Risk Assessment of Postoperative Pneumonia in Cancer Patients Using a Common Data Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
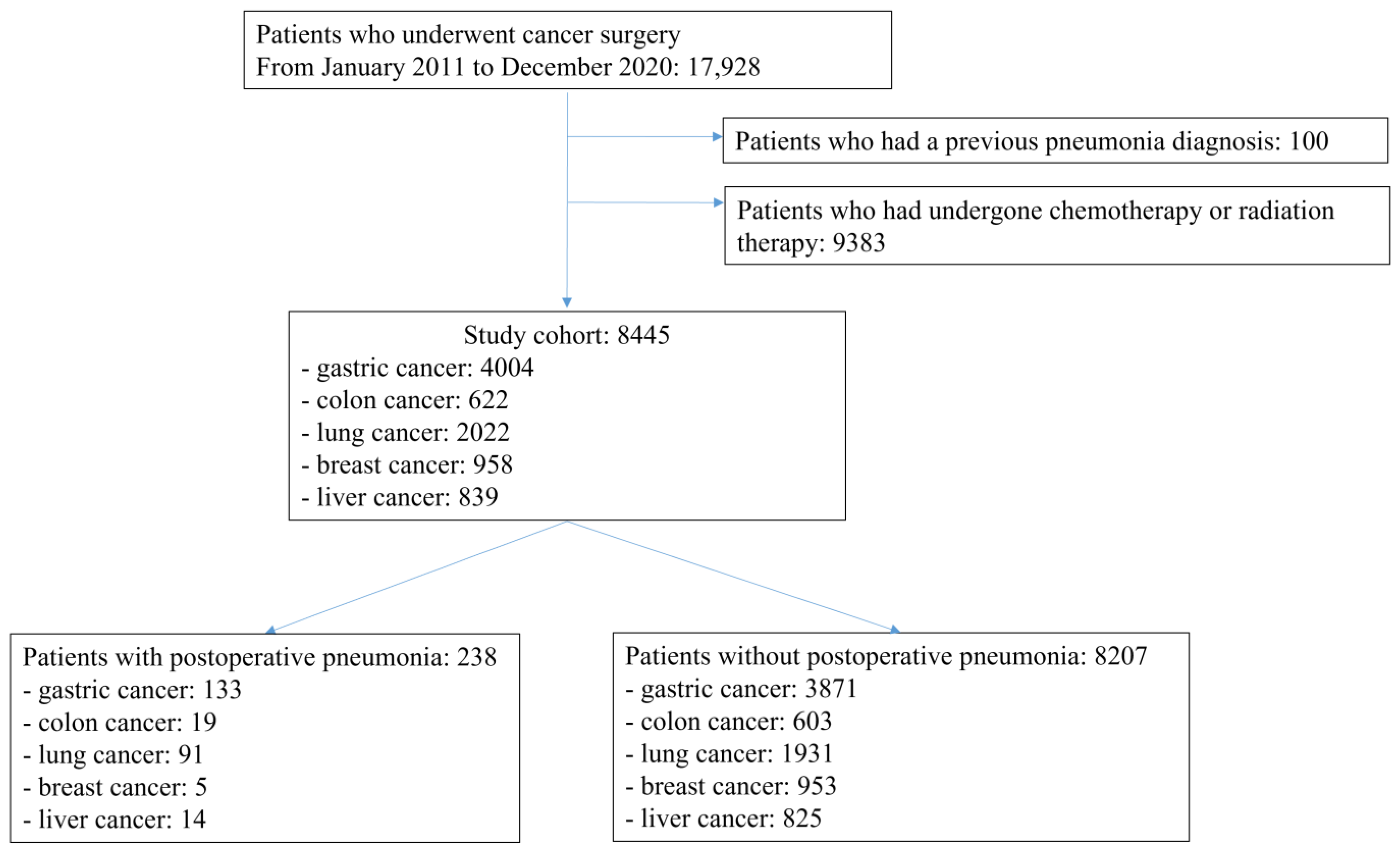
2.2. Registry-Based Patient Selection
2.3. Variables
2.4. Statistical Analyses
3. Results
3.1. Baseline and Demographic Data
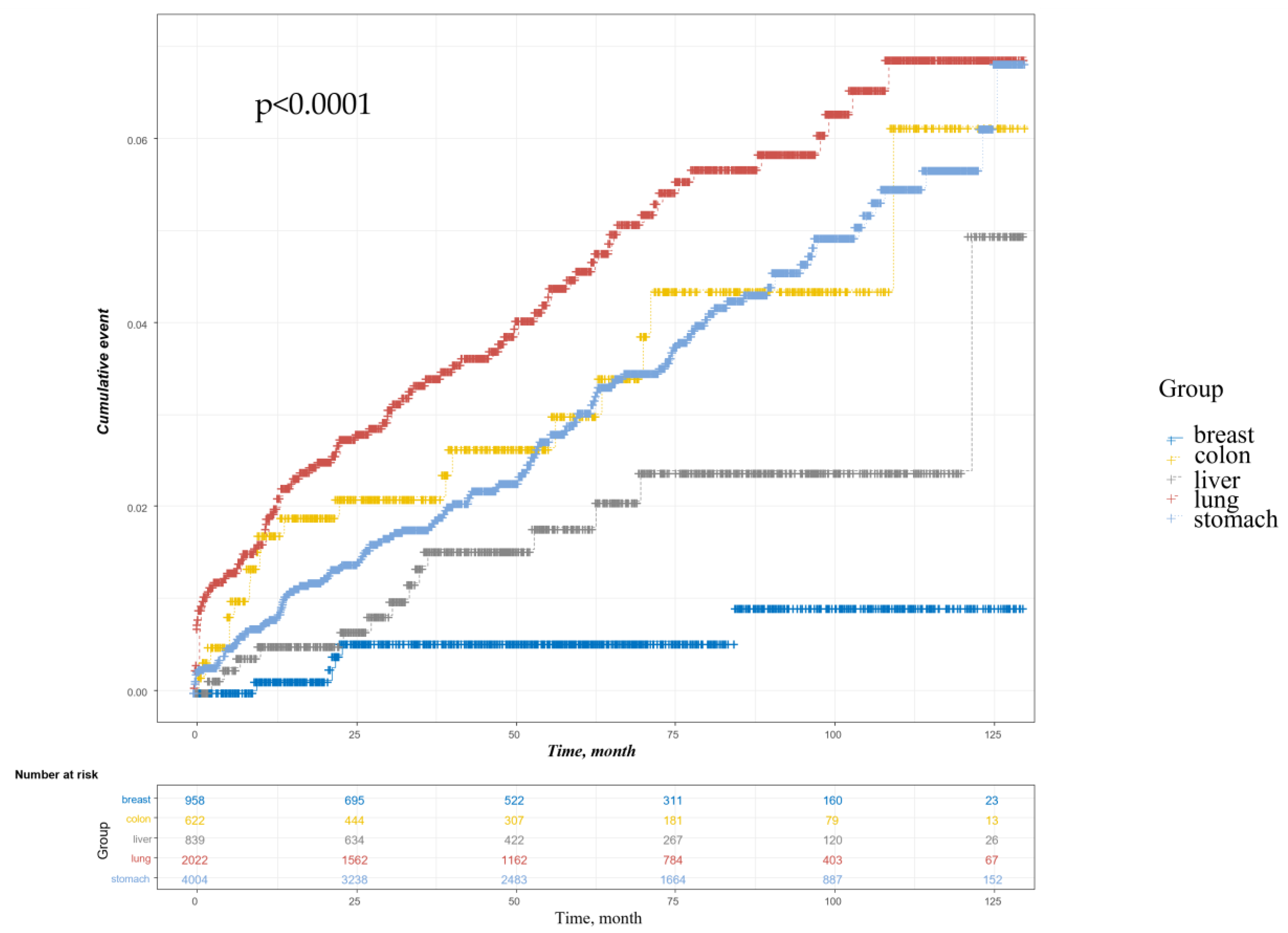
3.2. Pneumonia Incidence
3.3. Risk Factors for POP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walder, B.; Story, D.A. Postoperative pneumonia: Can this important complication be predicted and anticipated? Eur. J. Anaesthesiol. 2019, 36, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Russotto, V.; Sabate, S.; Canet, J. Development of a prediction model for postoperative pneumonia: A multicentre prospective observational study. Eur. J. Anaesthesiol. 2019, 36, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Tusman, G.; Bohm, S.H.; Warner, D.O.; Sprung, J. Atelectasis and perioperative pulmonary complications in high-risk patients. Curr. Opin. Anaesthesiol. 2012, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Hollmann, M.W.; Jaber, S.; Kozian, A.; Licker, M.; et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: A systematic review and meta-analysis. Lancet Respir. Med. 2014, 2, 1007–1015. [Google Scholar] [CrossRef]
- Chughtai, M.; Gwam, C.U.; Mohamed, N.; Khlopas, A.; Newman, J.M.; Khan, R.; Nadhim, A.; Shaffiy, S.; Mont, M.A. The Epidemiology and Risk Factors for Postoperative Pneumonia. J. Clin. Med. Res. 2017, 9, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Evans, S.E. Bacterial Pneumonia in Patients with Cancer: Novel Risk Factors and Management. Clin. Chest Med. 2017, 38, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Safdar, A. Pneumonia in the Cancer Patient. In Principles and Practice of Cancer Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2011; pp. 143–152. [Google Scholar] [CrossRef]
- Yao, L.; Luo, J.; Liu, L.; Wu, Q.; Zhou, R.; Li, L.; Zhang, C. Risk factors for postoperative pneumonia and prognosis in lung cancer patients after surgery: A retrospective study. Medicine 2021, 100, e25295. [Google Scholar] [CrossRef] [PubMed]
- Trinh, V.Q.; Ravi, P.; Abd-El-Barr, A.E.; Jhaveri, J.K.; Gervais, M.K.; Meyer, C.P.; Hanske, J.; Sammon, J.D.; Trinh, Q.D. Pneumonia after Major Cancer Surgery: Temporal Trends and Patterns of Care. Can. Respir. J. 2016, 2016, 6019416. [Google Scholar] [CrossRef][Green Version]
- Kim, D.H.; Lee, J.E.; Kim, Y.G.; Lee, Y.; Seo, D.W.; Lee, K.H.; Lee, J.H.; Kim, W.S.; Kim, Y.H.; Oh, J.S. High-Throughput Algorithm for Discovering New Drug Indications by Utilizing Large-Scale Electronic Medical Record Data. Clin. Pharmacol. Ther. 2020, 108, 1299–1307. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.H.; Kim, D.M.; Kholinne, E.; Lee, E.S.; Alzahrani, W.M.; Kim, J.W.; Jeon, I.H.; Koh, K.H. Do Nonsteroidal Anti-Inflammatory or COX-2 Inhibitor Drugs Increase the Nonunion or Delayed Union Rates After Fracture Surgery?: A Propensity-Score-Matched Study. J. Bone Jt. Surg. Am. 2021, 103, 1402–1410. [Google Scholar] [CrossRef]
- Morales, D.R.; Conover, M.M.; You, S.C.; Pratt, N.; Kostka, K.; Duarte-Salles, T.; Fernandez-Bertolin, S.; Aragon, M.; DuVall, S.L.; Lynch, K.; et al. Renin-angiotensin system blockers and susceptibility to COVID-19: An international, open science, cohort analysis. Lancet Digit. Health 2021, 3, e98–e114. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.W.; Seo, S.I.; Kim, Y.; Yoo, J.J.; Shin, W.G.; Kim, J.; You, S.C.; Park, R.W.; Park, Y.M.; Kim, K.J.; et al. Impact of pitavastatin on new-onset diabetes mellitus compared to atorvastatin and rosuvastatin: A distributed network analysis of 10 real-world databases. Cardiovasc. Diabetol. 2022, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Schuemie, M.J.; Krumholz, H.M.; You, S.C.; Chen, R.; Pratt, N.; Reich, C.G.; Duke, J.; Madigan, D.; Hripcsak, G.; et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: A systematic, multinational, large-scale analysis. Lancet 2019, 394, 1816–1826. [Google Scholar] [CrossRef]
- Vashisht, R.; Jung, K.; Schuler, A.; Banda, J.M.; Park, R.W.; Jin, S.; Li, L.; Dudley, J.T.; Johnson, K.W.; Shervey, M.M.; et al. Association of Hemoglobin A1c Levels With Use of Sulfonylureas, Dipeptidyl Peptidase 4 Inhibitors, and Thiazolidinediones in Patients With Type 2 Diabetes Treated With Metformin: Analysis From the Observational Health Data Sciences and Informatics Initiative. JAMA Netw. Open 2018, 1, e181755. [Google Scholar] [CrossRef]
- Jung, J.; Moon, S.M.; Jang, H.C.; Kang, C.I.; Jun, J.B.; Cho, Y.K.; Kang, S.J.; Seo, B.J.; Kim, Y.J.; Park, S.B.; et al. Incidence and risk factors of postoperative pneumonia following cancer surgery in adult patients with selected solid cancer: Results of “Cancer POP” study. Cancer Med. 2018, 7, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cabello, H.; Torres, A.; Celis, R.; El-Ebiary, M.; Puig de la Bellacasa, J.; Xaubet, A.; Gonzalez, J.; Agusti, C.; Soler, N. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: A bronchoscopic study. Eur. Respir. J. 1997, 10, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Sekine, Y.; Suzuki, H.; Iwata, T.; Chiyo, M.; Nakajima, T.; Yasufuku, K.; Yoshida, S. Trends of bacterial colonisation and the risk of postoperative pneumonia in lung cancer patients with chronic obstructive pulmonary disease. Eur. J. Cardiothorac. Surg. 2010, 37, 752–757. [Google Scholar] [CrossRef]
- Schussler, O.; Alifano, M.; Dermine, H.; Strano, S.; Casetta, A.; Sepulveda, S.; Chafik, A.; Coignard, S.; Rabbat, A.; Regnard, J.F. Postoperative pneumonia after major lung resection. Am. J. Respir. Crit. Care Med. 2006, 173, 1161–1169. [Google Scholar] [CrossRef]
- Simonsen, D.F.; Sogaard, M.; Bozi, I.; Horsburgh, C.R.; Thomsen, R.W. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir. Med. 2015, 109, 1340–1346. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jin, S.M.; Lee, C.H.; Lee, B.J.; Kang, C.H.; Yim, J.J.; Kim, Y.T.; Yang, S.C.; Yoo, C.G.; Han, S.K.; et al. Risk factors of postoperative pneumonia after lung cancer surgery. J. Korean Med. Sci. 2011, 26, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Sibila, O.; Anzueto, A. Pneumonia in Patients with Chronic Obstructive Pulmonary Disease. Tuberc. Respir. Dis. 2018, 81, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Mohri, Y.; Tonouchi, H.; Miki, C.; Kobayashi, M.; Kusunoki, M.; Mie Surgical Infection Research, G. Incidence and risk factors for hospital-acquired pneumonia after surgery for gastric cancer: Results of prospective surveillance. World J. Surg. 2008, 32, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, J.; Yu, P.; Wang, W.; Hu, X.; Hou, J.; Fang, S.; Liu, X. Perioperative risk factors for postoperative pneumonia after major oral cancer surgery: A retrospective analysis of 331 cases. PLoS ONE 2017, 12, e0188167. [Google Scholar] [CrossRef]
- Falagas, M.E.; Mourtzoukou, E.G.; Vardakas, K.Z. Sex differences in the incidence and severity of respiratory tract infections. Respir. Med. 2007, 101, 1845–1863. [Google Scholar] [CrossRef]
- Janele, D.; Lang, T.; Capellino, S.; Cutolo, M.; Da Silva, J.A.; Straub, R.H. Effects of testosterone, 17beta-estradiol, and downstream estrogens on cytokine secretion from human leukocytes in the presence and absence of cortisol. Ann. N. Y. Acad. Sci. 2006, 1069, 168–182. [Google Scholar] [CrossRef]
- McBride, K.E.; Solomon, M.J.; Bannon, P.G.; Glozier, N.; Steffens, D. Surgical outcomes for people with serious mental illness are poorer than for other patients: A systematic review and meta-analysis. Med. J. Aust. 2021, 214, 379–385. [Google Scholar] [CrossRef]
- Kheir, M.M.; Kheir, Y.N.P.; Tan, T.L.; Ackerman, C.T.; Rondon, A.J.; Chen, A.F. Increased Complications for Schizophrenia and Bipolar Disorder Patients Undergoing Total Joint Arthroplasty. J. Arthroplast. 2018, 33, 1462–1466. [Google Scholar] [CrossRef]
- Seminog, O.O.; Goldacre, M.J. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax 2013, 68, 171–176. [Google Scholar] [CrossRef]
- Oyesanmi, O.; Kunkel, E.J.; Monti, D.A.; Field, H.L. Hematologic side effects of psychotropics. Psychosomatics 1999, 40, 414–421. [Google Scholar] [CrossRef]
- Caruso, R.; Nanni, M.G.; Riba, M.; Sabato, S.; Mitchell, A.J.; Croce, E.; Grassi, L. Depressive spectrum disorders in cancer: Prevalence, risk factors and screening for depression: A critical review. Act. Oncol. 2017, 56, 146–155. [Google Scholar] [CrossRef] [PubMed]
| Variables | Non-POP Group (n = 8183) | POP Group (n = 262) | p-Value |
|---|---|---|---|
| Sex * | <0.001 | ||
| Female | 3596 (43.9) | 64 (24.4) | |
| Male | 4587 (56.1) | 198 (75.6) | |
| Age (years) * | 60.67 ± 14.10 | 68.69 ± 10.22 | <0.001 |
| Hypertension * | 320 (3.9) | 23 (8.8) | <0.001 |
| Diabetes mellitus | 481 (5.9) | 22 (8.4) | 0.118 |
| Renal disease | 138 (1.7) | 8 (3.1) | 0.153 |
| Mild liver disease | 390 (4.8) | 10 (3.8) | 0.573 |
| Heart failure | 59 (0.7) | 4 (1.5) | 0.26 |
| Chronic pulmonary disease * | 329 (4.0) | 29 (11.1) | <0.001 |
| Mood disorder * | 63 (0.8) | 7 (2.7) | 0.003 |
| Dementia | 16 (0.2) | 2 (0.8) | 0.2 |
| Hypothyroidism | 96 (1.2) | 2 (0.8) | 0.751 |
| Parkinson’s disease * | 26 (0.3) | 4 (1.5) | 0.007 |
| Peripheral vascular disease | 64 (0.8) | 3 (1.1) | 0.766 |
| Peptic ulcer disease | 271 (3.3) | 12 (4.6) | 0.343 |
| Cerebrovascular disease * | 357 (4.4) | 28 (10.7) | <0.001 |
| No. of Patients | No. of POP Cases | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Total | 8445 | 238 | ||
| Gastric cancer | 4004 | 133 | 5.67 (2.32–13.85) | <0.001 |
| Colon cancer | 622 | 19 | 6.14 (2.29–16.44) | <0.001 |
| Lung cancer | 2022 | 91 | 8.08 (3.29–19.89) | <0.001 |
| Liver cancer | 839 | 14 | 3.22 (1.16–8.94) | 0.025 |
| Breast cancer | 958 | 5 | Reference |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age | 1.06 (1.05–1.07) | <0.001 | 1.05 (1.04–1.06) | <0.001 |
| Sex (Ref: Female) | 2.32 (1.75–3.07) | <0.001 | 2.19 (1.65–2.91) | <0.001 |
| Hypertension | 2.04 (1.33–3.13) | 0.001 | ||
| Chronic pulmonary disease | 3.11 (2.11–4.58) | <0.001 | 2.10 (1.42–3.10) | <0.001 |
| Mood disorder | 3.84 (1.81–8.14) | <0.001 | 3.16 (1.46–6.83) | 0.003 |
| Parkinson’s disease | 4.46 (1.66–11.98) | 0.003 | ||
| Cerebrovascular disease | 3.18 (2.15–4.72) | <0.001 | 1.84 (1.22–2.77) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.H.; Kim, D.-H.; Kim, J.; Lee, J. Risk Assessment of Postoperative Pneumonia in Cancer Patients Using a Common Data Model. Cancers 2022, 14, 5988. https://doi.org/10.3390/cancers14235988
Lee YH, Kim D-H, Kim J, Lee J. Risk Assessment of Postoperative Pneumonia in Cancer Patients Using a Common Data Model. Cancers. 2022; 14(23):5988. https://doi.org/10.3390/cancers14235988
Chicago/Turabian StyleLee, Yong Hoon, Do-Hoon Kim, Jisun Kim, and Jaetae Lee. 2022. "Risk Assessment of Postoperative Pneumonia in Cancer Patients Using a Common Data Model" Cancers 14, no. 23: 5988. https://doi.org/10.3390/cancers14235988
APA StyleLee, Y. H., Kim, D.-H., Kim, J., & Lee, J. (2022). Risk Assessment of Postoperative Pneumonia in Cancer Patients Using a Common Data Model. Cancers, 14(23), 5988. https://doi.org/10.3390/cancers14235988






