Hubbing the Cancer Cell
Abstract
Simple Summary
Abstract
1. Introduction
2. The Need for Communication between Compartments
2.1. The Interactions between Mitochondria and the Nucleus
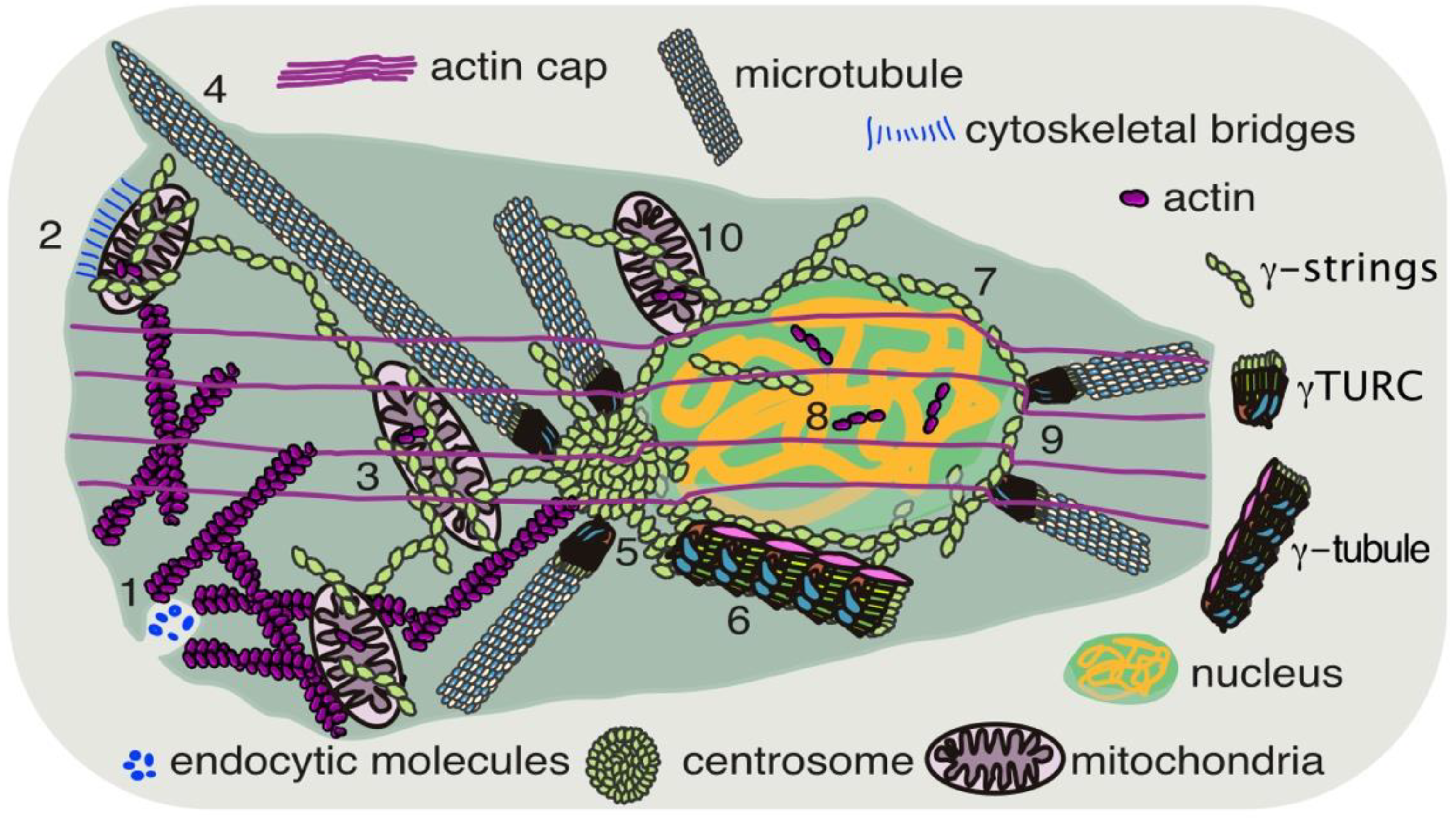
2.2. Cellular Skeleton
2.2.1. Actin
| Function | References | Meshwork |
|---|---|---|
| ATPase | [64] | actin |
| GTPase | [65] | microtubules, γ-tubulin 1 |
| Nuclear shape | [21,22,66,67,68] | actin, microtubules, γ-tubulin 1 |
| Nuclear formation | [61] | γ-tubulin 1 |
| Vesicle formation | [29] | actin |
| Vesicle trafficking | [30,69] | actin, microtubules |
| Location of organelles | [35,36,37,52,53,63,68] | actin, microtubules, γ-tubulin 1 |
| Mitochondrial shape | [49,63] | actin, γ-tubulin 1 |
| Transcriptional regulation | [43,44,46,48,63,70,71] | actin, γ-tubulin 1 |
| DNA replication | [70] | γ-tubulin 1 |
| Nucleation activity | [57,58,59,61] | γ-tubulin 1 |
| Membrane component | [61,63,70] | γ-tubulin 1 |
2.2.2. Microtubules
2.2.3. The γ-Tubulin Meshwork
3. In Cancer Cells
Cancer Progression
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| MO | mitochondrial outer membrane |
| MIM | mitochondrial inner membrane |
| Mt | mitochondria |
| DNA | deoxyribonucleic acid |
| TCA | tricarboxylic acid |
| RNA | ribonucleic acid |
| NIM | nuclear inner membrane |
| NOM | nuclear outer membrane |
| NPC | nuclear pore complex |
| ER | endoplasmic reticulum |
| F-actin | filament actin |
| LINC | linkers of nucleoskeleton and cytoskeleton |
| Drp1 | dynamin-related protein 1 |
| Mfn1 | mitofusin 1 |
| OPA1 | optic atrophy 1 |
| CK | creatinine kinase |
| PCr | phosphocreatine |
| VDAC | voltage-dependent anion channels |
| PCNA | proliferating cell nuclear antigen |
| SUN | Sad1 and UNC-84 |
| KASH | Klarsicht, ANC-1, Syne homology |
| γ-TuRC | γ-tubulin ring complex |
| E2F | E2 promoter binding factor |
| RB | retinoblastoma |
References
- Mankoff, D.A.; Eary, J.F.; Link, J.M.; Muzi, M.; Rajendran, J.G.; Spence, A.M.; Krohn, K.A. Tumor-Specific Positron Emission Tomography Imaging in Patients: [18F] Fluorodeoxyglucose and Beyond. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 3460–3469. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Harvey, A.J.; Kind, K.L.; Thompson, J.G. REDOX regulation of early embryo development. Reproduction 2002, 123, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate Metabolism in Human Lung Tumors. Cell 2017, 171, 358–371.e9. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.; Owen, O. Gluconeogenesis. In Metabolism Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2013; pp. 381–386. [Google Scholar] [CrossRef]
- Gustafsson, C.M.; Falkenberg, M.; Larsson, N.-G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Newton, A.C.; Bootman, M.D.; Scott, J.D. Second Messengers. Cold Spring Harb. Perspect. Biol. 2016, 8, a005926. [Google Scholar] [CrossRef]
- Frieden, B.R.; Gatenby, R.A. Signal transmission through elements of the cytoskeleton form an optimized information network in eukaryotic cells. Sci. Rep. 2019, 9, 6110. [Google Scholar] [CrossRef]
- De Duve, C. The origin of eukaryotes: A reappraisal. Nat. Rev. Genet. 2007, 8, 395–403. [Google Scholar] [CrossRef]
- Mannella, C.A. Consequences of Folding the Mitochondrial Inner Membrane. Front. Physiol. 2020, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Macara, I.G. Transport into and out of the Nucleus. Microbiol. Mol. Biol. Rev. 2001, 65, 570–594. [Google Scholar] [CrossRef]
- Kafkia, E.; Andres-Pons, A.; Ganter, K.; Seiler, M.; Smith, T.S.; Andrejeva, A.; Jouhten, P.; Pereira, F.; Franco, C.; Kuroshchenkova, A.; et al. Operation of a TCA cycle subnetwork in the mammalian nucleus. Sci. Adv. 2022, 8, eabq5206. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta 2014, 1841, 595–609. [Google Scholar] [CrossRef]
- Galmes, R.; Houcine, A.; van Vliet, A.R.; Agostinis, P.; Jackson, C.L.; Giordano, F. ORP5/ORP8 localize to endoplasmic reticulum–mitochondria contacts and are involved in mitochondrial function. EMBO Rep. 2016, 17, 800–810. [Google Scholar] [CrossRef]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER Tubules Mark Sites of Mitochondrial Division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nag, S.; Mason, A.B.; Barroso, M.M. Endosome–mitochondria interactions are modulated by iron release from transferrin. J. Cell Biol. 2016, 214, 831–845. [Google Scholar] [CrossRef]
- Desai, R.; East, D.A.; Hardy, L.; Faccenda, D.; Rigon, M.; Crosby, J.; Alvarez, M.S.; Singh, A.; Mainenti, M.; Hussey, L.K.; et al. Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci. Adv. 2020, 6, eabc9955. [Google Scholar] [CrossRef] [PubMed]
- Gilloteaux, J.; Kashouty, R.; Yono, N. The perinuclear space of pancreatic acinar cells and the synthetic pathway of zymogen in Scorpaena scrofa L.: Ultrastructural aspects. Tissue Cell 2008, 40, 7–20. [Google Scholar] [CrossRef]
- Kim, D.-H.; Khatau, S.B.; Feng, Y.; Walcott, S.; Sun, S.X.; Longmore, G.D.; Wirtz, D. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci. Rep. 2012, 2, 555. [Google Scholar] [CrossRef]
- Khatau, S.B.; Hale, C.M.; Stewart-Hutchinson, P.J.; Patel, M.S.; Stewart, C.L.; Searson, P.C.; Hodzic, D.; Wirtz, D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA 2009, 106, 19017–19022. [Google Scholar] [CrossRef] [PubMed]
- Khatau, S.B.; Kim, D.-H.; Hale, C.M.; Bloom, R.J.; Wirtz, D. The perinuclear actin cap in health and disease. Nucleus 2010, 1, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, I.; Tsukita, S.; Tsukita, S.; Sawai, T. Cleavage furrow isolated from newt eggs: Contraction, organization of the actin filaments, and protein components of the furrow. Proc. Natl. Acad. Sci. USA 1988, 85, 5966–5970. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-S.; Chen, Y.-J.; Chang, C.-L.; Lee, W.-R.; Liou, J. Cortical actin contributes to spatial organization of ER–PM junctions. Mol. Biol. Cell 2017, 28, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Palade, G.E. Studies on the endoplasmic reticulum: III. Its form and distribution in striated muscle cells. J. Biophys. Biochem. Cytol. 1957, 3, 269–300. [Google Scholar] [CrossRef]
- Chang, C.-L.; Liou, J. Phosphatidylinositol 4,5-Bisphosphate Homeostasis Regulated by Nir2 and Nir3 Proteins at Endoplasmic Reticulum-Plasma Membrane Junctions. J. Biol. Chem. 2015, 290, 14289–14301. [Google Scholar] [CrossRef]
- Chang, C.-L.; Hsieh, T.-S.; Yang, T.T.; Rothberg, K.G.; Azizoglu, D.B.; Volk, E.; Liao, J.-C.; Liou, J. Feedback Regulation of Receptor-Induced Ca2+ Signaling Mediated by E-Syt1 and Nir2 at Endoplasmic Reticulum-Plasma Membrane Junctions. Cell Rep. 2013, 5, 813–825. [Google Scholar] [CrossRef]
- Akamatsu, M.; Vasan, R.; Serwas, D.; Ferrin, M.A.; Rangamani, P.; Drubin, D.G. Principles of self-organization and load adaptation by the actin cytoskeleton during clathrin-mediated endocytosis. eLife 2020, 9, e49840. [Google Scholar] [CrossRef]
- Schuh, M. An actin-dependent mechanism for long-range vesicle transport. Nat. Cell Biol. 2011, 13, 1431–1436. [Google Scholar] [CrossRef]
- Trevisan, T.; Pendin, D.; Montagna, A.; Bova, S.; Ghelli, A.M.; Daga, A. Manipulation of Mitochondria Dynamics Reveals Separate Roles for Form and Function in Mitochondria Distribution. Cell Rep. 2018, 23, 1742–1753. [Google Scholar] [CrossRef]
- Moore, A.S.; Wong, Y.C.; Simpson, C.L.; Holzbaur, E.L.F. Dynamic actin cycling through mitochondrial subpopulations locally regulates the fission–fusion balance within mitochondrial networks. Nat. Commun. 2016, 7, 12886. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.J.; Boucher, E.; Mandato, C.A. Mitochondria-cytoskeleton associations in mammalian cytokinesis. Cell Div. 2016, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca Balderas, P. Mitochondria–plasma membrane interactions and communication. J. Biol. Chem. 2021, 297, 101164. [Google Scholar] [CrossRef] [PubMed]
- Senning, E.N.; Marcus, A.H. Actin polymerization driven mitochondrial transport in mating S. cerevisiae. Proc. Natl. Acad. Sci. USA 2009, 107, 721–725. [Google Scholar] [CrossRef]
- Basu, H.; Pekkurnaz, G.; Falk, J.; Wei, W.; Chin, M.; Steen, J.; Schwarz, T.L. FHL2 anchors mitochondria to actin and adapts mitochondrial dynamics to glucose supply. J. Cell Biol. 2021, 220, e201912077. [Google Scholar] [CrossRef]
- Gutnick, A.; Banghart, M.R.; West, E.R.; Schwarz, T.L. The light-sensitive dimerizer zapalog reveals distinct modes of immobilization for axonal mitochondria. Nat. Cell Biol. 2019, 21, 768–777. [Google Scholar] [CrossRef]
- Han, S.M.; Baig, H.S.; Hammarlund, M. Mitochondria Localize to Injured Axons to Support Regeneration. Neuron 2016, 92, 1308–1323. [Google Scholar] [CrossRef]
- Ellington, W.R. Evolution and Physiological Roles of Phosphagen Systems. Annu. Rev. Physiol. 2001, 63, 289–325. [Google Scholar] [CrossRef]
- Arnold, H.; Pette, D. Binding of Glycolytic Enzymes to Structure Proteins of the Muscle. Eur. J. Biochem. 1968, 6, 163–171. [Google Scholar] [CrossRef]
- Gellerich, F.N.; Wagner, M.; Kapischke, M.; Wicker, U.; Brdiczka, D. Effect of macromolecules on the regulation of the mitochondrial outer membrane pore and the activity of adenylate kinase in the inter-membrane space. Biochim. Biophys. Acta 1993, 1142, 217–227. [Google Scholar] [CrossRef]
- Roman, I.; Figys, J.; Steurs, G.; Zizi, M. Direct measurement of VDAC–actin interaction by surface plasmon resonance. Biochim. Biophys. Acta 2006, 1758, 479–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bohnsack, M.T.; Stüven, T.; Kuhn, C.; Cordes, V.C.; Görlich, D. A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes. Nat. Cell Biol. 2006, 8, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Dopie, J.; Skarp, K.-P.; Rajakylä, E.K.; Tanhuanpää, K.; Vartiainen, M.K. Active maintenance of nuclear actin by importin 9 supports transcription. Proc. Natl. Acad. Sci. USA 2012, 109, E544–E552. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Li, W.Y.; Hatano, Y.; Takasu, A.; Sakamoto, Y.; Yamamoto, M.; Ikeda, Z.; Shindo, T.; Plessner, M.; Morita, K.; et al. Zygotic Nuclear F-Actin Safeguards Embryonic Development. Cell Rep. 2020, 31, 107824. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Thuraisingam, T.; de Lima Morais, D.A.; Rola-Pleszczynski, M.; Radzioch, D. Nuclear Translocation of β-Actin Is Involved in Transcriptional Regulation during Macrophage Differentiation of HL-60 Cells. Mol. Biol. Cell 2010, 21, 811–820. [Google Scholar] [CrossRef]
- Xie, X.; Jankauskas, R.; Mazari, A.M.A.; Drou, N.; Percipalle, P. β-actin regulates a heterochromatin landscape essential for optimal induction of neuronal programs during direct reprograming. PLoS Genet. 2018, 14, e1007846. [Google Scholar] [CrossRef]
- Le, H.Q.; Ghatak, S.; Yeung, C.-Y.C.; Tellkamp, F.; Günschmann, C.; Dieterich, C.; Yeroslaviz, A.; Habermann, B.; Pombo, A.; Niessen, C.M.; et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 2016, 18, 864–875. [Google Scholar] [CrossRef]
- Xie, X.; Venit, T.; Drou, N.; Percipalle, P. In Mitochondria β-Actin Regulates mtDNA Transcription and Is Required for Mitochondrial Quality Control. iScience 2018, 3, 226–237. [Google Scholar] [CrossRef]
- Reyes, A.; He, J.; Mao, C.C.; Bailey, L.J.; Di Re, M.; Sembongi, H.; Kazak, L.; Dzionek, K.; Holmes, J.B.; Cluett, T.J.; et al. Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Res. 2011, 39, 5098–5108. [Google Scholar] [CrossRef]
- Schroer, T.A.; Steuer, E.R.; Sheetz, M.P. Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell 1989, 56, 937–946. [Google Scholar] [CrossRef]
- Vale, R.D.; Schnapp, B.J.; Mitchison, T.; Steuer, E.; Reese, T.S.; Sheetz, M.P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell 1985, 43, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.-H.; Xiao, H.; Wu, J.-M.; He, K.-M.; Lv, Z.-Z.; Li, Z.-J.; Xu, M.; Zhang, Y.-Y. Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis. 2018, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Hollenbeck, P. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 1993, 104, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Laan, L.; Husson, J.; Munteanu, E.L.; Kerssemakers, J.W.J.; Dogterom, M. Force-generation and dynamic instability of microtubule bundles. Proc. Natl. Acad. Sci. USA 2008, 105, 8920–8925. [Google Scholar] [CrossRef]
- Inoué, S.; Salmon, E.D. Force Generation by Microtubule Assembly/Disassembly in Mitosis and Related Movements. Mol. Biol. Cell 1995, 6, 1619–1640. [Google Scholar] [CrossRef]
- Farina, F.; Gaillard, J.; Guérin, C.; Couté, Y.; Sillibourne, J.; Blanchoin, L.; Théry, M. The centrosome is an actin-organizing centre. Nat. Cell Biol. 2016, 18, 65–75. [Google Scholar] [CrossRef]
- Kollman, J.M.; Merdes, A.; Mourey, L.; Agard, D.A. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 2011, 12, 709–721. [Google Scholar] [CrossRef]
- Lindström, L.; Alvarado-Kristensson, M. Characterization of gamma-tubulin filaments in mammalian cells. Biochim. Biophys. Acta 2018, 1865, 158–171. [Google Scholar] [CrossRef]
- Alvarado-Kristensson, M. γ-tubulin as a signal-transducing molecule and meshwork with therapeutic potential. Signal Transduct. Target. Ther. 2018, 3, 24. [Google Scholar] [CrossRef]
- Rosselló, C.A.; Lindström, L.; Glindre, J.; Eklund, G.; Alvarado-Kristensson, M. Gamma-tubulin coordinates nuclear envelope assembly around chromatin. Heliyon 2016, 2, e00166. [Google Scholar] [CrossRef]
- Rosselló, C.A.; Lindström, L.; Eklund, G.; Corvaisier, M.; Kristensson, M.A. γ-Tubulin–γ-Tubulin Interactions as the Basis for the Formation of a Meshwork. Int. J. Mol. Sci. 2018, 19, 3245. [Google Scholar] [CrossRef] [PubMed]
- Lindström, L.; Li, T.; Malycheva, D.; Kancharla, A.; Nilsson, H.; Vishnu, N.; Mulder, H.; Johansson, M.; Rosselló, C.A.; Alvarado-Kristensson, M. The GTPase domain of gamma-tubulin is required for normal mitochondrial function and spatial organization. Commun. Biol. 2018, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Frieden, C.; Patane, K. Differences in G-actin containing bound ATP or ADP: The magnesium-induced conformational change requires ATP. Biochemistry 1985, 24, 4192–4196. [Google Scholar] [CrossRef] [PubMed]
- Kristensson, M. The Game of Tubulins. Cells 2021, 10, 745. [Google Scholar] [CrossRef]
- Tariq, Z.; Zhang, H.; Chia-Liu, A.; Shen, Y.; Gete, Y.; Xiong, Z.-M.; Tocheny, C.; Campanello, L.; Wu, D.; Losert, W.; et al. Lamin A and microtubules collaborate to maintain nuclear morphology. Nucleus 2017, 8, 433–446. [Google Scholar] [CrossRef]
- Paonessa, F.; Evans, L.D.; Solanki, R.; Larrieu, D.; Wray, S.; Hardy, J.; Jackson, S.P.; Livesey, F.J. Microtubules Deform the Nuclear Membrane and Disrupt Nucleocytoplasmic Transport in Tau-Mediated Frontotemporal Dementia. Cell Rep. 2019, 26, 582–593.e5. [Google Scholar] [CrossRef]
- Zhao, T.; Graham, O.S.; Raposo, A.; Johnston, D.S. Growing Microtubules Push the Oocyte Nucleus to Polarize the Drosophila Dorsal-Ventral Axis. Science 2012, 336, 999–1003. [Google Scholar] [CrossRef]
- Fourriere, L.; Jimenez, A.J.; Perez, F.; Boncompain, G. The role of microtubules in secretory protein transport. J. Cell Sci. 2020, 133, jcs237016. [Google Scholar] [CrossRef]
- Corvaisier, M.; Zhou, J.; Malycheva, D.; Cornella, N.; Chioureas, D.; Gustafsson, N.M.S.; Rosselló, C.A.; Ayora, S.; Li, T.; Ekström-Holka, K.; et al. The γ-tubulin meshwork assists in the recruitment of PCNA to chromatin in mammalian cells. Commun. Biol. 2021, 4, 767. [Google Scholar] [CrossRef]
- Höög, G.; Zarrizi, R.; von Stedingk, K.; Jonsson, K.; Alvarado-Kristensson, M. Nuclear localization of γ-tubulin affects E2F transcriptional activity and S-phase progression. FASEB J. 2011, 25, 3815–3827. [Google Scholar] [CrossRef]
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic Nuclear Envelope Collapse in Cancer Cell Micronuclei. Cell 2013, 154, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Karlseder, J. DNA damage associated with mitosis and cytokinesis failure. Oncogene 2013, 32, 4593–4601. [Google Scholar] [CrossRef] [PubMed]
- Beaudouin, J.; Gerlich, D.; Daigle, N.; Eils, R.; Ellenberg, J. Nuclear Envelope Breakdown Proceeds by Microtubule-Induced Tearing of the Lamina. Cell 2002, 108, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guan, T.; Gerace, L. Integral Membrane Proteins of the Nuclear Envelope Are Dispersed throughout the Endoplasmic Reticulum during Mitosis. J. Cell Biol. 1997, 137, 1199–1210. [Google Scholar] [CrossRef]
- Alvarado-Kristensson, M.; Rosselló, C.A. The Biology of the Nuclear Envelope and Its Implications in Cancer Biology. Int. J. Mol. Sci. 2019, 20, 2586. [Google Scholar] [CrossRef]
- Lu, L.; Ladinsky, M.S.; Kirchhausen, T. Cisternal Organization of the Endoplasmic Reticulum during Mitosis. Mol. Biol. Cell 2009, 20, 3471–3480. [Google Scholar] [CrossRef]
- Wilson, E.B. The Distribution of the Chondriosomes to the Spermatozoa in Scorpions. Proc. Natl. Acad. Sci. USA 1916, 2, 321–324. [Google Scholar] [CrossRef]
- Xue, J.; Woo, E.M.; Postow, L.; Chait, B.T.; Funabiki, H. Chromatin-Bound Xenopus Dppa2 Shapes the Nucleus by Locally Inhibiting Microtubule Assembly. Dev. Cell 2013, 27, 47–59. [Google Scholar] [CrossRef]
- Hamaguchi, Y. Microinjection of Colchicine into Sea Urchin Eggs. Dev. Growth Differ. 1975, 17, 111–117. [Google Scholar] [CrossRef]
- Wheatley, S.P. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J. Cell Biol. 1996, 135, 981–989. [Google Scholar] [CrossRef]
- Canman, J.C.; Hoffman, D.B.; Salmon, E. The role of pre- and post-anaphase microtubules in the cytokinesis phase of the cell cycle. Curr. Biol. 2000, 10, 611–614. [Google Scholar] [CrossRef]
- Danilchik, M.; Funk, W.C.; Brown, E.; Larkin, K. Requirement for Microtubules in New Membrane Formation during Cytokinesis of Xenopus Embryos. Dev. Biol. 1998, 194, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; Tapia-Monsalves, C.; Vergara, E.H.; Pérez, M.J.; Aranguiz, A. Truncated Tau Induces Mitochondrial Transport Failure through the Impairment of TRAK2 Protein and Bioenergetics Decline in Neuronal Cells. Front. Cell. Neurosci. 2020, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Chacko, L.A.; Chug, M.K.; Jhunjhunwala, S.; Ananthanarayanan, V. Association of mitochondria with microtubules inhibits mitochondrial fission by precluding assembly of the fission protein Dnm1. J. Biol. Chem. 2019, 294, 3385–3396. [Google Scholar] [CrossRef] [PubMed]
- Kaldis, P.; Kamp, G.; Piendl, T.; Wallimann, T. Functions of Creatine Kinase Isoenzymes in Spermatozoa. Adv. Dev. Biol. 1997, 5, 275–312. [Google Scholar] [CrossRef]
- Koons, S.J.; Eckert, B.S.; Zobel, C. Immunofluorescence and inhibitor studies on creatine kinase and mitosis. Exp. Cell Res. 1982, 140, 401–409. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Gurnev, P.A.; Hoogerheide, D.P.; Rovini, A.; Sirajuddin, M.; Bezrukov, S.M. Sequence diversity of tubulin isotypes in regulation of the mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2018, 293, 10949–10962. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar] [CrossRef]
- Levine, M.S.; Bakker, B.; Boeckx, B.; Moyett, J.; Lu, J.; Vitre, B.; Spierings, D.C.; Lansdorp, P.M.; Cleveland, D.W.; Lambrechts, D.; et al. Centrosome Amplification Is Sufficient to Promote Spontaneous Tumorigenesis in Mammals. Dev. Cell 2017, 40, 313–322.e5. [Google Scholar] [CrossRef]
- Lingle, W.L.; Barrett, S.L.; Negron, V.C.; D’Assoro, A.B.; Boeneman, K.; Liu, W.; Whitehead, C.M.; Reynolds, C.; Salisbury, J.L. Centrosome amplification drives chromosomal instability in breast tumor development. Proc. Natl. Acad. Sci. USA 2002, 99, 1978–1983. [Google Scholar] [CrossRef]
- Kerketta, L.S.; Ghosh, K.; Nadkarni, A.; Madkaikar, M.; Vundinti, B.R. Centrosome Aberration Frequency and Disease Association in B-Acute Lymphoblastic Leukemia. In Vivo 2017, 31, 215–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, Y.; Matsuyama, H.; Furuya, T.; Oga, A.; Yoshihiro, S.; Okuda, M.; Kawauchi, S.; Sasaki, K.; Naito, K. Centrosome Hyperamplification Predicts Progression and Tumor Recurrence in Bladder Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 6449–6455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, C.K.; Jung, J.H.; Lee, K.Y.; Kang, C.S.; Kim, M.; Ko, Y.H.; Oh, C.S. Centrosome abnormalities in non-small cell lung cancer: Correlations with DNA aneuploidy and expression of cell cycle regulatory proteins. Pathol.-Res. Pract. 2007, 203, 839–847. [Google Scholar] [CrossRef] [PubMed]
- LoMastro, G.M.; Holland, A.J. The Emerging Link between Centrosome Aberrations and Metastasis. Dev. Cell 2019, 49, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Trevor, K.; McGuire, J.; Leonova, E. Association of vimentin intermediate filaments with the centrosome. J. Cell Sci. 1995, 108, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Piel, M.; Nordberg, J.; Euteneuer, U.; Bornens, M. Centrosome-Dependent Exit of Cytokinesis in Animal Cells. Science 2001, 291, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Kristensson, M. Choreography of the centrosome. Heliyon 2020, 6, e03238. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, K.; Yuan, X.; Wu, X.; Zhuang, Y.; Xu, T.; Xu, R.; Han, M. SUN1/2 and Syne/Nesprin-1/2 Complexes Connect Centrosome to the Nucleus during Neurogenesis and Neuronal Migration in Mice. Neuron 2009, 64, 173–187. [Google Scholar] [CrossRef]
- Meyerzon, M.; Fridolfsson, H.N.; Ly, N.; McNally, F.J.; Starr, D.A. UNC-83 is a nuclear-specific cargo adaptor for kinesin-1-mediated nuclear migration. Development 2009, 136, 2725–2733. [Google Scholar] [CrossRef]
- Puckelwartz, M.J.; Kessler, E.; Zhang, Y.; Hodzic, D.; Randles, K.N.; Morris, G.; Earley, J.U.; Hadhazy, M.; Holaska, J.M.; Mewborn, S.K.; et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum. Mol. Genet. 2009, 18, 607–620. [Google Scholar] [CrossRef]
- Starr, D.A.; Han, M. Role of ANC-1 in Tethering Nuclei to the Actin Cytoskeleton. Science 2002, 298, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Santino, D.; Tikhonenko, I.; Magidson, V.; Khodjakov, A.; Koonce, M.P. Rules of engagement: Centrosome–nuclear connections in a closed mitotic system. Biol. Open 2012, 1, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Koonce, M.P.; Köhler, J.; Neujahr, R.; Schwartz, J.; Tikhonenko, I.; Gerisch, G. Dynein motor regulation stabilizes interphase microtubule arrays and determines centrosome position. EMBO J. 1999, 18, 6786–6792. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zheng, Y. Lamins position the nuclear pores and centrosomes by modulating dynein. Mol. Biol. Cell 2015, 26, 3379–3389. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Gaillard, J.; Vianay, B.; Guerin, C.; Orhant-Prioux, M.; Blanchoin, L.; Théry, M. Actin network architecture can ensure robust centering or sensitive decentering of the centrosome. EMBO J. 2022, 41, e111631. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Hyman, A.A.; Beyer, A. Coiled-Coil Proteins Facilitated the Functional Expansion of the Centrosome. PLoS Comput. Biol. 2014, 10, e1003657. [Google Scholar] [CrossRef]
- Alvarado-Kristensson, M. A simple and fast method for fixation of cultured cell lines that preserves cellular structures containing gamma-tubulin. MethodsX 2018, 5, 227–233. [Google Scholar] [CrossRef]
- Dictenberg, J.B.; Zimmerman, W.; Sparks, C.A.; Young, A.; Vidair, C.; Zheng, Y.; Carrington, W.; Fay, F.S.; Doxsey, S.J. Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 1998, 141, 163–174. [Google Scholar] [CrossRef]
- Chumová, J.; Trögelová, L.; Kourová, H.; Volc, J.; Sulimenko, V.; Halada, P.; Kučera, O.; Benada, O.; Kuchařová, A.; Klebanovych, A.; et al. γ-Tubulin has a conserved intrinsic property of self-polymerization into double stranded filaments and fibrillar networks. Biochim. Biophys. Acta 2018, 1865, 734–748. [Google Scholar] [CrossRef]
- Lesca, C.; Germanier, M.; Raynaud-Messina, B.; Pichereaux, C.; Etievant, C.; Emond, S.; Burlet-Schiltz, O.; Monsarrat, B.; Wright, M.; Defais, M. DNA damage induce γ-tubulin-RAD51 nuclear complexes in mammalian cells. Oncogene 2005, 24, 5165–5172. [Google Scholar] [CrossRef]
- Ríos, R.M.; Sanchís, A.; Tassin, A.M.; Fedriani, C.; Bornens, M. GMAP-210 Recruits γ-Tubulin Complexes to cis-Golgi Membranes and Is Required for Golgi Ribbon Formation. Cell 2004, 118, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Pouchucq, L.; Lobos-Ruiz, P.; Araya, G.; Valpuesta, J.M.; Monasterio, O. The chaperonin CCT promotes the formation of fibrillar aggregates of γ-tubulin. Biochim. Biophys. Acta 2018, 1866, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Kristensson, M.; Rodríguez, M.J.; Silió, V.; Valpuesta, J.M.; Carrera, A.C. SADB phosphorylation of γ-tubulin regulates centrosome duplication. Nat. Cell Biol. 2009, 11, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Carrera, A.C.; Alvarado-Kristensson, M. SADB kinases license centrosome replication. Cell Cycle 2009, 8, 4005–4006. [Google Scholar] [CrossRef]
- Eklund, G.; Lang, S.; Glindre, J.; Ehlén, Å.; Alvarado-Kristensson, M. The Nuclear Localization of γ-Tubulin Is Regulated by SadB-mediated Phosphorylation. J. Biol. Chem. 2014, 289, 21360–21373. [Google Scholar] [CrossRef] [PubMed]
- Ehlén, Å.; Rosselló, C.A.; von Stedingk, K.; Höög, G.; Nilsson, E.; Pettersson, H.M.; Jirström, K.; Alvarado-Kristensson, M. Tumors with Nonfunctional Retinoblastoma Protein Are Killed by Reduced γ-Tubulin Levels. J. Biol. Chem. 2012, 287, 17241–17247. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Koch, B.; Walczak, R.; Ciray-Duygu, F.; González-Sánchez, J.C.; Devos, D.; Mattaj, I.; Gruss, O.J. The nucleoporin MEL-28 promotes RanGTP-dependent γ-tubulin recruitment and microtubule nucleation in mitotic spindle formation. Nat. Commun. 2014, 5, 3270. [Google Scholar] [CrossRef]
- Dráberová, E.; Sulimenko, V.; Vinopal, S.; Sulimenko, T.; Sládková, V.; D’Agostino, L.; Sobol, M.; Hozák, P.; Křen, L.; Katsetos, C.D.; et al. Differential expression of human γ-tubulin isotypes during neuronal development and oxidative stress points to a γ-tubulin-2 prosurvival function. FASEB J. 2017, 31, 1828–1846. [Google Scholar] [CrossRef]
- Zheng, L.; Cardaci, S.; Jerby, L.; MacKenzie, E.D.; Sciacovelli, M.; Johnson, T.I.; Gaude, E.; King, A.; Leach, J.D.G.; Edrada-Ebel, R.; et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 2015, 6, 6001. [Google Scholar] [CrossRef]
- Semaan, A.; Munkarah, A.R.; Arabi, H.; Bandyopadhyay, S.; Seward, S.; Kumar, S.; Qazi, A.; Hussein, Y.; Morris, R.T.; Ali-Fehmi, R. Expression of GLUT-1 in epithelial ovarian carcinoma: Correlation with tumor cell proliferation, angiogenesis, survival and ability to predict optimal cytoreduction. Gynecol. Oncol. 2011, 121, 181–186. [Google Scholar] [CrossRef]
- Bauer, D.E.; Hatzivassiliou, G.; Zhao, F.; Andreadis, C.; Thompson, C.B. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 2005, 24, 6314–6322. [Google Scholar] [CrossRef] [PubMed]
- Hatzivassiliou, G.; Zhao, F.; Bauer, D.E.; Andreadis, C.; Shaw, A.N.; Dhanak, D.; Hingorani, S.R.; Tuveson, D.A.; Thompson, C.B. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 2005, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Pizer, E.S.; Wood, F.D.; Heine, H.S.; Romantsev, F.E.; Pasternack, G.R.; Kuhajda, F.P. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996, 56, 1189–1193. [Google Scholar] [PubMed]
- Davidson, S.M.; Papagiannakopoulos, T.; Olenchock, B.A.; Heyman, J.E.; Keibler, M.A.; Luengo, A.; Bauer, M.R.; Jha, A.K.; O’Brien, J.P.; Pierce, K.A.; et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016, 23, 517–528. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Arismendi-Morillo, G. Electron microscopy morphology of the mitochondrial network in human cancer. Int. J. Biochem. Cell Biol. 2009, 41, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wu, Q.; Horbinski, C.M.; Flavahan, W.A.; Yang, K.; Zhou, W.; Dombrowski, S.M.; Huang, Z.; Fang, X.; Shi, Y.; et al. Mitochondrial control by DRP1 in brain tumor initiating cells. Nat. Neurosci. 2015, 18, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Kashatus, J.A.; Nascimento, A.; Myers, L.J.; Sher, A.; Byrne, F.L.; Hoehn, K.L.; Counter, C.M.; Kashatus, D.F. Erk2 Phosphorylation of Drp1 Promotes Mitochondrial Fission and MAPK-Driven Tumor Growth. Mol. Cell 2015, 57, 537–551. [Google Scholar] [CrossRef]
- Serasinghe, M.N.; Wieder, S.Y.; Renault, T.; Elkholi, R.; Asciolla, J.J.; Yao, J.L.; Jabado, O.; Hoehn, K.; Kageyama, Y.; Sesaki, H.; et al. Mitochondrial Division Is Requisite to RAS-Induced Transformation and Targeted by Oncogenic MAPK Pathway Inhibitors. Mol. Cell 2015, 57, 521–536. [Google Scholar] [CrossRef]
- Yang, M.; Liu, S.; Xiong, Y.; Zhao, J.; Deng, W. An integrative pan-cancer analysis of molecular characteristics and oncogenic role of mitochondrial creatine kinase 1A (CKMT1A) in human tumors. Sci. Rep. 2022, 12, 10025. [Google Scholar] [CrossRef]
- Corvaisier, M.; Alvarado-Kristensson, M. Non-Canonical Functions of the Gamma-Tubulin Meshwork in the Regulation of the Nuclear Architecture. Cancers 2020, 12, 3102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Yu, M.; Xie, Y.; Huang, Y.; Wolff, D.W.; Abel, P.W.; Tu, Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 2013, 32, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ye, F.; Jiang, X.; Guo, H.; Xie, W.; Zhang, Y.; Sheng, X. Drp1 mediates high glucose-induced mitochondrial dysfunction and epithelial-mesenchymal transition in endometrial cancer cells. Exp. Cell Res. 2020, 389, 111880. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.G.; Wikman, H.; Pantel, K.; Haigis, M.C.; De Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef]
- Garcin, C.; Straube, A. Microtubules in cell migration. Essays Biochem. 2019, 63, 509–520. [Google Scholar] [CrossRef]
- Kriebel, P.W.; Barr, V.A.; Rericha, E.C.; Zhang, G.; Parent, C.A. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J. Cell Biol. 2008, 183, 949–961. [Google Scholar] [CrossRef]
- Gomes, E.R.; Jani, S.; Gundersen, G.G. Nuclear Movement Regulated by Cdc42, MRCK, Myosin, and Actin Flow Establishes MTOC Polarization in Migrating Cells. Cell 2005, 121, 451–463. [Google Scholar] [CrossRef]
- Barnhart, E.L.; Allen, G.M.; Jülicher, F.; Theriot, J.A. Bipedal Locomotion in Crawling Cells. Biophys. J. 2010, 98, 933–942. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Corvaisier, M.; Malycheva, D.; Alvarado-Kristensson, M. Hubbing the Cancer Cell. Cancers 2022, 14, 5924. https://doi.org/10.3390/cancers14235924
Zhou J, Corvaisier M, Malycheva D, Alvarado-Kristensson M. Hubbing the Cancer Cell. Cancers. 2022; 14(23):5924. https://doi.org/10.3390/cancers14235924
Chicago/Turabian StyleZhou, Jingkai, Matthieu Corvaisier, Darina Malycheva, and Maria Alvarado-Kristensson. 2022. "Hubbing the Cancer Cell" Cancers 14, no. 23: 5924. https://doi.org/10.3390/cancers14235924
APA StyleZhou, J., Corvaisier, M., Malycheva, D., & Alvarado-Kristensson, M. (2022). Hubbing the Cancer Cell. Cancers, 14(23), 5924. https://doi.org/10.3390/cancers14235924






