Simple Summary
Cholangiocarcinoma (CCA) is a heterogenous and aggressive malignancy of the intra- and extrahepatic biliary tract, marked by a steeply rising incidence on a global scale. While surgery remains the only curative treatment option, most patients present with advanced or unresectable disease, and are, therefore, treated with systemic therapy, albeit with limited benefit. Biomarkers obtained from either the patients’ serum or tumor tissue might facilitate therapy guidance by selecting patients who would benefit the most from surgical and adjuvant treatment strategies, as well as by identifying those with higher risk of disease recurrence. Furthermore, several genetic aberrations in CCA have been linked with improved response upon targeted therapies, thus highlighting their role as predictive biomarkers. In this review we provide an overview of currently known prognostic and predictive biomarkers and discuss their role in CCA.
Abstract
Cholangiocarcinoma (CCA) is the second most common primary liver cancer and subsumes a heterogeneous group of malignant tumors arising from the intra- or extrahepatic biliary tract epithelium. A rising mortality from CCA has been reported worldwide during the last decade, despite significant improvement of surgical and palliative treatment. Over 50% of CCAs originate from proximal extrahepatic bile ducts and constitute the most common CCA entity in the Western world. Clinicopathological characteristics such as lymph node status and poor differentiation remain the best-studied, but imperfect prognostic factors. The identification of prognostic molecular markers as an adjunct to traditional staging systems may not only facilitate the selection of patients who would benefit the most from surgical, adjuvant or palliative treatment strategies, but may also be helpful in defining the aggressiveness of the disease and identifying patients at high-risk for tumor recurrence. The purpose of this review is to provide an overview of currently known molecular prognostic and predictive markers and their role in CCA.
1. Introduction
Cholangiocarcinoma (CCA) is a highly aggressive malignancy and the second most common primary liver tumor, accounting for three percent of all gastrointestinal malignancies [1]. The classification is based upon the anatomical site of origin, thus differentiating between intrahepatic (iCCA), perihilar (pCCA) (also called Klatskin tumor), and distal (dCCA) cholangiocarcinoma, with dCCA and pCCA being summarized as extrahepatic cholangiocarcinomas (eCCA). The most common entities are dCCA and pCCA, accounting for about 30% and 50% of all CCAs, respectively. In contrast, iCC represents only about 10% of all CCAs [2]. The anatomical boundary defining eCCA is the cystic duct, with dCCA arising distal and pCCA proximal of the junction of the cystic duct, comprising the right and left hepatic ducts up to the second order biliary branches. Tumors located above the second order bile ducts are termed iCCA [1,3,4]. Different types of CCA do not only differ in their etiology, pathophysiology, and treatment, but also possess unique biological and pathological features, providing the opportunity for individualized prognosis determination and targeted therapies.
Many CCA cases are sporadic, arising in the absence of known risk factors such as chronic biliary inflammation (e.g., in primary sclerosing cholangitis—PSC), cholestasis, hepatobiliary parasitic infections (liver flukes like Clonorchis sinensis and Opisthorchis viverrini), and liver cirrhosis (Figure 1) [1]. Furthermore, emerging evidence over the past years has suggested an important role of the gut microbiome on development of CCA, especially in patients with inflammatory bowel disease and PSC [5]. Epigenetic and environmental factors seem to have a significant impact on the development the disease, since there is a great difference in prevalence of CCA between Southeast Asia (e.g., 85 per 100,000 in Northeast Thailand) and the Western hemisphere (<6 per 100,000 population). Symptoms of CCA are usually vague and arise late in already-advanced disease, resulting in median survival of less than two years from the timepoint of diagnosis. So far, surgery remains the only curative treatment option, with a 5-year survival rate ranging between 25 and 50% after surgical resection [6,7,8,9,10,11]. Due to high risk of recurrence, especially in patients with lymph node metastasis, tumor-positive resection borders, and low-grade CCA, adjuvant chemotherapy is recommended by current guidelines [12,13,14]. Patients with metastatic or inoperable disease are treated with systemic chemotherapy, albeit with dismal benefit [7,15,16,17]. Advancements in organ preservation and poor survival outcomes following surgical resection have promoted consideration of liver transplantation (LT) as a curative approach for patients suffering from pCCA and iCCA [18,19]. In fact, LT in patients adhering to the so-called Mayo clinic protocol, compromising neoadjuvant chemoradiation in patients with unresectable pCCA, size <3 cm and without extrahepatic or lymph node metastasis, offered promising results [20]. A French multicenter randomized, intent-to-treat study (NCT02232932) comparing the 5-year survival following capecitabine-based chemoradiotherapy with subsequent LT to standard liver resection in patients suffering pCCA is ongoing [21]. In contrast to pCCA, iCCA is a contraindication in most centers worldwide due to historically poor outcome. However, recent data suggest LT as an effective treatment in highly selected patients with localized and early iCCA or patients with disease stability after neoadjuvant chemotherapy [11]. Results from a multicenter, single-arm, prospective study (NCT02878473) evaluating the 5-year survival in patients with single iCC ≤ 2 cm in size, liver cirrhosis, and CA 19-9 ≤ 100 ng/mL, undergoing LT are eagerly awaited. While the number of patients suffering from CCA continues to rise, the need for novel diagnostic strategies and therapeutic options has become of utmost clinical importance. As such, the identification of preoperatively available molecular markers of prognosis as an adjunct to traditional staging systems has emerged as a promising strategy to select patients who may benefit the most from surgical and adjuvant therapy. Furthermore, the identification of predictive markers might result in precise and effective systemic treatment.
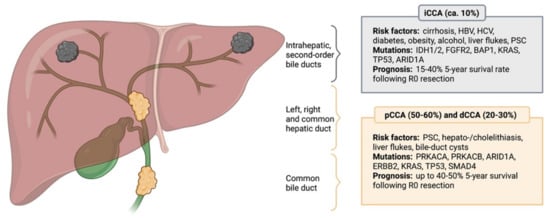
Figure 1.
Anatomical classification of cholangiocarcinoma. CCA is anatomically divided into intrahepatic (iCCA), perihillar (pCCA) and distal (dCCA) cholangiocarcinoma, with pCCA and dCCA being summarized as extrahepatic cholangiocarcinoma (eCCA). Different CCA subtypes possess distinct molecular aberrations and differ in terms of their etiology, while certain risk factors and genetic mutations are not subtype-specific. The most common risk factors and prevailing genetic alterations are presented. HBV: Hepatitis B virus; HCV: Hepatitis C virus; PSC: Primary sclerosing cholangitis; IDH1/2: Isocitrate dehydrogenase 1/2; FGFR2: Fibroblast growth factor receptor 2; BAP1: BRCA1 associated protein 1; KRAS: Kirsten rat sarcoma virus; TP53: Tumor suppressor protein 53; ARID1A: AT-rich interactive domain-containing protein 1A; PRKACA: Protein kinase cAMP-activated catalytic subunit alpha; PRKACB: Protein kinase cAMP-activated catalytic subunit beta; ERBB2: Erb-B2 receptor tyrosine kinase 2; SMAD4: Mothers against decapentaplegic homolog 4.
2. Biomarkers in CCA
Biomarkers can have either prognostic or predictive value, while certain biomarkers are known to possess both. Prognostic biomarkers inform about the likelihood of certain cancer-associated events, e.g., disease-recurrence or progression of disease, and overall survival. Predictive biomarkers provide information about treatment benefit and are used to identify individuals who are more likely to have a favorable or unfavorable effect from a particular therapy compared to individuals without the biomarker [22,23,24]. The utility of molecular markers for clinical practice has been assessed by defining their levels of evidence, ranging from level I as the highest evidence level, obtained from high-powered, prospective, randomized controlled trials (RCT), to level V, which possess the weakest evidence and are derived from single clinical cases. Importantly, in order for a novel biomarker to be implemented into the routine clinical practice, at least level II evidence is required [25].
Different types of CCA do not only show distinct anatomical and histological features, but also possess individual molecular profiles and genetic aberrations. In this context, Nakamura et al. identified a total of 32 significantly altered genes in about 40% of cases by molecularly characterizing biliary tract cancers from 260 patients [26]. Another research group analyzed 410 cancer-associated genes in tumor samples of 195 patients and discovered genetic alterations with potential therapeutic implications in 47% these of patients [27]. The spectrum of actionable genomic targets in CCA embraces various kinases (FGFR1, FGFR2, FGFR3, PIK3CA, ALK, EGFR, ERBB2, BRAF and AKT3), oncogenes (IDH1, IDH2, CCND1, CCND3 and MDM2) and tumor-suppressor genes (BRCA1 and BRCA2) [26]. The most common genetic alterations shared between iCCA and eCCA are KRAS mutations (15%–20%), TP53 mutations (15 %–25%) and AT-rich interactive domain-containing protein 1A (ARID1A) mutations (approx. 20%) [28]. Certain alterations, such as FGFR1/2 fusions or IDH1 mutations, are unique to iCCA and rarely occur in eCCA. Mutations in BRCA1 associated protein 1 (BAP1) are also enriched in iCCA and present in less than 1% of eCCA. In contrast, mutations in ERBB2 seem to be exclusive to eCCA [26,29]. An overview of common signaling pathways involved in the development and progression of CCA is displayed in Figure 2.
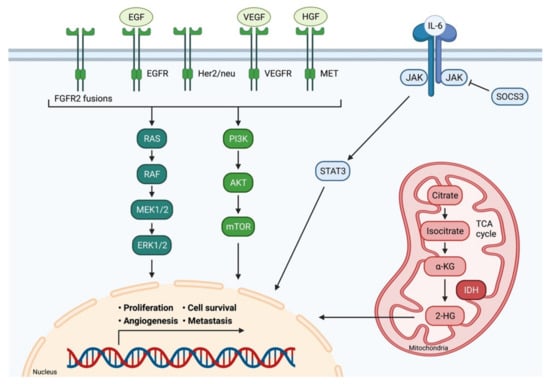
Figure 2.
Signaling pathways in cholangiocarcinoma. Multiple signaling pathways are involved in the development and progression of CCA. Receptor tyrosine kinases activate the RAS-MAPK pathway and the PI3K-AKT pathway. IL-6 induces the JAK/STAT signaling pathway. Consequently, these pathways impact important cellular processes, such as cell proliferation, differentiation, survival, and angiogenesis. IDH 1/2 mutations lead to the accumulation of the oncometabolite intracellular 2-hydroxyglutarate (2-HG). Adapted from [30]. FGFR2: Fibroblast growth factor receptor 2; EGF: Epidermal growth factor; EGFR: Epidermal growth factor receptor; Her2/neu: Human epidermal growth factor receptor 2; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; HGF: Hepatocyte growth factor; MET: C-met-encoded receptor for hepatocyte growth factor; IL-6: Interleukin-6; JAK: Janus kinase; SOCS3: Suppressor of cytokine signaling 3; STAT3: Signal transducer and activator of transcription protein; RAS: Rat sarcoma; RAF: Rat fibrosarcoma; MEK1/2: Mitogen-activated protein kinase kinase; ERK1/2: Extracellular signal-regulated kinase 1/2; PI3K: Phosphatidylinositol 3 kinase; AKT: Protein kinase B; mTOR: Mammalian target of rapamycin; α-KG: α-Ketoglutaric acid; 2-HG: 2- hydroxyglutarate; IDH: Isocitrate dehydrogenase; TCA cycle: Citric acid cycle.
In the following paragraphs, we will provide an overview of the most relevant biomarkers used for prognosis prediction in patients with CCA. Additionally, currently known molecular aberrations, which were identified as predictive biomarkers of treatment response, will be discussed.
3. Prognostic Serum Biomarkers
Prognostic markers in patients with CCA are currently based on clinical factors, such as tumor extent, existence of metastasis, surgical resection margin or histological tumor differentiation. Over the last years, various molecular markers of prognosis, obtained mainly from peripheral blood and tumor tissue, have been proposed for the detection and prediction of prognosis in CCA (Table 1).
3.1. Serum Proteins
So far, carbohydrate antigen (CA19-9) and carcinoembryonic antigen (CEA) are the most widely used biomarkers for diagnosis and surveillance of CCA. CA 19-9, a glycoprotein mainly produced by biliary and pancreatic duct cells, has been associated with poor prognosis, while preoperatively elevated levels of CA 19-9 proved to be a negative independent prognostic factor in CCA [31,32]. Furthermore, CA 19-9 decline ≥ 50% under chemotherapy with gemcitabine correlated with improved therapy response and increased survival in patients with advanced CCA [33]. However, the sensitivity and specificity of this biomarker is 72% and 84%, respectively, thus limiting its diagnostic and prognostic value [34]. CEA is a commonly used tumor marker in colorectal cancer but has evolved as a relevant biomarker in CCA as well [35]. In fact, studies have reported high variations in terms of sensitivity and specificity of CEA in patients with CCA, ranging from approximately 40%–80% and 50%–90%, respectively [33,36,37,38]. CEA has been identified as an independent prognostic marker in CCA, especially in combination with CA 19-9 [36,38,39,40,41,42]. Nonetheless, the partially low sensitivity and specificity reported should be kept in mind when using this biomarker for diagnosis and surveillance of CCA. Other promising serum diagnostic and prognostic biomarkers include cytokeratin-19 fragment (CYFRA 21-1), matrix metalloproteinase-7 (MMP-7) and osteopontin. CYFRA 21-1 showed a negative correlation with one-year outcome in patients with iCCA and gallbladder carcinoma, but no association with eCCA was found [43]. Interestingly, CYFRA 21-1 and MMP-7 levels were both elevated in patients with CCA compared to those with benign biliary disease, emphasizing their putative diagnostic value [44]. The role of osteopontin, a secreted extracellular matrix glycophosphoprotein, remains inconclusive. While Loosen et al. showed that serum osteopontin levels are increased in patients with CCA and associated with impaired survival, another study demonstrated the opposite [45,46]. Although data on these biomarkers seem promising, further studies are required to validate current findings.
3.2. Inflammatory Biomarkers
Circulating cytokines have been associated with disease progression, tumor stage or treatment response in numerous malignancies. In the context of CCA, increased levels of interleukin-6 (IL-6) were found in patients with CCA, compared to healthy individuals, showing a rather moderate sensitivity (73%) and specificity of 92%. Furthermore, high expression of IL-6 in serum and tumor tissue, as well as marked expression of interleukin-17 (IL-17) in peritumoral cells negatively correlated with overall survival (OS) and disease-free survival (DFS) in patients who underwent surgical resection of iCCA [47].
The urokinase plasminogen activator receptor (suPAR) is an inflammatory mediator and the soluble form of the cell surface receptor uPAR (CD87). suPAR was demonstrated to be an independent prognostic biomarker in various cancer types. In terms of CCA, increased uPAR expression in tumor tissue and suPAR expression in patients’ serum has been associated with impaired survival, whereas another study showed a positive correlation between uPAR expression and lymphatic invasion and metastasis, respectively [48,49]. Furthermore, elevated baseline levels of uPAR were predictive of poor survival in patients treated with palliative chemotherapy due to inoperable CCA [50].
Since a combination of two prognostic markers may improve the power of prognosis, the ratio of certain serum proteins with high diagnostic and predictive power was applied in several cancer types. As such, various ratios of myeloid and lymphatic cells have been proposed as prognostic markers in CCA. Indeed, the neutrophil-to-lymphocyte ratio, a well know prognostic factor in gastric and lung cancer, was associated with impaired survival in patients with resected CCA, as well as in those under chemotherapy due to advanced disease. Moreover, C-reactive-protein to albumin ratio negatively correlated with OS and DFS in CCA [51]. The albumin to gamma-glutamyltransferase ratio (AGR) proved to be an independent prognostic indicator for iCCA following curative resection, demonstrating improved predictive accuracy compared with the TNM staging alone [52]. Other inflammatory biomarker ratios calculated from peripheral blood measurements are still under investigation. While relatively simple to determine, these putative outcome-predicting ratios have not become part of the clinical routine yet.
3.3. Circulating Nucleic Acids
In recent years, circulating nucleic acids, such as cell-free DNA (cfDNA) or RNA, mostly microRNA (miRNA), have emerged as promising biomarkers for detection and prognosis prediction in multiple cancer types due to their abundance and stability in biofluids. In the case of CCA, the sensitivity and specificity of pooled miRNAs were calculated at up to 80% and 90%, respectively, in several metanalyses [53,54,55,56]. Notably, miRNA measured in bile samples showed the highest diagnostic efficiency. Increased serum and plasma levels of miR-21 positively correlated with the TNM stage and poor survival and decreased after surgical resection of the tumor. Notably, estimation of miR-21 enabled differentiation between patients with CCA and healthy controls, however miR-21 was increased in other malignancies as well, thus limiting its specificity [57]. Further studies investigating other miRNAs are controversial. While reduced levels of miR-150 were observed in individuals with CCA, another study reported an upregulation of miR-150 in patients suffering iCCA. Remarkably, the combination of reduced miR-150 and increased CA19-9 seemed to improve the accuracy of CCA diagnosis [58]. Expression of miRNAs was further investigated in bile samples and correlated with CCA occurrence; however, their prognostic value remains inconclusive [57].
cfDNA represents a fragment of DNA which is released upon cell apoptosis or necrosis, a common process in tumorigenesis. The most appealing fact about cfDNA determination is the ability to screen for overall mutation patterns of the respective malignancy without the need for obtaining primary tumor tissue. To support these findings, plasma samples from 31 patients with CCA were screened for oncogenic mutations. The results showed that the same mutation patterns could be observed in the tumor itself [59]. Hence, cfDNA might facilitate the detection of specific genomic alterations and subsequently the establishment of effective, mutation-based therapies.
3.4. Single-Nucleotide Polymorphisms
Single-nucleotide polymorphisms (SNPs) are genetic modifications defined by a substitution of a single nucleotide at a specific position in the genome. Depending on the function of the affected genetic region, SNPs have been associated with clinical outcomes and cancer susceptibility in a large variety of malignancies, making them potential prognostic or therapeutic targets [60,61,62,63,64]. Most recently, a single-center analysis of multiple genes involved in tumor inflammation and angiogenesis revealed CXCR1 (interleukine-8-receptor alpha—IL-8RA) +860 C>G heterozygous polymorphism to be an independent prognostic factor for DFS, cancer-specific survival and OS in patients with pCCA [65]. Further studies have linked SNPs to CCA. The G protein subunit-β 3 (GNB3) 825 C>T polymorphism was associated with longer OS in patients with eCCA [66]. Other variants, such as the enhancer of zeste homolog 2 (EZH2), nuclear factor (erythroid-derived 2)-like 2 (NRF2), x-ray repair cross-complementing group (XRCC1), ATP binding cassette subfamily C member 2 (ABCB2), ATPase Phospholipid Transporting 8B1 (ATP8B1), natural killer cell receptor G2D (NKG2D), and alpha1-antitrypsin (α1AT) deficiency Z heterozygosity, have been linked to increased risk of CCA or associated with the outcome of patients with bile duct tumors [64,67,68]. Nevertheless, biomarker-embedded clinical trials and validation in independent cohorts of patients are required to confirm these preliminary findings.
3.5. Other Biomarkers with Potentially Prognostic Value
Circulating tumor cells (CTC), various metabolites found in bile, blood, or urine, as well as extracellular vesicles represent some of other biomarkers currently evaluated for diagnosis and outcome prediction in patients with CCA [69]. While metabolites and extracellular vesicles have been investigated mostly for diagnostic purpose and differentiation between CCA and other hepatic malignancies or non-malignant biliary diseases, CTC have shown an association with DFS and OS in numerous malignancies. However, the number of studies suggesting that CTCs may have an impact on clinical outcome in CCA are scarce [57].
Taken together, numerous molecular markers of prognosis in CCA with promising results from clinical studies have been identified over recent years. Before implementation into clinical routine, larger patient cohorts and biomarker-embedded clinical trials are needed. Furthermore, novel technologies enabling the measurement of cf-DNA or miRNAs, will facilitate the detection of novel biomarkers in the future. Even though determination of CA19-9 and CEA has certain limitations, they remain the most frequently used and best evaluated serum biomarkers in CCA.
4. Prognostic Tumor Tissue Biomarkers
Biomarkers from tumor tissue present a valuable source of potential factors for outcome prediction in terms of both survival and treatment response, or individualized therapies in patients suffering CCA (Table 2). In fact, CCA was described as a highly genomic heterogeneous malignancy, with most of the genetic alterations being related to DNA repair mechanism, chromatin remodeling or cancer cell proliferation and growth [57]. While some mutations are specific to either iCCA or eCCA, others are found in tumor tissue irrespective of the anatomical localization. The latter include mutations in the KRAS proto-oncogene (15–22%), TP53 tumor suppressor gene (25–40%), ARID1A chromatin remodeling complex (12–18%), and BRCA1/2 (3–5%). Mutations in KRAS and TP53 have been associated with impaired outcome and tumor recurrence following surgical resection of CCA, thus highlighting their prognostic value [70]. The role of ARID1A as a prognostic marker remains inconclusive since it has been described as both an oncogene and tumor-suppressor gene. Inactivation of ARID1A correlated with tumor metastasis and was common in liver fluke-associated CCA [71]. Another study confirmed the prognostic role of ARID1A by showing a correlation between low ARID1A expression and impaired outcome in patients with iCCA [72]. In contrast, increased expression of ARID1A was associated with a higher risk of mortality and disease recurrence in patients with iCCA [73]. In a large metanalysis, including 4,126 patients from 73 studies, that analyzed 77 known biomarkers, EGFR, MUC1, MUC4, fascin, and p27 showed an association with OS of patients suffering from CCA [74]. Another metanalysis assessed prognostic biomarkers associated with OS in patients with eCCA in a univariate analysis. While six markers (VEGF, COX-2, GLUT-1, cyclin D1, fascin, and Ki-67) correlated with impaired survival, p16, p27, and E-Cadherin had positive prognostic effects [75]. Moreover, the analysis of 53 patients with surgical tumor resection due to CCA revealed 39 transcriptomic prognostic biomarkers. Interestingly, all of them showed a relation with T-cell activation and immune response. For instance, the expression levels of cytotoxic T-lymphocyte antigen 4 (CTL4) and forkhead box P3 (FOXP3) correlated with recurrence-free survival [76].
4.1. Cell Surface Molecules
Several cell surface molecules are known to have significant impact on cancer progression by regulating cell motility and transcellular signaling. As such, expression of CD155, an immunoglobulin-like transmembrane glycoprotein, was associated with shorter DFS and OS in patients suffering CCA. Upregulation of CD155 correlated with histological grading, lymph node metastasis, expression of vascular endothelial growth factor (VEGF), and microvascular density, and was suggested as an independent prognostic marker for CCA [77]. High expression of CD44 correlated with significantly shorter OS compared to low intratumoral expression of CD44 in patients with liver fluke-associated CCA [78]. Furthermore, CD55 and CD97 showed an association with poor histological grading, lymph node metastasis, venous invasion, and shorter OS, while CD98 was proposed as an independent prognostic factor in CCA [79].
4.2. Signaling Molecules
Diverse signaling molecules, mainly cytokines and intracellular signaling molecules, are directly involved in carcinogenesis and have been associated with patients’ outcome in multiple cancer types. As such, increased levels of IL-6 in tumor tissue and IL-17 in peritumoral cells correlated with impaired OS and DFS in patients with iCCA. Furthermore, multivariate analysis revealed that IL6 and peritumoral IL17 are independent prognostic factors for DFS, while preoperatively increased levels of IL-6 in serum were associated with significantly reduced DFS [47]. Suppressor of cytokine signaling 3 (SOCS3) is an antagonist of the JAK/STAT pathway, thus playing an integral role in shaping the inflammatory environment and tumorigenesis in CCA. The expression of SOCS3 was significantly downregulated in tumor tissue of patients with CCA, while the upstream regulator tumor necrosis factor α-induced protein 3 (TNFAIP3 or A20) was increased. Notably, patients with low intratumoral expression of SOCS3 and high expression of A20 showed a dramatically reduced OS rate. Moreover, both proteins were associated with lymph node metastasis and postoperative disease recurrence, thereby suggesting their role as prognostic markers in CCA [80].
Ring finger protein 43 (RNF43), which has been described as both an oncogene and tumorsuppressor gene, was downregulated in tumor tissue of patients with iCCA and correlated with poor prognosis. Furthermore, RNF43 was shown to be an independent prognostic factor in uni- and multivariate analysis [81].
LIM and SH3 protein 1 (LASP-1) is a focal adhesion protein, known to play a key role in cell migration, invasion, and proliferation in a wide variety of tumors. Analysis of human CCA tissue samples revealed that LASP-1 was markedly overexpressed in tumor compared to healthy tissue. Moreover, expression of LASP-1 positively correlated with tumor size, poor histological differentiation, lymph node metastasis, advanced TNM stage, and poor prognosis in CCA patients. In contrast, downregulation of LASP-1 resulted in cancer cell apoptosis and suppressed cell migration, invasion, and proliferation in vitro [82].
Similarly, the expression of B7-H4, which is a member of the B7 superfamily of ligands and regulator of T cell-mediated antitumor immune response, was upregulated in cancer tissue in approximately 50% of cases in a cohort of 137 patients suffering from CCA and was associated with poor histological differentiation, lymph node metastasis, staging, reduced OS, and early recurrence of tumor. Additionally, B7-H4 suppressed the peritumoral infiltration of CD8+ cytotoxic T lymphocytes [83]. Hepatoma-derived growth factor (HDGF) is another biomarker that has been associated with poor outcome and tumor progression in patients suffering CCA [84]. High expression of Ki-67, a well-known proliferation marker, and p73 was associated with shorter OS of pCCA patients, while Ki-67 correlated with tumor stage [85].
Induction of epithelial mesenchymal transition (EMT) results in increased cancer cell proliferation and metastasis. Indeed, SOX4 transcription factor, a member of the SOX (SRY-related HMG-box) family, has been shown to promote EMT in vitro, while SOX4 overexpression in tumor tissue indicated poor prognosis in patients with iCCA [86]. Moreover, elevated serum level of nardilysin (N-arginine dibasic convertase, NRDC), a soluble cytosolic protein, correlated with increased NRDC mRNA expression in tumor tissue and EMT-inducing transcription factors, and was associated with shorter OS and DFS in patients with iCCA [87].
4.3. Mucins
Mucins (MUC) are heavily O-glycosylated proteins, mainly expressed by ductal and glandular epithelial tissues. Vast production of mucus is frequently found in various carcinomas and has been described in CCA multiple times. In this context, MUC5AC was aberrantly expressed in tumor tissue and associated with larger tumor size and advanced stage in liver fluke-associated CCA [88]. Another study showed an association between MUC5AC expression in iCCA tissue and lymph node metastasis in patients who underwent curative-intent hepatectomy. Additionally, increased MUC5AC expression was identified as an independent prognostic marker of poor survival in patients with iCCA [89].
4.4. Tumor Stroma and Microenvironment
The tumor microenvironment plays a crucial role in shaping the growth, proliferation, and metastasis of malignant cells. Thus, certain molecules localized in tumoral stroma might be used as prognostic markers. Indeed, overexpression of epithelial cell adhesion molecule (EpCAM) in the stroma of ICC proved to be an independent risk factor for OS and DFS. Besides expression in iCCA tissue, overexpression of EpCAM in nontumor fibrous liver tissue correlated with reduced DFS as well [90]. Furthermore, high expression of Lysyl oxidase-like 2 (LOXL2), a matrix-remodeling enzyme that has already been associated with metastasis in hepatocellular carcinoma (HCC), in peritumoral stroma proved to be an independent prognostic factor of worse OS and DFS in patients with iCCA [91]. The metalloproteinases (MMP) are enzymes that degrade extracellular matrices, thus facilitating tumor cell progression and metastasis. MMP-9 and MMP-11 were markedly expressed in CCA tissue, and their expression correlated with decreased OS [92,93]. Tissue expression of MMP-9 was associated with IL-8 tissue expression, while the latter proved to be an independent prognostic factor of OS in patients with CCA [94].
4.5. Non-Coding RNA
The emerging role of serum-derived non-coding RNAs as prognostic biomarkers has been described in the previous section. Similarly, non-coding RNA, mostly microRNA (miRNA), small interfering RNA (siRNA), and long non-coding RNA (lncRNA), can be measured in tumor tissue. In this context, different studies reported an overexpression of lncRNA H19 and lncRNA-PANDRA in CCA tissue, as well as a significant correlation with tumor progression, TNM staging and OS of patients. Accordingly, in vitro analysis demonstrated the involvement of both RNAs in cell growth and proliferation, EMT and anti-apoptosis [95,96]. Hence, lncRNA H19 and PANDRA may serve as poor prognostic markers for CCA.
Using a custom microarray, the expression levels of three miRNAs, miR-675-5p, miR-652-3p and miR-338-3p, were identified in CCA tissue and strongly correlated with the prognosis of patients with iCCA. Risk scores, defined by using regression coefficients risk, revealed significantly shorter OS and DFS medians in patients with high-risk scores compared to those with low-risk scores. This three-miRNA signature was marked as an independent prognostic predictor for iCCA [97]. Upregulation of tissue miR-29a was suggested as a beneficial prognostic marker for CCA [98].
In conclusion, tumor tissue biomarkers may be of particular value for resected CCA, as they can have both prognostic (e.g., KRAS, TP53), as well as predictive (e.g., IDH1/2, FGFR2) implications by predicting the individual patient’s prognosis and response to targeted therapies. The major drawback remains the necessity of an invasive procedure for tissue sample collection, especially in patients with primary unresectable disease. Alternatively, a preliminary study by Ikeno et al. suggested non-invasive assessment of tumor metabolic activity by 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) to be associated with impaired survival in patients with iCCA and KRAS mutations [99]. In comparison, serum biomarkers are less invasive to obtain, thus being more suitable for diagnosis and monitoring of disease recurrence. The ideal combination of both biomarker types, as well as sampling timepoints are yet to defined. The detection of genetic aberrations in CCA tissue renders the development of custom and more effective therapies possible and will be discussed in the following paragraphs.
5. Predictive Biomarkers of Treatment Response and Novel Therapeutic Strategies
While patients with metastatic or locally advanced non-resectable disease are treated to with palliative chemotherapy, surgical resection with adjuvant chemotherapy represents the gold standard in patients with resectable CCA. According to current guidelines, first-line adjuvant chemotherapy should be conducted with capecitabin, whereas gemcitabin/cisplatin is recommended for patients with advanced disease [100,101,102]. However, the survival rates in both patient groups remain poor, thus prompting the development of targeted and more effective therapies (Supplementary Tables S1–S7). CCA is marked by a high rate of genomic alterations. Even though the most common ones, such as KRAS, TP53 and ARID1A are not easily druggable, >40% of CCA still bear a targetable genetic alteration [28].
5.1. Fibroblast Growth Factor Receptor
Fibroblast growth factor receptor 2 (FGFR2) fusions are unique to iCCA and represent one of the main druggable targets. Fibroblast growth factor receptor tyrosine kinases (FGFR) play a key role in activating important signaling pathways, such as RAS-RAF-MEK-ERK and PI3K-AKT-mTOR cascade, thus regulating cellular growth, survival, and differentiation [103,104]. Although the prognostic value of FGFR2 remains controversial, clinical studies evaluating FGFR inhibitors as targeted therapy for patients with advanced CCA show promising results [103,105,106,107,108,109,110,111]. Mazzaferro et al. published in 2019 a multicenter, phase I/II, open-label study exploring safety and effectivity of derazantinib, a pan-FGFR inhibitor, in patients with FGFR2 gen fusion-positive iCCA [112]. With an overall response rate of 20.7% and a disease control rate of 82.8%, derazantinib offered a promising anti-tumor activity, resulting in initiation of a larger study for this patient population (NCT03230318) [112]. Based on a promising phase II trial reporting a 14.8% response rate with a 75.4% disease control rate (NCT02150967; [113,114,115]), the selective FGFR1-3 inhibitor infigratinib is currently evaluated in comparison to gemcitabine and cisplatin in patients with advanced or metastatic CCA and FGFR2 gene fusion in a running phase III RCT (NCT03773302) [116]. Futibatinib and pemigatinib are further selective FGFR kinase inhibitors under current investigation in phase III RCT (NCT04093362 [117]; NCT03656536 [118]).
5.2. C-met-Encoded Receptor for Hepatocyte Growth Factor
Another signaling pathway, impacting cell proliferation, motility, and sensitivity to apoptotic cell death, that may be impaired in CCA, is the c-met-encoded receptor (MET) for hepatocyte growth factor signaling pathway [105]. MET is a heterodimeric tyrosine kinase transmembrane protein and receptor of the hepatocyte growth factor [119]. Overexpression of MET occurs in up to 60% of iCCA, whereas mutations in MET axis can be detected in 7% of iCCA [105]. In 2013 Pant et al. examined the combination of tivantinib, an inhibitor of the c-MET tyrosine kinase, and gemcitabine in patients with solid tumors, among them CCA, showing promising results in terms of antitumor activity [120]. In 2017 the research group Goyal et al. demonstrated in a phase II study the limited activity and toxicity of cabozantinib, another multikinase inhibitor targeting MET and vascular endothelial growth factor receptor 2 (VEGFR2) [121]. Further studies are still ongoing (NCT02496208, NCT02711553).
5.3. Tyrosine Kinases
Tyrosine kinases are enzymes responsible for the activation of signal transduction cascades through phosphorylation of participative proteins, a step that is inhibited by tyrosine kinase inhibitors. Representative members of the tyrosine kinase family are HER2/neu, VEGFR, platelet-derived growth factor receptor (PDGFR), and FGFR2, all playing an important role in tumorigenesis, cancer progression, and survival. In this context, recent studies have highlighted their use as targeted therapies in treatment of CCA [122]. EGFR is a transmembrane protein of the ErbB tyrosine kinase receptor family, compromising four distinct membrane receptors: EGFR (ERBB1), HER2/neu (ERBB2), ERBB3, and ERBB4 [123]. Overexpression of EGFR and HER2/neu has been detected in up to 30% of iCCA, resulting in promoted tumor cell proliferation, migration, and angiogenesis [123]. In fact, multivariate analysis showed that EGFR expression was a significant prognostic factor and risk factor for tumor recurrence in CCA [124]. Consequently, one could imagine that inhibition of EGFR tyrosine kinase activity might results in decreased progression of CCA, thus making it a promising therapeutical approach. Drugs targeting EGFR can be divided in tyrosine kinase inhibitors, like gefitinib and erlotinib, and monoclonal antibodies, such as cetuximab and panitumumab [125]. Various HER2/neu and EGFR inhibitors are currently being studied, either as single-agents, in addition to established chemotherapy or in combination with other targeted agents, such as the VEGF inhibitor bevacizumab [126,127,128]. Even though initial trials investigating the use of anti-EGFR and anti-HER2/neu inhibitors in patients with advanced CCA were promising [128,129], data from subsequent phase II RCTs were disappointing. In fact, neither the application of lapatinib (EGFR and HER2/neu double inhibitor), panitumumab (anti-EGFR antibody), nor cetuximab (anti-EGFR inhibitor), as monotherapy or in addition to traditional chemotherapy (gemcitabine and oxaliplatin), significantly increased the OS or progression free survival (PFS) [130,131,132,133,134]. Interestingly, the addition of erlotinib, a drug targeting EGFR by tyrosinase kinase inhibition, to chemotherapy with gemcitabine and oxaliplatin significantly prolonged PFS in a subgroup of patients suffering from CCA [135]. Furthermore, the combination of erlotinib with the VEGF antibody bevacizumab showed promising results for treatment of advanced biliary cancer in a multicenter phase II clinical trial [127]. HER2/neu overexpression was found to be higher in eCCA than in iCC [136]. However, studies investigating the use of HER2/neu antibodies, such as pertuzumab and trastuzumab, did not provide convincing results in terms of oncological outcome [137,138]. Additional studies investigating targeted therapies directed at HER2/neu or the use of multikinase inhibitors are ongoing.
5.4. Angiogenesis
VEGF and its receptors play a pivotal role in tumor angiogenesis. In fact, overexpression of VEGF has been reported in up to 50% of iCCA and 60% of eCCA [124]. However, data on antitumorigenic effects of several VEGF inhibitors, such as bevacizumab and sorafenib, were rather disappointing [139,140,141,142,143,144,145]. Recently, the multikinase inhibitor regorafenib showed promising efficiency in two phase II trials [146,147]. Based on these encouraging results, Demols et al. conducted a multicenter phase II RCT for patients with nonresectable or metastatic biliary tract cancer and progression on first-line chemotherapy, revealing a significant improvement of PFS and tumor control in patients treated with regorafenib [148]. The VEGFR2 inhibitor apatinib is evaluated in an ongoing RCT as well (NCT03609489).
5.5. Isocitrate Dehydrogenase-1 and -2
Isocitrate Dehydrogenase 1 or 2 (IDH) catalytic site mutations exclusively occur in iCCA with a percentage of 18–30% [26,29]. IDH mutations contribute to accumulation of oncometabolite intracellular 2-hydroxyglutarate (2-HG), which in turn disrupts several regulatory cellular pathways that are relevant for epigenetic remodeling and DNA repair [30,149,150]. Elevated levels of circulating 2-HG have been measured in IDH1/2 mutant CCA patients, suggesting the role of 2-HG as a surrogate biomarker of IDH mutation status, tumor burden or treatment response [151]. Although there are data proclaiming that IDH mutations do not have any prognostic or therapeutic significance [2,29,110], recent results demonstrated IDH as a possible target for specific therapy of iCCA [150]. In this context, AG-120 (ivosidenib) is known to inhibit IDH1, whereas AG-221 (enasidenib) inhibits IDH2 [105,149]. In 2020, Abou-Alfa et al. published a multicenter phase III RCT including CCA patients with mutated IDH1 and disease progression upon standard chemotherapy, who were randomly assigned to ivodesinib or placebo treatment. Treatment with ivosidenib significantly improved PFS in this population, while effects on overall survival remain unclear. The optimal use will be explored in future trials [150].
5.6. KRAS
Mutations of KRAS and aberrant activation of KRAS signaling pathways occur in up to 40% of CCA, with 42% of mutations appearing in eCCA and 22% in iCCA [105,152]. Currently, KRAS cannot be targeted directly, but rather through inhibition of downstream PI3K-AKT-mTOR and Raf-MEK-ERK pathways, both known to play an important role in cell proliferation, growth and angiogenesis. In 2011, Tanios et al. published a multi-institutional phase II study about the MEK 1/2 inhibitor selumetinib as a treatment for patients suffering from metastatic biliary cancer and demonstrated selumetinib as a well-tolerated drug in combination with current treatment strategies [153]. Furthermore, Kim et al. recently started the first prospective randomized trial on MEK inhibitor trametinib in comparison to chemotherapy with 5-fluorouracil or capecitabine in refractory advanced biliary cancer. However, the study had to be paused because of a lack of clinical activity of trametinib therapy [152]. The combination of trametinib with dabrafenib, a specific BRAF inhibitor, resulted in an overall response rate (ORR) of 47% [154]. Another study investigating the effect of dabrafenib and trametinib in patients with solid tumors is ongoing (NCT02465060). Recent trials evaluating the combination of two KRAS pathway inhibitors offer some promising results [109,155].
5.7. Immunotherapy
Inflammation and subsequent activation of the immune system play an important role in carcinogenesis and development of multiple tumors. As such, tumor cell-mediated upregulation of immune checkpoint molecules, e.g., programmed cell death protein 1 (PD-1), results in suppressed immune reaction, facilitating the survival and progression of tumor cells. In fact, the PD-1 antibody pembrolizumab has recently been approved for treatment of unresectable or metastatic microsatellite instability (MSI) high or deficient solid tumors. MSI develops upon loss of DNA mismatch repair mechanism, resulting in stronger immune response and increased expression of PD-1 ligand (PD-L1). While the role of MSI in CCA remains inconclusive, a phase II trial of pembrolizumab efficacy in MMR-deficient tumors demonstrated a 100% disease control rate (DCR) with one complete response and three stable diseases in four patients with CCA [156]. The results of the KEYNOTE-028 phase 1b trial (NCT02054808) revealed the safety and efficacy of pembrolizumab in patients with PD-L1-positive advanced biliary tract cancer, while results from the KEYNOTE-158 phase II trial showed a 40.9% ORR in a subset of 22 patients with MSI high CCA treated by pembrolizumab [157]. Recently the TOPAZ-1 study was able to demonstrate a clinical meaningful OS benefit by adding durvalumab, a PD-L1 inhibitor, to a standard chemotherapy consisting of gemcitabine plus cisplatin (Astra-Zeneca press release October 25, 2021). Further phase-III trials (e.g., Keynote-966, NCT04003636) investigating the role of immunotherapy in CCA are ongoing.
6. Conclusions
Cholangiocarcinoma is an anatomically distinct and genetically heterogeneous tumor, with rising incidence worldwide, dismal prognosis and highly limited therapy options. The vast spectrum of genomic alterations differs greatly between each CCA subtype and offers the opportunity for development of prognostic and predictive biomarkers, as well as novel therapeutic strategies. Preoperative identification of prognostic biomarkers may help to identify patients who would profit the most from radical surgical resection, while preventing the risk of postoperative complications and chemotherapy delay. Furthermore, serum and tissue biomarkers could help to identify the subgroup of patients with high recurrence risk, thus necessitating closer follow-up or prolonged chemotherapy regimen. Although numerous options have been developed over the last years, including miRNAs, lncRNAs, SNPs and various signaling molecules from both patients’ serum and tumor tissue, CA19-9 and CEA remain the most applied prognostic biomarkers. In either case, palliative or curative intent, development of custom and patient-directed therapies based on unique genetic alterations will increase the therapeutic effectiveness and consecutively patients’ survival rates. So far, emerging targeted therapies with promising effect include FGFR inhibitors and IDH1/2 inhibitors, as well as immunotherapies. While several other biomarkers and clinical studies have shown promising results, validation in larger patient cohorts and international trials is necessary and has already been initiated to a certain extent. Over the next years, results from multicentric RCTs may fundamentally impact the diagnostic and therapeutic management of CCA and will hopefully improve patient outcomes.

Table 1.
Serum biomarkers associated with prognosis in CCA.
Table 1.
Serum biomarkers associated with prognosis in CCA.
| Name | Occurrence | Expression | Associated Prognostic Value | Reference |
|---|---|---|---|---|
| Proteins/Cytokines | ||||
| CA19-9 | CCA (all subtypes) | Increased | OS | [31,32,33,34] |
| CEA | mostly iCCA, but also all CCA subtypes | Increased | OS | [36,38,39,40,41,42] |
| CYFRA | iCCA, gallbladder cancer | Increased | OS | [43,158] |
| Osteopontin | CCA (all subtypes) | Increased | OS | [45] |
| iCCA | Low level of circulating osteopontin/volume; Decreased expression in tumor tissue | OS | [46] | |
| Urokinase plasminogen activator receptor (uPAR) | CCA (all subtypes) | Elevated serum levels; Increased expression in tumor tissue | OS | [48] |
| 2-hydroxyglutarate (2-HG) | iCCA | Elevated serum levels | IDH1/2 mutation status, tumor burden | [151] |
| Nardilysin (NRDC) | iCCA | Elevated serum levels and mRNA expression in tumor tissue | OS, DFS | [87] |
| IL-6 | iCCA | Elevated serum levels | DFS | [47] |
| Circulating Nucleic Acids | ||||
| miR-21 | CCA (all subtypes) | Elevated serum levels | OS, clinical staging, metastasis | [159] |
| miR-192 | Liver fluke-associated CCA | Elevated serum levels | OS, lymph node metastasis | [160] |
| miR-106a | CCA | Decreased serum levels | OS, lymph node metastasis | [161] |
| miR-26a | CCA | Elevated serum levels | OS, clinical stage, metastasis, differentiation status | [162] |
| Panel (miR-29, miR-122, miR-155, miR-192 | CCA | Elevated serum levels | OS | [56] |
| Single-Nucleotide Polymorphisms (SNPs) | ||||
| CXCR1 +860 C>G | pCCA | Heterozygous polymorphism | OS, DFS | [65] |
| G protein subunit-β 3 (GNB3) 825 C>T | eCCA | Heterozygous polymorphism | OS | [66] |
| EZH2 rs887569 TT genotype | CCA | Homozygous polymorphism | OS | [163] |
| NRF2 rs6726395 GG genotype | CCA | Homozygous polymorphism | OS | [164] |

Table 2.
Tumor tissue biomarkers associated with prognosis in CCA.
Table 2.
Tumor tissue biomarkers associated with prognosis in CCA.
| Name | Occurrence | Expression | Associated Prognostic Value | Reference |
|---|---|---|---|---|
| Cell Surface Molecules | ||||
| CD 155 | CCA | Increased | OS, DFS, histological grading, lymph node metastasis | [77] |
| CD44 | Liver fluke-associated CCA | Increased | OS | [78] |
| CD55, CD97 | iCCA | Increased | OS, histological grading, lymph node metastasis, venous invasion | [165] |
| CD98 | CCA | Increased | OS | [166] |
| Signaling Molecules, Growth Pathways, Angiogenesis | ||||
| IL-6 | iCCA | Increased | OS, DFS | [47] |
| IL-17 | iCCA | Increased peritumoral expression | OS, DFS | [47] |
| SOCS3 | CCA | Low intratumoral expression | OS, lymph node metastasis, postoperative disease recurrence | [80] |
| Tumor necrosis factor α-induced protein 3 (TNFAIP3 or A20) | CCA | Increased intratumoral expression | OS, lymph node metastasis, postoperative disease recurrence | [80] |
| RNF43 | iCCA | Low intratumoral expression | OS | [81] |
| LIM and SH3 protein 1 (LASP-1) | CCA | Increased intratumoral expression | OS, tumor size, histological differentiation, lymph node metastasis, TNM stage | [82] |
| B7-H4 | CCA | Increased | OS, histological differentiation, lymph node metastasis, staging, early recurrence of tumor | [83] |
| Hepatoma-derived growth factor (HDGF) | iCCA | Increased | OS, lymph node metastasis, TNM stage | [84] |
| Ki-67, p73 | pCCA | Increased | OS, TNM stage | [85] |
| Sex-determining region Y-box 4 (SOX4) | iCCA | Increased | OS | [86] |
| Sex-determining region Y-box 9 (SOX9) | iCCA | Increased | OS | [86] |
| KRAS | CCA | Increased | OS | [167] |
| TP53 | CCA | Increased | OS | [167] |
| ARID1A | CCA, mostly fluke-associated iCCA | Decreased | OS | [71,72] |
| iCCA | Increased | OS, recurrence rate | [73] | |
| EGFR, MUC1, MUC4, fascin | CCA | Increased | OS | Metanalysis by [74] |
| VEGF, COX-2, GLUT-1, cyclin D1, Ki67 | eCCA | Increased | OS | Metanalysis by [75] |
| p16, p27, E-cadherin | eCCA | Increased | OS | Metanalysis by [75] |
| c-MET | CCA | Increased | OS, DFS | [168] |
| DKK1 | iCCA, pCCA | Increased | OS, lymph-node metastasis | [169,170] |
| BAP1 | CCA | Retained expression | OS, DFS | [25,169,170] |
| Loss of expression | Trend towards improved OS, histological differentiation, lymph-node metastasis | |||
| PBRM1 | CCA | Retained expression | OS, DFS | [29,171] |
| Mucins | ||||
| MUC5AC | Liver fluke-associated iCCA, iCCA | Increased | OS, lymph node metastasis, TNM stage, tumor size | [88,89] |
| MUC4 | CCA | Increased | OS | [172] |
| MUC16 | iCCA | Increased | OS | [173] |
| Tumor Stroma and Microenvironment | ||||
| Epithelial cell adhesion molecule (EpCAM) | iCCA | Increased expression in peritumoral stroma | OS, DFS | [90] |
| Lysil oxidase-like 2 (LOXL2) | iCCA | Increased expression in peritumoral stroma | OS, DFS | [91] |
| Matrix metalloproteinase -9 (MMP-9) | pCCA | Increased tissue expression | OS | [92] |
| Matrix metalloproteinase -11 (MMP-11) | CCA | Increased tissue expression | OS | [93] |
| Non-Coding RNA | ||||
| lncRNA H19 | CCA | Increased tissue expression | OS, DFS, tumor size, TNM stage | [95] |
| lncRNA-PANDRA | CCA | Increased tissue expression | OS, DFS, lymph node metastasis, TNM stage | [96] |
| Panel (miR-675-5p, miR-652-3p and miR-338-3p) | iCCA | Overexpression | OS, DFS | [97] |
| miR-29a | CCA | Overexpression | OS, lymph node metastasis, histological differentiation, clinical staging | [98] |
| miR-21 | Liver fluke-associated iCCA | Overexpression | OS, lymph-node metastasis | [174] |
| miR-92b | CCA | Overexpression | OS | [175] |
| miR-34a | eCCA | Decreased expression | OS, lymph-node metastasis, clinical stage | [176] |
| miR-181a | CCA | Overexpression | OS | [177] |
| miR-191 | iCCA | Overexpression | OS, DFS | [178] |
| miR-203, miR-373 | CCA | Decreased expression | OS, DFS | [178,179] |
| miR-221 | eCCA | Overexpression | DFS | [180] |
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers14041026/s1, Table S1: Overview of current and ongoing clinical trials for therapies targeting IDH1/2 in CCA, Table S2: Overview of current and ongoing clinical trials for therapies targeting KRAS in CCA, Table S3: Overview of current and ongoing clinical trials for therapies targeting FGFR in CCA, Table S4: Overview of current and ongoing clinical trials for therapies targeting EGFR and VEGF in CCA, Table S5: Overview of current and ongoing clinical trials for targeted therapies including multikinase inhibitors in CCA, Table S6: Overview of current and ongoing clinical trials for therapies targeting the MET, notch, and chromatin remodeling pathways in CCA, Table S7: Overview of current and ongoing clinical trials for immunotherapies in CCA. References [181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199] are cited in the supplementary materials.
Author Contributions
S.P. and G.L. wrote the manuscript; S.P., S.R. and G.L. acquired the data; S.P. and G.L. drafted and revised the manuscript; Further authors (D.U., I.L., C.E., D.P.M., U.P., F.K., N.R., C.B., I.M.S., S.S., F.T., W.S., M.S. and J.P.) have substantially contributed to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Park, R.; Al-Jumayli, M.; Al-Rajabi, R.; Sun, W. Biologics, Immunotherapy, and Future Directions in the Treatment of Advanced Cholangiocarcinoma. Clin. Color. Cancer 2019, 18, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 7–18. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Özdirik, B.; Müller, T.; Wree, A.; Tacke, F.; Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021, 22, 6975. [Google Scholar] [CrossRef] [PubMed]
- Lurje, G.; Bednarsch, J.; Czigany, Z.; Lurje, I.; Schlebusch, I.K.; Boecker, J.; Meister, F.A.; Tacke, F.; Roderburg, C.; Dulk, M.D.; et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur. J. Surg. Oncol. 2019, 45, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Tacke, F.; Strnad, P.; Ulmer, T.F.; Gaisa, N.T.; Bruners, P.; Neumann, U.P.; Lurje, G. Left- versus right-sided hepatectomy with hilar en-bloc resection in perihilar cholangiocarcinoma. HPB 2020, 22, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moustafa, M.; Linecker, M.; Lurje, G.; Capobianco, I.; Baumgart, J.; Ratti, F.; Rauchfuss, F.; Balci, D.; Fernandes, E.; et al. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-Center Study. Ann. Surg. Oncol. 2020, 27, 1372–1384. [Google Scholar] [CrossRef]
- Haber, P.K.; Wabitsch, S.; Kästner, A.; Andreou, A.; Krenzien, F.; Schöning, W.; Pratschke, J.; Schmelzle, M. Laparoscopic Liver Resection for Intrahepatic Cholangiocarcinoma: A Single-Center Experience. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 1354–1359. [Google Scholar] [CrossRef]
- Jonas, S.; Krenzien, F.; Atanasov, G.; Hau, H.-M.; Gawlitza, M.; Moche, M.; Wiltberger, G.; Pratschke, J.; Schmelzle, M. Hilar en bloc resection for hilar cholangiocarcinoma in patients with limited liver capacities—Preserving parts of liver segment 4. Eur. Surg. 2018, 50, 22–29. [Google Scholar] [CrossRef]
- Krenzien, F.; Nevermann, N.; Krombholz, A.; Benzing, C.; Haber, P.; Fehrenbach, U.; Lurje, G.; Pelzer, U.; Pratschke, J.; Schmelzle, M.; et al. Treatment of Intrahepatic Cholangiocarcinoma—A Multidisciplinary Approach. Cancers 2022, 14, 362. [Google Scholar] [CrossRef]
- Lurje, G.; Bednarsch, J.; Roderburg, C.; Trautwein, C.; Neumann, U.P. Aktueller Therapiealgorithmus des intrahepatischen cholangiozellulären Karzinoms. Der Chir. 2018, 89, 858–864. [Google Scholar] [CrossRef]
- Bednarsch, J.; Neumann, U.P.; Lurje, G. Reply to: Does lymphovascular invasion really associate with decreased overall survival for patients with resected cholangiocarcinoma? Eur. J. Surg. Oncol. 2019, 45, 1513–1514. [Google Scholar] [CrossRef]
- Schmelzle, M.; Schöning, W.; Pratschke, J. Chirurgische Therapie maligner Gallengangserkrankungen. Der Chirurg 2019, 91, 3–10. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Amygdalos, I.; Strnad, P.; Halm, P.; Wiltberger, G.; Ulmer, T.F.; Schulze-Hagen, M.; Bruners, P.; et al. Insufficient future liver remnant and preoperative cholangitis predict perioperative outcome in perihilar cholangiocarcinoma. HPB 2020, 23, 99–108. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Strnad, P.; Bruners, P.; Ulmer, T.F.; den Dulk, M.; Lurje, G.; Neumann, U.P. The role of ALPPS in intrahepatic cholangiocarcinoma. Langenbeck’s Arch. Surg. 2019, 404, 885–894. [Google Scholar] [CrossRef]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation from Donation After Brain Death. Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]
- Pavicevic, S.; Uluk, D.; Reichelt, S.; Fikatas, P.; Globke, B.; Raschzok, N.; Schmelzle, M.; Öllinger, R.; Schöning, W.; Eurich, D.; et al. Hypothermic oxygenated machine perfusion for extended criteria donor allografts—Preliminary experience with extended organ preservation times in the setting of organ reallocation. Artif. Organs 2021, 46, 306–311. [Google Scholar] [CrossRef]
- Lauterio, A.; De Carlis, R.; Centonze, L.; Buscemi, V.; Incarbone, N.; Vella, I.; De Carlis, L. Current Surgical Management of Peri-Hilar and Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 3657. [Google Scholar] [CrossRef]
- Vibert, E.; Boleslawski, E. Transplantation Versus Resection for Hilar Cholangiocarcinoma. Ann. Surg. 2019, 269, e5–e6. [Google Scholar] [CrossRef]
- Ballman, K.V. Biomarker: Predictive or Prognostic? J. Clin. Oncol. 2015, 33, 3968–3971. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource [Internet]; Food and Drug Administration (US): Silver Spring, MD, USA; National Institutes of Health (US): Bethesda, MD, USA, 2016.
- Czigany, Z.; Lurje, I.; Schmelzle, M.; Schöning, W.; Öllinger, R.; Raschzok, N.; Sauer, I.M.; Tacke, F.; Strnad, P.; Trautwein, C.; et al. Ischemia-Reperfusion Injury in Marginal Liver Grafts and the Role of Hypothermic Machine Perfusion: Molecular Mechanisms and Clinical Implications. J. Clin. Med. 2020, 9, 846. [Google Scholar] [CrossRef]
- Hayes, D.F.; Bast, R.; Desch, C.E.; Fritsche, H.; Kemeny, N.E.; Jessup, J.M.; Locker, G.Y.; Macdonald, J.S.; Mennel, R.G.; Norton, L.; et al. Tumor Marker Utility Grading System: A Framework to Evaluate Clinical Utility of Tumor Markers. J. Natl. Cancer Inst. 1996, 88, 1456–1466. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; ElZawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Lowery, M.; Ptashkin, R.N.; Jordan, E.J.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Nault, J.; Villanueva, A. Biomarkers for Hepatobiliary Cancers. Hepatology 2020, 73, 115–127. [Google Scholar] [CrossRef]
- Churi, C.R.; Shroff, R.; Wang, Y.; Rashid, A.; Kang, H.; Weatherly, J.; Zuo, M.; Zinner, R.; Hong, D.; Meric-Bernstam, F.; et al. Mutation Profiling in Cholangiocarcinoma: Prognostic and Therapeutic Implications. PLoS ONE 2014, 9, e115383. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Bolm, L.; Petrova, E.; Weitz, J.; Rückert, F.; Wittel, U.A.; Makowiec, F.; Lapshyn, H.; Bronsert, P.; Rau, B.M.; Khatkov, I.E.; et al. Prognostic relevance of preoperative bilirubin-adjusted serum carbohydrate antigen 19-9 in a multicenter subset analysis of 179 patients with distal cholangiocarcinoma. HPB 2019, 21, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-K.; Lin, J.-J.; He, G.-H.; Wang, H.; Lu, J.-H.; Yang, G.-S. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 7890–7898. [Google Scholar] [PubMed]
- Lee, B.S.; Lee, S.H.; Son, J.H.; Jang, D.K.; Chung, K.H.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T. Prognostic value of CA 19-9 kinetics during gemcitabine-based chemotherapy in patients with advanced cholangiocarcinoma. J. Gastroenterol. Hepatol. 2016, 31, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, L.; He, Q.; Wang, S.; Pan, Z.; Wang, T.; Zhao, Y. Diagnostic Accuracy of Serum CA19-9 in Patients with Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2015, 21, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Koch, A.; Vucur, M.; Schneider, A.T.; Binnebösel, M.; Ulmer, T.F.; Lurje, G.; Schoening, W.; et al. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci. Rep. 2017, 7, 16975. [Google Scholar] [CrossRef]
- Fang, T.; Wang, H.; Wang, Y.; Lin, X.; Cui, Y.; Wang, Z. Clinical Significance of Preoperative Serum CEA, CA125, and CA19-9 Levels in Predicting the Resectability of Cholangiocarcinoma. Dis. Markers 2019, 2019, e6016931. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.-J.; Chen, J.; Liu, W.; Li, J.-W.; Jiang, P.; Zhao, X.; Guo, F.; Li, X.-W.; Wang, S.-G.; et al. Application of Joint Detection of AFP, CA19-9, CA125 and CEA in Identification and Diagnosis of Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 3451–3455. [Google Scholar] [CrossRef]
- Qiu, Y.; He, J.; Chen, X.; Huang, P.; Hu, K.; Yan, H. The diagnostic value of five serum tumor markers for patients with cholangiocarcinoma. Clin. Chim. Acta 2018, 480, 186–192. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, L.; Wang, Y.; Ge, R.; Sun, Y.; Wei, G. Survival Outcomes and Prognostic Factors of Surgical Therapy for All Potentially Resectable Intrahepatic Cholangiocarcinoma: A Large Single-Center Cohort Study. J. Gastrointest. Surg. 2014, 18, 562–572. [Google Scholar] [CrossRef]
- Qiang, Z.-Y.; Zhang, W.-H.; Jin, S.; Dai, K.-F.; He, Y.-T.; Tao, L.-Y.; Yu, H.-B. Carcinoembryonic antigen, α-fetoprotein, and Ki67 as biomarkers and prognostic factors in intrahepatic cholangiocarcinoma: A retrospective cohort study. Ann. Hepatol. 2020, 20, 100242. [Google Scholar] [CrossRef]
- Moro, A.; Mehta, R.; Sahara, K.; Tsilimigras, D.I.; Paredes, A.Z.; Farooq, A.; Hyer, J.M.; Endo, I.; Shen, F.; Guglielmi, A.; et al. The Impact of Preoperative CA19-9 and CEA on Outcomes of Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 2888–2901. [Google Scholar] [CrossRef]
- He, C.; Zhang, Y.; Song, Y.; Wang, J.; Xing, K.; Lin, X.; Li, S. Preoperative CEA levels are supplementary to CA19-9 levels in predicting prognosis in patients with resectable intrahepatic cholangiocarcinoma. J. Cancer 2018, 9, 3117–3128. [Google Scholar] [CrossRef]
- Huang, L.; Chen, W.; Liang, P.; Hu, W.; Zhang, K.; Shen, S.; Chen, J.; Zhang, Z.; Chen, B.; Han, Y.; et al. Serum CYFRA 21-1 in Biliary Tract Cancers: A Reliable Biomarker for Gallbladder Carcinoma and Intrahepatic Cholangiocarcinoma. Am. J. Dig. Dis. 2014, 60, 1273–1283. [Google Scholar] [CrossRef]
- Itatsu, K.; Zen, Y.; Ohira, S.; Ishikawa, A.; Sato, Y.; Harada, K.; Ikeda, H.; Sasaki, M.; Nimura, Y.; Nakanuma, Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007, 27, 1174–1184. [Google Scholar] [CrossRef]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Pombeiro, I.; Leyh, C.; Benz, F.; Vucur, M.; Longerich, T.; Koch, A.; Braunschweig, T.; et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J. Hepatol. 2017, 67, 749–757. [Google Scholar] [CrossRef]
- Zhou, K.-Q.; Liu, W.-F.; Yang, L.-X.; Sun, Y.-F.; Hu, J.; Chen, F.-Y.; Zhou, C.; Zhang, X.-Y.; Peng, Y.-F.; Yu, L.; et al. Circulating osteopontin per tumor volume as a prognostic biomarker for resectable intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2019, 8, 582–596. [Google Scholar] [CrossRef]
- Asukai, K.; Kawamoto, K.; Eguchi, H.; Konno, M.; Nishida, N.; Koseki, J.; Noguchi, K.; Hasegawa, S.; Ogawa, H.; Yamada, D.; et al. Prognostic Impact of Peritumoral IL-17-Positive Cells and IL-17 Axis in Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22, 1524–1531. [Google Scholar] [CrossRef]
- Loosen, S.H.; Breuer, A.; Tacke, F.; Kather, J.N.; Gorgulho, J.; Alizai, P.H.; Bednarsch, J.; Roeth, A.A.; Lurje, G.; Schmitz, S.M.; et al. Circulating levels of soluble urokinase plasminogen activator receptor predict outcome after resection of biliary tract cancer. JHEP Rep. 2020, 2, 100080. [Google Scholar] [CrossRef]
- Thummarati, P.; Wijitburaphat, S.; Prasopthum, A.; Menakongka, A.; Sripa, B.; Tohtong, R.; Suthiphongchai, T. High level of urokinase plasminogen activator contributes to cholangiocarcinoma invasion and metastasis. World J. Gastroenterol. 2012, 18, 244–250. [Google Scholar] [CrossRef]
- Grunnet, M.; Christensen, I.; Lassen, U.; Jensen, L.; Lydolph, M.; Lund, I.; Thurison, T.; Høyer-Hansen, G.; Mau-Sørensen, M. Prognostic significance of circulating intact and cleaved forms of urokinase plasminogen activator receptor in inoperable chemotherapy treated cholangiocarcinoma patients. Clin. Biochem. 2014, 47, 599–604. [Google Scholar] [CrossRef]
- Lang, S.A.; Bednarsch, J.; Joechle, K.; Amygdalos, I.; Czigany, Z.; Heij, L.; Ulmer, T.F.; Neumann, U.P. Prognostic biomarkers for cholangiocarcinoma (CCA): State of the art. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 497–510. [Google Scholar] [CrossRef]
- Jing, C.-Y.; Fu, Y.-P.; Shen, H.-J.; Zheng, S.-S.; Lin, J.-J.; Yi, Y.; Huang, J.-L.; Xu, X.; Zhang, J.; Zhou, J.; et al. Albumin to gamma-glutamyltransferase ratio as a prognostic indicator in intrahepatic cholangiocarcinoma after curative resection. Oncotarget 2016, 8, 13293–13303. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, J.; Wu, B.; Chen, J.; Zhu, Z.; Cai, P.; Guo, W.; Gu, Z.; Wang, J.; Huang, S. Diagnostic and prognostic value of microRNAs in cholangiocarcinoma: A systematic review and meta-analysis. Cancer Manag. Res. 2018, 10, 2125–2139. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Yang, S.; Li, X. Identification of microRNAs as biomarkers for cholangiocarcinoma detection: A diagnostic meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 156–162. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, X.; Zhang, Q.; Wang, C.; Zhao, Y. Diagnostic value of microRNAs as biomarkers for cholangiocarcinoma. Dig. Liver Dis. 2016, 48, 1227–1232. [Google Scholar] [CrossRef]
- Loosen, S.H.; Lurje, G.; Wiltberger, G.; Vucur, M.; Koch, A.; Kather, J.N.; Paffenholz, P.; Tacke, F.; Ulmer, F.T.; Trautwein, C.; et al. Serum levels of miR-29, miR-122, miR-155 and miR-192 are elevated in patients with cholangiocarcinoma. PLoS ONE 2019, 14, e0210944. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Salem, P.E.S.; Ghazala, R.A.; El Gendi, A.M.; Emara, D.M.; Ahmed, N.M. The association between circulating MicroRNA-150 level and cholangiocarcinoma. J. Clin. Lab. Anal. 2020, 34, e23397. [Google Scholar] [CrossRef]
- Andersen, R.F.; Jakobsen, A. Screening for circulating RAS/RAF mutations by multiplex digital PCR. Clin. Chim. Acta 2016, 458, 138–143. [Google Scholar] [CrossRef]
- Lurje, G.; Nagashima, F.; Zhang, W.; Yang, D.; Chang, H.M.; Gordon, M.A.; El-Khoueiry, A.; Husain, H.; Wilson, P.M.; Ladner, R.D.; et al. Polymorphisms in cyclooxygenase-2 and epidermal growth factor receptor are associated with progression-free survival independent of K-ras in metastatic colorectal cancer patients treated with single-agent cetuximab. Clin. Cancer Res. 2008, 14, 7884–7895. [Google Scholar] [CrossRef][Green Version]
- Lurje, G.; Zhang, W.; Schultheis, A.M.; Yang, D.; Groshen, S.; Hendifar, A.E.; Husain, H.; Gordon, M.A.; Nagashima, F.; Chang, H.M.; et al. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann. Oncol. 2008, 19, 1734–1741. [Google Scholar] [CrossRef]
- Lurje, G.; Husain, H.; Power, D.; Yang, D.; Groshen, S.; Pohl, A.; Zhang, W.; Ning, Y.; Manegold, P.; El-Khoueiry, A.; et al. Genetic variations in angiogenesis pathway genes associated with clinical outcome in localized gastric adenocarcinoma. Ann. Oncol. 2010, 21, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Lurje, G.; Leers, J.M.; Pohl, A.; Oezcelik, A.; Zhang, W.; Ayazi, S.; Winder, T.; Ning, Y.; Yang, D.; Klipfel, N.E.; et al. Genetic Variations in Angiogenesis Pathway Genes Predict Tumor Recurrence in Localized Adenocarcinoma of the Esophagus. Ann. Surg. 2010, 251, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.V.; Hamesch, K.; Gross, A.; Mandorfer, M.; Moeller, L.S.; Pereira, V.; Pons, M.; Kuca, P.; Reichert, M.C.; Benini, F.; et al. Liver Phenotypes of European Adults Heterozygous or Homozygous for Pi∗Z Variant of AAT (Pi∗MZ vs. Pi∗ZZ genotype) and Noncarriers. Gastroenterology 2020, 159, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Gaisa, N.T.; Dahl, E.; Knüchel, R.; Miller, H.; Ulmer, T.F.; Strnad, P.; Trautwein, C.; et al. Genetic variant of CXCR1 (rs2234671) associates with clinical outcome in perihilar cholangiocarcinoma. Liver Cancer 2022, in press. [CrossRef]
- Fingas, C.D.; Katsounas, A.; Kahraman, A.; Siffert, W.; Jochum, C.; Gerken, G.; Nückel, H.; Canbay, A. Prognostic Assessment of Three Single-Nucleotide Polymorphisms (GNB3825C>T,BCL2-938C>A,MCL1-386C>G) in Extrahepatic Cholangiocarcinoma. Cancer Investig. 2009, 28, 472–478. [Google Scholar] [CrossRef]
- Braconi, C.; Roessler, S.; Kruk, B.; Lammert, F.; Krawczyk, M.; Andersen, J.B. Molecular perturbations in cholangiocarcinoma: Is it time for precision medicine? Liver Int. 2019, 39 (Suppl. S1), 32–42. [Google Scholar] [CrossRef]
- Rhodes, K.; Zhang, W.; Yang, D.; Press, O.; Gordon, M.; Vallbohmer, D.; Schultheis, A.; Lurje, G.; Ladner, R.; Fazzone, W.; et al. ABCB1, SLCO1B1 and UGT1A1 gene polymorphisms are associated with toxicity in metastatic colorectal cancer patients treated with first-line irinotecan. Drug Metab. Lett. 2007, 1, 23–30. [Google Scholar] [CrossRef]
- Lurje, G.; Schiesser, M.; Hoffmann, A.C.; Schneider, P.M. Circulating Tumor Cells in Gastrointestinal Malignancies: Current Techniques and Clinical Implications. J. Oncol. 2009, 2010, e392652. [Google Scholar] [CrossRef]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.; et al. Genomic and Genetic Characterization of Cholangiocarcinoma Identifies Therapeutic Targets for Tyrosine Kinase Inhibitors. Gastroenterology 2012, 142, 1021–1031.e15. [Google Scholar] [CrossRef]
- Namjan, A.; Techasen, A.; Loilome, W.; Sa-Ngaimwibool, P.; Jusakul, A. ARID1A alterations and their clinical significance in cholangiocarcinoma. PeerJ 2020, 8, e10464. [Google Scholar] [CrossRef]
- Sasaki, M.; Nitta, T.; Sato, Y.; Nakanuma, Y. Loss of ARID1A Expression Presents a Novel Pathway of Carcinogenesis in Biliary Carcinomas. Am. J. Clin. Pathol. 2016, 145, 815–825. [Google Scholar] [CrossRef]
- Bi, C.; Liu, M.; Rong, W.; Wu, F.; Zhang, Y.; Lin, S.; Liu, Y.; Wu, J.; Wang, L. High Beclin-1 and ARID1A expression corelates with poor survival and high recurrence in intrahepatic cholangiocarcinoma: A histopathological retrospective study. BMC Cancer 2019, 19, 213. [Google Scholar] [CrossRef]
- Ruys, A.T.; Koerkamp, B.G.; Wiggers, J.K.; Klümpen, H.-J.; Kate, F.J.T.; Van Gulik, T.M. Prognostic Biomarkers in Patients with Resected Cholangiocarcinoma: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2013, 21, 487–500. [Google Scholar] [CrossRef]
- Jones, R.P.; Bird, N.T.; Smith, R.A.; Palmer, D.H.; Fenwick, S.W.; Poston, G.J.; Malik, H.Z. Prognostic molecular markers in resected extrahepatic biliary tract cancers: A systematic review and meta-analysis of immunohistochemically detected biomarkers. Biomarkers Med. 2015, 9, 763–775. [Google Scholar] [CrossRef]
- Ghidini, M.; Cascione, L.; Carotenuto, P.; Lampis, A.; Trevisani, F.; Previdi, M.C.; Hahne, J.C.; Said-Huntingford, I.; Raj, M.; Zerbi, A.; et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur. J. Cancer 2017, 86, 158–165. [Google Scholar] [CrossRef]
- Huang, D.-W.; Huang, M.; Lin, X.-S.; Huang, Q. CD155 expression and its correlation with clinicopathologic characteristics, angiogenesis, and prognosis in human cholangiocarcinoma. OncoTargets Ther. 2017, ume 10, 3817–3825. [Google Scholar] [CrossRef]
- Thanee, M.; Loilome, W.; Techasen, A.; Sugihara, E.; Okazaki, S.; Abe, S.; Ueda, S.; Masuko, T.; Namwat, N.; Khuntikeo, N.; et al. CD44 variant-dependent redox status regulation in liver fluke-associated cholangiocarcinoma: A target for cholangiocarcinoma treatment. Cancer Sci. 2016, 107, 991–1000. [Google Scholar] [CrossRef]
- Saengboonmee, C.; Sawanyawisuth, K.; Chamgramol, Y.; Wongkham, S. Prognostic biomarkers for cholangiocarcinoma and their clinical implications. Expert Rev. Anticancer Ther. 2018, 18, 579–592. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, M.; Zhou, Q.; Wang, H.; Wang, Z.; Zhong, X.; Zhang, L.; Tai, S.; Cui, Y. The Prognostic Role of SOCS3 and A20 in Human Cholangiocarcinoma. PLoS ONE 2015, 10, e0141165. [Google Scholar] [CrossRef]
- Talabnin, C.; Janthavon, P.; Thongsom, S.; Suginta, W.; Talabnin, K.; Wongkham, S. Ring finger protein 43 expression is associated with genetic alteration status and poor prognosis among patients with intrahepatic cholangiocarcinoma. Hum. Pathol. 2016, 52, 47–54. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Chu, B.; Zhang, F.; Zhang, Y.; Ke, F.; Chen, Y.; Xu, Y.; Liu, S.; Zhao, S.; et al. Upregulated LASP-1 correlates with a malignant phenotype and its potential therapeutic role in human cholangiocarcinoma. Tumor Biol. 2016, 37, 8305–8315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, F.; Li, Z.; Jiang, P.; Deng, X.; Tian, F.; Li, X.; Wang, S. Aberrant expression of B7-H4 correlates with poor prognosis and suppresses tumor-infiltration of CD8+ T lymphocytes in human cholangiocarcinoma. Oncol. Rep. 2016, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, H.-D.; Liu, Y.-F.; Liu, L.; Sun, Q.; Cui, X.-J. Hepatoma-derived growth factor: A novel prognostic biomarker in intrahepatic cholangiocarcinoma. Tumor Biol. 2014, 36, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, B.; Guo, X.; Zhang, X.; Hu, J.; Hu, X.; Lu, Y. Expression of Ki-67, Bax and p73 in patients with hilar cholangiocarcinoma. Cancer Biomark. 2014, 14, 197–202. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Zhan, X.; Lin, T.; Yang, M.; Hu, J.; Han, B.; Hu, S. SOX4 is associated with poor prognosis in cholangiocarcinoma. Biochem. Biophys. Res. Commun. 2014, 452, 614–621. [Google Scholar] [CrossRef]
- Yoh, T.; Hatano, E.; Kasai, Y.; Fuji, H.; Nishi, K.; Toriguchi, K.; Sueoka, H.; Ohno, M.; Seo, S.; Iwaisako, K.; et al. Serum Nardilysin, a Surrogate Marker for Epithelial–Mesenchymal Transition, Predicts Prognosis of Intrahepatic Cholangiocarcinoma after Surgical Resection. Clin. Cancer Res. 2018, 25, 619–628. [Google Scholar] [CrossRef]
- Boonla, C.; Sripa, B.; Thuwajit, P.; Cha-On, U.; Puapairoj, A.; Miwa, M.; Wongkham, S. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2005, 11, 4939–4946. [Google Scholar] [CrossRef]
- Abe, T.; Amano, H.; Shimamoto, F.; Hattori, M.; Kuroda, S.; Kobayashi, T.; Tashiro, H.; Ohdan, H. Prognostic evaluation of mucin-5AC expression in intrahepatic cholangiocarcinoma, mass-forming type, following hepatectomy. Eur. J. Surg. Oncol. 2015, 41, 1515–1521. [Google Scholar] [CrossRef]
- Sulpice, L.; Rayar, M.; Turlin, B.; Boucher, E.; Bellaud, P.; Desille, M.; Meunier, B.; Clément, B.; Boudjema, K.; Coulouarn, C. Epithelial cell adhesion molecule is a prognosis marker for intrahepatic cholangiocarcinoma. J. Surg. Res. 2014, 192, 117–123. [Google Scholar] [CrossRef]
- Bergeat, D.; Fautrel, A.; Turlin, B.; Merdrignac, A.; Rayar, M.; Boudjema, K.; Coulouarn, C.; Sulpice, L. Impact of stroma LOXL2 overexpression on the prognosis of intrahepatic cholangiocarcinoma. J. Surg. Res. 2016, 203, 441–450. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, C.; Xia, L.; He, Z.; Lu, Z.; Liu, C.; Jia, M.; Wang, J.; Niu, J. High expression of matrix metalloproteinase-9 indicates poor prognosis in human hilar cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 6157–6164. [Google Scholar]
- Tongtawee, T.; Kaewpitoon, S.J.; Loyd, R.; Chanvitan, S.; Leelawat, K.; Praditpol, N.; Jujinda, S.; Kaewpitoon, N. High Expression of Matrix Metalloproteinase-11 indicates Poor Prognosis in Human Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 3697–3701. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Li, F.; Sun, F.; Niu, J. Interleukin-8 is a prognostic indicator in human hilar cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 8376–8384. [Google Scholar]
- Xu, Y.; Wang, Z.; Jiang, X.; Cui, Y. Overexpression of long noncoding RNA H19 indicates a poor prognosis for cholangiocarcinoma and promotes cell migration and invasion by affecting epithelial-mesenchymal transition. Biomed. Pharmacother. 2017, 92, 17–23. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, X.; Cui, Y. Upregulated long noncoding RNA PANDAR predicts an unfavorable prognosis and promotes tumorigenesis in cholangiocarcinoma. OncoTargets Ther. 2017, 10, 2873–2883. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Li, S.-H.; Huang, G.-L.; Lin, G.-H.; Shuang, Z.-Y.; Lao, X.-M.; Xu, L.; Lin, X.-J.; Wang, H.-Y.; Li, S.-P. Identification of a novel microRNA signature associated with intrahepatic cholangiocarcinoma (ICC) patient prognosis. BMC Cancer 2015, 15, 64. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, Y. Increased Expression of miR-29a and Its Prognostic Significance in Patients with Cholangiocarcinoma. Oncol. Res. Treat. 2017, 40, 128–132. [Google Scholar] [CrossRef]
- Ikeno, Y.; Seo, S.; Iwaisako, K.; Yoh, T.; Nakamoto, Y.; Fuji, H.; Taura, K.; Okajima, H.; Kaido, T.; Sakaguchi, S.; et al. Preoperative metabolic tumor volume of intrahepatic cholangiocarcinoma measured by 18F-FDG-PET is associated with the KRAS mutation status and prognosis. J. Transl. Med. 2018, 16, 95. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Stein, A.; Arnold, D.; Bridgewater, J.; Goldstein, D.; Jensen, L.H.; Klümpen, H.-J.; Lohse, A.W.; Nashan, B.; Primrose, J.; Schrum, S.; et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial)—A randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015, 15, 564. [Google Scholar] [CrossRef]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2013, 25, 391–398. [Google Scholar] [CrossRef]
- Voss, M.H.; Hierro, C.; Heist, R.S.; Cleary, J.M.; Meric-Bernstam, F.; Tabernero, J.; Janku, F.; Gandhi, L.; Iafrate, A.J.; Borger, D.R.; et al. A Phase I, Open-Label, Multicenter, Dose-escalation Study of the Oral Selective FGFR Inhibitor Debio 1347 in Patients with Advanced Solid Tumors Harboring FGFR Gene Alterations. Clin. Cancer Res. 2019, 25, 2699–2707. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion–Positive Cholangiocarcinoma. Cancer Discov. 2016, 7, 252–263. [Google Scholar] [CrossRef]
- Mertens, J.C.; Rizvi, S.; Gores, G.J. Targeting cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1864, 1454–1460. [Google Scholar] [CrossRef]
- Borad, M.J.; Gores, G.J.; Roberts, L. Fibroblast growth factor receptor 2 fusions as a target for treating cholangiocarcinoma. Curr. Opin. Gastroenterol. 2015, 31, 264–268. [Google Scholar] [CrossRef]
- Saka, H.; Kitagawa, C.; Kogure, Y.; Takahashi, Y.; Fujikawa, K.; Sagawa, T.; Iwasa, S.; Takahashi, N.; Fukao, T.; Tchinou, C.; et al. Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: A Phase I study. Investig. New Drugs 2017, 35, 451–462. [Google Scholar] [CrossRef]
- Bahleda, R.; Italiano, A.; Hierro, C.; Mita, A.; Cervantes, A.; Chan, N.; Awad, M.; Calvo, E.; Moreno, V.; Govindan, R.; et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin. Cancer Res. 2019, 25, 4888–4897. [Google Scholar] [CrossRef]
- Kurzrock, R.; Ball, D.W.; Zahurak, M.L.; Nelkin, B.D.; Subbiah, V.; Ahmed, S.; O’Connor, A.; Karunsena, E.; Parkinson, R.M.; Bishop, J.A.; et al. A Phase I Trial of the VEGF Receptor Tyrosine Kinase Inhibitor Pazopanib in Combination with the MEK Inhibitor Trametinib in Advanced Solid Tumors and Differentiated Thyroid Cancers. Clin. Cancer Res. 2019, 25, 5475–5484. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; He, Y.; Soni, N.; et al. FOENIX-CCA2: A phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J. Clin. Oncol. 2020, 38, 108. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Busset, M.D.D.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2018, 120, 165–171. [Google Scholar] [CrossRef]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients with FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39, 265. [Google Scholar] [CrossRef]
- Javle, M.; Kelley, R.; Roychowdhury, S.; Weiss, K.; Abou-Alfa, G.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.; Goyal, L.; et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann. Oncol. 2018, 29, viii720. [Google Scholar] [CrossRef]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.-Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Futur. Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef]
- Borad, M.J.; Bridgewater, J.A.; Morizane, C.; Shroff, R.T.; Oh, D.-Y.; Moehler, M.H.; Furuse, J.; Benhadji, K.A.; He, H.; Valle, J.W. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J. Clin. Oncol. 2020, 38, TPS600. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Futur. Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef]
- Terada, T.; Nakanuma, Y.; Sirica, A.E. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum. Pathol. 1998, 29, 175–180. [Google Scholar] [CrossRef]
- Pant, S.; Saleh, M.; Bendell, J.; Infante, J.; Jones, S.; Kurkjian, C.; Moore, K.; Kazakin, J.; Abbadessa, G.; Wang, Y.; et al. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann. Oncol. 2014, 25, 1416–1421. [Google Scholar] [CrossRef]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef]
- Sirica, A.-E. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2008, 14, 7033–7058. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2007, 98, 418–425. [Google Scholar] [CrossRef]
- Lurje, G.; Lenz, H.-J. EGFR Signaling and Drug Discovery. Oncology 2009, 77, 400–410. [Google Scholar] [CrossRef]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.-N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; LoConte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.-P.; Horvath, L.; et al. Report of a Multicenter Phase II Trial Testing a Combination of Biweekly Bevacizumab and Daily Erlotinib in Patients with Unresectable Biliary Cancer: A Phase II Consortium Study. J. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef]
- Philip, P.A.; Mahoney, M.R.; Allmer, C.; Thomas, J.; Pitot, H.C.; Kim, G.; Donehower, R.C.; Fitch, T.; Picus, J.; Erlichman, C. Phase II Study of Erlotinib in Patients with Advanced Biliary Cancer. J. Clin. Oncol. 2006, 24, 3069–3074. [Google Scholar] [CrossRef]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Belani, C.; Singh, D.A.; Tanaka, M.; Lenz, H.-J.; Yen, Y.; Kindler, H.L.; Iqbal, S.; Longmate, J.; Mack, P.C.; et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother. Pharmacol. 2009, 64, 777–783. [Google Scholar] [CrossRef]
- Peck, J.; Wei, L.; Zalupski, M.; O’Neil, B.; Calero, M.V.; Bekaii-Saab, T. HER2/neu May Not Be an Interesting Target in Biliary Cancers: Results of an Early Phase II Study with Lapatinib. Oncology 2012, 82, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.H.; Lindebjerg, J.; Ploen, J.; Hansen, T.; Jakobsen, A. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann. Oncol. 2012, 23, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-typeKRASadvanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2015, 122, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ole Larsen, F.; Taksony Solyom Hoegdall, D.; Hoegdall, E.; Nielsen, D. Gemcitabine, capecitabine and oxaliplatin with or without cetuximab in advanced biliary tract carcinoma. Acta Oncol. 2015, 55, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Jang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; Mcnamara, M.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2016, 36, 141–157. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Javle, M.; Churi, C.; Kang, H.; Shroff, R.T.; Janku, F.; Surapaneni, R.; Zuo, M.; Barrera, C.; Alshamsi, H.O.; Krishnan, S.; et al. HER2/neu-directed therapy for biliary tract cancer. J. Hematol. Oncol. 2015, 8, 58. [Google Scholar] [CrossRef]
- Larsen, F.O.; Markussen, A.; Diness, L.V.; Nielsen, D. Efficacy and Safety of Capecitabine, Irinotecan, Gemcitabine, and Bevacizumab as Second-Line Treatment in Advanced Biliary Tract Cancer: A Phase II Study. Oncology 2017, 94, 19–24. [Google Scholar] [CrossRef]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; A Chan, J.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2009, 11, 48–54. [Google Scholar] [CrossRef]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am. J. Clin. Oncol. 2018, 41, 649–655. [Google Scholar] [CrossRef]
- Bengala, C.; Bertolini, F.; Malavasi, N.; Boni, C.; Aitini, E.; Dealis, C.; Zironi, S.; Depenni, R.; Fontana, A.; Del Giovane, C.; et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br. J. Cancer 2009, 102, 68–72. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.J.; Ben-Josef, E.; Lenz, H.-J.; Gold, P.J.; Hamilton, R.D.; Govindarajan, R.; Eng, C.; Blanke, C.D. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Investig. New Drugs 2011, 30, 1646–1651. [Google Scholar] [CrossRef]
- Lee, J.K.; Capanu, M.; O’Reilly, E.M.; Ma, J.; Chou, J.F.; Shia, J.; Katz, S.; Gansukh, B.; Reidylagunes, D.; Segal, N.H.; et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br. J. Cancer 2013, 109, 915–919. [Google Scholar] [CrossRef]
- Moehler, M.; Maderer, A.; Schimanski, C.; Kanzler, S.; Denzer, U.; Kolligs, F.; Ebert, M.; Distelrath, A.; Geissler, M.; Trojan, J.; et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: A double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur. J. Cancer 2014, 50, 3125–3135. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2018, 125, 902–909. [Google Scholar] [CrossRef]
- Kim, R.D.; Sanoff, H.K.; Poklepovic, A.S.; Soares, H.; Kim, J.; Lyu, J.; Liu, Y.; Nixon, A.B.; Kim, D.W. A multi-institutional phase 2 trial of regorafenib in refractory advanced biliary tract cancer. Cancer 2020, 126, 3464–3470. [Google Scholar] [CrossRef]
- Demols, A.; Borbath, I.; Eynde, M.V.D.; Houbiers, G.; Peeters, M.; Marechal, R.; Delaunoit, T.; Goemine, J.-C.; Laurent, S.; Holbrechts, S.; et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann. Oncol. 2020, 31, 1169–1177. [Google Scholar] [CrossRef]
- Golub, D.; Iyengar, N.; Dogra, S.; Wong, T.; Bready, D.; Tang, K.; Modrek, A.S.; Placantonakis, D.G. Mutant Isocitrate Dehydrogenase Inhibitors as Targeted Cancer Therapeutics. Front. Oncol. 2019, 9, 417. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Borger, D.R.; Goyal, L.; Yau, T.C.C.; Poon, R.T.; Ancukiewicz, M.; Deshpande, V.; Christiani, D.C.; Liebman, H.M.; Yang, H.; Kim, H.; et al. Circulating Oncometabolite 2-Hydroxyglutarate Is a Potential Surrogate Biomarker in Patients with Isocitrate Dehydrogenase-Mutant Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2014, 20, 1884–1890. [Google Scholar] [CrossRef]
- Kim, R.D.; McDonough, S.; El-Khoueiry, A.B.; Bekaii-Saab, T.S.; Stein, S.M.; Sahai, V.; Keogh, G.P.; Kim, E.J.; Baron, A.D.; Siegel, A.B.; et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib Versus 5-fluorouracil or capecitabine in refractory advanced biliary cancer. Eur. J. Cancer 2020, 130, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.W.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-Institutional Phase II Study of Selumetinib in Patients with Metastatic Biliary Cancers. J. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Jinawath, A.; Akiyama, Y.; Sripa, B.; Yuasa, Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J. Cancer Res. Clin. Oncol. 2006, 133, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, T.; Yamazaki, O.; Tanaka, H.; Takemura, S.; Yamamoto, T.; Tanaka, S.; Nishiguchi, S.; Kubo, S. Serum Cytokeratin 19 Fragment (CYFRA21-1) as a Prognostic Factor in Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2007, 15, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Huang, Q.; Jin, Z.-Y.; Xie, F.; Zhu, C.-L.; Liu, Z.; Wang, C. Circulating microRNA-21 as a prognostic, biological marker in cholangiocarcinoma. J. Cancer Res. Ther. 2018, 14, 220. [Google Scholar] [CrossRef]
- Silakit, R.; Loilome, W.; Yongvanit, P.; Chusorn, P.; Techasen, A.; Boonmars, T.; Khuntikeo, N.; Chamadol, N.; Pairojkul, C.; Namwat, N. Circulating miR-192 in liver fluke-associated cholangiocarcinoma patients: A prospective prognostic indicator. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 864–872. [Google Scholar] [CrossRef]
- Cheng, Q.; Feng, F.; Zhu, L.; Zheng, Y.; Luo, X.; Liu, C.; Yi, B.; Jiang, X. Circulating miR-106a is a Novel Prognostic and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci. Rep. 2015, 5, 16103. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, K.-L.; Zhang, N.; Ma, X.-W.; Yan, S.-W.; Cao, D.-H.; Shi, S.-J. Serum miR-26a as a diagnostic and prognostic biomarker in cholangiocarcinoma. Oncotarget 2015, 6, 18631–18640. [Google Scholar] [CrossRef] [PubMed]
- Paolicchi, E.; Pacetti, P.; Giovannetti, E.; Mambrini, A.; Orlandi, M.; Crea, F.; Romani, A.A.; Tartarini, R.; Danesi, R.; Peters, G.J.; et al. A single nucleotide polymorphism in EZH2 predicts overall survival rate in patients with cholangiocarcinoma. Oncol. Lett. 2013, 6, 1487–1491. [Google Scholar] [CrossRef]
- Khunluck, T.; Kukongviriyapan, V.; Puapairoj, A.; Khuntikeo, N.; Senggunprai, L.; Zeekpudsa, P.; Prawan, A. Association of NRF2 polymorphism with cholangiocarcinoma prognosis in Thai patients. Asian Pac. J. Cancer Prev. 2014, 15, 299–304. [Google Scholar] [CrossRef]
- Meng, Z.-W.; Liu, M.-C.; Hong, H.-J.; Du, Q.; Chen, Y.-L. Expression and prognostic value of soluble CD97 and its ligand CD55 in intrahepatic cholangiocarcinoma. Tumor Biol. 2017, 39, 1010428317694319. [Google Scholar] [CrossRef]
- Kaira, K.; Sunose, Y.; Oriuchi, N.; Kanai, Y.; Takeyoshi, I. CD98 is a promising prognostic biomarker in biliary tract cancer. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 654–657. [Google Scholar] [CrossRef]
- Nepal, C.; O’Rourke, C.J.; Oliveira, D.N.P.; Taranta, A.; Shema, S.; Gautam, P.; Calderaro, J.; Barbour, A.; Raggi, C.; Wennerberg, K.; et al. Genomic perturbations reveal distinct regulatory networks in intrahepatic cholangiocarcinoma. Hepatology 2018, 68, 949–963. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ojima, H.; Iwasaki, M.; Shimizu, H.; Kokubu, A.; Hiraoka, N.; Kosuge, T.; Yoshikawa, D.; Kono, T.; Furukawa, H.; et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br. J. Cancer 2011, 105, 131–138. [Google Scholar] [CrossRef]
- Shi, X.-D.; Yu, X.-H.; Wu, W.-R.; Xu, X.-L.; Wang, J.-Y.; Xu, L.-B.; Zhang, R.; Liu, C. Dickkopf-1 expression is associated with tumorigenity and lymphatic metastasis in human hilar cholangiocarcinoma. Oncotarget 2016, 7, 70378–70387. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Yang, X.-R.; Shen, Q.-J.; Yang, L.-X.; Xu, Y.; Qiu, S.-J.; Sun, Y.-F.; Zhang, X.; Wang, Z.; Zhu, K.; et al. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer 2012, 119, 993–1003. [Google Scholar] [CrossRef]
- Sarcognato, S.; Gringeri, E.; Fassan, M.; Di Giunta, M.; Maffeis, V.; Guzzardo, V.; Cillo, U.; Guido, M. Prognostic role of BAP-1 and PBRM-1 expression in intrahepatic cholangiocarcinoma. Virchows Arch. 2018, 474, 29–37. [Google Scholar] [CrossRef]
- Li, B.; Tang, H.; Zhang, A.; Dong, J. Prognostic Role of Mucin Antigen MUC4 for Cholangiocarcinoma: A Meta-Analysis. PLoS ONE 2016, 11, e0157878. [Google Scholar] [CrossRef]
- Higashi, M.; Yamada, N.; Yokoyama, S.; Kitamoto, S.; Tabata, K.; Koriyama, C.; Batra, S.K.; Yonezawa, S. Pathobiological Implications of MUC16/CA125 Expression in Intrahepatic Cholangiocarcinoma-Mass Forming Type. Pathobiology 2012, 79, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Chusorn, P.; Namwat, N.; Loilome, W.; Techasen, A.; Pairojkul, C.; Khuntikeo, N.; Dechakhamphu, A.; Talabnin, C.; Chan-On, W.; Ong, C.K.; et al. Overexpression of microRNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis. Tumor Biol. 2013, 34, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-H.; Zhou, H.-W.; Liu, M.; Sun, J.-Z. The role of miR-92b in cholangiocarcinoma patients. Int. J. Biol. Markers 2018, 33, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Li, G.; Bi, W.; Yang, L.; Yao, L.; Wu, D. microRNA-34a inhibits epithelial mesenchymal transition in human cholangiocarcinoma by targeting Smad4 through transforming growth factor-beta/Smad pathway. BMC Cancer 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, C.; Pan, S.; Liang, Y.; Han, J.; Lan, Y.; Sun, J.; Li, K.; Sun, B.; Yang, G. N-myc downstream-regulated gene 2 inhibits human cholangiocarcinoma progression and is regulated by leukemia inhibitory factor/MicroRNA-181c negative feedback pathway. Hepatology 2016, 64, 1606–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Z.-Q.; Yang, Z.-R.; Tong, D.-N.; Guan, J.; Shi, B.-J.; Nie, J.; Ding, X.-T.; Li, B.; Zhou, G.-W.; et al. MicroRNA-191 acts as a tumor promoter by modulating the TET1-p53 pathway in intrahepatic cholangiocarcinoma. Hepatology 2017, 66, 136–151. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Tian, R.; Sun, H.; Zou, S. miR-373 Negatively Regulates Methyl-CpG-Binding Domain Protein 2 (MBD2) in Hilar Cholangiocarcinoma. Am. J. Dig. Dis. 2010, 56, 1693–1701. [Google Scholar] [CrossRef]
- Li, J.; Yao, L.; Li, G.; Ma, D.; Sun, C.; Gao, S.; Zhang, P.; Gao, F. miR-221 Promotes Epithelial-Mesenchymal Transition through Targeting PTEN and Forms a Positive Feedback Loop with β-catenin/c-Jun Signaling Pathway in Extra-Hepatic Cholangiocarcinoma. PLoS ONE 2015, 10, e0141168. [Google Scholar] [CrossRef]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; McEntee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 153. [Google Scholar] [CrossRef]
- Ikeda, M.; Ioka, T.; Fukutomi, A.; Morizane, C.; Kasuga, A.; Takahashi, H.; Todaka, A.; Okusaka, T.; Creasy, C.L.; Gorman, S.; et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2017, 109, 215–224. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Goff, L.W.; Cardin, D.B.; Whisenant, J.G.; Du, L.; Koyama, T.; Dahlman, K.B.; Salaria, S.N.; Young, R.T.; Ciombor, K.K.; Gilbert, J.; et al. A phase I trial investigating pulsatile erlotinib in combination with gemcitabine and oxaliplatin in advanced biliary tract cancers. Investig. New Drugs 2016, 35, 95–104. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Ramasubbaiah, R.; Yu, M.; Picus, J.; Bufill, J.A.; Tong, Y.; Coleman, N.; Johnston, E.L.; Currie, C.; Loehrer, P.J. Phase II Trial of Erlotinib and Docetaxel in Advanced and Refractory Hepatocellular and Biliary Cancers: Hoosier Oncology Group GI06-101. Oncologist 2012, 17, 13. [Google Scholar] [CrossRef][Green Version]
- Kim, S.T.; Jang, K.-T.; Lee, S.J.; Jang, H.-L.; Lee, J.; Park, S.H.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Park, J.O. Tumour shrinkage at 6 weeks predicts favorable clinical outcomes in a phase III study of gemcitabine and oxaliplatin with or without erlotinib for advanced biliary tract cancer. BMC Cancer 2015, 15, 530. [Google Scholar] [CrossRef][Green Version]
- Moehler, M.; Maderer, A.; Ehrlich, A.; Foerster, F.; Schad, A.; Nickolay, T.; Ruckes, C.; Weinmann, A.; Sivanathan, V.; Marquardt, J.U.; et al. Safety and efficacy of afatinib as add-on to standard therapy of gemcitabine/cisplatin in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, phase I trial with an extensive biomarker program. BMC Cancer 2019, 19, 55. [Google Scholar] [CrossRef]
- Jeong, H.; Jeong, J.; Kim, K.-P.; Lee, S.; Oh, D.; Park, D.; Song, T.; Park, Y.; Hong, S.-M.; Ryoo, B.-Y.; et al. Feasibility of HER2-Targeted Therapy in Advanced Biliary Tract Cancer: A Prospective Pilot Study of Trastuzumab Biosimilar in Combination with Gemcitabine Plus Cisplatin. Cancers 2021, 13, 161. [Google Scholar] [CrossRef]
- Arkenau, H.-T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist 2018, 23, 1407-e136. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, H.; Hao, M.; Zhou, Y.; Chen, Q.; Chen, Z. Efficacy and Safety of Apatinib in Treatment of Unresectable Intrahepatic Cholangiocarcinoma: An Observational Study. Cancer Manag. Res. 2020, 12, 5345–5351. [Google Scholar] [CrossRef]
- Zhang, G.; Gong, S.; Pang, L.; Hou, L.; He, W. Efficacy and Safety of Apatinib Treatment for Advanced Cholangiocarcinoma After Failed Gemcitabine-Based Chemotherapy: An Open-Label Phase II Prospective Study. Front. Oncol. 2021, 11, 3965. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Long, J.; Lin, J.; Mao, J.; Xie, F.; Wang, Y.; Wang, Y.; Xun, Z.; Bai, Y.; et al. The Efficacy and Safety of Apatinib Plus Camrelizumab in Patients with Previously Treated Advanced Biliary Tract Cancer: A Prospective Clinical Study. Front. Oncol. 2021, 11, e646979. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, J.; Aravantinos, G.; Koliou, G.-A.; Pentheroudakis, G.; Zagouri, F.; Psyrri, A.; Lampropoulou, D.I.; Demiri, S.; Pectasides, D.; Razis, E.; et al. First Line Gemcitabine/Pazopanib in Locally Advanced and/or Metastatic Biliary Tract Carcinoma. A Hellenic Cooperative Oncology Group Phase II Study. Anticancer Res. 2020, 40, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Bajardi, E.A.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. 2014, 26, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Assenat, E.; Portales, F.; Ychou, M. A multicenter phase ib-IIR trial assessing activity of regorafenib in combination with modified gemcitabine-oxaliplatin (mGEMOX) in patients with advanced biliary tract cancer (aBTC). J. Clin. Oncol. 2018, 36, 427. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Sun, H.; Bai, C.; Jia, R.; Li, Y.; Zhang, W.; Liu, L.; Huang, C.; Guan, M.; et al. A single-arm, multicenter, open-label phase 2 trial of surufatinib in patients with unresectable or metastatic biliary tract cancer. Cancer 2021, 127, 3975–3984. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Ikezawa, H.; Hayata, N.; Nakajima, R.; Morizane, C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: Primary analysis results. BMC Cancer 2020, 20, v246. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Yamamoto, N.; Naito, Y.; Kuboki, Y.; Koyama, T.; Piao, Y.; Tsujimoto, N.; Asou, H.; Inoue, K.; Kondo, S. Merestinib monotherapy or in combination for japanese patients with advanced and/or metastatic cancer: A phase 1 study. Cancer Med. 2021, 10, 6579–6589. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.P.; Verret, W.; Mulla, S.; Liu, B.; Wang, Y.; Macarulla, T.; Ren, Z.; El-Khoueiry, A.B.; Zhu, A.X. IMbrave 151: A randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer. Ther. Adv. Med Oncol. 2021, 13, 17588359211036544. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).