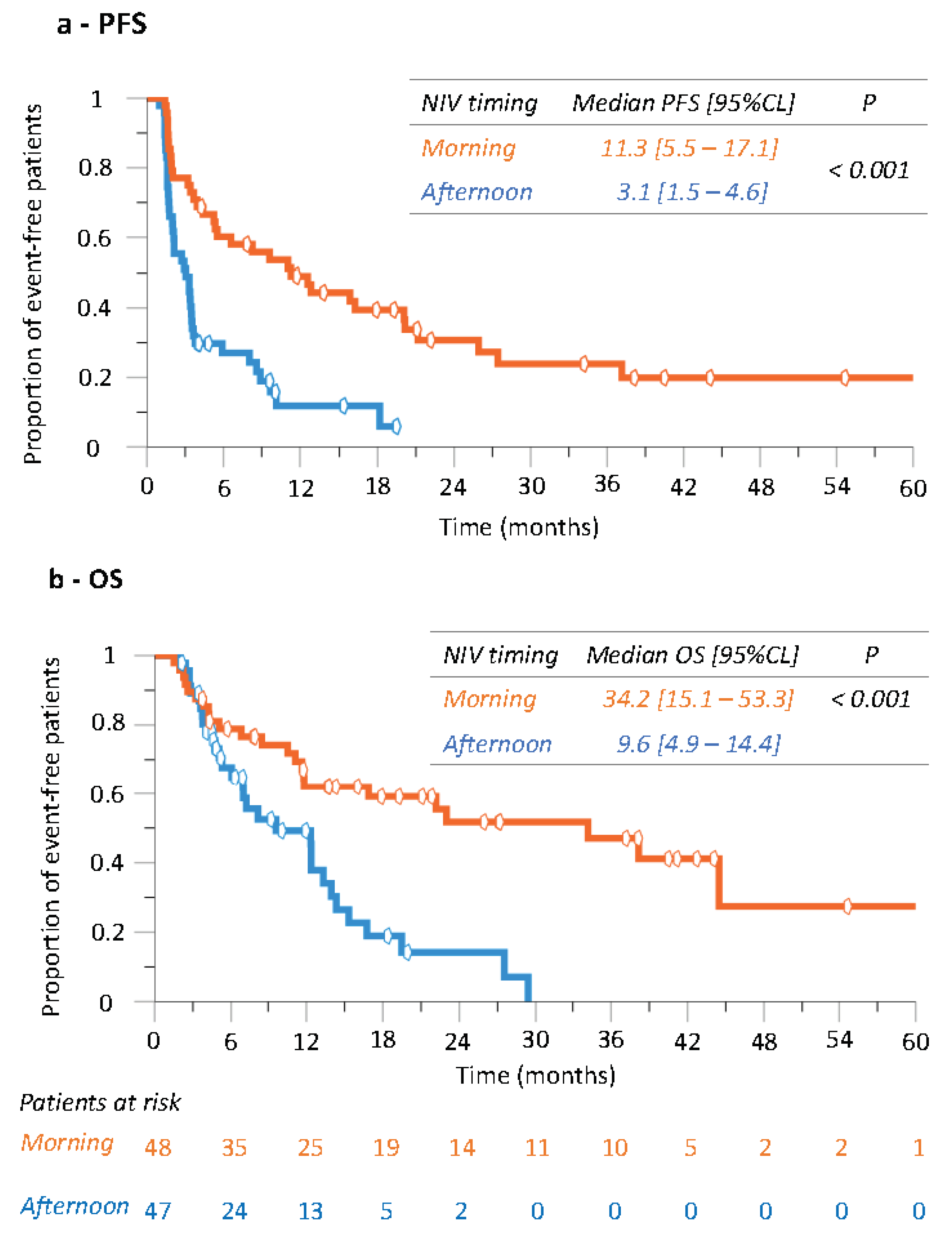
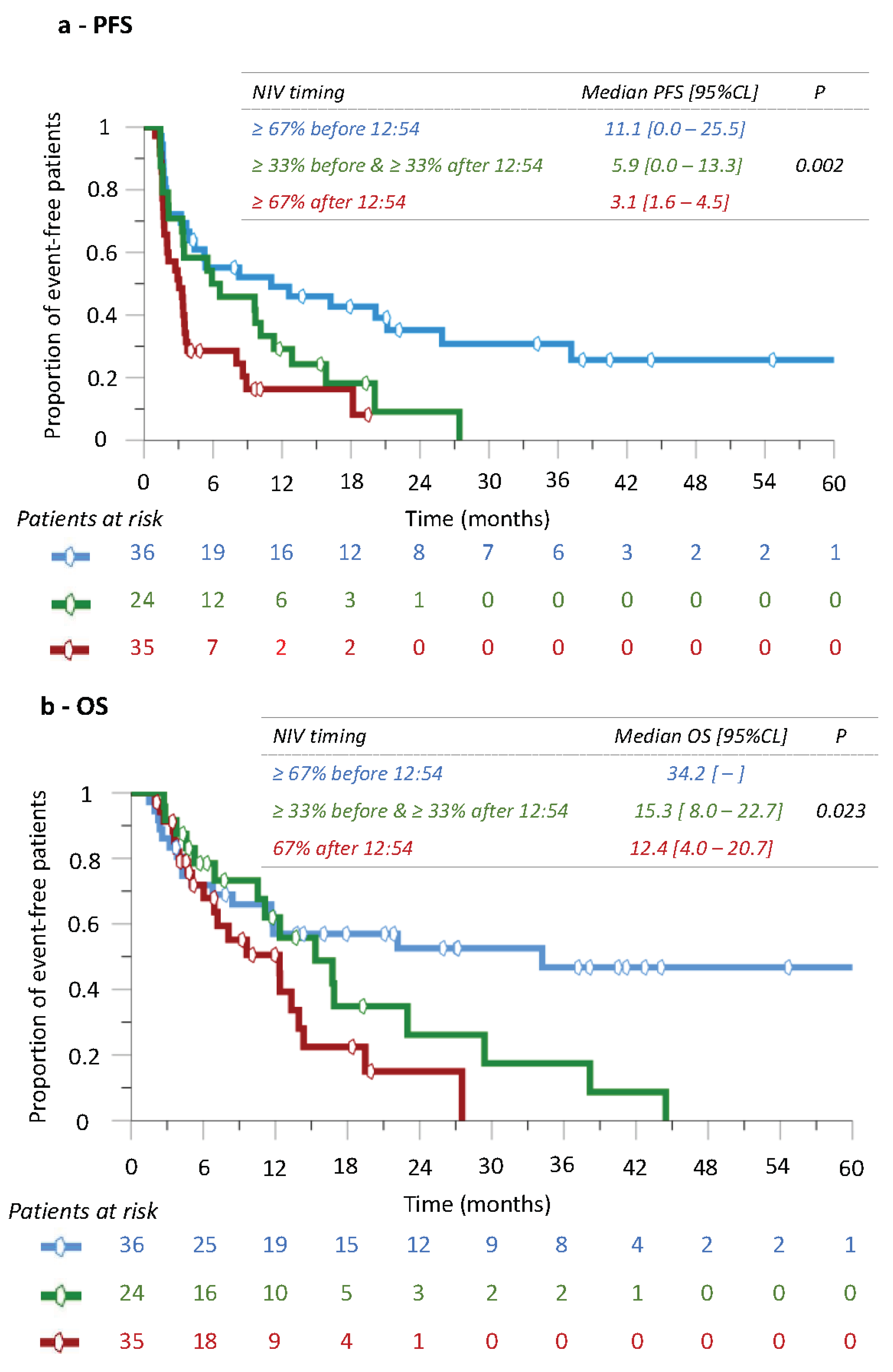
3.4. Progression-Free Survival (PFS) and Overall Survival According to Nivolumab Timing
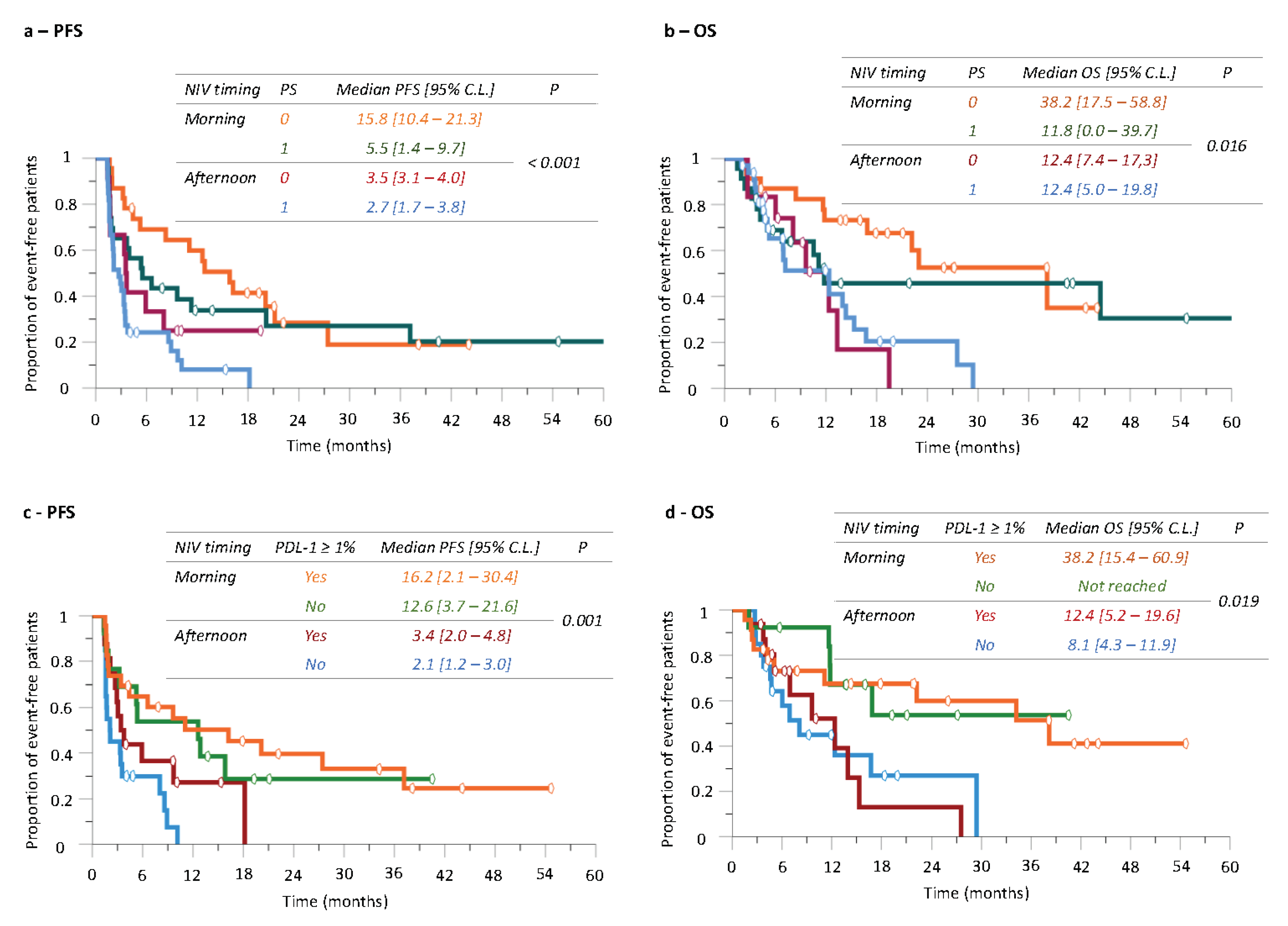
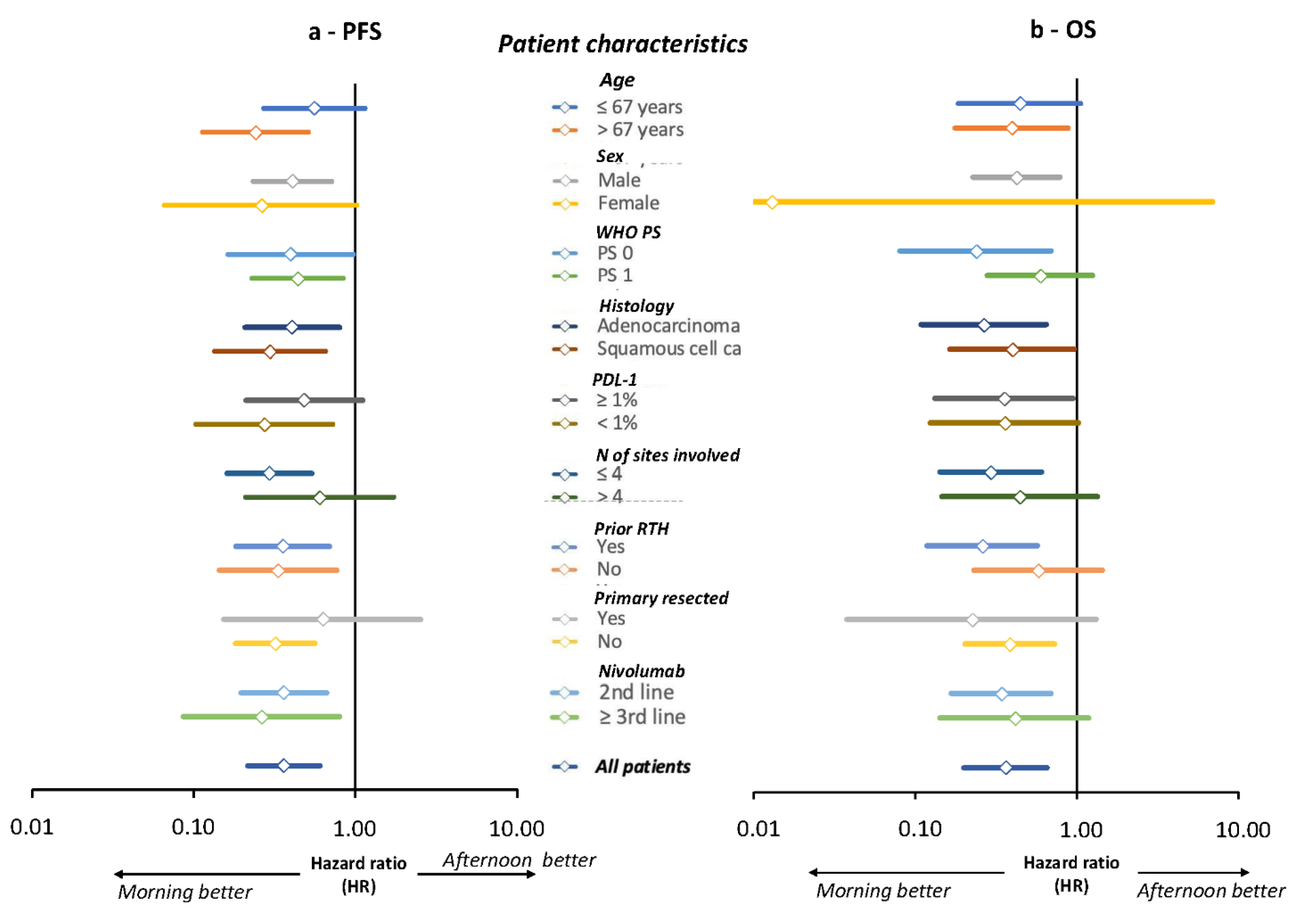
3.5. Did Patient Performance Status Influence Nivolumab Timing-Dependent Efficacy?
3.6. Did Tumor PD-L1 Expression Influence Nivolumab Timing-Dependent Efficacy?
3.7. Univariate and Multivariate Cox Model Analyses of PFS and OS
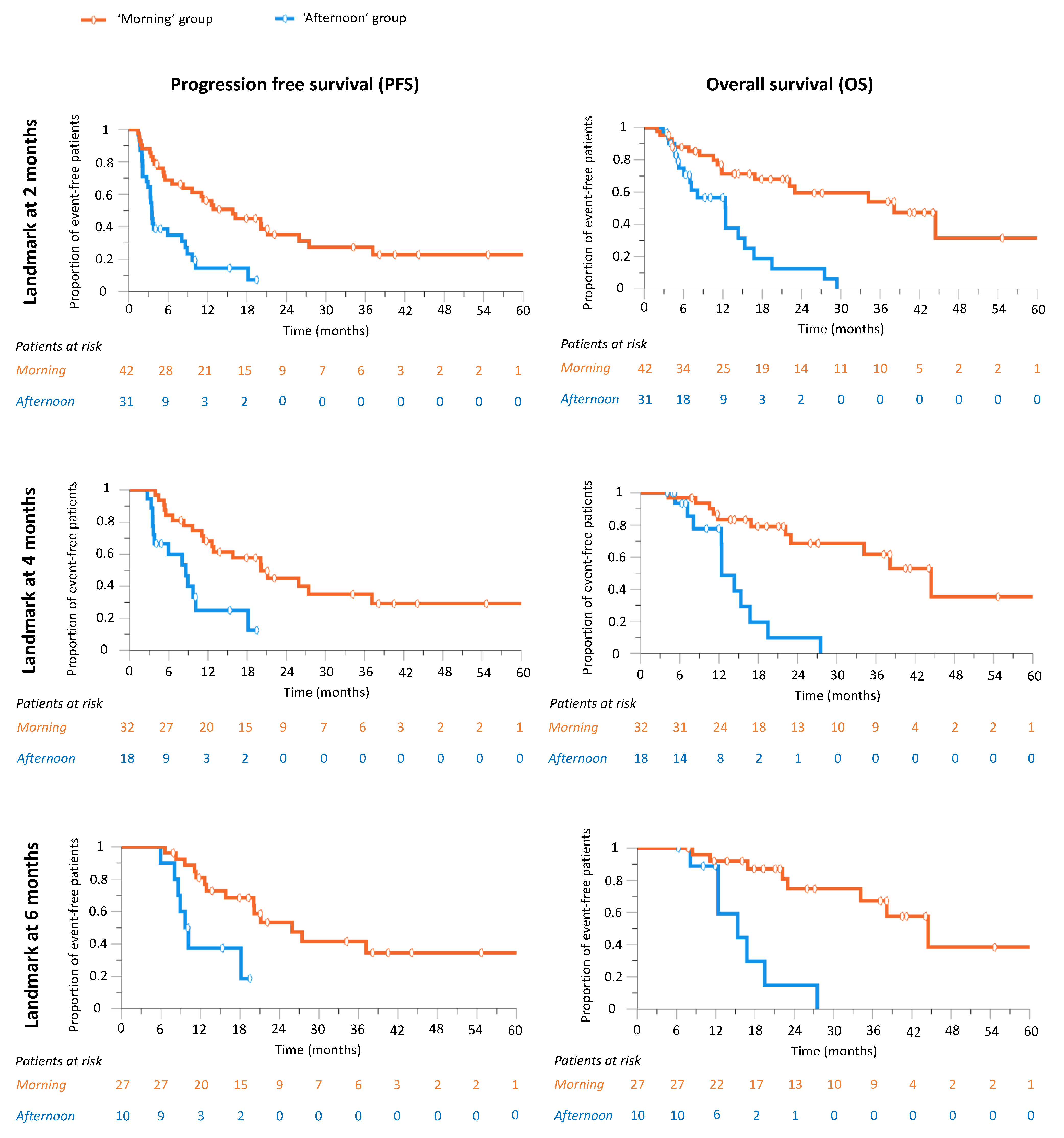
3.8. Nivolumab’s Efficacy According to Infusion Time following Trichotomization of Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Perez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2015, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Aguiar, P.N., Jr.; Santoro, I.L.; Tadokoro, H.; Lopes, G.D.L.; Filardi, B.A.; Oliveira, P.; Mountzios, G.; de Mello, R.A. The role of PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: A network meta-analysis. Immunotherapy 2016, 8, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Herbst, R.S. Nivolumab and Pembrolizumab for Non–Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 3713–3717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Cash, E.; Sephton, S.; Woolley, C.; Elbehi, A.M.; Anu, R.I.; Ekine-Afolabi, B.; Kok, V.C. The role of the circadian clock in cancer hallmark acquisition and immune-based cancer therapeutics. J. Exp. Clin. Cancer Res. 2021, 40, 119. [Google Scholar] [CrossRef]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian Clock Proteins and Immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bree, L.C.J.; Mourits, V.P.; Koeken, V.A.; Moorlag, S.J.; Janssen, R.; Folkman, L.; Barreca, D.; Krausgruber, T.; Fife-Gernedl, V.; Novakovic, B.; et al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J. Clin. Investig. 2020, 130, 5603–5617. [Google Scholar] [CrossRef] [PubMed]
- Downton, P.; Early, J.O.; Gibbs, J.E. Circadian rhythms in adaptive immunity. Immunology 2019, 161, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, E.E.; Rooney, J.; Dardente, H.; Hardy, M.-P.; Labrecque, N.; Cermakian, N. Circadian Variation of the Response of T Cells to Antigen. J. Immunol. 2011, 187, 6291–6300. [Google Scholar] [CrossRef]
- Fukuda, R.; Ichikawa, Y.; Takaya, M.; Ogawa, Y.; Masumoto, A. Circadian Variations and Prednisolone-Induced Alterations of Circulating Lymphocyte Subsets in Man. Intern. Med. 1994, 33, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, P.; Gao, W.; Zhou, Q.; Feng, T.; Tian, X. Circadian clock: A regulator of the immunity in cancer. Cell Commun. Signal. 2021, 19, 37. [Google Scholar] [CrossRef]
- Nobis, C.C.; Dubeau Laramée, G.; Kervezee, L.; Maurice De Sousa, D.; Labrecque, N.; Cermakian, N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc. Natl. Acad. Sci USA 2019, 116, 20077–20086. [Google Scholar] [CrossRef] [Green Version]
- Lévi, F.; Canon, C.; Dipalma, M.; Florentin, I.; Misset, J.L. When should the immune clock be reset? From circadian pharmacodynamics to temporally optimized drug delivery. Ann. N. Y. Acad. Sci. 1991, 618, 312–329. [Google Scholar] [CrossRef]
- Chen, J.; Liu, A.; Lin, Z.; Wang, B.; Chai, X.; Chen, S.; Lu, W.; Zheng, M.; Cao, T.; Zhong, M.; et al. Downregulation of the circadian rhythm regulator HLF promotes multiple-organ distant metastases in non-small cell lung cancer through PPAR/NF-κb signaling. Cancer Lett. 2020, 482, 56–71. [Google Scholar] [CrossRef]
- Lévi, F.; Focan, C.; Karaboué, A.; De La Valette, V.; Focan-Henrard, D.; Baron, B.; Kreutz, F.; Giacchetti, S. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, Z.; Jiang, T.; Yang, X.; Du, Y.; Liang, J.; Wang, L.; Xi, J.; Lin, M.; Feng, M. Circadian rhythm-associated clinical relevance and Tumor Microenvironment of Non-small Cell Lung Cancer. J. Cancer 2021, 12, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Chen, Y.-B.; Jin, S.; Fang, X.-F.; He, X.-X.; Xiong, Z.-F.; Yang, S.-L. Research on circadian clock genes in non-small-cell lung carcinoma. Chronobiol. Int. 2019, 36, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Sothern, R.B.; Parrella, P.; Muscarella, L.A.; Fazio, V.M.; Giuliani, F.; Polyakova, V.; Kvetnoy, I.M. Comparison of circadian characteristics for cytotoxic lymphocyte subsets in non-small cell lung cancer patients versus controls. Clin. Exp. Med. 2011, 12, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Grilli, M.; Carughi, S.; Puzzolante, F.; De Cata, A.; La Viola, M.; Giuliani, A.; Urbano, N.; Tarquini, R.; Perfetto, F. Immune System Alterations in Lung Cancer Patients. Int. J. Immunopathol. Pharmacol. 2003, 16, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, D.C.; Kleber, T.; Brammer, B.; Xu, K.M.; Switchenko, J.M.; Janopaul-Naylor, J.R.; Zhong, J.; Yushak, M.L.; Harvey, R.D.; Paulos, C.M.; et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): A propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 2021, 22, 1777–1786. [Google Scholar] [CrossRef]
- Lévi, F.; Giacchetti, S.; Adam, R.; Zidani, R.; Metzger, G.; Misset, J.-L. Chronomodulation of chemotherapy against metastatic colorectal cancer. Eur. J. Cancer 1995, 31A, 1264–1270. [Google Scholar] [CrossRef]
- Lévi, F.A.; Zidani, R.; Misset, J.-L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 1997, 350, 681–686. [Google Scholar] [CrossRef]
- Lévi, F.A.; Zidani, R.; Vannetzel, J.M.; Perpoint, B.; Focan, C.; Faggiuolo, R.; Chollet, P.; Garufi, C.; Itzhaki, M.; Dogliotti, L.; et al. Chronomodulated Versus Fixed-Infusion—Rate Delivery of Ambulatory Chemotherapy with Oxaliplatin, Fluorouracil, and Folinic Acid (Leucovorin) in Patients With Colorectal Cancer Metastases: A Randomized Multi-institutional Trial. JNCI J. Natl. Cancer Inst. 1994, 86, 1608–1617. [Google Scholar] [CrossRef]
- Karaboué, A.; Bisseux, L.; Pavese, I.; Collon, T. Circadian variation in nivolumab efficacy in patients with advanced non-small cell lung cancer. J. Clin. Oncol. 2020, 38, e21585. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; Borghaei, H.; Ramalingam, S.S.; Horn, L.; De Castro Carpeño, J.; Pluzanski, A.; Burgio, M.A.; Garassino, M.; Chow, L.Q.M.; Gettinger, S.; et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet Oncol. 2019, 20, 1395–1408. [Google Scholar] [CrossRef]
- Chen, R.; Tao, Y.; Xu, X.; Shan, L.; Jiang, H.; Yin, Q.; Pei, L.; Cai, F.; Ma, L.; Yu, Y. The efficacy and safety of nivolumab, pembrolizumab, and atezolizumab in treatment of advanced non-small cell lung cancer. Discov. Med. 2018, 26, 155–166. [Google Scholar] [PubMed]
- Gettinger, S.; Horn, L.; Jackman, D.; Spigel, D.; Antonia, S.; Hellmann, M.; Powderly, J.; Heist, R.; Sequist, L.V.; Smith, D.C.; et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non–Small-Cell Lung Cancer: Results From the CA209-003 Study. J. Clin. Oncol. 2018, 36, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Vokes, E.; Ready, N.; Felip, E.; Horn, L.; Burgio, M.; Antonia, S.; Frontera, O.A.; Gettinger, S.; Holgado, E.; Spigel, D.; et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann. Oncol. 2018, 29, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.N., Jr.; Santoro, I.L.; Tadokoro, H.; Lopes, G.D.L.; Filardi, B.A.; Oliveira, P.; Castelo-Branco, P.; Mountzios, G.; de Mello, R.A. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker. Immunotherapy 2016, 8, 1011–1019. [Google Scholar] [CrossRef]
- Carbognin, L.; Pilotto, S.; Milella, M.; Vaccaro, V.; Brunelli, M.; Caliò, A.; Cuppone, F.; Sperduti, I.; Giannarelli, D.; Chilosi, M.; et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS ONE 2015, 10, e0130142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berner, F.; Bomze, D.; Diem, S.; Ali, O.H.; Fässler, M.; Ring, S.; Niederer, R.; Ackermann, C.J.; Baumgaertner, P.; Pikor, N.; et al. Association of Checkpoint Inhibitor–Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non–Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1043–1047. [Google Scholar] [CrossRef]
- Gettinger, S.; Rizvi, N.A.; Chow, L.Q.; Borghaei, H.; Brahmer, J.; Ready, N.; Gerber, D.; Shepherd, F.A.; Antonia, S.; Goldman, J.W.; et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 2980–2987. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Owonikoko, T.K.; Kamphorst, A.O.; Pakkala, S.; Belani, C.P.; Khuri, F.R.; Ahmed, R.; Ramalingam, S.S. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2017, 124, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Lévi, F.A. Systems Chronotherapeutics. Pharmacol. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallmann, R.; Okyar, A.; Lévi, F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol. Med. 2016, 22, 430–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévi, F. Circadian chronotherapy for human cancers. Lancet Oncol. 2001, 2, 307–315. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, A.W.; Oswald, I.; Micklem, H.S.; Boyd, J.E.; Elton, R.A.; Jazwinska, E.; James, K. Circadian variation of lymphocyte subpopulations: A study with monoclonal antibodies. BMJ 1983, 286, 1773–1775. [Google Scholar] [CrossRef] [Green Version]
- Tavadia, H.B.; Fleming, A.K.; Hume, P.D.; Simpson, H.W. Circadian rhythmicity of human plasma cortisol and PHA-induced lymphocyte transformation. Clin. Exp. Immunol. 1975, 22, 190–193. [Google Scholar]
- Druzd, D.; Matveeva, O.; Ince, L.; Harrison, U.; He, W.; Schmal, C.; Herzel, H.; Tsang, A.H.; Kawakami, N.; Leliavski, A.; et al. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 2017, 46, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Long, J.E.; Drayson, M.; Taylor, A.E.; Toellner, K.-M.; Lord, J.; Phillips, A.C. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 2016, 34, 2679–2685. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020, 9, 8086–8121. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Khan, F.; James, C.D.; Heimberger, A.B.; Lesniak, M.S.; Chen, P. Circadian regulation of cancer cell and tumor microenvironment crosstalk. Trends Cell Biol. 2021, 31, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X.; Quesnel, B. Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers 2021, 13, 3034. [Google Scholar] [CrossRef] [PubMed]
- Brocco, D.; Lanuti, P.; Pieragostino, D.; Cufaro, M.; Simeone, P.; Bologna, G.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; Irtelli, L.; et al. Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers 2021, 13, 585. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients (N = 95) | ‘Morning’ Group (n = 48) | ‘Afternoon’ Group (n = 47) | p-Value |
|---|---|---|---|---|
| Timing of >50% of infusions | 09:27 to 12:54 | 12:55 to 17:14 | ||
| Actual NIV a timing | ||||
| Median time, hh:min | 12:54 | 11:55 | 13:34 | <0.001 |
| (range) | (9:27–17:14) | (9:27–16:52) | (10:10–17:14) | |
| (IQR) | (11:55–13:34) | (11:11–12:26) | (13:10–14:44) | |
| Median intra-patient CV b | 10% | 11% | 8% | |
| (range) | (2%–21%) | (2%–21%) | (2%–19%) | |
| Age, years | ||||
| Median (range) | 66.9 (41.2–82.5) | 66.2 (41.2–80.8) | 69.2 (48.8–82.5) | 0.012 |
| Sex | ||||
| Female | 16 (16.8%) | 4 (8.3%) | 12 (25.5%) | 0.025 |
| Male | 79 (83.2%) | 44 (91.7%) | 35 (74.5%) | |
| Tobacco-induced COPD c | 74 (77.9%) | 37 (77.1%) | 37 (78.7%) | 0.847 |
| Histological type | ||||
| Adenocarcinoma | 55 (57.9%) | 26 (54.2%) | 29 (61.7%) | 0.696 |
| Squamous cell carcinoma | 37 (38.9%) | 20 (41.7%) | 17 (36.2%) | |
| NSCLC unspecified | 3 (3.2%) | 2 (4.2%) | 1 (2.1%) | |
| Tumor PD-L1 expression | 0.098 | |||
| ≥1% | 39 (41.1%) | 23 (47.9%) | 16 (34.0%) | |
| <1% | 33 (34.7%) | 13 (27.1%) | 20 (42.6%) | |
| Not assessed | 23 (24.2%) | 12 (25.0%) | 11 (23.4%) | |
| Primary resected | 15 (15.8%) | 9 (18.8%) | 6 (12.8%) | 0.424 |
| Prior adjuvant chemo. | 11 (11.6%) | 7 (14.6%) | 4 (8.5%) | 0.355 |
| Prior radiotherapy | 57 (60.0%) | 27 (56.3%) | 30 (63.8%) | 0.451 |
| N of prior chemo. lines | ||||
| 0 | 1 (1.1%) | 1 (2.1%) | 0 (0.0%) | 0.955 |
| 1 | 72 (75.8%) | 36 (75.0%) | 36 (76.6%) | |
| 2–5 | 22 (23.2%) | 11 (22.9%) | 11 (23.4%) | |
| Number of sites involved | ||||
| Median (range) | 4 (1–8) | 4 (1–7) | 3 (2–8) | 0.854 |
| Site of metastases | ||||
| Brain | 23 (24.2%) | 12 (25.0%) | 11 (23.4%) | 0.856 |
| Pleura | 39 (41.1%) | 16 (33.3%) | 23 (48.9%) | 0.122 |
| Bone | 49 (51.6%) | 21 (43.8%) | 28 (59.6%) | 0.123 |
| Liver | 24 (25.3%) | 15 (31.3%) | 9 (19.1%) | 0.175 |
| Adrenal gland | 19 (20.0%) | 10 (20.8%) | 9 (19.1%) | 0.837 |
| Pericardium | 7 (7.4%) | 4 (8.3%) | 3 (6.4%) | 1 |
| WHO performance status | ||||
| 0 | 35 (36.8%) | 23 (47.9%) | 12 (25.5%) | 0.061 |
| 1 | 56 (58.9%) | 23 (47.9%) | 33 (70.2%) | |
| 2 | 4 (4.2%) | 2 (4.2%) | 2 (4.3%) |
| Toxicities | Toxicity Grade | All (n = 95) | Morning’ Group (n = 48) | Afternoon’ Group (n = 47) | p-Value |
|---|---|---|---|---|---|
| Fatigue | Grade 0 | 43 (45.3%) | 20 (41.7%) | 23 (48.9%) | 0.024 |
| Grade 1 | 13 (13.7%) | 11 (22.9%) | 2 (4.3%) | ||
| Grade 2 | 29 (30.5%) | 14 (29.2%) | 15 (31.9%) | ||
| Grade 3 | 9 (9.5%) | 2 (4.2%) | 7 (14.9%) | ||
| Grade 4 | 1 (1.1%) | 1 (2.1%) | 0 (0.0%) | ||
| Skin toxicity | Grade 0 | 70 (74.5%) | 30 (63.8%) | 40 (85.1%) | 0.049 |
| Grade 1 | 3 (3.2%) | 2 (4.3%) | 1 (2.1%) | ||
| Grade 2 | 19 (20.2%) | 14 (29.8%) | 5 (10.6%) | ||
| Grade 3 | 2 (2.1%) | 1 (2.1%) | 1 (2.1%) | ||
| Anorexia | Grade 0 | 71 (74.7%) | 36 (75.0%) | 35 (74.5%) | 0.856 |
| Grade 1 | 11 (11.6%) | 6 (12.5%) | 5 (10.6%) | ||
| Grade 2 | 9 (9.5%) | 5 (10.4%) | 4 (8.5%) | ||
| Grade 3 | 4 (4.2%) | 1 (2.1%) | 3 (6.4%) | ||
| Anemia | Grade 0 | 72 (75.8%) | 39 (81.3%) | 33 (70.2%) | 0.193 |
| Grade 1 | 6 (6.3%) | 1 (2.1%) | 5 (10.6%) | ||
| Grade 2 | 16 (16.8%) | 7 (14.6%) | 9 (19.1%) | ||
| Grade 3 | 1 (1.1%) | 1 (2.1%) | 0 (0%) | ||
| Nausea | Grade 0 | 75 (78.9%) | 40 (83.3%) | 35 (74.5%) | 0.633 |
| Grade 1 | 15 (15.8%) | 6 (12.5%) | 9 (19.1%) | ||
| Grade 2 | 4 (4.2%) | 2 (4.2%) | 2 (4.3%) | ||
| Grade 4 | 1 (1.1%) | 0 (0.0%) | 1 (2.1%) | ||
| Hepatitis | Grade 0 | 80 (84.2%) | 39 (81.3%) | 41 (87.2%) | 0.828 |
| Grade 1 | 13 (13.7%) | 7 (14.6%) | 6 (12.8%) | ||
| Grade 2 | 1 (1.1%) | 1 (2.1%) | 0 (0%) | ||
| Grade 3 | 1 (1.1%) | 1 (2.1%) | 0 (0.0%) | ||
| Renal failure | Grade 0 | 80 (84.2%) | 38 (79.2%) | 42 (89.4%) | 0.173 |
| Grade 1 | 15 (15.8%) | 10 (20.8%) | 5 (10.6%) | ||
| Vomiting | Grade 0 | 81 (85.3%) | 42 (87.5%) | 39 (83.0%) | 0.75 |
| Grade 1 | 9 (9.5%) | 4 (8.3%) | 5 (10.6%) | ||
| Grade 2 | 3 (3.2%) | 1 (2.1%) | 2 (4.3%) | ||
| Grade 3 | 1 (1.1%) | 1 (2.1%) | 0 (0.0%) | ||
| Grade 4 | 1 (1.1%) | 0 (0.0%) | 1 (2.1%) | ||
| Hypothyroidism | Grade 0 | 83 (87.4%) | 40 (83.3%) | 43 (91.5%) | 0.353 |
| Grade 1 | 8 (8.4%) | 6 (12.5%) | 2 (4.3%) | ||
| Grade 2 | 4 (4.2%) | 2 (4.2%) | 2 (4.3%) | ||
| Muscle toxicity | Grade 0 | 83 (87.4%) | 41 (85.4%) | 42 (89.4%) | 0.102 |
| Grade 1 | 4 (4.2%) | 4 (8.3%) | 0 (0.0%) | ||
| Grade 2 | 6 (6.3%) | 3 (6.3%) | 3 (6.4%) | ||
| Grade 3 | 2 (2.1%) | 0 (0.0%) | 2 (4.3%) | ||
| Diarrhea | Grade 0 | 84 (88.4%) | 43 (89.6%) | 41 (87.2%) | 0.197 |
| Grade 1 | 6 (6.3%) | 4 (8.3%) | 2 (4.3%) | ||
| Grade 2 | 3 (3.2%) | 0 (0.0%) | 3 (6.4%) | ||
| Grade 3 | 1 (1.1%) | 1 (2.1%) | 0 (0.0%) | ||
| Grade 4 | 1 (1.1%) | 0 (0.0%) | 1 (2.1%) | ||
| Abdominal pain | Grade 0 | 87 (91.6%) | 43 (89.6%) | 44 (93.6%) | 0.24 |
| Grade 1 | 3 (3.2%) | 2 (4.2%) | 1 (2.1%) | ||
| Grade 2 | 3 (3.2%) | 3 (6.3%) | 0 (0.0%) | ||
| Grade 3 | 2 (2.1%) | 0 (0.0%) | 2 (4.3%) |
| PFS | ||||
| 95% CL of hazard ratio | ||||
| Prognostic factors of PFS | p-values | Hazard ratio | CL inf | CL sup |
| NIV dosing time | 0.001 | 0.258 | 0.115 | 0.580 |
| 2nd line NIV | 0.009 | 0.324 | 0.140 | 0.752 |
| OS | ||||
| 95% CL of hazard ratio | ||||
| Prognostic factors of OS | p-value | Hazard ratio | CL inf | CL sup |
| NIV dosing time | <0.001 | 0.174 | 0.082 | 0.370 |
| 2nd line NIV | 0.004 | 0.390 | 0.204 | 0.746 |
| Adenocarcinoma | <0.014 | 0.460 | 0.248 | 0.854 |
| Bone not involved | 0.005 | 0.420 | 0.230 | 0.765 |
| Liver not involved | <0.001 | 0.300 | 0.153 | 0.588 |
| WHO PS 0 | 0.048 | 2.005 | 1.007 | 3.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaboué, A.; Collon, T.; Pavese, I.; Bodiguel, V.; Cucherousset, J.; Zakine, E.; Innominato, P.F.; Bouchahda, M.; Adam, R.; Lévi, F. Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results from a Pilot Study in Patients with Metastatic Non-Small-Cell Lung Cancer. Cancers 2022, 14, 896. https://doi.org/10.3390/cancers14040896
Karaboué A, Collon T, Pavese I, Bodiguel V, Cucherousset J, Zakine E, Innominato PF, Bouchahda M, Adam R, Lévi F. Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results from a Pilot Study in Patients with Metastatic Non-Small-Cell Lung Cancer. Cancers. 2022; 14(4):896. https://doi.org/10.3390/cancers14040896
Chicago/Turabian StyleKaraboué, Abdoulaye, Thierry Collon, Ida Pavese, Viviane Bodiguel, Joel Cucherousset, Elda Zakine, Pasquale F. Innominato, Mohamed Bouchahda, René Adam, and Francis Lévi. 2022. "Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results from a Pilot Study in Patients with Metastatic Non-Small-Cell Lung Cancer" Cancers 14, no. 4: 896. https://doi.org/10.3390/cancers14040896
APA StyleKaraboué, A., Collon, T., Pavese, I., Bodiguel, V., Cucherousset, J., Zakine, E., Innominato, P. F., Bouchahda, M., Adam, R., & Lévi, F. (2022). Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results from a Pilot Study in Patients with Metastatic Non-Small-Cell Lung Cancer. Cancers, 14(4), 896. https://doi.org/10.3390/cancers14040896









