A Tailored Approach for Appendicular Impending and Pathologic Fractures in Solid Cancer Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology of Metastatic Bone Disease
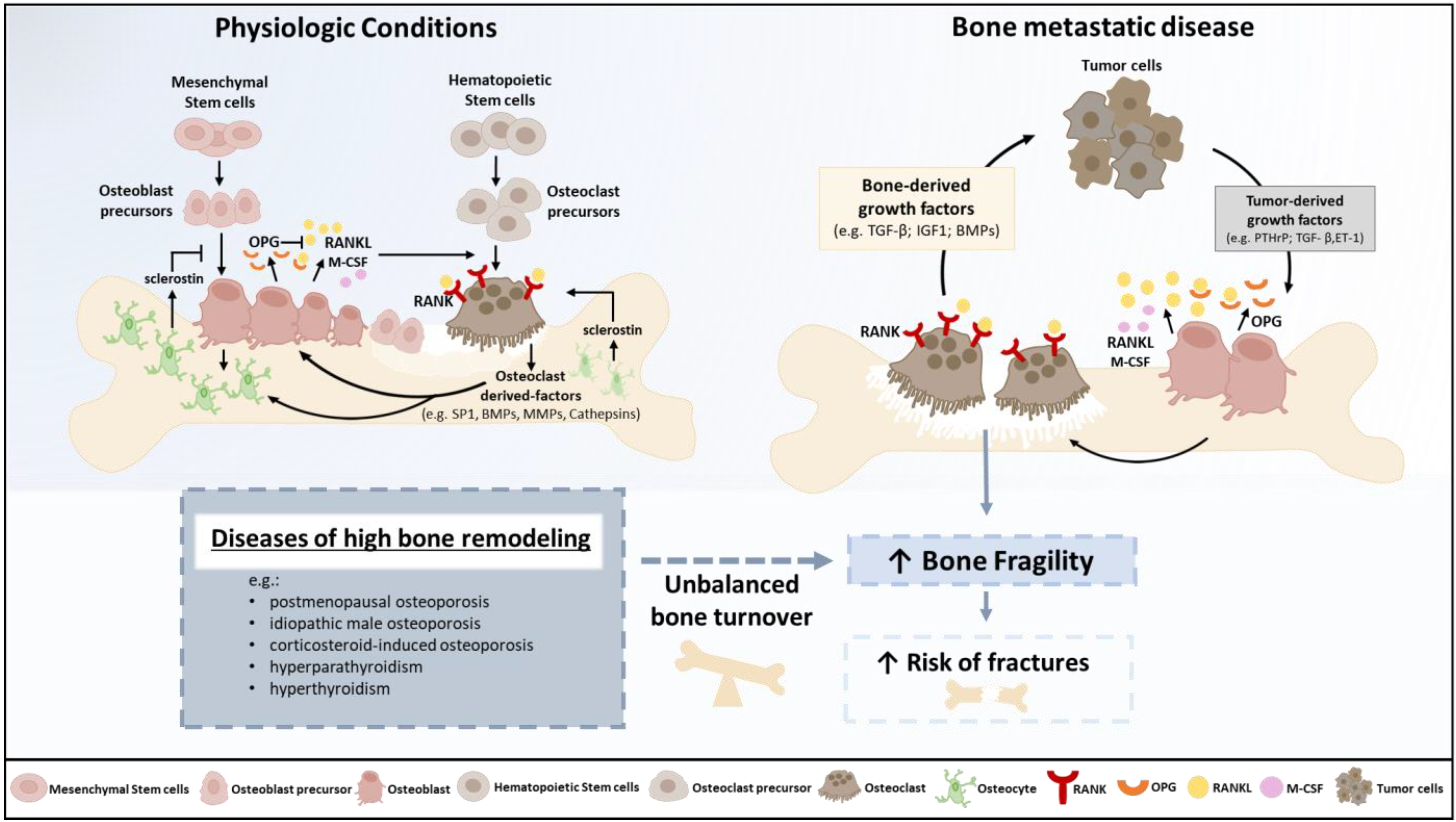
3. Physiopathology of Osteolysis and Bone Fragility
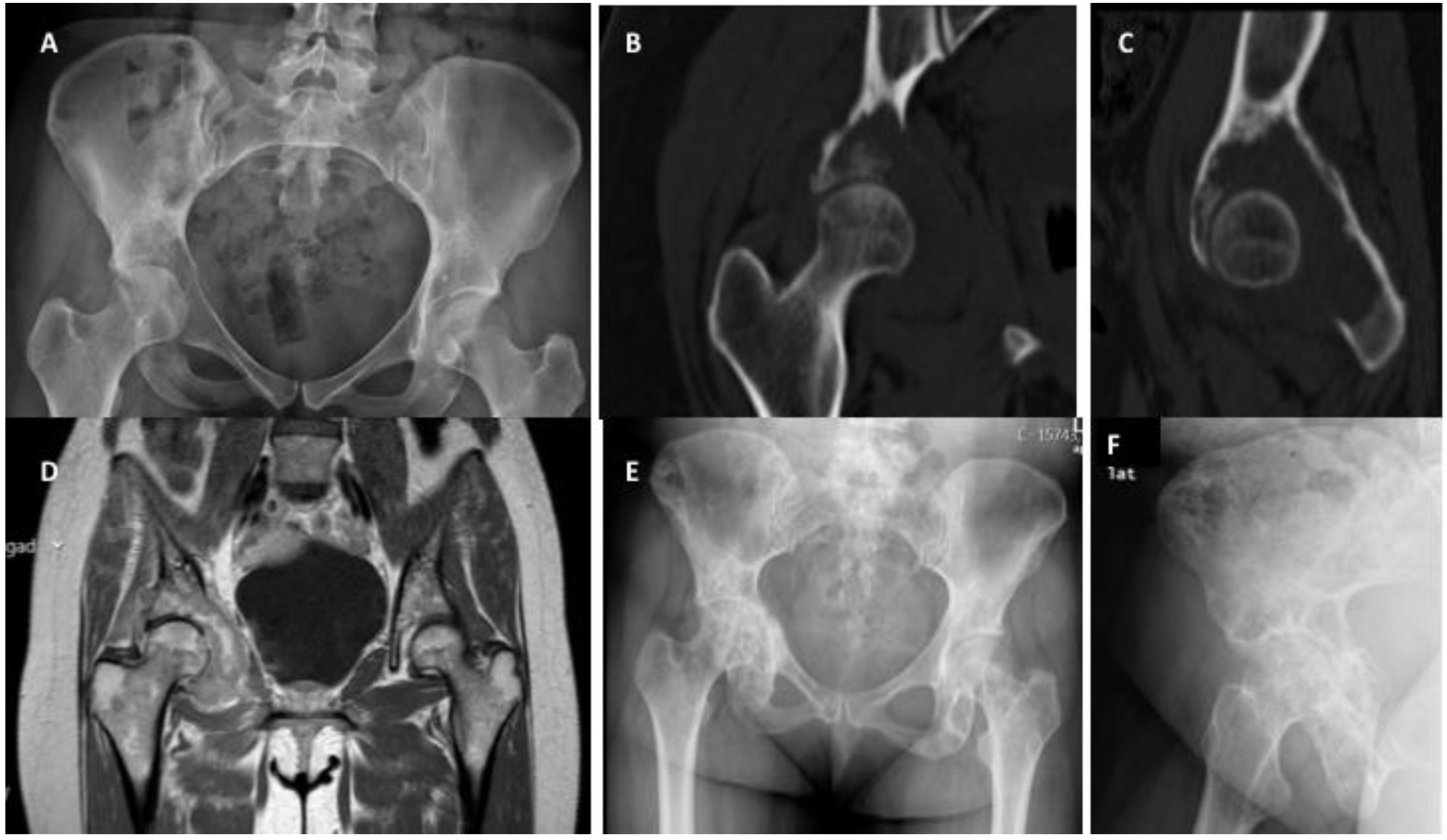
4. Impending and Pathologic Fractures Associated with Solid Metastases
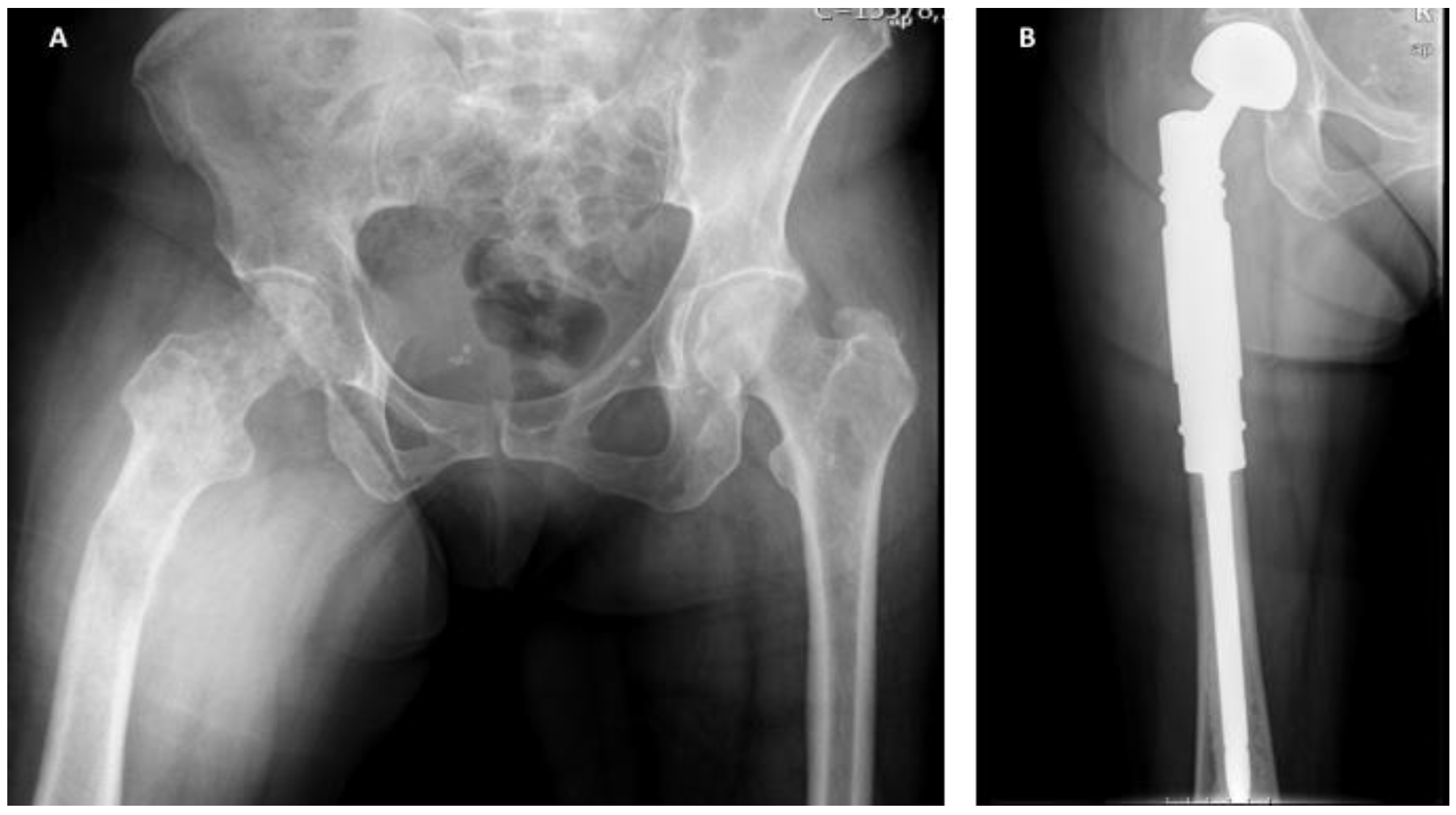
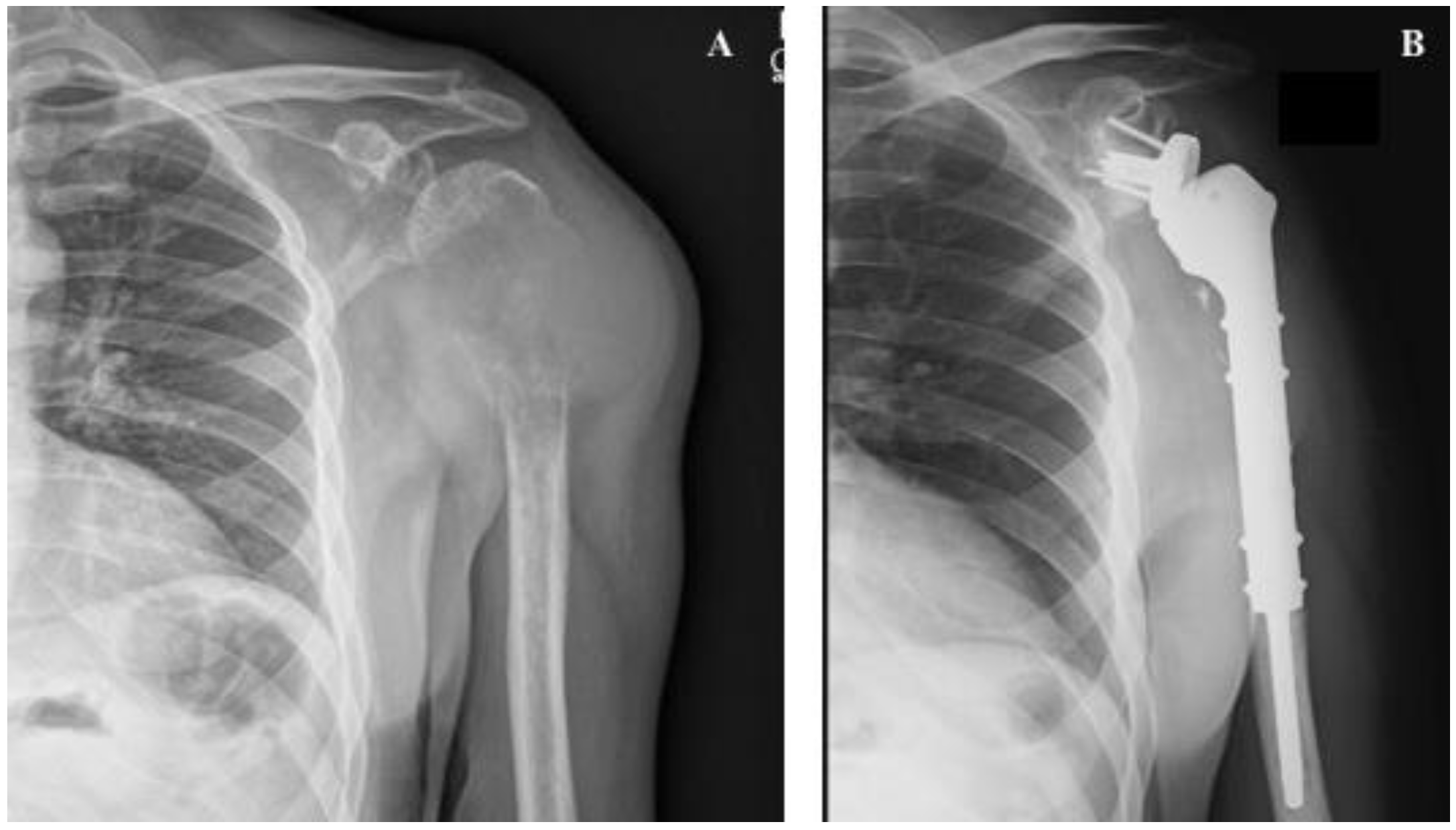
5. The Treatment of Impending and Pathologic Fractures Secondary to Bone Metastases
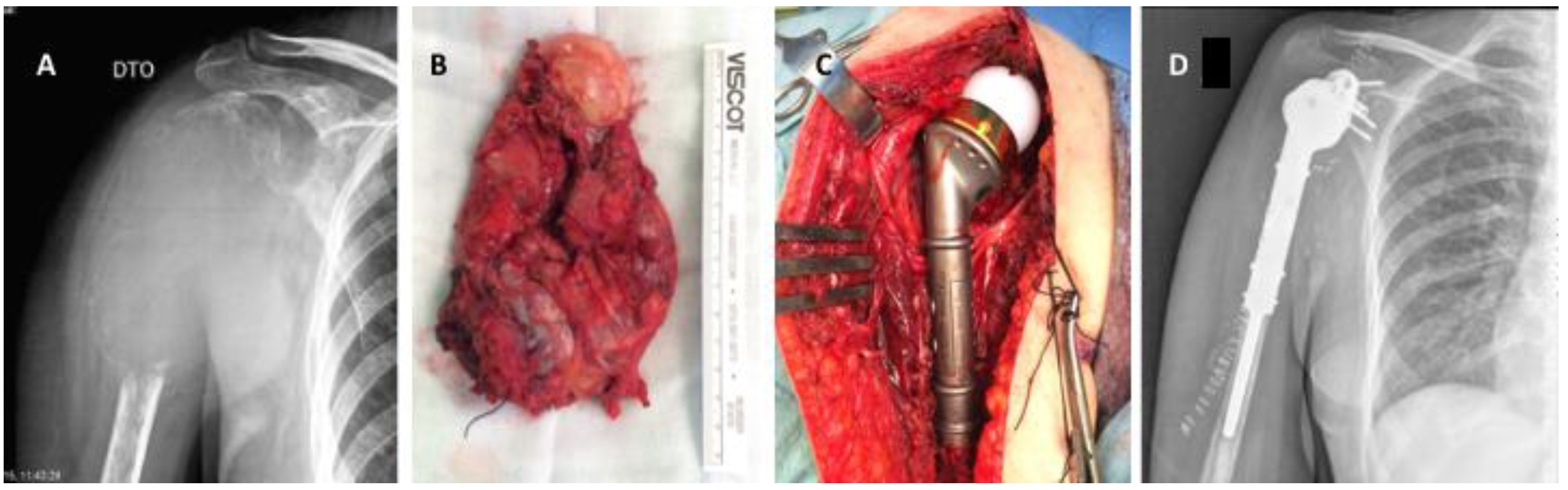
6. The Management of Appendicular Impending and Pathologic Fractures Based on Tumor Histology
6.1. Breast Cancer Metastases
6.2. Prostate Cancer Metastases
6.3. Lung Cancer Metastases
6.4. Renal Cancer Metastases
6.5. Thyroid Cancer Metastases
7. Primary Malignant Bone Tumors and Bone Metastases
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benca, E.; Patsch, J.M.; Mayr, W.; Pahr, D.H.; Windhager, R. The insufficiencies of risk analysis of impending pathological fractures in patients with femoral metastases: A literature review. Bone Rep. 2016, 5, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Jensen, A.; Jacobsen, J.B.; Nørgaard, M.; Fryzek, J.P.; Sørensen, H.T. Survival in breast cancer patients with bone metastases and skeletal-related events: A population-based cohort study in Denmark (1999–2007). Breast Cancer Res. Treat. 2011, 129, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Jensen, A.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sørensen, H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef]
- DePuy, V.; Anstrom, K.J.; Castel, L.D.; Schulman, K.A.; Weinfurt, K.P.; Saad, F. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Supportive Care Cancer 2007, 15, 869–876. [Google Scholar] [CrossRef]
- Weinfurt, K.P.; Li, Y.; Castel, L.D.; Saad, F.; Timbie, J.W.; Glendenning, G.A.; Schulman, K.A. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann. Oncol. 2005, 16, 579–584. [Google Scholar] [CrossRef]
- Body, J.-J.; Pereira, J.; Sleeboom, H.; Maniadakis, N.; Terpos, E.; Acklin, Y.P.; Finek, J.; Gunther, O.; Hechmati, G.; Mossman, T.; et al. Health resource utilization associated with skeletal-related events: Results from a retrospective European study. Eur. J. Health Econ. 2016, 17, 711–721. [Google Scholar] [CrossRef][Green Version]
- Barlev, A.; Song, X.; Ivanov, B.; Setty, V.; Chung, K. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. JMCP 2010, 16, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.T.; Lerman, D.M.; Patel, N.M.; Rapp, T.B. Is Prophylactic Intervention More Cost-effective Than the Treatment of Pathologic Fractures in Metastatic Bone Disease? Clin. Orthop. Relat. Res. 2016, 474, 1563–1570. [Google Scholar] [CrossRef]
- Mirels, H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin. Orthop. Relat. Res. 1989, 256–264. [Google Scholar] [CrossRef]
- Damron, T.A.; Morgan, H.; Prakash, D.; Grant, W.; Aronowitz, J.; Heiner, J. Critical evaluation of Mirels’ rating system for impending pathologic fractures. Clin. Orthop. Relat. Res. 2003, 415, S201–S207. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R.U.; Randall, R.L. Bone Metastases: Epidemiology and Societal Effect. In Metastatic Bone Disease: An Integrated Approach to Patient Care; Randall, R.L., Ed.; Springer: New York, NY, USA, 2016; pp. 3–11. [Google Scholar] [CrossRef]
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80, 1588–1594. [Google Scholar] [CrossRef]
- Hess, K.R.; Varadhachary, G.R.; Taylor, S.H.; Wei, W.; Raber, M.N.; Lenzi, R.; Abbruzzese, J.L. Metastatic patterns in adenocarcinoma. Cancer 2006, 106, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Y.; Weinhandl, E.D.; Blaes, A.H.; Cetin, K.; Chia, V.M.; Stryker, S.; Pinzone, J.J.; Acquavella, J.F.; Arneson, T.J. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin. Epidemiol. 2012, 4, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, B.; Subramaniam, D.; Joy, J. Skeletal Metastasis—An Epidemiological Study. Indian J. Surg. Oncol. 2018, 9, 46–51. [Google Scholar] [CrossRef]
- Jacofsky, D.J.; Haidukewych, G.J. Management of pathologic fractures of the proximal femur: State of the art. J. Orthop. Trauma 2004, 18, 459–469. [Google Scholar] [CrossRef]
- Nikkel, L. Hospitalizations for fracture in patients with metastatic disease: Primary source lesions in the United States. J. Community Supportive Oncol. 2017, 15, e14–e20. [Google Scholar] [CrossRef]
- Jensen, A.Ø.; Jacobsen, J.B.; Nørgaard, M.; Yong, M.; Fryzek, J.P.; Sørensen, H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark. BMC Cancer 2011, 11, 29. [Google Scholar] [CrossRef]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef]
- Joshi, A.D.; Carter, J.A.; Botteman, M.F.; Kaura, S. Cost-effectiveness of zoledronic acid in the management of skeletal metastases in patients with lung cancer in France, Germany, Portugal, the Netherlands, and the United kingdom. Clin. Ther. 2011, 33, 291–304.e298. [Google Scholar] [CrossRef]
- Lipton, A.; Colombo-Berra, A.; Bukowski, R.M.; Rosen, L.; Zheng, M.; Urbanowitz, G. Skeletal complications in patients with bone metastases from renal cell carcinoma and therapeutic benefits of zoledronic acid. Clin. Cancer Res. 2004, 10, 6397s–6403s. [Google Scholar] [CrossRef] [PubMed]
- Oefelein, M.G.; Ricchiuti, V.; Conrad, W.; Resnick, M.I. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol. 2002, 168, 1005–1007. [Google Scholar] [CrossRef]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.M.; Smith, M.; Coleman, R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Leali, P.T.; Muresu, F.; Melis, A.; Ruggiu, A.; Zachos, A.; Doria, C. Skeletal fragility definition. Clin. Cases Miner. Bone Metab. 2011, 8, 11–13. [Google Scholar] [PubMed]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. Bonekey Rep. 2014, 3, 481. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef]
- Kozlow, W.; Guise, T.A. Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. J. Mammary Gland Biol. Neoplasia 2005, 10, 169–180. [Google Scholar] [CrossRef]
- Chavassieux, P.; Seeman, E.; Delmas, P.D. Insights into material and structural basis of bone fragility from diseases associated with fractures: How determinants of the biomechanical properties of bone are compromised by disease. Endocr. Rev. 2007, 28, 151–164. [Google Scholar] [CrossRef]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clezardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Primers 2020, 6, 83. [Google Scholar] [CrossRef]
- Ali, S.M.; Demers, L.M.; Leitzel, K.; Harvey, H.A.; Clemens, D.; Mallinak, N.; Engle, L.; Chinchilli, V.; Costa, L.; Brady, C.; et al. Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastasis. Ann. Oncol. 2004, 15, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Rachner, T.D.; Coleman, R.E.; Jakob, F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014, 2, 500–512. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Loberg, R.; Taichman, R.S. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006, 25, 573–587. [Google Scholar] [CrossRef]
- Taichman, R.S.; Cooper, C.; Keller, E.T.; Pienta, K.J.; Taichman, N.S.; McCauley, L.K. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002, 62, 1832–1837. [Google Scholar]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef]
- Chu, G.C.; Zhau, H.E.; Wang, R.; Rogatko, A.; Feng, X.; Zayzafoon, M.; Liu, Y.; Farach-Carson, M.C.; You, S.; Kim, J.; et al. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr. Relat. Cancer 2014, 21, 311–326. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Dang, L.; Liang, C.; Lu, A.; Zhang, G. RANKL/RANK System-Based Mechanism for Breast Cancer Bone Metastasis and Related Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 76. [Google Scholar] [CrossRef]
- Casimiro, S.; Guise, T.A.; Chirgwin, J. The critical role of the bone microenvironment in cancer metastases. Mol. Cell. Endocrinol. 2009, 310, 71–81. [Google Scholar] [CrossRef]
- Casimiro, S.; Ferreira, A.R.; Mansinho, A.; Alho, I.; Costa, L. Molecular Mechanisms of Bone Metastasis: Which Targets Came from the Bench to the Bedside? Int. J. Mol. Sci. 2016, 17, 1415. [Google Scholar] [CrossRef]
- Yin, J.J.; Mohammad, K.S.; Kakonen, S.M.; Harris, S.; Wu-Wong, J.R.; Wessale, J.L.; Padley, R.J.; Garrett, I.R.; Chirgwin, J.M.; Guise, T.A. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. USA 2003, 100, 10954–10959. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.B.; Hedican, S.P.; George, D.J.; Reddi, A.H.; Piantadosi, S.; Eisenberger, M.A.; Simons, J.W. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat. Med. 1995, 1, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Guise, T.A.; Yin, J.J.; Elliott, J.; Horwood, N.J.; Martin, T.J.; Gillespie, M.T. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 1999, 140, 4451–4458. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef]
- Fidler, M. Incidence of fracture through metastases in long bones. Acta Orthop. Scand. 1981, 52, 623–627. [Google Scholar] [CrossRef]
- Menck, H.; Schulze, S.; Larsen, E. Metastasis size in pathologic femoral fractures. Acta Orthop. Scand. 1988, 59, 151–154. [Google Scholar] [CrossRef]
- Harrington, K.D. Impending pathologic fractures from metastatic malignancy: Evaluation and management. Instr. Course Lect. 1986, 35, 357–381. [Google Scholar]
- Van der Wal, C.; Eggermont, F.; Fiocco, M.; Kroon, H.M.; Ayu, O.; Slot, A.; Snyers, A.; Rozema, T.; Verdonschot, N.J.J.; Dijkstra, P.D.S.; et al. Axial cortical involvement of metastatic lesions to identify impending femoral fractures; a clinical validation study. Radiother. Oncol. 2020, 144, 59–64. [Google Scholar] [CrossRef]
- Liebl, H.; Garcia, E.G.; Holzner, F.; Noel, P.B.; Burgkart, R.; Rummeny, E.J.; Baum, T.; Bauer, J.S. In-Vivo Assessment of Femoral Bone Strength Using Finite Element Analysis (FEA) Based on Routine MDCT Imaging: A Preliminary Study on Patients with Vertebral Fractures. PLoS ONE 2015, 10, e0116907. [Google Scholar] [CrossRef]
- Koivumäki, J.E.; Thevenot, J.; Pulkkinen, P.; Kuhn, V.; Link, T.M.; Eckstein, F.; Jämsä, T. Ct-based finite element models can be used to estimate experimentally measured failure loads in the proximal femur. Bone 2012, 50, 824–829. [Google Scholar] [CrossRef]
- De Felice, F.; Piccioli, A.; Musio, D.; Tombolini, V. The role of radiation therapy in bone metastases management. Oncotarget 2017, 8, 25691–25699. [Google Scholar] [CrossRef]
- Agarwal, J.; Baum, R.; Hoefnagel, C.; Hoskin, P.; Mount Kim, E.; Mariani, G. Criteria for Palliation of Bone Metastases—Clinical Applications; IAEA: Vienna, Austria, 2007. [Google Scholar]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef]
- Wu, J.S.; Wong, R.K.; Lloyd, N.S.; Johnston, M.; Bezjak, A.; Whelan, T. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases—An evidence-based practice guideline. BMC Cancer 2004, 4, 71. [Google Scholar] [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W.M. Palliative radiotherapy trials for bone metastases: A systematic review. J. Clin. Oncol. 2007, 25, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. 2012, 24, 112–124. [Google Scholar] [CrossRef]
- Rich, S.E.; Chow, R.; Raman, S.; Liang Zeng, K.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Willeumier, J.J.; van der Linden, Y.M.; Dijkstra, P.D. Lack of clinical evidence for postoperative radiotherapy after surgical fixation of impending or actual pathologic fractures in the long bones in patients with cancer; a systematic review. Radiother. Oncol. 2016, 121, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Gralow, J.R.; Biermann, J.S.; Farooki, A.; Fornier, M.N.; Gagel, R.F.; Kumar, R.; Litsas, G.; McKay, R.; Podoloff, D.A.; Srinivas, S.; et al. NCCN Task Force Report: Bone Health In Cancer Care. J. Natl. Compr. Cancer Netw. 2013, 11 (Suppl. 3), S-1–S-50. [Google Scholar] [CrossRef] [PubMed]
- Capanna, R.; Piccioli, A.; Di Martino, A.; Daolio, P.A.; Ippolito, V.; Maccauro, G.; Piana, R.; Ruggieri, P.; Gasbarrini, A.; Spinelli, M.S.; et al. Management of long bone metastases: Recommendations from the Italian Orthopaedic Society bone metastasis study group. Expert Rev. Anticancer Ther. 2014, 14, 1127–1134. [Google Scholar] [CrossRef]
- Bickels, J.; Dadia, S.; Lidar, Z. Surgical management of metastatic bone disease. J. Bone Jt. Surg. 2009, 91, 1503–1516. [Google Scholar] [CrossRef]
- Katzer, A.; Meenen, N.M.; Grabbe, F.; Rueger, J.M. Surgery of skeletal metastases. Arch. Orthop. Trauma Surg. 2002, 122, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Imanishi, J.; Charoenlap, C.; Choong, P.F.M. Intramedullary nailing has sufficient durability for metastatic femoral fractures. World J. Surg. Oncol. 2016, 14, 80. [Google Scholar] [CrossRef]
- Dijstra, S.; Wiggers, T.; van Geel, B.N.; Boxma, H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur. J. Surg. Acta Chir. 1994, 160, 535–542. [Google Scholar]
- Cheung, F.H. The practicing orthopedic surgeon’s guide to managing long bone metastases. Orthop. Clin. N. Am. 2014, 45, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Denaro, V.; Di Martino, A.; Piccioli, A. (Eds.) Management of Bone Metastases: A Multidisciplinary Guide, 1st ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Müller, D.A.; Capanna, R. The Surgical Treatment of Pelvic Bone Metastases. Adv. Orthop. 2015, 2015, 525363. [Google Scholar] [CrossRef]
- Wunder, J.S.; Ferguson, P.C.; Griffin, A.M.; Pressman, A.; Bell, R.S. Acetabular metastases: Planning for reconstruction and review of results. Clin. Orthop. Relat. Res. 2003, 415, S187–S197. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Crockard, A.; Antonietti, P.; Boriani, S.; Bünger, C.; Gasbarrini, A.; Grejs, A.; Harms, J.; Kawahara, N.; Mazel, C.; et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J. Neurosurg. Spine 2008, 8, 271–278. [Google Scholar] [CrossRef]
- Bauer, H.C.; Wedin, R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop. Scand. 1995, 66, 143–146. [Google Scholar] [CrossRef]
- Kieser, D.C.; Parker, J.; Reynolds, J. En Bloc Resection of Isolated Spinal Metastasis: A Systematic Review Update. Clin. Spine Surg. 2020, 34, 103–106. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Clezardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone Metastasis: Mechanisms, Therapies and Biomarkers. Physiol. Rev. 2020, 101, 797–855. [Google Scholar] [CrossRef]
- Singh, T.; Kaur, V.; Kumar, M.; Kaur, P.; Murthy, R.S.; Rawal, R.K. The critical role of bisphosphonates to target bone cancer metastasis: An overview. J. Drug Target. 2015, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Ferrari, S.; Russell, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Henry, D.; Vadhan-Raj, S.; Hirsh, V.; von Moos, R.; Hungria, V.; Costa, L.; Woll, P.J.; Scagliotti, G.; Smith, G.; Feng, A.; et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: An analysis of data from patients with solid tumors. Supportive Care Cancer 2014, 22, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P. Denosumab: A comprehensive review. South Asian J. Cancer 2013, 2, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Mengistu, G.; Edessa, D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: Potential targets for anticancer therapy. Onco Targets Ther. 2017, 10, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Dougall, W.C. Molecular pathways: Osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 2012, 18, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Cronin, S.J.F.; Penninger, J.M. Targeting the RANKL/RANK/OPG Axis for Cancer Therapy. Front. Oncol. 2020, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Schramek, D.; Widschwendter, M.; Fourkala, E.O.; Zaikin, A.; Jones, A.; Jaeger, B.; Rack, B.; Janni, W.; Scholz, C.; et al. Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer. Oncotarget 2017, 8, 3811–3825. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Kuo, C.H.; Lai, T.Y.; Lin, Y.M.; Su, C.C.; Hsu, H.H.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Tang, C.H. RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. J. Cell. Biochem. 2011, 112, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Wu, B.; Dong, Z.; Wang, Y.; Lu, J.; Shi, P.; Bai, W.; Wang, Z. Potential role of the OPG/RANK/RANKL axis in prostate cancer invasion and bone metastasis. Oncol. Rep. 2014, 32, 2605–2611. [Google Scholar] [CrossRef]
- Van Poznak, C.; Somerfield, M.R.; Barlow, W.E.; Biermann, J.S.; Bosserman, L.D.; Clemons, M.J.; Dhesy-Thind, S.K.; Dillmon, M.S.; Eisen, A.; Frank, E.S.; et al. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J. Clin. Oncol. 2017, 35, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Saylor, P.J.; Rumble, R.B.; Tagawa, S.; Eastham, J.A.; Finelli, A.; Reddy, P.S.; Kungel, T.M.; Nissenberg, M.G.; Michalski, J.M. Bone Health and Bone-Targeted Therapies for Prostate Cancer: ASCO Endorsement of a Cancer Care Ontario Guideline. J. Clin. Oncol. 2020, 38, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Campagnaro, E.; Reimers, M.A.; Qin, A.; Alva, A.S.; Schneider, B.J.; Van Poznak, C.H. Use of Bone-Modifying Agents in Myeloma and Bone Metastases: How Recent Dosing Interval Studies Have Affected Our Practice. J. Oncol. Pract. 2018, 14, 457–464. [Google Scholar] [CrossRef]
- Leng, S.; Lentzsch, S. Bone-Modifying Agents: Complicated to Use. J. Oncol. Pract. 2018, 14, 469–470. [Google Scholar] [CrossRef]
- Glantschnig, H.; Fisher, J.E.; Wesolowski, G.; Rodan, G.A.; Reszka, A.A. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003, 10, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Coleman, R.; Gnant, M. Bone effects of mammalian target of rapamycin (mTOR) inhibition with everolimus. Crit. Rev. Oncol. Hematol. 2013, 87, 101–111. [Google Scholar] [CrossRef]
- Lee, R.J.; Smith, M.R. Targeting MET and vascular endothelial growth factor receptor signaling in castration-resistant prostate cancer. Cancer J. 2013, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Xu, C.; Li, B.; Chen, J.; Chen, J.; Wang, Z. Immune Checkpoint Inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J. Immunol. Res. 2021, 2021, 8970173. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Flamini, E.; Fabbri, L.; Serra, P.; Mercatali, L.; Ricci, R.; Sacanna, E.; Falasconi, M.C.; Casadei, R.; Galassi, R.; et al. Multidisciplinary approach to the treatment of bone metastases: Osteo-Oncology Center, a new organizational model. Tumori J. 2009, 95, 291–297. [Google Scholar] [CrossRef]
- Bongiovanni, A.; Recine, F.; Fausti, V.; Foca, F.; Casadei, R.; Falasconi, M.C.; Oboldi, D.; Sansoni, E.; Fabbri, L.; Micheletti, S.; et al. Ten-year experience of the multidisciplinary Osteoncology Center. Supportive Care Cancer 2019, 27, 3395–3402. [Google Scholar] [CrossRef]
- Kuchuk, I.; Hutton, B.; Moretto, P.; Ng, T.; Addison, C.L.; Clemons, M. Incidence, consequences and treatment of bone metastases in breast cancer patients-Experience from a single cancer centre. J. Bone Oncol. 2013, 2, 137–144. [Google Scholar] [CrossRef]
- Lin, P.P.; Mirza, A.N.; Lewis, V.O.; Cannon, C.P.; Tu, S.M.; Tannir, N.M.; Yasko, A.W. Patient survival after surgery for osseous metastases from renal cell carcinoma. J. Bone Jt. Surg. 2007, 89, 1794–1801. [Google Scholar] [CrossRef]
- Ratasvuori, M.; Wedin, R.; Hansen, B.H.; Keller, J.; Trovik, C.; Zaikova, O.; Bergh, P.; Kalen, A.; Laitinen, M. Prognostic role of en-bloc resection and late onset of bone metastasis in patients with bone-seeking carcinomas of the kidney, breast, lung, and prostate: SSG study on 672 operated skeletal metastases. J. Surg. Oncol. 2014, 110, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kido, A.; Tanaka, Y.; Facchini, G.; Peta, G.; Rossi, G.; Mavrogenis, A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021, 28, 3347–3372. [Google Scholar] [CrossRef]
- Lin, S.C.; Yu-Lee, L.Y.; Lin, S.H. Osteoblastic Factors in Prostate Cancer Bone Metastasis. Curr. Osteoporos. Rep. 2018, 16, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Cho, Y.M.; Kim, S.H.; Shin, K.-H.; Jung, S.-T.; Kim, H.S. Clinical analysis of patients with skeletal metastasis of lung cancer. BMC Cancer 2019, 19, 303. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019, 15, 100205. [Google Scholar] [CrossRef]
- Ruatta, F.; Derosa, L.; Escudier, B.; Colomba, E.; Guida, A.; Baciarello, G.; Loriot, Y.; Fizazi, K.; Albiges, L. Prognosis of renal cell carcinoma with bone metastases: Experience from a large cancer centre. Eur. J. Cancer 2019, 107, 79–85. [Google Scholar] [CrossRef]
- Umer, M.; Mohib, Y.; Atif, M.; Nazim, M. Skeletal metastasis in renal cell carcinoma: A review. Ann. Med. Surg. 2018, 27, 9–16. [Google Scholar] [CrossRef]
- Geraets, S.E.W.; Bos, P.K.; van der Stok, J. Preoperative embolization in surgical treatment of long bone metastasis: A systematic literature review. EFORT Open Rev. 2020, 5, 17–25. [Google Scholar] [CrossRef]
- Iñiguez-Ariza, N.M.; Bible, K.C.; Clarke, B.L. Bone metastases in thyroid cancer. J. Bone Oncol. 2020, 21, 100282. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
- De Pinieux, G.; Karanian, M.; Le Loarer, F.; Le Guellec, S.; Chabaud, S.; Terrier, P.; Bouvier, C.; Batistella, M.; Neuville, A.; Robin, Y.M.; et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS ONE 2021, 16, e0246958. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Soft Tissue and Bone Tumours; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Lalam, R.; Bloem, J.L.; Noebauer-Huhmann, I.M.; Wörtler, K.; Tagliafico, A.; Vanhoenacker, F.; Nikodinovska, V.V.; Sanal, H.T.; Woude, H.V.; Papakonstantinou, O.; et al. ESSR Consensus Document for Detection, Characterization, and Referral Pathway for Tumors and Tumorlike Lesions of Bone. Semin. Musculoskelet. Radiol. 2017, 21, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.E.; Madewell, J.E.; Ragsdale, B.D. Radiologic and pathologic analysis of solitary bone lesions. Part III: Matrix patterns. Radiol. Clin. N. Am. 1981, 19, 785–814. [Google Scholar] [PubMed]
- Ragsdale, B.D.; Madewell, J.E.; Sweet, D.E. Radiologic and pathologic analysis of solitary bone lesions. Part II: Periosteal reactions. Radiol. Clin. N. Am. 1981, 19, 749–783. [Google Scholar] [PubMed]
- Monfardini, L.; Preda, L.; Aurilio, G.; Rizzo, S.; Bagnardi, V.; Renne, G.; Maccagnoni, S.; Vigna, P.D.; Davide, D.; Bellomi, M. CT-guided bone biopsy in cancer patients with suspected bone metastases: Retrospective review of 308 procedures. Radiol. Med. 2014, 119, 852–860. [Google Scholar] [CrossRef]
- Zustovich, F.; Pastorelli, D. Therapeutic management of bone metastasis in prostate cancer: An update. Expert Rev. Anticancer Ther. 2016, 16, 1199–1211. [Google Scholar] [CrossRef]
- Zhiyu, W.; Rui, Z.; Shuai, W.; Hui, Z. Surgical treatment of patients with lung cancer and bone metastases: A prospective, observational study. Lancet 2016, 388, S42. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M.J. Molecular mechanisms of action of bisphosphonates: Current status. Clin. Cancer Res. 2006, 12, 6222s–6230s. [Google Scholar] [CrossRef]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Graham, R.; Russell, G.; Rogers, M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 1998, 13, 581–589. [Google Scholar] [CrossRef]
- Van der Linden, Y.M.; Kroon, H.M.; Dijkstra, S.P.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.; Marijnen, C.A. Simple radiographic parameter predicts fracturing in metastatic femoral bone lesions: Results from a randomised trial. Radiother. Oncol. 2003, 69, 21–31. [Google Scholar] [CrossRef]
- Wernle, J.D.; Damron, T.A.; Allen, M.J.; Mann, K.A. Local irradiation alters bone morphology and increases bone fragility in a mouse model. J. Biomech. 2010, 43, 2738–2746. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ito, T.; Mizuno, Y.; Sudo, Y.; Kim, Y.; Tsunoda, R.; Takai, S. Effect of Orthopedics Promotional Activities on Multidisciplinary Management of Patients with Bone Metastases. J. Nippon Med. Sch. 2019, 86, 327–335. [Google Scholar] [CrossRef] [PubMed]


| Parameter | 1 | 2 | 3 |
|---|---|---|---|
| Site | Upper limb | Lower limb | Peritrochanteric |
| Size | <1/3 | 1/3 to 2/3 | >2/3 |
| Lesion | Blastic | Mixed | Lytic |
| Pain | Mild | Moderate | Functional |
| Tumor Histology | Breast | Prostate | Lung | Kidney | Thyroid |
|---|---|---|---|---|---|
| External beam radiotherapy | +++ | +++ | +++ | + | + |
| Surgery | ++ | + | ++ | +++ | +++ |
| Bone-targeted agents | +++ | +++ | ++ | ++ | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares do Brito, J.; Lopes-Brás, R.; Abrunhosa-Branquinho, A.; Fernandes, I.; Gomes, I.; Casimiro, S.; Costa, L. A Tailored Approach for Appendicular Impending and Pathologic Fractures in Solid Cancer Metastases. Cancers 2022, 14, 893. https://doi.org/10.3390/cancers14040893
Soares do Brito J, Lopes-Brás R, Abrunhosa-Branquinho A, Fernandes I, Gomes I, Casimiro S, Costa L. A Tailored Approach for Appendicular Impending and Pathologic Fractures in Solid Cancer Metastases. Cancers. 2022; 14(4):893. https://doi.org/10.3390/cancers14040893
Chicago/Turabian StyleSoares do Brito, Joaquim, Raquel Lopes-Brás, André Abrunhosa-Branquinho, Isabel Fernandes, Inês Gomes, Sandra Casimiro, and Luís Costa. 2022. "A Tailored Approach for Appendicular Impending and Pathologic Fractures in Solid Cancer Metastases" Cancers 14, no. 4: 893. https://doi.org/10.3390/cancers14040893
APA StyleSoares do Brito, J., Lopes-Brás, R., Abrunhosa-Branquinho, A., Fernandes, I., Gomes, I., Casimiro, S., & Costa, L. (2022). A Tailored Approach for Appendicular Impending and Pathologic Fractures in Solid Cancer Metastases. Cancers, 14(4), 893. https://doi.org/10.3390/cancers14040893







