Digital Quantification of Intratumoral CD8+ T-Cells Predicts Relapse and Unfavorable Outcome in Uveal Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Case Selection, Clinical and Pathological Data Collection
2.2. Immunohistochemistry
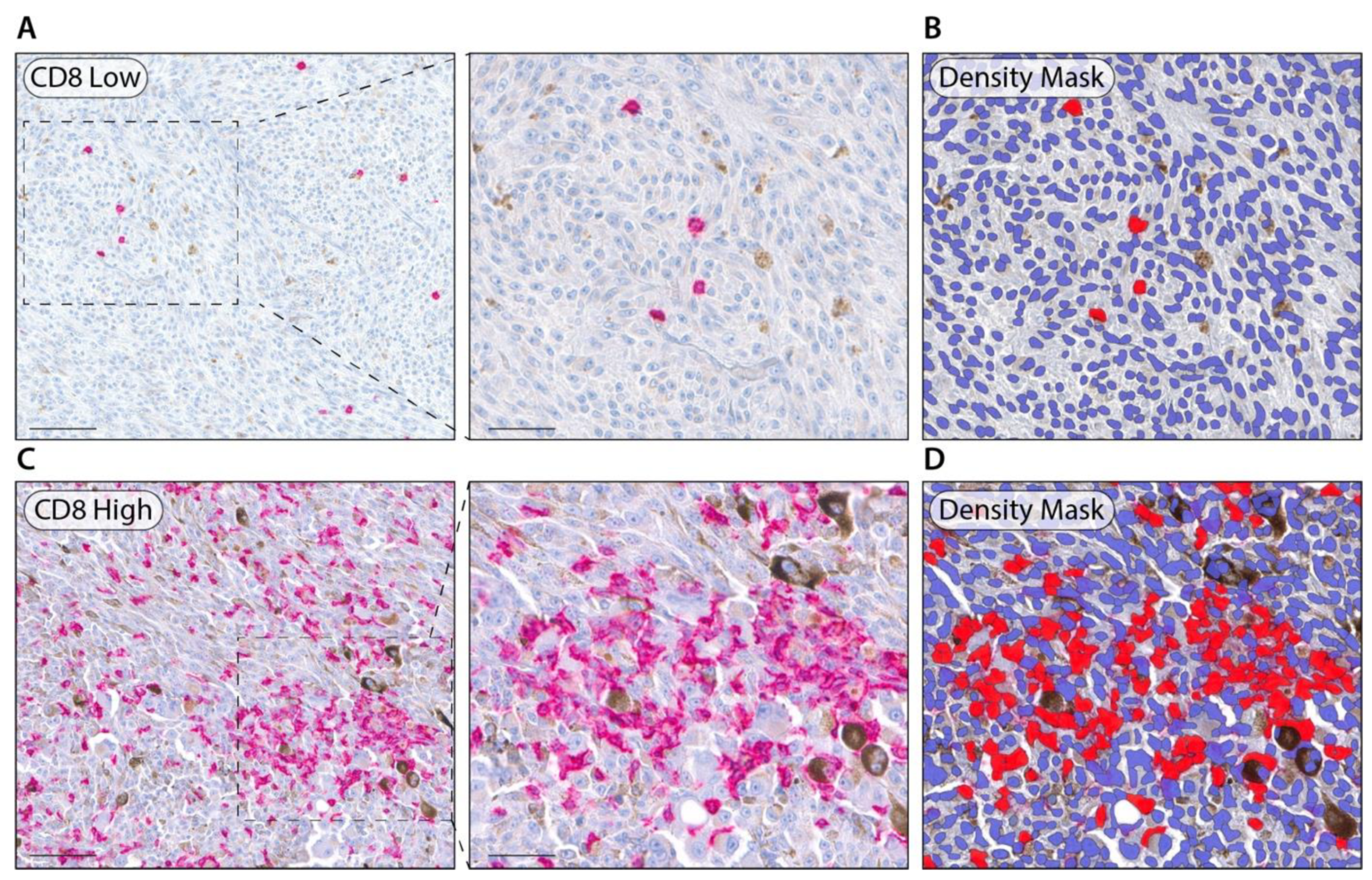
2.3. Digital Image Analysis
2.4. Statistical Analyses
3. Results
3.1. Clinical Features
3.2. Histopathology
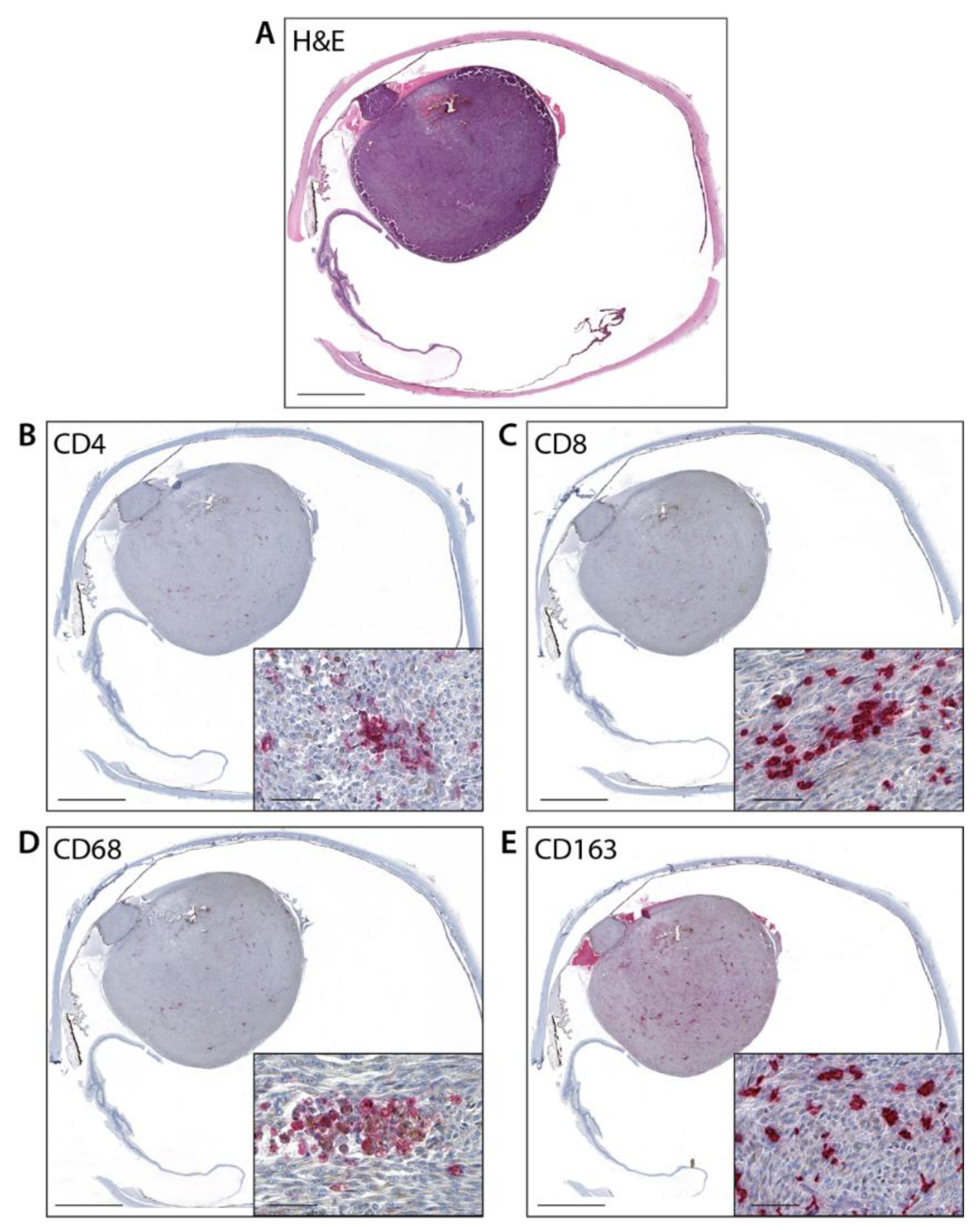
3.3. Immunohistochemistry and Digital Image Analysis
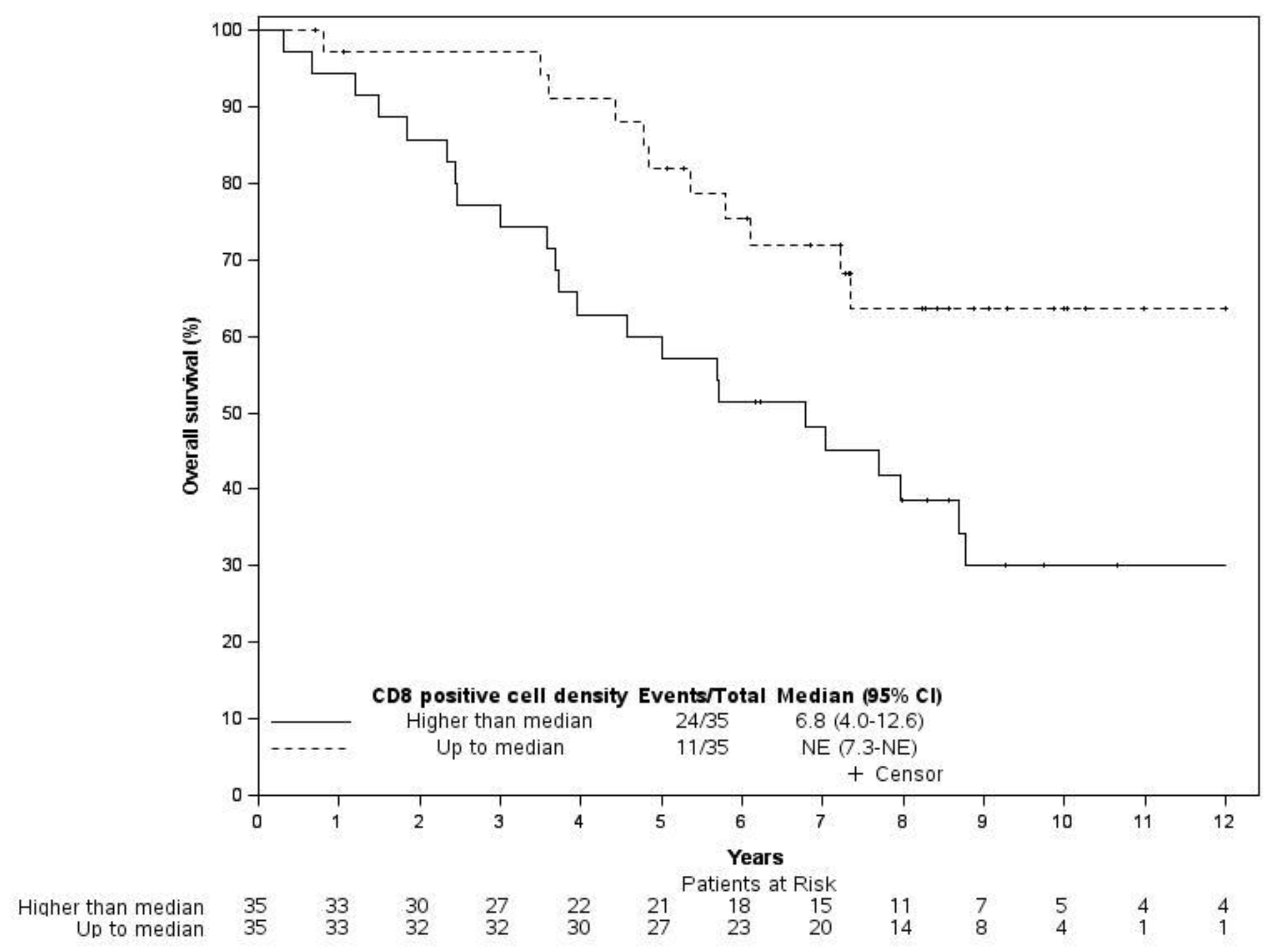
3.4. Follow-Up and Survival Information
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L.; Shields, J.A. Uveal melanoma: Estimating prognosis. Indian J. Ophthalmol. 2015, 63, 93. [Google Scholar] [CrossRef]
- Kashyap, S.; Meel, R.; Singh, L.; Singh, M. Uveal melanoma. Semin. Diagn. Pathol. 2016, 33, 141–147. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef]
- Griewank, K.G.; Murali, R. Pathology and genetics of uveal melanoma. Pathology 2013, 45, 18–27. [Google Scholar] [CrossRef]
- Berus, T.; Halon, A.; Markiewicz, A.; Orlowska-Heitzman, J.; Romanowska-Dixon, B.; Donizy, P. Clinical, histopathological and cytogenetic prognosticators in uveal melanoma–A comprehensive review. Anticancer Res. 2017, 37, 6541–6549. [Google Scholar]
- Shammas, H.F.; Blodi, F.C. Prognostic factors in choroidal and ciliary body melanomas. Arch. Ophthalmol. 1977, 95, 63–69. [Google Scholar] [CrossRef]
- Sarubi, H.C.; Pereira, N.B.; Gomes, C.C.; Gomez, R.S.; Carmo, A.; Melo, F.M.; Bastos Rodrigues, L.; Pedrosa, M.S.; Friedman, E.; Marco, L.D. Molecular and immunohistochemical analyses of uveal melanoma patient cohort. Melanoma Res. 2019, 29, 248–253. [Google Scholar] [CrossRef]
- Kuk, D.; Shoushtari, A.N.; Barker, C.A.; Panageas, K.S.; Munhoz, R.R.; Momtaz, P.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bogatch, K.; et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 2016, 21, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. Ocular immune privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Ocular immune privilege and ocular melanoma: Parallel universes or immunological plagiarism? Front. Immunol. 2012, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Schinzari, G.; Zizzari, I.G.; Maiorano, B.A.; Pagliara, M.M.; Sammarco, M.G.; Fiorentino, V.; Petrone, G.; Cassano, A.; Rindi, G.; et al. Immunological backbone of uveal melanoma: Is there a rationale for immunotherapy? Cancers 2019, 11, 1055. [Google Scholar] [CrossRef]
- Laplane, L.; Duluc, D.; Bikfalvi, A.; Larmonier, N.; Pradeu, T. Beyond the tumour microenvironment. Int. J. Cancer 2019, 145, 2611–2618. [Google Scholar] [CrossRef]
- Triozzi, P.L.; Schoenfield, L.; Plesec, T.; Saunthararajah, Y.; Tubbs, R.R.; Singh, A.D. Molecular profiling of primary uveal melanomas with tumor-infiltrating lymphocytes. Oncoimmunology 2014, 3, e947169. [Google Scholar] [CrossRef] [PubMed]
- Lachota, M.; Lennikov, A.; Malmberg, K.J.; Zagozdzon, R. Bioinformatic analysis reveals central role for tumor-infiltrating immune cells in uveal melanoma progression. J. Immunol. Res. 2021, 2021, 9920234. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Yuan, Y.; Lu, Y.; Luo, M.; Lin, L.; Ma, S. Construction of a promising tumor-infiltrating CD8+ T cells gene signature to improve prediction of the prognosis and immune response of uveal melanoma. Front. Cell Dev. Biol. 2021, 9, 673838. [Google Scholar] [CrossRef]
- Krishna, Y.; McCarthy, C.; Kalirai, H.; Coupland, S.E. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum. Pathol. 2017, 66, 159–166. [Google Scholar] [CrossRef]
- Gezgin, G.; Dogrusöz, M.; van Essen, T.H.; Kroes, W.G.M.; Luyten, G.P.M.; van der Velden, P.A.; Walter, V.; Verdijk, R.M.; van Hall, T.; van der Burg, S.H.; et al. Genetic evolution of uveal melanoma guides the development of an inflammatory microenvironment. Cancer Immunol. Immunother. 2017, 66, 903–912. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.G.; Jager, M.J. Uveal melanoma: The inflammatory microenvironment. J. Innate Immun. 2012, 4, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Mä, T.; Summanen, P.; Tarkkanen, A.; Kivelä, T. Tumor-infiltrating macrophages (CD68+ cells) and prognosis in malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1414–1421. [Google Scholar]
- Ribas, A.; Shin, D.S.; Zaretsky, J.; Frederiksen, J.; Cornish, A.; Avramis, E.; Seja, E.; Kivork, C.; Siebert, J.; Kaplan-Lefko, P.; et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol. Res. 2016, 4, 194–203. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowsky, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- la de Cruz, P.O., Jr.; Specht, C.S.; McLean, I.W. Lymphocytic infiltration in uveal malignant melanoma. Cancer 1990, 65, 112–115. [Google Scholar] [CrossRef]
- de Lange, M.J.; van Pelt, S.I.; Versluis, M.; Jordanova, E.S.; Kroes, W.G.M.; Ruivenkamp, C.; van der Burg, S.H.; Luyten, G.P.M.; van Hall, T.; Jager, M.J.; et al. Heterogeneity revealed by integrated genomic analysis uncovers a molecular switch in malignant uveal melanoma. Oncotarget 2015, 6, 37824. [Google Scholar] [CrossRef]
- Narasimhaiah, D.; Legrand, C.; Damotte, D.; Remark, R.; Munda, M.; de Potter, P.; Coulie, P.G.; Vikkula, M.; Godfraind, C. DNA alteration-based classification of uveal melanoma gives better prognostic stratification than immune infiltration, which has a neutral effect in high-risk group. Cancer Med. 2019, 8, 3036–3046. [Google Scholar] [CrossRef]
- Durante, M.A.; Rodriguez, D.A.; Kurtenbach, S.; Kuznetsov, J.N.; Sanchez, M.I.; Decatur, C.L.; Snyder, H.; Feun, L.G.; Livingstone, A.S.; Harbour, J.W. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat. Commun. 2020, 11, 496. [Google Scholar] [CrossRef]
- Croci, D.O.; Zacarías Fluck, M.F.; Rico, M.J.; Matar, P.; Rabinovich, G.A.; Scharovsky, O.G. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol. Immunother. 2007, 56, 1687–1700. [Google Scholar] [CrossRef]
- Buscher, K.; Ehinger, E.; Gupta, P.; Pramod, A.B.; Wolf, D.; Tweet, G.; Pan, C.; Mills, C.D.; Lusis, A.J.; Ley, K. Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival. Nat. Commun. 2017, 8, 16041. [Google Scholar] [CrossRef]
- Ley, K. M1 means kill; M2 means heal. J. Immunol. 2017, 199, 2191–2193. [Google Scholar] [CrossRef]
- Maat, W.; Ly, L.V.; Jordanova, E.S.; de Wolff-Rouendaal, D.; Schalij-Delfos, N.E.; Jager, M.J. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 505–510. [Google Scholar] [CrossRef]
- de Lange, M.J.; Nell, R.J.; Lalai, R.N.; Versluis, M.; Jordanova, E.S.; Luyten, G.P.M.; Jager, M.J.; van der Burg, S.H.; Zoutman, V.H.; van Hall, T. Digital PCR-based t-cell quantification–assisted deconvolution of the microenvironment reveals that activated macrophages drive tumor inflammation in uveal melanoma. Mol. Cancer Res. 2018, 16, 1902–1911. [Google Scholar] [CrossRef]
- Marshall, E.; Romaniuk, C.; Ghaneh, P.; Wong, H.; McKay, M.; Chopra, M.; Coupland, S.E.; Damato, B.E. MRI in the detection of hepatic metastases from high-risk uveal melanoma: A prospective study in 188 patients. Br. J. Ophthalmol. 2013, 97, 159–163. [Google Scholar] [CrossRef]
- Kujala, E.; Mä, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Lorenzo, D.; Piulats, J.M.; Ochoa, M.; Arias, L.; Gutiérrez, C.; Català, J.; Cobos, E.; Garcia-Bru, P.; Dias, B.; Padrón-Pérez, N.; et al. Clinical predictors of survival in metastatic uveal melanoma. Jpn. J. Ophthalmol. 2019, 63, 197–209. [Google Scholar] [CrossRef]
- Bergman, L.; Seregard, S.; Nilsson, B.; Lundell, G.; Ringborg, U.; Ragnarsson-Olding, B. Uveal melanoma survival in Sweden from 1960 to 1998. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3282–3287. [Google Scholar] [CrossRef]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal melanoma: 5-year update on incidence, treatment, and survival (SEER 1973-2013). Ocul. Oncol. Pathol. 2018, 4, 145–151. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.G.; Ly, L.V.; Jordanova, E.S.; Vrolijk, J.; Versluis, M.; Luyten, G.P.M.; Jager, M.J. Detection of M2-macrophages in uveal melanoma and relation with survival. Investig. Ophthalmol. Vis. Sci. 2011, 52, 643–650. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Mashayekhi, A.; Ganesh, A.; Furuta, M.; Shields, J.A. Influence of age on prognosis of young patients with uveal melanoma: A matched retrospective cohort study. Eur. J. Ophthalmol. 2013, 23, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Stacey, A.W.; Dedania, V.S.; Materin, M.; Demirci, H. Improved prognostic precision in uveal melanoma through a combined score of clinical stage and molecular prognostication. Ocul. Oncol. Pathol. 2022, 8, 35–41. [Google Scholar] [CrossRef] [PubMed]
| Overall N = 72 | |
|---|---|
| CD4+ intratumoral score | |
| 0 | 37 (51.4) |
| 1+ | 31 (43.1) |
| 2+ | 4 (5.6) |
| CD4+ density (cells/mm2) * | |
| Mean (SD) | 126.2 (199.8) |
| Median (Q1–Q3) | 39.4 (8.6–122.2) |
| Min–Max | 0.0–760.1 |
| CD8+ intratumoral score | |
| 0 | 17 (23.6) |
| 1+ | 39 (54.2) |
| 2+ | 13 (18.1) |
| 3+ | 3 (4.2) |
| CD8+ density (cells/mm2) † | |
| Mean (SD) | 113.3 (213.0) |
| Median (Q1–Q3) | 13.3 (3.0–124.7) |
| Min–Max | 0.0–939.0 |
| CD68+ intratumoral score * | |
| 0 | 12 (16.9) |
| 1+ | 34 (47.9) |
| 2+ | 22 (31.0) |
| 3+ | 3 (4.2) |
| CD68+ density (cells/mm2) * | |
| Mean (SD) | 99.3 (167.4) |
| Median (Q1–Q3) | 46.1 (4.1–106.8) |
| Min–Max | 0.0–846.4 |
| CD163+ intratumoral score | |
| 0 | 2 (2.8) |
| 1+ | 10 (13.9) |
| 2+ | 32 (44.4) |
| 3+ | 28 (38.9) |
| CD163+ density (cells/mm2) †† | |
| Mean (SD) | 337.5 (295.2) |
| Median (Q1–Q3) | 260.6 (96.4–524.6) |
| Min–Max | 0.0–1188.0 |
| Multivariable Model Including CD4+ | Multivariable Model Including CD8+ | Multivariable Model Including CD68+ | Multivariable Model Including CD163+ | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| CD4+ density (cells/mm2) (>39.4 vs. ≤39.4) | 0.96 (0.51–1.79) | 0.891 | ||||||
| CD8+ density (cells/mm2) (>13.3 vs. ≤13.3) | 2.08 (1.09–3.99) | 0.027 | ||||||
| CD68+ density (cells/mm2) (>46.1 vs. ≤46.1) | 1.12 (0.58–2.17) | 0.745 | ||||||
| CD163+ density (cells/mm2) (>260.6 vs. ≤260.6) | 1.73 (0.91–3.27) | 0.094 | ||||||
| Age (1 year increase) | 1.03 (1.01–1.06) | 0.008 | 1.04 (1.01–1.06) | 0.003 | 1.03 (1.01–1.06) | 0.007 | 1.03 (1.01–1.05) | 0.015 |
| Sex (Male vs. Female) | 1.17 (0.63–2.19) | 0.623 | 1.21 (0.64–2.28) | 0.564 | 1.15 (0.62–2.16) | 0.656 | 1.07 (0.57–2.00) | 0.832 |
| Stage at diagnosis (III vs. II) | 2.43 (1.25–4.71) | 0.009 | 2.86 (1.44–5.68) | 0.003 | 2.37 (1.21–4.65) | 0.012 | 2.88 (1.44–5.76) | 0.003 |
| Multivariable Model Including CD4+ | Multivariable Model Including CD8+ | Multivariable Model Including CD68+ | Multivariable Model Including CD163+ | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| CD4+ density (cells/mm2) (>39.4 vs. ≤39.4) | 1.12 (0.57–2.21) | 0.745 | ||||||
| CD8+ density (cells/mm2) (>13.3 vs. ≤13.3) | 3.30 (1.58–6.88) | 0.001 * | ||||||
| CD68+ density (cells/mm2) (>46.1 vs. ≤46.1) | 1.26 (0.61–2.61) | 0.528 | ||||||
| CD163+ density (cells/mm2) (>260.6 vs. ≤260.6) | 1.93 (0.96–3.90) | 0.067 | ||||||
| Age (1 year increase) | 1.05 (1.02–1.08) | 0.001 | 1.05 (1.02–1.08) | <0.001 | 1.05 (1.02–1.08) | 0.001 | 1.05 (1.02–1.08) | 0.001 |
| Sex (Male vs. Female) | 1.34 (0.67–2.67) | 0.412 | 1.58 (0.79–3.17) | 0.196 | 1.32 (0.66–2.64) | 0.436 | 1.26 (0.63–2.52) | 0.521 |
| Stage at diagnosis (III vs. II) | 2.68 (1.27–5.64) | 0.010 | 2.89 (1.36–6.12) | 0.006 | 2.59 (1.21–5.52) | 0.014 | 3.22 (1.49–6.98) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurdogan, O.; De Logu, F.; Galli, F.; Tuncer, S.; Ugolini, F.; Simi, S.; Portelli, F.; Nassini, R.; Massi, D.; Buyukbabani, N. Digital Quantification of Intratumoral CD8+ T-Cells Predicts Relapse and Unfavorable Outcome in Uveal Melanoma. Cancers 2022, 14, 5959. https://doi.org/10.3390/cancers14235959
Hurdogan O, De Logu F, Galli F, Tuncer S, Ugolini F, Simi S, Portelli F, Nassini R, Massi D, Buyukbabani N. Digital Quantification of Intratumoral CD8+ T-Cells Predicts Relapse and Unfavorable Outcome in Uveal Melanoma. Cancers. 2022; 14(23):5959. https://doi.org/10.3390/cancers14235959
Chicago/Turabian StyleHurdogan, Ozge, Francesco De Logu, Francesca Galli, Samuray Tuncer, Filippo Ugolini, Sara Simi, Francesca Portelli, Romina Nassini, Daniela Massi, and Nesimi Buyukbabani. 2022. "Digital Quantification of Intratumoral CD8+ T-Cells Predicts Relapse and Unfavorable Outcome in Uveal Melanoma" Cancers 14, no. 23: 5959. https://doi.org/10.3390/cancers14235959
APA StyleHurdogan, O., De Logu, F., Galli, F., Tuncer, S., Ugolini, F., Simi, S., Portelli, F., Nassini, R., Massi, D., & Buyukbabani, N. (2022). Digital Quantification of Intratumoral CD8+ T-Cells Predicts Relapse and Unfavorable Outcome in Uveal Melanoma. Cancers, 14(23), 5959. https://doi.org/10.3390/cancers14235959






