Use of Selective Serotonin Reuptake Inhibitors Is Associated with a Lower Risk of Colorectal Cancer among People with Family History
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Assessment of Selective Serotonin Reuptake Inhibitors Use
2.3. Assessment of Outcome
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Boyle, P.; Langman, J.S. ABC of colorectal cancer: Epidemiology. BMJ 2000, 321, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, S.; Higurashi, T.; Komiya, Y.; Arimoto, J.; Horita, N.; Kaneko, T.; Iwasaki, M.; Nakagama, H.; Nakajima, A. Chemoprevention of colorectal cancer: Past, present, and future. Cancer Sci. 2019, 110, 3018–3026. [Google Scholar] [CrossRef]
- Zhang, N.; Sundquist, J.; Sundquist, K.; Zhang, Z.G.; Ji, J. Combined Use of Aspirin and Selective Serotonin Reuptake Inhibitors Is Associated with Lower Risk of Colorectal Cancer: A Nested Case-Control Study. Am. J. Gastroenterol. 2021, 116, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Coogan, P.F.; Strom, B.L.; Rosenberg, L. Antidepressant use and colorectal cancer risk. Pharmacoepidemiol. Drug Saf. 2009, 18, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Huang, C.W.; Chen, Y.L.; Yang, Y.H.; Chen, V.C. Association between selective serotonin reuptake inhibitors and kidney cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2021, 148, 1331–1337. [Google Scholar] [CrossRef]
- Chan, H.L.; Chiu, W.C.; Chen, V.C.; Huang, K.Y.; Wang, T.N.; Lee, Y.; McIntyre, R.S.; Hsu, T.C.; Lee, C.T.; Tzang, B.S. SSRIs associated with decreased risk of hepatocellular carcinoma: A population-based case-control study. Psychooncology 2018, 27, 187–192. [Google Scholar] [CrossRef]
- Morch, L.S.; Dehlendorff, C.; Baandrup, L.; Friis, S.; Kjaer, S.K. Use of antidepressants and risk of epithelial ovarian cancer. Int. J. Cancer 2017, 141, 2197–2203. [Google Scholar] [CrossRef]
- Marcinkute, M.; Afshinjavid, S.; Fatokun, A.A.; Javid, F.A. Fluoxetine selectively induces p53-independent apoptosis in human colorectal cancer cells. Eur. J. Pharmacol. 2019, 857, 172441. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.J.; Jung, S.K.; Vo, T.T.L.; Jeong, C.H. Anticancer activity of paroxetine in human colon cancer cells: Involvement of MET and ERBB3. J. Cell. Mol. Med. 2019, 23, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Park, S.; Kim, S.Y.; Cho, S.H.; Jeong, M.S.; Kim, S.R.; Seo, J.B.; Kim, S.H.; Kim, K.N. 1-Cinnamoyltrichilinin from Melia azedarach Causes Apoptosis through the p38 MAPK Pathway in HL-60 Human Leukemia Cells. Int. J. Mol. Sci. 2020, 21, 7506. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Sundquist, J.; Sundquist, K. Cholera Vaccine Use Is Associated with a Reduced Risk of Death in Patients with Colorectal Cancer: A Population-Based Study. Gastroenterology 2018, 154, 86–92.e1. [Google Scholar] [CrossRef]
- Wolbers, M.; Koller, M.T.; Witteman, J.C.; Steyerberg, E.W. Prognostic models with competing risks: Methods and application to coronary risk prediction. Epidemiology 2009, 20, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N.; Black, D.W. Bipolar Disorder and Suicide: A Review. Curr. Psychiatry. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Putter, H.; Fiocco, M.; Geskus, R.B. Tutorial in biostatistics: Competing risks and multi-state models. Stat. Med. 2007, 26, 2389–2430. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Kerber, R.A.; Neklason, D.W.; Samowitz, W.S.; Burt, R.W. Frequency of familial colon cancer and hereditary nonpolyposis colorectal cancer (Lynch syndrome) in a large population database. Fam. Cancer 2005, 4, 239–244. [Google Scholar] [CrossRef]
- Taylor, D.P.; Burt, R.W.; Williams, M.S.; Haug, P.J.; Cannon-Albright, L.A. Population-based family history-specific risks for colorectal cancer: A constellation approach. Gastroenterology 2010, 138, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.E.; Houlston, R.S. A systematic review and meta-analysis of familial colorectal cancer risk. Am. J. Gastroenterol. 2001, 96, 2992–3003. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tamim, H.; Shapiro, S.; Stang, M.R.; Collet, J.P. Use of antidepressants and risk of colorectal cancer: A nested case-control study. Lancet Oncol. 2006, 7, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chubak, J.; Boudreau, D.M.; Rulyak, S.J.; Mandelson, M.T. Colorectal cancer risk in relation to antidepressant medication use. Int. J. Cancer 2011, 128, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.P.; Riis, A.H.; Lash, T.L.; Dalton, S.O.; Friis, S.; Robertson, D.; Sorensen, H.T. Antidepressant use and colorectal cancer risk: A Danish population-based case-control study. Br. J. Cancer 2011, 104, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Haukka, J.; Sankila, R.; Klaukka, T.; Lonnqvist, J.; Niskanen, L.; Tanskanen, A.; Wahlbeck, K.; Tiihonen, J. Incidence of cancer and antidepressant medication: Record linkage study. Int. J. Cancer 2010, 126, 285–296. [Google Scholar] [CrossRef]
- Kiridly-Calderbank, J.F.; Sturgeon, S.R.; Kroenke, C.H.; Reeves, K.W. Antidepressant Use and Risk of Colorectal Cancer in the Women’s Health Initiative. Cancer Epidemiol. Biomark. Prev. 2018, 27, 892–898. [Google Scholar] [CrossRef]
- Lee, H.C.; Chiu, W.C.; Wang, T.N.; Liao, Y.T.; Chien, I.C.; Lee, Y.; McIntyre, R.S.; Chen, P.C.; Chen, V.C. Antidepressants and colorectal cancer: A population-based nested case-control study. J. Affect. Disord. 2017, 207, 353–358. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Matcham, F.; Dauchy, S.; Barbui, C.; Hotopf, M. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst. Rev. 2018, 4, CD011006. [Google Scholar] [CrossRef]
- Li, X.J.; Dai, Z.Y.; Zhu, B.Y.; Zhen, J.P.; Yang, W.F.; Li, D.Q. Effects of sertraline on executive function and quality of life in patients with advanced cancer. Med. Sci. Monit. 2014, 20, 1267–1273. [Google Scholar] [CrossRef]
- Christensen, D.K.; Armaiz-Pena, G.N.; Ramirez, E.; Matsuo, K.; Zimmerman, B.; Zand, B.; Shinn, E.; Goodheart, M.J.; Bender, D.; Thaker, P.H.; et al. SSRI use and clinical outcomes in epithelial ovarian cancer. Oncotarget 2016, 7, 33179–33191. [Google Scholar] [CrossRef] [PubMed]
- Nayan, M.; Juurlink, D.N.; Austin, P.C.; Macdonald, E.M.; Finelli, A.; Kulkarni, G.S.; Hamilton, R.J.; Canadian Drug Safety and Effectiveness Research Network (CDSERN). Medication use and kidney cancer survival: A population-based study. Int. J. Cancer 2018, 142, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Farley, N.N.; Kertesy, S.B.; Dubyak, G.R.; Cowen, D.S. Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J. Neurochem. 2005, 92, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Stepulak, A.; Rzeski, W.; Sifringer, M.; Brocke, K.; Gratopp, A.; Kupisz, K.; Turski, L.; Ikonomidou, C. Fluoxetine inhibits the extracellular signal regulated kinase pathway and suppresses growth of cancer cells. Cancer Biol. Ther. 2008, 7, 1685–1693. [Google Scholar] [CrossRef]
- Gil-Ad, I.; Zolokov, A.; Lomnitski, L.; Taler, M.; Bar, M.; Luria, D.; Ram, E.; Weizman, A. Evaluation of the potential anti-cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer-xenografted mice. Int. J. Oncol. 2008, 33, 277–286. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lin, S.S.; Hsu, F.T.; Chung, J.G. Fluoxetine Inhibits DNA Repair and NF-kB-modulated Metastatic Potential in Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 5201–5210. [Google Scholar] [CrossRef]
- Yuan, I.; Horng, C.T.; Chen, V.C.; Chen, C.H.; Chen, L.J.; Hsu, T.C.; Tzang, B.S. Escitalopram oxalate inhibits proliferation and migration and induces apoptosis in non-small cell lung cancer cells. Oncol. Lett. 2018, 15, 3376–3382. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.; Shen, X.; Wang, Q.; Lv, J.; Liu, M.; Cheng, F.; Zhao, Z.; Pang, X. Repurposing sertraline sensitizes non-small cell lung cancer cells to erlotinib by inducing autophagy. JCI Insight 2018, 3, e98921. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, L.; Zhao, Y.; Jiang, Y.; Chen, L.; Yu, Y.; Ouyang, L. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2018, 51, e12402. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Shim, J.S.; Kim, D.; Kwon, H.J. Antidepressant drug sertraline modulates AMPK-MTOR signaling-mediated autophagy via targeting mitochondrial VDAC1 protein. Autophagy 2021, 17, 2783–2799. [Google Scholar] [CrossRef] [PubMed]
- Khing, T.M.; Po, W.W.; Sohn, U.D. Fluoxetine Enhances Anti-tumor Activity of Paclitaxel in Gastric Adenocarcinoma Cells by Triggering Apoptosis and Necroptosis. Anticancer Res. 2019, 39, 6155–6163. [Google Scholar] [CrossRef] [PubMed]
- Kannen, V.; Marini, T.; Turatti, A.; Carvalho, M.C.; Brandao, M.L.; Jabor, V.A.; Bonato, P.S.; Ferreira, F.R.; Zanette, D.L.; Silva, W.A., Jr.; et al. Fluoxetine induces preventive and complex effects against colon cancer development in epithelial and stromal areas in rats. Toxicol. Lett. 2011, 204, 134–140. [Google Scholar] [CrossRef]
- Melino, G. Antidepressants synergize with chemotherapy against cancer stem cells. Aging 2015, 7, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Tian, Y.; Xu, S.; Chen, H. Oxaliplatin-induced peripheral neuropathy: Clinical features, mechanisms, prevention and treatment. J. Neurol. 2021, 268, 3269–3282. [Google Scholar] [CrossRef]
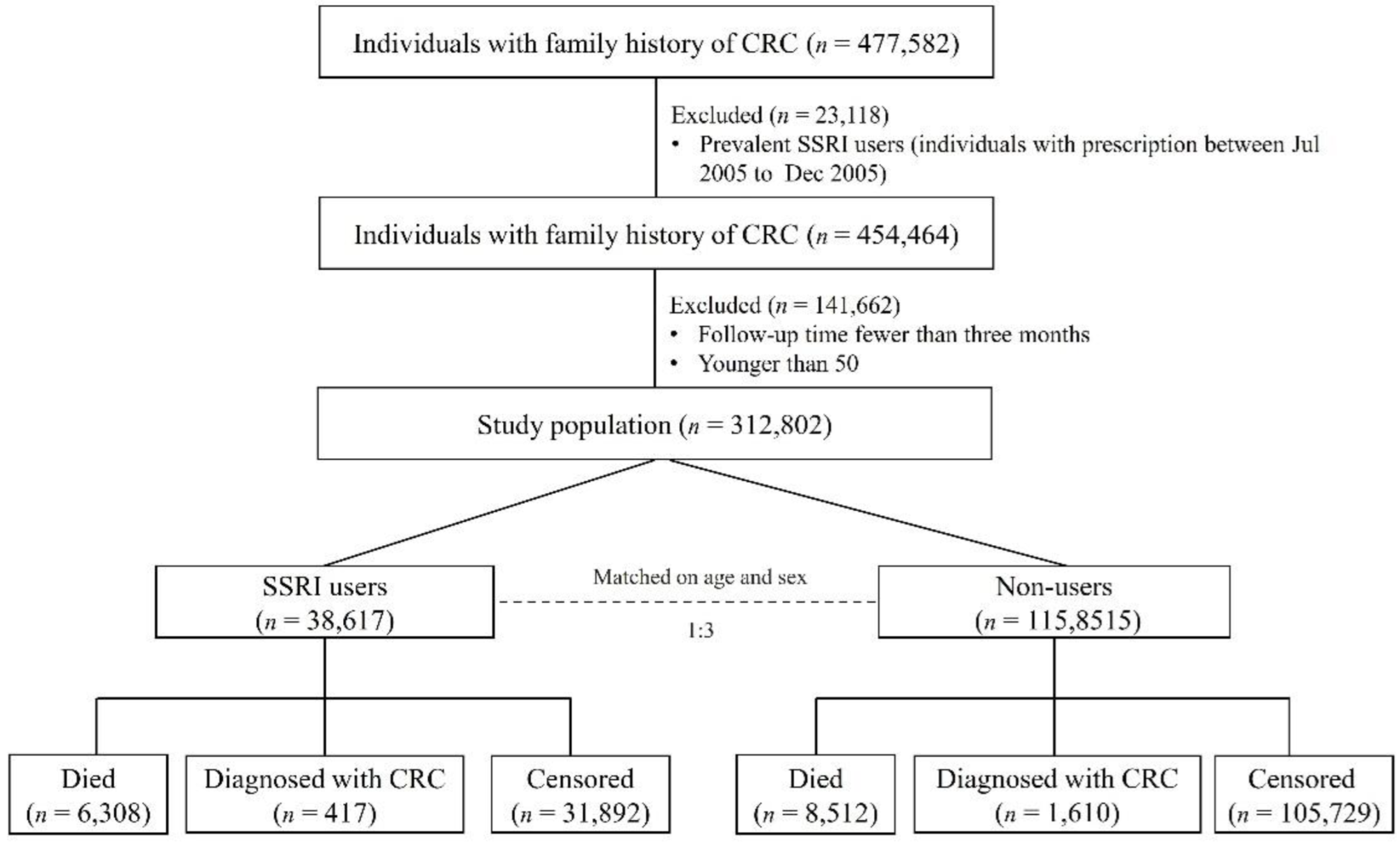
| SSRIs Users (n = 38,617) | Non-Users (n = 115,851) | |||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age at index | ||||
| 50–59 | 14,584 | 37.8 | 43,752 | 37.8 |
| 60–69 | 13,275 | 32.4 | 39,825 | 32.4 |
| ≥ 670 | 10,758 | 27.8 | 32,274 | 27.8 |
| Sex | ||||
| Males | 15,678 | 40.6 | 47,034 | 40.6 |
| Females | 22,939 | 59.4 | 68,817 | 59.4 |
| Birth country | ||||
| Sweden | 37,788 | 97.8 | 113,389 | 97.8 |
| Others | 829 | 2.2 | 2462 | 2.2 |
| Highest education level, year | ||||
| 1–9 | 9931 | 25.7 | 29,432 | 25.4 |
| 10–11 | 16,735 | 43.3 | 49,790 | 43.0 |
| ≥12 | 11,951 | 31.0 | 36,629 | 31.6 |
| Income | ||||
| Lowest | 7608 | 19.7 | 22,217 | 19.2 |
| Middle-low | 10,058 | 26.1 | 29,929 | 25.8 |
| Middle-high | 9577 | 24.8 | 27,139 | 23.4 |
| Highest | 11,374 | 29.4 | 36,566 | 31.6 |
| Inflammatory bowel disease | ||||
| No | 37,985 | 98.4 | 114,482 | 98.8 |
| Yes | 632 | 1.6 | 1369 | 1.2 |
| Obesity | ||||
| No | 37,703 | 97.6 | 114,430 | 98.8 |
| Yes | 914 | 2.4 | 1421 | 1.2 |
| COPD | ||||
| No | 34,925 | 90.4 | 108,899 | 94.0 |
| Yes | 3692 | 9.6 | 6952 | 6.0 |
| Colonoscopy | ||||
| No | 35,811 | 92.7 | 110,623 | 95.5 |
| Yes | 2806 | 7.3 | 5228 | 4.5 |
| CCI | ||||
| 0 | 25,667 | 66.5 | 91,290 | 78.8 |
| 1 | 7873 | 20.4 | 16,738 | 14.4 |
| 2 | 2809 | 7.3 | 5080 | 4.4 |
| ≥3 | 2268 | 5.8 | 2743 | 2.4 |
| Outpatient visits, per year | ||||
| 0 | 13,792 | 35.7 | 54,163 | 46.7 |
| 1 | 14,085 | 36.5 | 33,925 | 29.3 |
| 2 | 5633 | 14.6 | 12,723 | 11.0 |
| ≥3 | 5107 | 13.2 | 15,040 | 13.0 |
| Prescription of other medicines | ||||
| Aspirin | 8065 | 20.9 | 16,113 | 13.9 |
| Metformin | 2616 | 6.8 | 5872 | 5.1 |
| Statin | 10,700 | 27.7 | 23,667 | 20.4 |
| Individuals, n | Person-Years | Cancer Diagnoses, n | IR, Per 1000 Person-Year | Crude | Adjusted * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |||||
| SSRIs use | ||||||||||
| Non-users | 11,5851 | 804,265 | 1610 | 2.00 | 1 | 1 | ||||
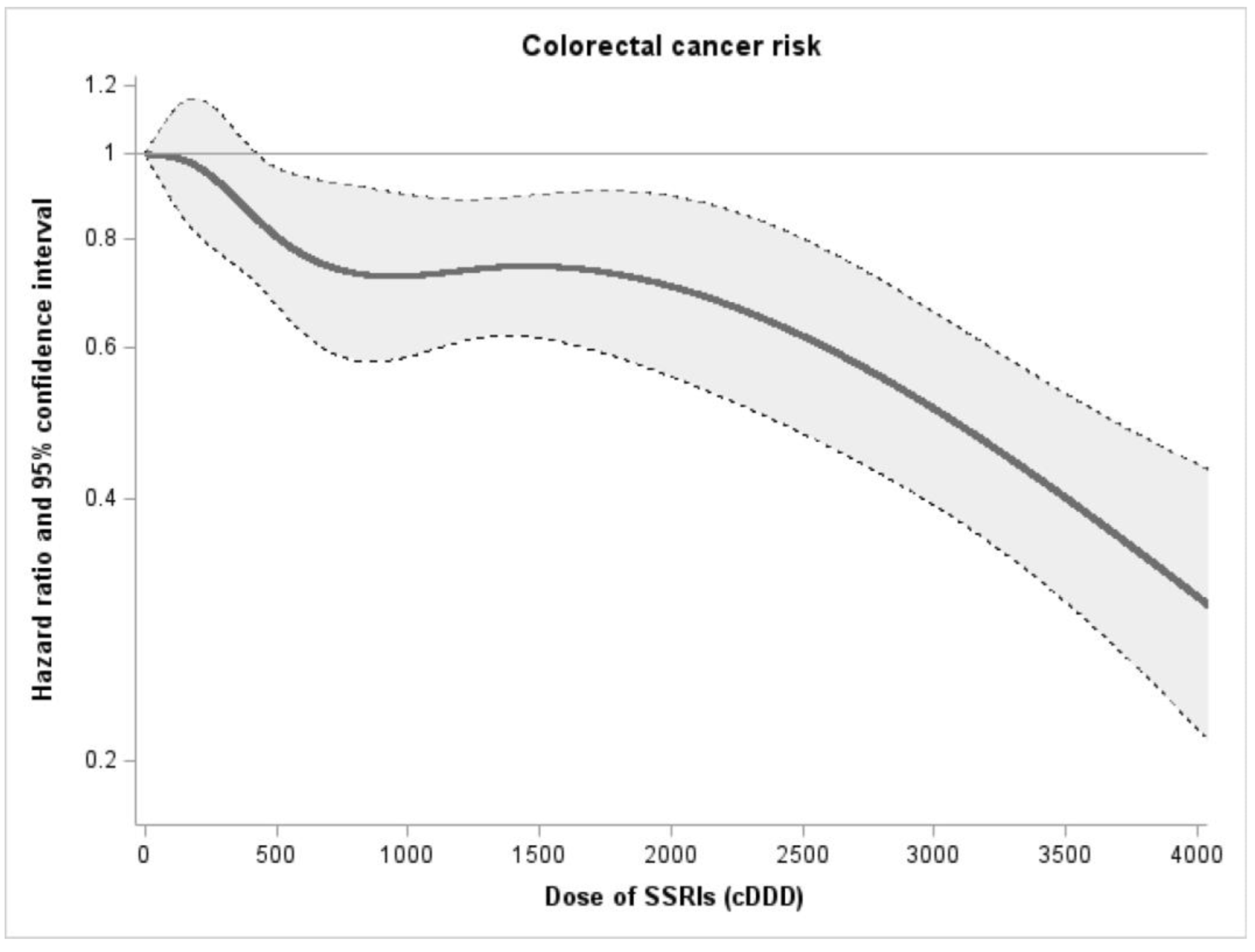
| SSRIs users | 38,617 | 253,019 | 417 | 1.65 | 0.77 | 0.70–0.85 | <0.001 | 0.77 | 0.70–0.85 | <0.001 |
| Subtype of SSRIs | ||||||||||
| Fluoxetine | 1515 | 14,224 | 21 | 1.47 | 0.74 | 0.49–1.13 | 0.164 | 0.75 | 0.47–1.18 | 0.210 |
| Citalopram | 15,587 | 129,042 | 227 | 1.76 | 0.77 | 0.68–0.88 | <0.001 | 0.77 | 0.67–0.88 | <0.001 |
| Paroxetine | 1116 | 10,759 | 13 | 1.21 | 0.64 | 0.38–1.09 | 0.103 | 0.66 | 0.36–1.20 | 0.172 |
| Sertraline | 9761 | 81,764 | 117 | 1.43 | 0.71 | 0.60–0.85 | <0.001 | 0.69 | 0.57–0.83 | <0.001 |
| Escitalopram | 3995 | 34,586 | 51 | 1.47 | 0.77 | 0.58–1.01 | 0.055 | 0.84 | 0.64–1.11 | 0.219 |
| Cancer site | ||||||||||
| Colon cancer | ||||||||||
| Non-users | 11,5851 | 804,265 | 1125 | 1.40 | 1 | 1 | ||||
| SSRIs users | 38,617 | 253,019 | 298 | 1.18 | 0.79 | 0.71–0.89 | <0.001 | 0.79 | 0.70–0.89 | <0.001 |
| Rectal cancer | ||||||||||
| Non-users | 115,851 | 804,265 | 485 | 0.6 | 1 | 1 | ||||
| SSRIs users | 38,617 | 253,019 | 119 | 0.47 | 0.73 | 0.61–0.87 | <0.001 | 0.73 | 0.63–0.91 | 0.003 |
| Cancer stage | ||||||||||
| Stage I and II | ||||||||||
| Non-users | 115,851 | 804,265 | 616 | 0.77 | 1 | 1 | ||||
| SSRIs users | 38,617 | 253,019 | 169 | 0.67 | 0.82 | 0.72–0.95 | 0.010 | 0.80 | 0.68–0.93 | 0.004 |
| Stage III and IV | ||||||||||
| Non-users | 115,851 | 804,265 | 785 | 0.98 | 1 | 1 | ||||
| SSRIs users | 38,617 | 253,019 | 186 | 0.74 | 0.71 | 0.61–0.82 | <0.001 | 0.73 | 0.63–0.85 | <0.001 |
| Individuals, n | Person-Years | Cancer Diagnoses, n | IR, Per 1000 Person-Year | Crude | Adjusted * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |||||
| Age | ||||||||||
| 50–59 | ||||||||||
| Non-users | 43,752 | 339,128 | 392 | 1.16 | 1 | 1 | ||||
| SSRIs users | 14,584 | 110,460 | 121 | 1.1 | 0.92 | 0.77–1.10 | 0.353 | 0.91 | 0.76–1.09 | 0.303 |
| 60–69 | ||||||||||
| Non-users | 39,825 | 296,312 | 657 | 2.22 | 1 | 1 | ||||
| SSRIs users | 13,275 | 92,807 | 134 | 1.44 | 0.61 | 0.52–0.73 | <0.001 | 0.61 | 0.51–0.73 | <0.001 |
| ≥70 | ||||||||||
| Non-users | 32,274 | 168,826 | 561 | 3.32 | 1 | 1 | ||||
| SSRIs users | 10,758 | 49,752 | 162 | 3.26 | 0.86 | 0.74–0.99 | 0.048 | 0.86 | 0.73–1.01 | 0.064 |
| Sex | ||||||||||
| Male | ||||||||||
| Non-users | 47,034 | 316,661 | 696 | 2.2 | 1 | 1 | ||||
| SSRIs users | 15,678 | 97,325 | 193 | 1.98 | 0.83 | 0.72–0.95 | 0.008 | 0.84 | 0.72–0.97 | 0.018 |
| Female | ||||||||||
| Non-users | 68,817 | 487,605 | 914 | 1.87 | 1 | 1 | ||||
| SSRIs users | 22,939 | 155,694 | 224 | 1.44 | 0.73 | 0.64–0.83 | <0.001 | 0.74 | 0.64–0.84 | <0.001 |
| Individuals, n | Person-Years | Cancer Diagnoses, n | IR, Per 1000 Person-Year | Crude | Adjusted * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |||||
| Sensitivity analysis 1 † | ||||||||||
| Non-users | 110,506 | 800,862 | 1458 | 1.82 | 1 | 1 | ||||
| SSRIs users | 36,138 | 251,473 | 374 | 1.49 | 0.82 | 0.73–0.93 | 0.001 | 0.82 | 0.73–0.93 | 0.001 |
| Sensitivity analysis 2 ‡ | ||||||||||
| SSRIs users | 113,007 | 784,061 | 1570 | 2.00 | 1 | 1 | ||||
| Non-users | 37,210 | 243,515 | 399 | 1.64 | 0.77 | 0.70–0.85 | <0.001 | 0.76 | 0.69–0.84 | <0.001 |
| Sensitivity analysis 3 § | ||||||||||
| SSRIs users | 115,851 | 804,265 | 1610 | 2.00 | 1 | 1 | ||||
| Non-users | 38,617 | 253,017 | 417 | 1.65 | 0.77 | 0.70–0.85 | <0.001 | 0.77 | 0.70–0.85 | <0.001 |
| Sensitivity analysis 4|| | ||||||||||
| Non-users | 24,930 | 235,566 | 415 | 1.76 | 1 | 1 | ||||
| TCA users | 8310 | 74,980 | 118 | 1.57 | 0.85 | 0.71–1.02 | 0.088 | 1.02 | 0.76–1.39 | 0.875 |
| Method | Beta | SE | Causal Effect (95%CI) | p Value |
|---|---|---|---|---|
| MR Egger | 5.345 × 10−3 | 7.102 × 10−3 | 1.005 (0.991–1.019) | 0.449 |
| Weighted median | 1.106 × 10−3 | 6.352 × 10−3 | 1.001 (0.989–1.013) | 0.862 |
| Inverse variance weighted | 3.836 × 10−3 | 4.043 × 10−3 | 1.004 (0.996–1.012) | 0.343 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Sundquist, J.; Sundquist, K.; Ji, J. Use of Selective Serotonin Reuptake Inhibitors Is Associated with a Lower Risk of Colorectal Cancer among People with Family History. Cancers 2022, 14, 5905. https://doi.org/10.3390/cancers14235905
Zhang N, Sundquist J, Sundquist K, Ji J. Use of Selective Serotonin Reuptake Inhibitors Is Associated with a Lower Risk of Colorectal Cancer among People with Family History. Cancers. 2022; 14(23):5905. https://doi.org/10.3390/cancers14235905
Chicago/Turabian StyleZhang, Naiqi, Jan Sundquist, Kristina Sundquist, and Jianguang Ji. 2022. "Use of Selective Serotonin Reuptake Inhibitors Is Associated with a Lower Risk of Colorectal Cancer among People with Family History" Cancers 14, no. 23: 5905. https://doi.org/10.3390/cancers14235905
APA StyleZhang, N., Sundquist, J., Sundquist, K., & Ji, J. (2022). Use of Selective Serotonin Reuptake Inhibitors Is Associated with a Lower Risk of Colorectal Cancer among People with Family History. Cancers, 14(23), 5905. https://doi.org/10.3390/cancers14235905






