Gastric Cancer Risk and Pathogenesis in BRCA1 and BRCA2 Carriers
Abstract
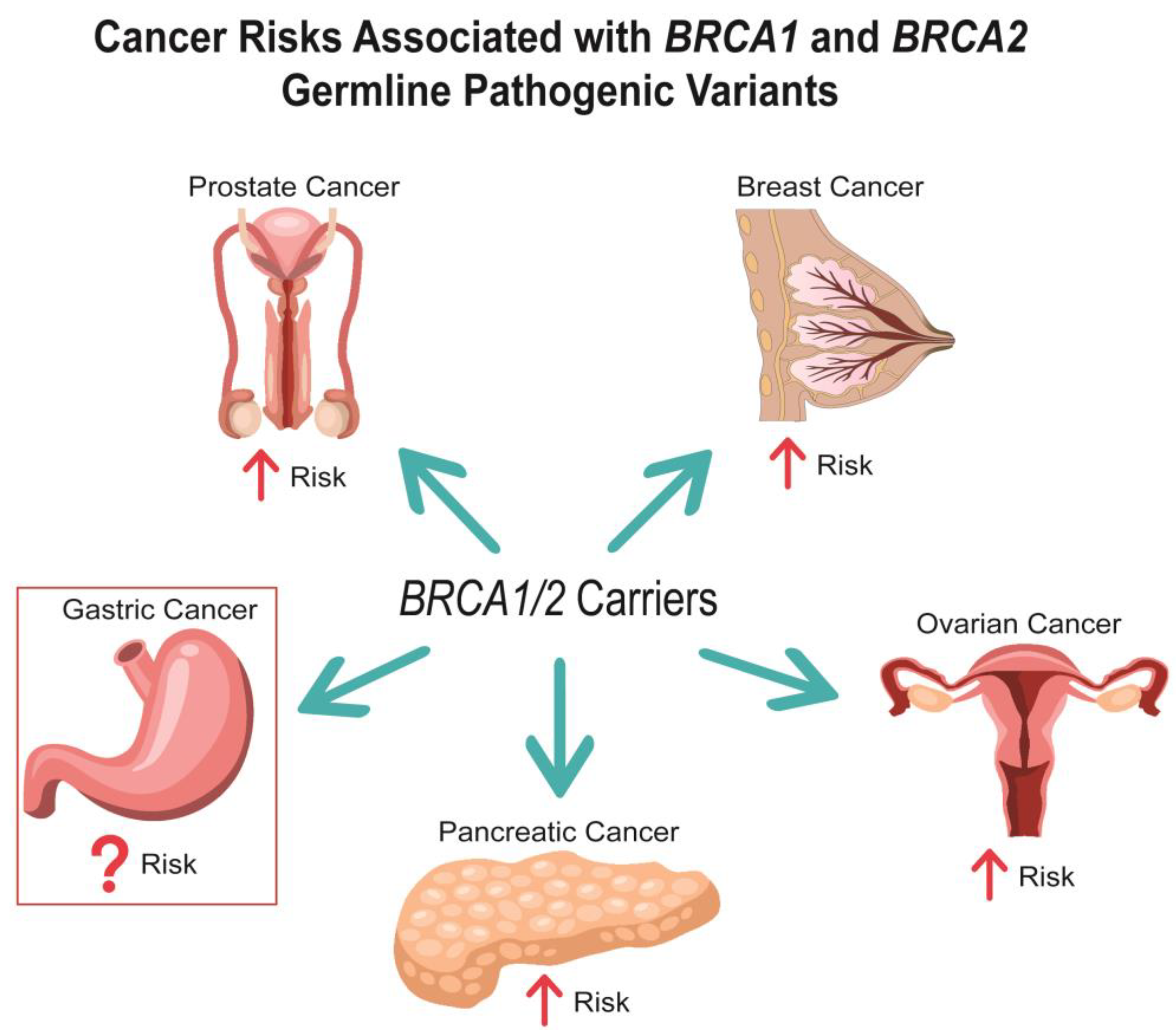
Simple Summary
Abstract
1. Introduction
2. Gastric Cancer Risk in BRCA1/2 Carriers
2.1. Gastric Cancer Risk among BRCA1 PV Carriers
2.2. Gastric Cancer Risk among BRCA2 PV Carriers
2.3. Gastric Cancer Risk among Cohorts Comprised of BRCA1 and BRCA2 PV Carriers
3. BRCA1/2-Associated Gastric Cancer
3.1. Classical Pathways of Gastric Carcinogenesis
3.2. Homologous Recombination Deficiency in Gastric Cancer
3.3. Prognostic Value of BRCA1/2 Expression in Gastric Cancer
3.4. Potential Therapeutic Interventions in BRCA1/2 Associated Gastric Cancer
4. Gastric Surveillance Considerations in BRCA1/2 Carriers
4.1. Non-Gastric Surveillance in BRCA1/2 Carriers
4.2. Gastric Surveillance in Other Hereditary Gastric Cancer Risk Syndromes
4.3. Gastric Cancer Risk Management in BRCA1/2 Carriers
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ackerson, S.M.; Romney, C.; Schuck, P.L.; Stewart, J.A. To Join or Not to Join: Decision Points Along the Pathway to Double-Strand Break Repair vs. Chromosome End Protection. Front. Cell Dev. Biol. 2021, 9, 708763. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, L.; Howard, S.M.; Cejka, P. Main steps in DNA double-strand break repair: An introduction to homologous recombination and related processes. Chromosoma 2018, 127, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Stinson, B.M.; Moreno, A.T.; Walter, J.C.; Loparo, J.L. A Mechanism to Minimize Errors during Non-homologous End Joining. Mol. Cell 2020, 77, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Prakash, R.; Zhang, Y.; Feng, W.; Jasin, M. Homologous recombination and human health: The roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb. Perspect. Biol. 2015, 7, a016600. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.N.; Kachnic, L.A. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene 2003, 22, 5784–5791. [Google Scholar] [CrossRef]
- Walsh, C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef]
- Snouwaert, J.N.; Gowen, L.C.; Latour, A.M.; Mohn, A.R.; Xiao, A.; DiBiase, L.; Koller, B.H. BRCA1 deficient embryonic stem cells display a decreased homologous recombination frequency and an increased frequency of non-homologous recombination that is corrected by expression of a BRCA1 transgene. Oncogene 1999, 18, 7900–7907. [Google Scholar] [CrossRef]
- Holloman, W.K. Unraveling the mechanism of BRCA2 in homologous recombination. Nat. Struct. Mol. Biol. 2011, 18, 748–754. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Functions of BRCA1 and BRCA2 in the biological response to DNA damage. J. Cell Sci. 2001, 114, 3591–3598. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. J. Am. Med. Assoc. 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated with BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef]
- Momozawa, Y.; Sasai, R.; Usui, Y.; Shiraishi, K.; Iwasaki, Y.; Taniyama, Y.; Parsons, M.T.; Mizukami, K.; Sekine, Y.; Hirata, M.; et al. Expansion of Cancer Risk Profile for BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2022, 8, 871–878. [Google Scholar] [CrossRef]
- Dullens, B.; de Putter, R.; Lambertini, M.; Toss, A.; Han, S.; Van Nieuwenhuysen, E.; Van Gorp, T.; Vanderstichele, A.; Van Ongeval, C.; Keupers, M.; et al. Cancer Surveillance in Healthy Carriers of Germline Pathogenic Variants in BRCA1/2: A Review of Secondary Prevention Guidelines. J. Oncol. 2020, 2020, 9873954. [Google Scholar] [CrossRef]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Bancroft, E.K.; Page, E.C.; Castro, E.; Lilja, H.; Vickers, A.; Sjoberg, D.; Assel, M.; Foster, C.S.; Mitchell, G.; Drew, K.; et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur. Urol. 2014, 66, 489–499. [Google Scholar] [CrossRef]
- Segal, N.; Ber, Y.; Benjaminov, O.; Tamir, S.; Yakimov, M.; Kedar, I.; Rosenbaum, E.; Sela, S.; Ozalvo, R.; Shavit-Grievink, L.; et al. Imaging-based prostate cancer screening among BRCA mutation carriers—Results from the first round of screening. Ann. Oncol. 2020, 31, 1545–1552. [Google Scholar] [CrossRef]
- Sun, P.; Li, Y.; Chao, X.; Li, J.; Luo, R.; Mei, L.; He, J. Clinical characteristics and prognostic implications of BRCA-associated tumors in males: A pan-tumor survey. BMC Cancer 2020, 20, 994. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Wakai, T.; Nagahashi, M.; Shimada, Y.; Hanyu, T.; Kanu, Y.; Muneoka, Y.; Ishikawa, T.; Takizawa, K.; Tajima, Y.; et al. Pathogenic germline BRCA1/2 mutations and familial predisposition to gastric cancer. JCO Precis. Oncol. 2018, 2, 18–00097. [Google Scholar]
- Moran, A.; O’Hara, C.; Khan, S.; Shack, L.; Woodward, E.; Maher, E.R.; Lalloo, F.; Evans, D.G. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam. Cancer 2012, 11, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, A.; Nej, K.; Huzarski, T.; Scott, R.J.; Lubinski, J. BRCA2 gene mutations in families with aggregations of breast and stomach cancers. Br. J. Cancer 2002, 87, 888–891. [Google Scholar] [CrossRef]
- Figer, A.; Irmin, L.; Geva, R.; Flex, D.; Sulkes, J.; Sulkes, A.; Friedman, E. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br. J. Cancer 2001, 84, 478–481. [Google Scholar] [CrossRef]
- Schlebusch, C.M.; Dreyer, G.; Sluiter, M.D.; Yawitch, T.M.; van den Berg, H.J.; van Rensburg, E.J. Cancer prevalence in 129 breast-ovarian cancer families tested for BRCA1 and BRCA2 mutations. S. Afr. Med. J. 2010, 100, 113–117. [Google Scholar] [CrossRef]
- Johannsson, O.; Loman, N.; Moller, T.; Kristoffersson, U.; Borg, A.; Olsson, H. Incidence of malignant tumours in relatives of BRCA1 and BRCA2 germline mutation carriers. Eur. J. Cancer 1999, 35, 1248–1257. [Google Scholar] [CrossRef]
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Sahasrabudhe, R.; Lott, P.; Bohorquez, M.; Toal, T.; Estrada, A.P.; Suarez, J.J.; Brea-Fernandez, A.; Cameselle-Teijeiro, J.; Pinto, C.; Ramos, I.; et al. Germline Mutations in PALB2, BRCA1, and RAD51C, Which Regulate DNA Recombination Repair, in Patients with Gastric Cancer. Gastroenterology 2017, 152, 983–986.e6. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Siu, H.C.; Leung, S.Y.; Stratton, M.R. A mutational signature in gastric cancer suggests therapeutic strategies. Nat. Commun. 2015, 6, 8683. [Google Scholar] [CrossRef]
- Ichikawa, H.; Nagahashi, M.; Shimada, Y.; Hanyu, T.; Ishikawa, T.; Kameyama, H.; Kobayashi, T.; Sakata, J.; Yabusaki, H.; Nakagawa, S.; et al. Actionable gene-based classification toward precision medicine in gastric cancer. Genome Med. 2017, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, J.; Li, X.; Li, J.; Zhou, L.; Qiu, T.; Zhang, M.; Liu, P. Prognostic significance of BRCA1 expression in gastric cancer. Med. Oncol. 2013, 30, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Liu, Y.J.; Yin, X.L.; Zhan, P.; Gu, Y.; Ni, X.Z. Loss of BRCA1 expression leads to worse survival in patients with gastric carcinoma. World J. Gastroenterol. 2013, 19, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Cho, H.J.; Kim, M.; Lee, K.H.; Kim, M.A.; Han, S.W.; Oh, D.Y.; Lee, H.J.; Im, S.A.; Kim, T.Y.; et al. Differing effects of adjuvant chemotherapy according to BRCA1 nuclear expression in gastric cancer. Cancer Chemother. Pharmacol. 2013, 71, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Zhao, C.M.; Huang, Y.; Wang, W.; Zhang, S.; Wang, X. BRCA1 and BRCA2 expression patterns and prognostic significance in digestive system cancers. Hum. Pathol. 2018, 71, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Pádua, J.D.B.; Mariano, C.F.A.; Fabro, A.T.; Tirapelli, D.P.D.C.; Sankarankutty, A.K.; Dos Santos, J.S.; Brunaldi, M.O. Prognostic Value of the Immunohistochemical Expression of RAD51 and BRCA2 in Gastric Adenocarcinoma. J. Histochem. Cytochem. 2022, 70, 199–210. [Google Scholar] [CrossRef]
- Moiseyenko, V.M.; Volkov, N.M.; Suspistin, E.N.; Yanus, G.A.; Iyevleva, A.G.; Kuligina, E.S.; Togo, A.V.; Kornilov, A.V.; Ivantsov, A.O.; Imyanitov, E.N. Evidence for predictive role of BRCA1 and bTUBIII in gastric cancer. Med. Oncol. 2013, 30, 545. [Google Scholar] [CrossRef]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.; Rosen, B.; Bradley, L.; Kwan, E.; Jack, E.; Vesprini, D.J.; Kuperstein, G.; Abrahamson, J.L.; et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 2001, 68, 700–710. [Google Scholar] [CrossRef]
- Brose, M.S.; Rebbeck, T.R.; Calzone, K.A.; Stopfer, J.E.; Nathanson, K.L.; Weber, B.L. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J. Natl. Cancer Inst. 2002, 94, 1365–1372. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.; Easton, D.F.; Bishop, D.T.; Narod, S.A.; Goldgar, D.E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994, 343, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Easton, D.F.; Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Mersch, J.; Jackson, M.A.; Park, M.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015, 121, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ławniczak, M.; Jakubowska, A.; Bialek, A.; Lubinski, J.; Jaworska-Bieniek, K.; Kaczmarek, K.; Starzynska, T. BRCA1 founder mutations do not contribute to increased risk of gastric cancer in the Polish population. Hered. Cancer Clin. Pract. 2016, 14, 3. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, Y.L.; Li, C.Y. BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies. Medicina 2021, 57, 905. [Google Scholar] [CrossRef]
- Tulinius, H.; Olafsdottir, G.H.; Sigvaldason, H.; Arason, A.; Barkardottir, R.B.; Eglisson, V.; Ogmundsdottir, H.M.; Tryggvadottir, L.; Gudlaugsdottir, S.; Eyfjord, J.E. The effect of a single BRCA2 mutation on cancer in Iceland. J. Med. Genet. 2002, 39, 457–462. [Google Scholar] [CrossRef]
- Jakubowska, A.; Scott, R.; Menkiszak, J.; Gronwald, J.; Byrski, T.; Huzarski, T.; Gorski, B.; Cybulski, C.; Debniak, T.; Kowalska, E.; et al. A high frequency of BRCA2 gene mutations in Polish families with ovarian and stomach cancer. Eur. J. Hum. Genet. 2003, 11, 955–958. [Google Scholar] [CrossRef]
- van Asperen, C.J.; Brohet, R.M.; Meijers-Heijboer, E.J.; Hoogerbrugge, N.; Verhoef, S.; Vasen, H.F.; Ausems, M.G.; Menko, F.H.; Gomez Garcia, E.B.; Klijn, J.G.; et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J. Med. Genet. 2005, 42, 711–719. [Google Scholar] [CrossRef]
- Bermejo, J.L.; Hemminki, K. Risk of cancer at sites other than the breast in Swedish families eligible for BRCA1 or BRCA2 mutation testing. Ann. Oncol. 2004, 15, 1834–1841. [Google Scholar] [CrossRef]
- Kim, H.; Choi, D.H.; Park, W.; Im, Y.H.; Ahn, J.S.; Park, Y.H.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.H.; et al. The association between non-breast and ovary cancers and BRCA mutation in first- and second-degree relatives of high-risk breast cancer patients: A large-scale study of Koreans. Hered. Cancer Clin. Pract. 2019, 17, 1. [Google Scholar] [CrossRef]
- Noh, J.M.; Choi, D.H.; Baek, H.; Nam, S.J.; Lee, J.E.; Kim, J.W.; Ki, C.S.; Park, W.; Huh, S.J. Associations between BRCA Mutations in High-Risk Breast Cancer Patients and Familial Cancers Other than Breast or Ovary. J. Breast Cancer 2012, 15, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar] [PubMed]
- Ansari, S.; Gantuya, B.; Tuan, V.P.; Yamaoka, Y. Diffuse gastric cancer: A summary of analogous contributing factors for its molecular pathogenicity. Int. J. Mol. Sci. 2018, 19, 2424. [Google Scholar] [CrossRef] [PubMed]
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Cancer Genome Research Atlas Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Tavakoli, A.; Monavari, S.H.; Mohammadi, F.S.; Kiani, S.J.; Armat, S.; Farahmand, M. Association between Epstein-Barr virus infection and gastric cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Hansford, S.; Kaurah, P.; Li-Chang, H.; Woo, M.; Senz, J.; Pinheiro, H.; Schrader, K.A.; Schaeffer, D.F.; Shumansky, K.; Zogopoulos, G.; et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015, 1, 23–32. [Google Scholar] [CrossRef]
- Lu, C.; Xie, M.; Wendl, M.C.; Wang, J.; McLellan, M.D.; Leiserson, M.D.; Huang, K.L.; Wyczalkowski, M.A.; Jayasinghe, R.; Banerjee, T.; et al. Patterns and functional implications of rare germline variants across 12 cancer types. Nat. Commun. 2015, 6, 10086. [Google Scholar] [CrossRef]
- Halpern, N.; Grinshpun, A.; Boursi, B.; Golan, T.; Margalit, O.; Aderka, D.; Friedman, E.; Laitman, Y.; Hubert, A.; Kadouri, L.; et al. Clinical Characteristics and Prognosis of Gastric Cancer Patients with BRCA 1/2 Germline Mutations: Report of Ten Cases and a Literature Review. Onco Targets Ther. 2020, 13, 11637–11644. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Gronwald, J.; Zuziak, D.; Cybulski, C.; Kladny, J.; Gorski, B.; Lubinski, J.; Narod, S.A. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 2009, 115, 359–363. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Schelman, W.R.; Wilding, G.; Moreno, V.; Baird, R.D.; Miranda, S.; Hylands, L.; Riisnaes, R.; Forster, M.; Omlin, A.; et al. The poly (ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2013, 14, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Balmaña, J.; Tung, N.M.; Isakoff, S.J.; Grana, B.; Ryan, P.D.; Saura, C.; Lowe, E.S.; Frewer, P.; Winer, E.; Baselga, J.; et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann. Oncol. 2014, 25, 1656–1663. [Google Scholar] [CrossRef]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Cadadei, S.; Nord, A.S.; et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly (ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Wubbenhorst, B.; Wenz, B.M.; De Sloover, D.; Pluta, J.; Emery, L.; Barrett, A.; Kraya, A.A.; Anastopoulos, I.N.; Yu, S.; et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat. Commun. 2017, 8, 319. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 1.2023—7 September 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed on 1 October 2022).
- National Comprehensive Cancer Network. Prostate Cancer Early Detection. Version 1.2022—16 February 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf (accessed on 1 October 2022).
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Long, J.M.; Ahmad, N.A.; Attalla, S.; Bradbury, A.R.; Carpenter, E.L.; Clark, D.F.; Constantino, G.; Das, K.K.; Domcheck, S.M.; et al. EUS-based Pancreatic Cancer Surveillance in BRCA1/BRCA2/PALB2/ATM Carriers without a Family History of Pancreatic Cancer. Cancer Prev. Res. 2021, 14, 1033–1040. [Google Scholar] [CrossRef]
- Wang, L.; Domchek, S.M.; Kochman, M.L.; Katona, B.W. Reaching beyond family history as inclusion criteria for pancreatic cancer surveillance in high-risk populations. Genes Cancer 2022, 13, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, M.S.; Calderwood, A.H.; Thosani, N.C.; Rebbeck, T.R.; Wani, S.; Canto, M.I.; Fishman, D.S.; Golan, T.; Hidalgo, M.; Kwon, R.S.; et al. ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Summary and recommendations. Gastrointest. Endosc. 2022, 95, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Ebrahimzadeh, J.; Stanich, P.P.; Katona, B.W. Endoscopic Surveillance in Patients with the Highest Risk of Gastric Cancer: Challenges and Solutions. Cancer Manag. Res. 2022, 14, 2953–2969. [Google Scholar] [CrossRef]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef] [PubMed]
- Coudert, M.; Drouet, Y.; Delhomelle, H.; Svrcek, M.; Benusiglio, P.R.; Coulet, F.; Clark, D.F.; Katona, B.W.; van Hest, L.P.; van der Kolk, L.E.; et al. First estimates of diffuse gastric cancer risks for carriers of CTNNA1 germline pathogenic variants. J. Med. Genet. 2022, 59, 1189–1195. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2022—8 June 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf (accessed on 1 October 2022).
- Foretová, L.; Navratilova, M.; Svoboda, M.; Grell, P.; Nemec, L.; Sirotek, L.; Obermannova, R.; Novotny, I.; Sachlova, M.; Fabian, P.; et al. GAPPS—Gastric Adenocarcinoma and Proximal Polyposis of the Stomach Syndrome in 8 Families Tested at Masaryk Memorial Cancer Institute—Prevention and Prophylactic Gastrectomies. Klin. Onkol. 2019, 32, 109–117. [Google Scholar] [CrossRef]
- Worthley, D.L.; Phillips, K.D.; Wayte, N.; Schrader, K.A.; Healey, S.; Kaurah, P.; Shulkes, A.; Grimpen, F.; Clouston, A.; Moore, D.; et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): A new autosomal dominant syndrome. Gut 2012, 61, 774–779. [Google Scholar] [CrossRef]
- Boland, C.R.; Idos, G.E.; Durno, C.; Giardiello, F.M.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gross, S.; Gupta, S.; Jacobson, B.C.; et al. Diagnosis and Management of Cancer Risk in the Gastrointestinal Hamartomatous Polyposis Syndromes: Recommendations from the US Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2022, 117, 846–864. [Google Scholar] [CrossRef]
| Author | Year | Population Location | Patient Population | Comparator Cohort | Risk Estimates | Gastric Cancer Risk Increased? |
|---|---|---|---|---|---|---|
| Gastric Cancer Risk in BRCA1 PV Carriers | ||||||
| Ford et al. [41] | 1994 | North America and Western Europe | 464 BRCA1 PV carriers | General Population | 1 observed case vs. 0.76 cases expected; RR 1.11, p > 0.05 | No |
| Johannsson et al. [27] | 1999 | Sweden | 1145 relatives from 29 families with a proband with a BRCA1 PV | General Population | All: SMR 2.76, 95% CI 1.01–6.00; F: SMR 5.16, 95% CI 1.14–13.22; M: SMR 1.43, 95% CI 0.17–5.15 | Yes |
| Risch et al. [38] | 2001 | Canada | 39 BRCA1 PV carriers and 291 FDRs | 4378 FDRs of ovarian cancer patients without BRCA1 or BRCA2 PVs | Incidence: 4.9% vs. 0.8%; RR 6.2, 95% CI 2.0–19 | Yes |
| Brose et al. [39] | 2002 | United States | 483 BRCA1 PV carriers | General Population | Age-adjusted lifetime risk: 5.5% vs. 0.8%, 95% CI 3.4–7.5% | Yes |
| Thompson et al. [42] | 2002 | North America and Western Europe | 2245 BRCA1 PV carriers | General Population | RR 1.56, 95% CI 0.91–2.68 | No |
| Schlebusch et al. [26] | 2010 | South Africa | 793 individuals from 26 families with a BRCA1 PV | General Population | 7 cases observed vs. 6.62 cases expected, p = 0.8829 | No |
| Moran et al. [23] | 2012 | England | 631 BRCA1 PV carriers and 1184 FDRs from 268 families with a BRCA1 PV | General Population | RR 2.4, 95% CI 1.2–4.3 | Yes |
| Mersch et al. [43] | 2014 | United States | 613 BRCA1 PV carriers | General Population | SIR 1.736, 95% CI 0.023–9.661 | No |
| Li et al. [15] | 2022 | Multinational (>10 countries) | 8884 BRCA1 PV carriers | General Population | RR 2.17, 95% CI 1.25–3.77 | Yes |
| BRCA1 PVs amongst gastric cancer patients | ||||||
| Ławniczak et al. [44] | 2016 | Poland | 317 patients with GC | 4570 controls | Mutation rate: 0.63% vs. 0.48%; OR 1.3, 95% CI 0.3–5.6 | No |
| Momozawa et al. [16] | 2022 | Japan | 10,705 cases of GC | 37,086 controls | OR 5.2, 95% CI 2.6–10.5 | Yes |
| Meta-analysis | ||||||
| Lee et al. [45] | 2021 | North America, Western Europe, South Africa | Meta-analysis, including 5 studies pertaining to BRCA1 PVs, all of which are cited in this sub-section of Table 1 [23,28,38,42,43] | Varied by study | BRCA1 PVs were not associated with increased risk of GC (RR 1.70, 95% CI 0.93–3.09) | No |
| Author | Year | Population Location | Patient Population | Comparator Cohort | Risk Estimates | Gastric Cancer Risk Increased? |
|---|---|---|---|---|---|---|
| Gastric Cancer Risk in BRCA2 PV Carriers | ||||||
| Breast Cancer Linkage Consortium [28] | 1999 | Europe and North America | 1152 confirmed or probable BRCA2 PV carriers from 173 families * | General Population | RR 2.59, 95% CI 1.46–4.61 | Yes |
| Johannsson et al. [27] | 1999 | Sweden | 728 relatives from 20 families with a proband with a BRCA2 PV | General Population | All: SMR 1.63, 95% CI 0.34–4.75; F: SMR 1.37, 95% CI 0.03–7.64; M: 1.79, 0.22–6.48 | No |
| Risch et al. [38] | 2001 | Canada | 21 BRCA2 PV carriers and 160 FDRs | 4378 FDRs of ovarian cancer patients without BRCA1 or BRCA2 PVs | Incidence: 1.8% vs. 0.80%; RR 2.3, 95% CI 0.30–18 | No |
| Tulinius et al. [46] | 2002 | Iceland | 90 families with a proband with a BRCA2 PV | General Population | F FDRs: RR 1.78, 95% CI 0.57–4.10; F SDRs: RR 3.08, 95% CI 2.09–4.34; M FDRs: RR 2.40, 95% CI 1.29–4.05; M SDRs: RR 1.91, 95% CI 1.33–2.63 | Yes |
| van Asperen et al. [48] | 2005 | Netherlands | 1811 individuals with a 50% probability of having a BRCA2 PV | General Population | RR 1.2, 95% CI 0.6–2.0 | No |
| Schlebusch et al. [26] | 2010 | South Africa | 1264 individuals from 43 families with a BRCA2 PV | General Population | 24 cases observed vs. 11.17 cases expected, p = 0.0001 | Yes |
| Moran et al. [23] | 2012 | England | 517 BRCA2 PV carriers and 1009 FDRs from 222 families with a BRCA2 PV | General Population | RR 2.7, 95% CI 1.3–4.8 | Yes |
| Mersch et al. [43] | 2014 | United States | 459 BRCA2 PV carriers | General Population | SIR 1.755, 95% CI 0.023–9.763 | No |
| Li et al. [15] | 2022 | Multinational (>10 countries) | 6095 BRCA2 PV carriers | General Population | RR 3.69, 95% CI 2.40–5.67 | Yes |
| BRCA2 PVs amongst gastric cancer patients | ||||||
| Figer et al. [25] | 2001 | Israel | 35 Ashkenazi Jewish patients with GC | General Ashkenazi Jewish Population | Mutation rate: 5.7% vs. 1.2%; OR 5.2, 95% CI 1.2–22 | Yes |
| Jakubowska et al. [24] | 2002 | Poland | 29 breast cancer patients from families with ≥1 female breast cancer diagnosed before age 50 and ≥1 male GC diagnosed before age 55 | 248 breast-ovarian cancer families | Mutation rate: 20.7% vs. 6.9%, p < 0.025 | Yes |
| Jakubowska et al. [47] | 2003 | Poland | 34 women with ovarian cancer and family history of GC | 75 women with ovarian cancer and family history of ovarian cancer but not GC | Mutation rate: 23.5% vs. 4.0%; OR 7.4, 95% CI 1.8–30 | Yes |
| Momozawa et al. [16] | 2022 | Japan | 10,705 cases of GC | 37,086 controls | OR 4.7, 95% CI 3.1–7.1 | Yes |
| Meta-analysis | ||||||
| Lee et al. [45] | 2021 | North America, Western Europe, South Africa | Meta-analysis, including 6 studies pertaining to BRCA2 PVs, all of which are cited in this sub-section of Table 2 [23,25,28,38,43,49] | Varied by study | BRCA2 PVs were associated with increased risk of GC (RR 2.15, 95% CI 1.98–2.33) | Yes |
| Author | Year | Population Location | Patient Population | Comparator Cohort | Risk Estimates | Gastric Cancer Risk Increased? |
|---|---|---|---|---|---|---|
| Gastric Cancer Risk in BRCA1/2 PV Carriers | ||||||
| Bermejo et al. [49] | 2004 | Sweden | 10,359 individuals from families with breast and ovarian cancer | General Population | Incidence: 1.88% (95% CI 1.05–3.12%) vs. 0.92% | Yes |
| Noh et al. [51] | 2012 | Korea | 49 BRCA1/2 PV carriers | 189 high-risk breast cancer patients without BRCA1/2 PVs | Proportional incidence of a family history of GC: 24.7% for comparator cohort vs. 20.5% for BRCA1/2 PV carriers; RR 0.947, 95% CI 0.822–1.091 | No |
| Kim et al. [50] | 2019 | Korea | 377 BRCA1/2 PV carriers | 2178 high-risk breast cancer patients without BRCA1/2 PVs | Proportional incidence of a family history of GC: 13.8% vs. 7.4%; OR 1.666, 95% CI 1.183–2.345 | Yes |
| Sun et al. [21] | 2020 | Multinational (>10 countries) | 294 male BRCA1/2 PV carriers with tumors | 4577 male patients with tumors who were not carriers of BRCA1/2 PVs | Proportional incidence of GC: 11.9% vs. 5.5%, p < 0.001 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckley, K.H.; Niccum, B.A.; Maxwell, K.N.; Katona, B.W. Gastric Cancer Risk and Pathogenesis in BRCA1 and BRCA2 Carriers. Cancers 2022, 14, 5953. https://doi.org/10.3390/cancers14235953
Buckley KH, Niccum BA, Maxwell KN, Katona BW. Gastric Cancer Risk and Pathogenesis in BRCA1 and BRCA2 Carriers. Cancers. 2022; 14(23):5953. https://doi.org/10.3390/cancers14235953
Chicago/Turabian StyleBuckley, Kole H., Blake A. Niccum, Kara N. Maxwell, and Bryson W. Katona. 2022. "Gastric Cancer Risk and Pathogenesis in BRCA1 and BRCA2 Carriers" Cancers 14, no. 23: 5953. https://doi.org/10.3390/cancers14235953
APA StyleBuckley, K. H., Niccum, B. A., Maxwell, K. N., & Katona, B. W. (2022). Gastric Cancer Risk and Pathogenesis in BRCA1 and BRCA2 Carriers. Cancers, 14(23), 5953. https://doi.org/10.3390/cancers14235953





