Oral Cancer in HSCT Pediatric Patients Arising on GVHD: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Yuan, W.; Li, M.; Cheng, L.; Yang, J.; Yin, B.; Huang, X. In situ buccal carcinoma in a teenager after hematopoietic stem cell transplantation: A case report. Medicine 2020, 99, e22781. [Google Scholar] [CrossRef]
- Adhikari, J.; Sharma, P.; Bhatt, V.R. Risk of secondary solid malignancies after allogeneic hematopoietic stem cell transplantation and preventive strategies. Future Oncol. (Lond. Engl.) 2015, 11, 3175–3185. [Google Scholar] [CrossRef]
- Bosi, A.; Bartolozzi, B. Safety of bone marrow stem cell donation: A review. Transplant. Proc. 2010, 42, 2192–2194. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Pagel, J.M.; Gooley, T.A.; Petersdorf, E.W.; Sorror, M.L.; Woolfrey, A.E.; Hansen, J.A.; Salter, A.I.; Lansverk, E.; Stewart, F.M.; et al. Comparison of matched unrelated and matched related donor myeloablative hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Leukemia 2010, 24, 1276–1282. [Google Scholar] [CrossRef]
- Bhatia, S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev. Hematol. 2011, 4, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Santarone, S.; Natale, A.; Angelini, S.; Papalinetti, G.; Vaddinelli, D.; Di Bartolomeo, A.; Di Bartolomeo, P. Secondary oral cancer following hematopoietic cell transplantation. Bone Marrow Transplant. 2021, 56, 1038–1046. [Google Scholar] [CrossRef]
- Gervazio, T.C.; Silva, J.K.; Evangelista, K.; Cavalcanti, M.; Silva, M.; Yamamoto-Silva, F.P.; Silva, B. Risk of oral cancer in patients with graft-vs-host disease: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Demarosi, F.; Soligo, D.; Lodi, G.; Moneghini, L.; Sardella, A.; Carrassi, A. Squamous cell carcinoma of the oral cavity associated with graft versus host disease: Report of a case and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.D.; Curtis, R.E.; Socié, G.; Sobocinski, K.A.; Gilbert, E.; Landgren, O.; Travis, L.B.; Travis, W.D.; Flowers, M.E.; Friedman, D.L.; et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood 2009, 113, 1175–1183. [Google Scholar] [CrossRef]
- Leuci, S.; Coppola, N.; Blasi, A.; Ruoppo, E.; Bizzoca, M.E.; Lo Muzio, L.; Marano, L.; Risitano, A.M.; Mignogna, M.D. Oral Dysplastic Complications after HSCT: Single Case Series of Multidisciplinary Evaluation of 80 Patients. Life 2020, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Aljitawi, O.; Zadik, Y. Oral Graft-Versus-Host Disease: A Pictorial Review and a Guide for Dental Practitioners. Int. Dent. J. 2021, 71, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Janowiak-Majeranowska, A.; Osowski, J.; Mikaszewski, B.; Majeranowski, A. Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review. Cancers 2022, 14, 2175. [Google Scholar] [CrossRef]
- Gomes, A.O.; Torres, S.R.; Maiolino, A.; Dos Santos, C.W.; Silva Junior, A.; Correa, M.E.; Moreira, M.C.; Gonçalves, L. Early and late oral features of chronic graft-versus-host disease. Rev. Bras. Hematol. Hemoter. 2014, 36, 43–49. [Google Scholar] [CrossRef]
- Abdelsayed, R.A.; Sumner, T.; Allen, C.M.; Treadway, A.; Ness, G.M.; Penza, S.L. Oral precancerous and malignant lesions associated with graft-versus-host disease: Report of 2 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Henry-Amar, M.; Cosset, J.M.; Devergie, A.; Girinsky, T.; Gluckman, E. Increased incidence of solid malignant tumors after bone marrow transplantation for severe aplastic anemia. Blood 1991, 78, 277–279. [Google Scholar] [CrossRef]
- Somers, G.R.; Tabrizi, S.N.; Tiedemann, K.; Chow, C.W.; Garland, S.M.; Venter, D.J. Squamous cell carcinoma of the tongue in a child with Fanconi anemia: A case report and review of the literature. Pediatr. Pathol. Lab. Med. J. Soc. Pediatr. Pathol. Affil. Int. Paediatr. Pathol. Assoc. 1995, 15, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Millen, F.J.; Rainey, M.G.; Hows, J.M.; Burton, P.A.; Irvine, G.H.; Swirsky, D. Oral squamous cell carcinoma after allogeneic bone marrow transplantation for Fanconi anaemia. Br. J. Haematol. 1997, 99, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, H.; Yokoe, H.; Miya, T.; Atsuta, F.; Miura, N.; Tanzawa, H.; Sato, K. Gingival squamous cell carcinoma in a patient with chronic graft-versus-host disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 171–174. [Google Scholar] [CrossRef]
- Shimada, K.; Yokozawa, T.; Atsuta, Y.; Kohno, A.; Maruyama, F.; Yano, K.; Taji, H.; Kitaori, K.; Goto, S.; Iida, H.; et al. Solid tumors after hematopoietic stem cell transplantation in Japan: Incidence, risk factors and prognosis. Bone Marrow Transplant. 2005, 36, 115–121. [Google Scholar] [CrossRef]
- Salum, F.G.; Martins, G.B.; de Figueiredo, M.A.; Cherubini, K.; Yurgel, L.S.; Torres-Pereira, C. Squamous cell carcinoma of the tongue after bone marrow transplantation in a patient with Fanconi anemia. Braz. Dent. J. 2006, 17, 161–165. [Google Scholar] [CrossRef]
- Byun, J.H.; Park, B.W.; Kim, J.R.; Lee, G.W.; Lee, J.H. Squamous cell carcinoma of the tongue after bone marrow transplant and graft-versus-host disease: A case report and review of the literature. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2008, 66, 144–147. [Google Scholar] [CrossRef]
- Masserot, C.; Peffault de Latour, R.; Rocha, V.; Leblanc, T.; Rigolet, A.; Pascal, F.; Janin, A.; Soulier, J.; Gluckman, E.; Socié, G. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer 2008, 113, 3315–3322. [Google Scholar] [CrossRef]
- Tomihara, K.; Dehari, H.; Yamaguchi, A.; Abe, M.; Miyazaki, A.; Nakamori, K.; Hareyama, M.; Hiratsuka, H. Squamous cell carcinoma of the buccal mucosa in a young adult with history of allogeneic bone marrow transplantation for childhood acute leukemia. Head Neck 2009, 31, 565–568. [Google Scholar] [CrossRef]
- Montebugnoli, L.; Gissi, D.B.; Marchetti, C.; Foschini, M.P. Multiple squamous cell carcinomas of the oral cavity in a young patient with graft-versus-host disease following allogenic bone marrow transplantation. Int. J. Oral Maxillofac. Surg. 2011, 40, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Islam, M.N.; Bhattacharyya, I.; Sandow, P.; Moreb, J.S. Oral squamous cell carcinoma positive for p16/human papilloma virus in post allogeneic stem cell transplantation: 2 cases and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, e74–e78. [Google Scholar] [CrossRef]
- Torres-Pereira, C.C.; Stramandinoli-Zanicotti, R.T.; Amenábar, J.M.; Sassi, L.M.; Galbiatti Pedruzzi, P.A.; Piazzetta, C.M.; Bonfim, C. Oral squamous cell carcinoma in two siblings with Fanconi anemia after allogeneic bone marrow transplantation. Spec. Care Dent. Off. Publ. Am. Assoc. Hosp. Dent. Acad. Dent. Handicap. Am. Soc. Geriatr. Dent. 2014, 34, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, C.; Ribeiro, L.; Nichele, S.; Bitencourt, M.; Loth, G.; Koliski, A.; Funke, V.; Pilonetto, D.V.; Pereira, N.F.; Flowers, M.; et al. Long-term Survival, Organ Function, and Malignancy after Hematopoietic Stem Cell Transplantation for Fanconi Anemia. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Cancer. Available online: https://www.cancer.net/cancer-types/oral-and-oropharyngeal-cancer/statistics (accessed on 15 August 2022).
- Majorana, A.; Schubert, M.M.; Porta, F.; Ugazio, A.G.; Sapelli, P.L. Oral complications of pediatric hematopoietic cell transplantation: Diagnosis and management. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2000, 8, 353–365. [Google Scholar]
- Nakasone, H.; Remberger, M.; Tian, L.; Brodin, P.; Sahaf, B.; Wu, F.; Mattsson, J.; Lowsky, R.; Negrin, R.; Miklos, D.B.; et al. Risks and benefits of sex-mismatched hematopoietic cell transplantation differ according to conditioning strategy. Haematologica 2015, 100, 1477–1485. [Google Scholar] [CrossRef]
- Kruse, A.L.; Grätz, K.W. Oral carcinoma after hematopoietic stem cell transplantation—A new classification based on a literature review over 30 years. Head Neck Oncol. 2009, 1, 29. [Google Scholar] [CrossRef]
- Anak, S.; Yalman, N.; Bilgen, H.; Sepet, E.; Deviren, A.; Gürtekin, B.; Tunca, F.; Başaran, B. Squamous cell carcinoma development in Fanconi anemia patients who underwent hematopoietic stem cell transplantation. Pediatr. Transplant. 2020, 24, e13706. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.E.; Metayer, C.; Rizzo, J.D.; Socié, G.; Sobocinski, K.A.; Flowers, M.E.; Travis, W.D.; Travis, L.B.; Horowitz, M.M.; Deeg, H.J. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: An international case-control study. Blood 2005, 105, 3802–3811. [Google Scholar] [CrossRef] [PubMed]
- Leisenring, W.; Friedman, D.L.; Flowers, M.E.; Schwartz, J.L.; Deeg, H.J. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Danylesko, I.; Shimoni, A. Second Malignancies after Hematopoietic Stem Cell Transplantation. Curr. Treat. Options Oncol. 2018, 19, 9. [Google Scholar] [CrossRef] [PubMed]
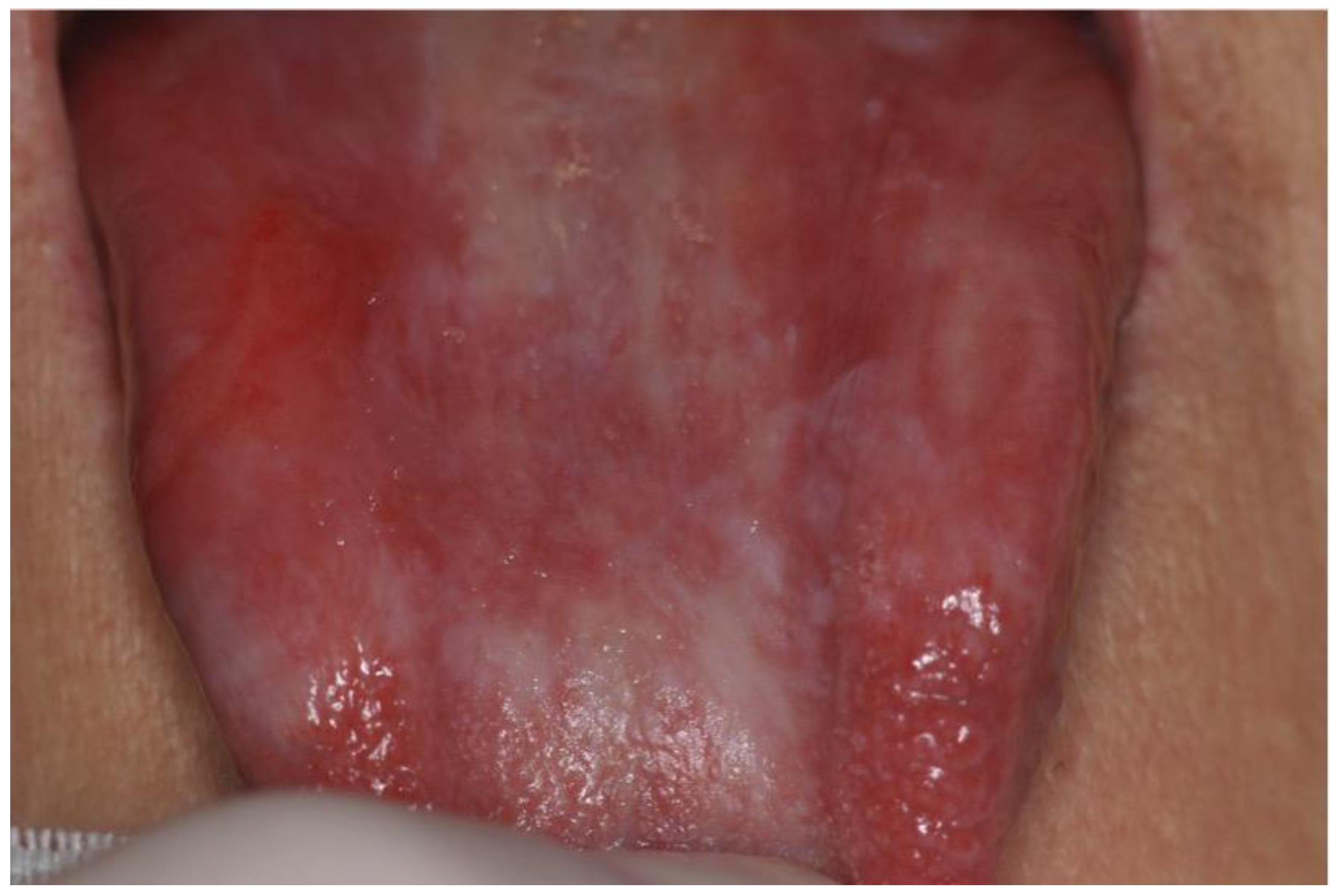
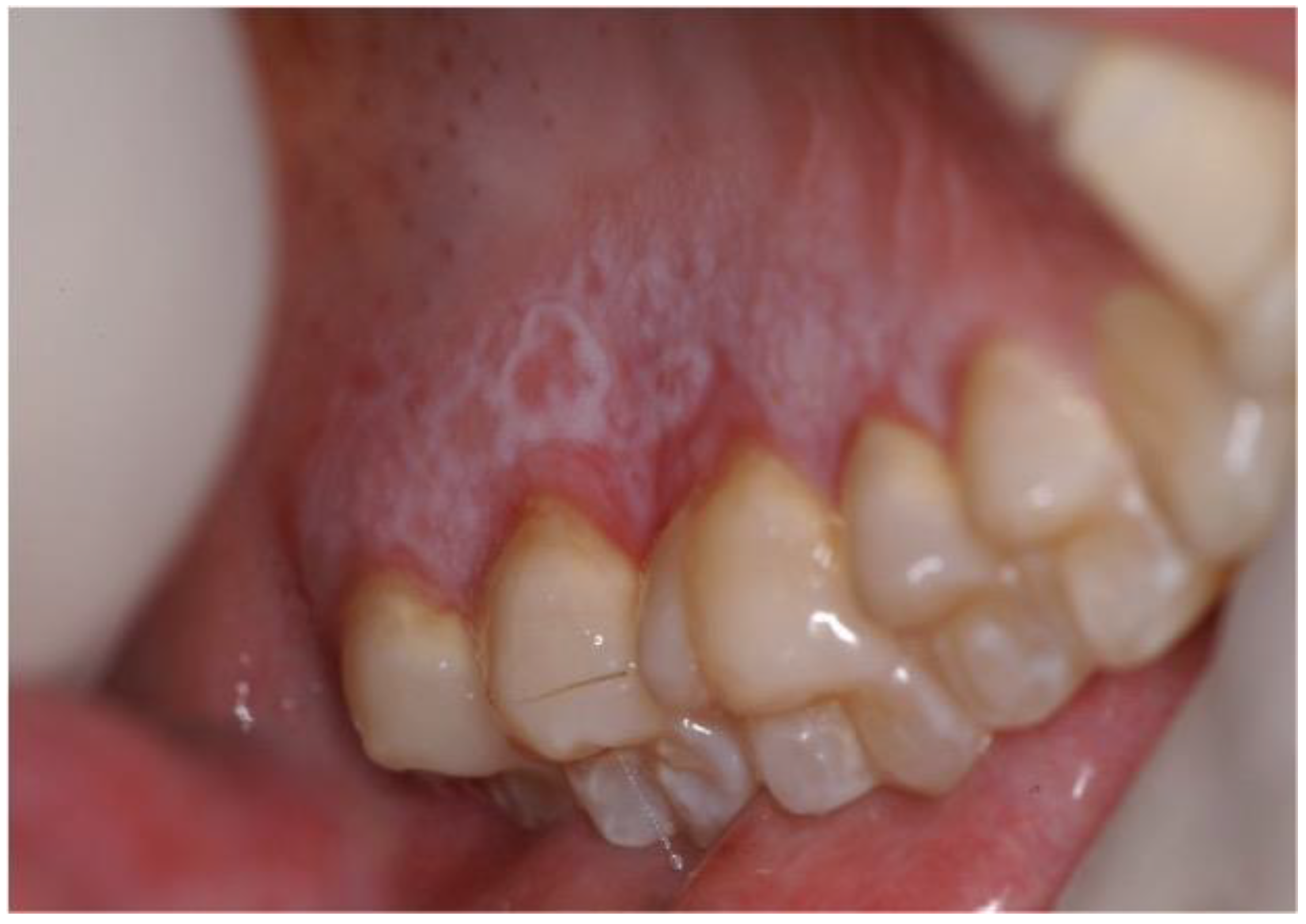
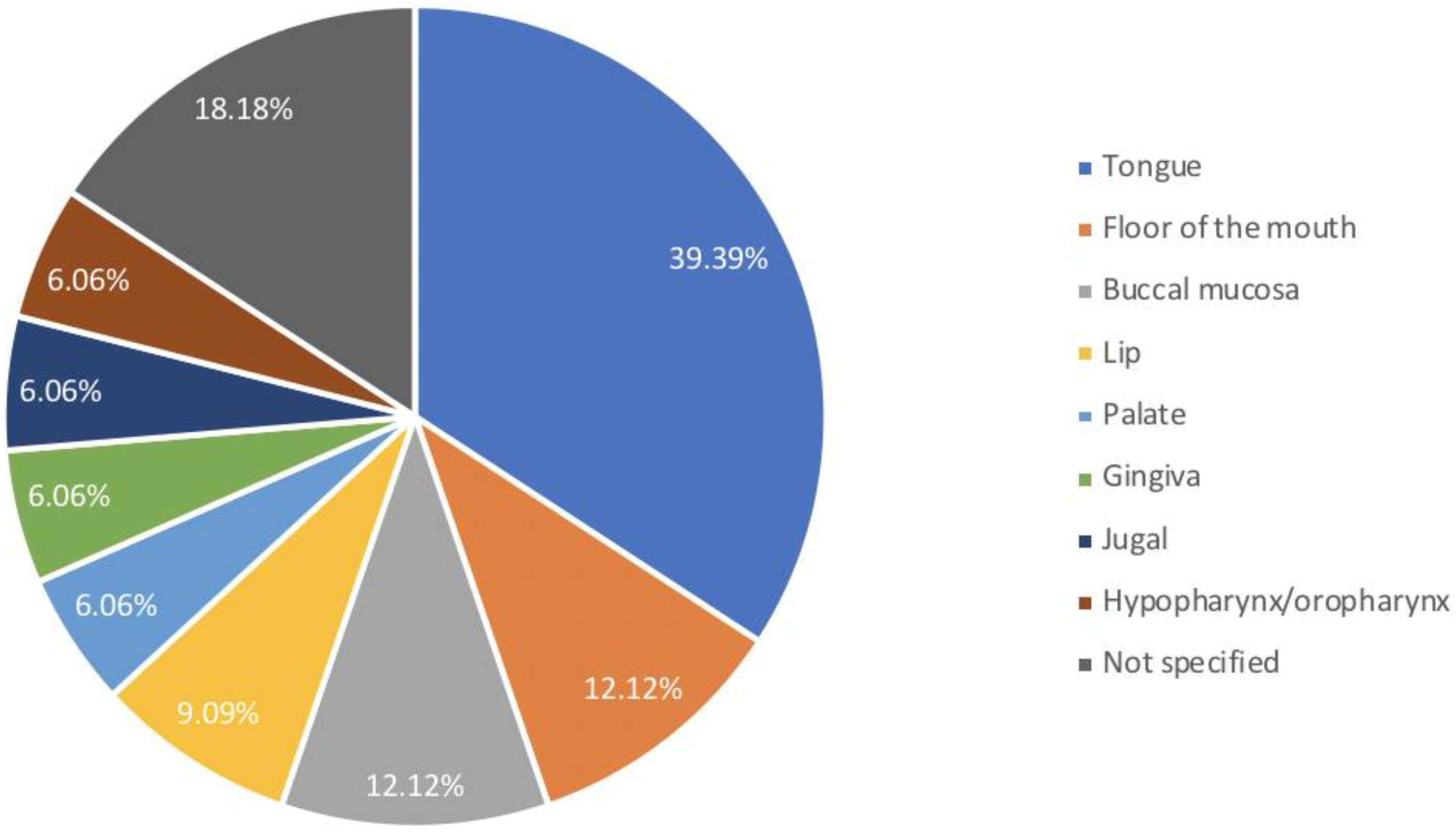
| Authors/Year of Publication/ Study Type | Disease | Age at Trasplantation/ Gender | Stem Cell Source | HLA Compatibility | Type of Conditioning Regimen | Conditioning Regimen | GVHD Prophylaxis | aGVHD | Time Lapse between HSCT and aGVHD (Months) | Sites Involved aGVHD | Grading aGVHD | Therapy aGVHD | cGVHD | Time Lapse between HSCT and cGVHD (Months) | Sites Involved cGVHD | Grading cGVHD | Therapy cGVHD | Time Lapse between HSCT and OSCC (Year) | Cancer Site | Staging (TNM) | Histology | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Socié et al., 1991 [15] Retrospective study | SAA | 12/M | - | HLA-id | RIC | CY, TAI | MTX | No | N/A | N/A | N/A | N/A | Yes | - | Oral cavity | Extensive | - | 7.9 | Lip | - | SCC | SE, RT | - |
| Somers et al., 1995 [16] Case report | FA | 8/M | - | - | RIC | CY, TAI | CS, S | Yes | - | - | - | - | Yes | 60 | Oral cavity | - | AZA | 8 | Tongue | - | SCC | SE | - |
| Tongue (after 6 months) | - | SE | |||||||||||||||||||||
| Millen et al., 1997 [17] Case report | FA | 8/F | BM | HLA-id | MAC | LD CY, TBI | - | Yes | - | Skin, Gut | III | - | Yes | - | Skin, Liver, Oral cavity | - | CS, AZA | 10 | Buccal mucosa | - | SCC | SE, ND, RT | Dead after 3 months |
| Otsubo et al., 1997 [18] Case report | SAA | 16/F | - | HLA-id | RIC | CY, TLI | CS, MTX | Yes | 1 | forearms | II | S | Yes | 4 | Oral cavity | - | CS, S | 4 | Gingiva | T3N0M0 | SCC | SE, ND | Alive (no information on follow-up) |
| Shimada et al., 2005 [19] Retrospective study | NHL | 17.5 M | BM | HLA-id | MAC | E, MEL, TBI | - | Yes | - | - | - | - | Yes | - | Oral cavity | Mild | - | 11.5 | Oral cavity | - | SCC | Dead after 3 months | |
| Salum et al., 2006 [20] Case report | FA | 5/M | BM | - | RIC | CY | CS, MTX | No | N/A | N/A | N/A | N/A | Yes | 10 | Buccal mucosa | - | SC | 11 | Tongue | T3N0M0 | SCC | CH, RT | Dead after 4 months |
| 84 | Tongue | - | No treatment | ||||||||||||||||||||
| 94 | Palate, Gingiva, Upper lip, Buccal mucosa | - | LC | ||||||||||||||||||||
| Byun et al., 2008 [21] Case report | CML | 12/F | BM | - | MAC | CY, BU | CS, S | No | N/A | N/A | N/A | N/A | Yes | 6 | Oral cavity, Skin, Liver, Eyes, Lungs | - | CS, S | 5 | Tongue | T2N0M0 | SCC* | SE, ND | Alive (follow-up: 5 months) |
| Masserot et al., 2008 [22] Case series | FA | 11.7/M | BM | MUD | MAC | CY LD, TBI, ATG | Yes (N/S) | No | N/A | N/A | N/A | N/A | Yes | - | Mucosal, Skin | - | - | 10 | Tongue, Palate | T1N0M0 | SCC | SE, ND | Alive (follow-up:9 months) |
| FA | 11.2/M | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | III | S, CS, ATG | Yes | - | Mucosal | - | - | 5.7 | Tongue | T2N+M0 | SCC | SE, RT | Dead after 5.5 months | |
| FA | 9.7/M | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | III | S, CS, ATG | Yes | - | Mucosal | - | - | 7.8 | Hypopharynxs | T4N2cMx | SCC | CH | Dead after 6 months | |
| FA | 8.9/F | BM | MUD | MAC | CY LD, TAI, ATG | Yes (N/S) | Yes | - | - | II | S, CS | Yes | - | Mucosal | - | - | 8.3 | Tongue | T1NxMx | SCC | SE | Dead after 6 months | |
| FA | 5.2/M | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | III | S, CS | Yes | - | Mucosal, Skin | - | - | 15.3 | Floor of the mouth | T1N0M0 | SCC | SE, ND | Alive (follow-up: 23 months) | |
| FA | 7.3/M | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | II | S, CS | Yes | - | Mucosal, Eye | - | - | 12.4 | Oropharynx | T4N2cMx | SCC | RT | Dead after 4.5 months | |
| FA | 11.2/M | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | II | S, CS | Yes | - | Mucosal | - | - | 7 | Floor of the mouth | T3N0Mx | SCC | SE, ND | Dead after 16 months | |
| FA | 4.6//M | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | IV | S, CS, ATG | Yes | - | Mucosal, Skin, Eye, Liver | - | - | 5.5 | Tongue | T3N0Mx | SCC | RT | Dead after 2.5 months | |
| FA | 6.5/F | BM | RD | MAC | CY HD, TBI | Yes (N/S) | Yes | - | - | III | S, ATG | Yes | - | Mucosal, Eye | - | - | 21.6 | Jugal, Floor of the mouth | T1NxMx | SCC | SE, CRY | Dead after 46.5 months | |
| FA | 10.3/M | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | II | S, CS | Yes | - | Mucosal | - | - | 14 | Palate, Jugal | T1NxMx | SCC | SE | Dead after 11 months | |
| FA | 14.3/F | BM | RD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | II | S, CS | Yes | - | Mucosal, Skin, Eye | - | - | 9.4 | Gingiva | T2N0Mx | SCC | SE, ND | Dead after 6.5 months | |
| FA | 7.5/F | BM | MUD | RIC | CY LD, TAI | Yes (N/S) | Yes | - | - | II | S, CS | Yes | - | Mucosal, Eye | - | 19.2 | Tongue | T1N0M0 | SCC | SE, ND | Dead after 41 months | ||
| Tomihara et al., 2009 [23] Case report | ALL | 11/M | BM | MMD | MAC | TBI | - | No | N/A | N/A | N/A | N/A | Yes | - | Oral cavity | - | - | 13 | Buccal mucosa | SCC | RT, ND | Alive (no information on follow-up) | |
| Montebugnoli et al., 2011 [24] Case report | TM | 9/F | - | - | - | - | CS, S | No | N/A | N/A | N/A | N/A | Yes | 6 | Buccal mucosa | - | LC, AMD | 17 | Tongue | T3N0M0 | SCC | SE, ND | Alive (follow-up: 2 years) |
| Floor of the mouth (after 4 months) | T2N0M0 | SE | |||||||||||||||||||||
| Katz et al., 2014 [25] Case report | AML | 18/M | - | MUD | - | - | - | No | N/A | N/A | N/A | N/A | Yes | - | Lower lip | - | - | 9 | Upper lip | T1N0M0 | SCC* | RT | Alive (no information on follow-up) |
| Torres-Pereira et al., 2014 [26] Case report | FA | 8/F | - | HLA-id | RIC | CY | CS, MTX | No | N/A | N/A | N/A | N/A | Yes | - | Buccal mucosa | Mild | No treatment | 10 | Tongue | - | SCC | SE, ND | Alive (follow-up: 5 years) |
| Bonfim et al., 2016 [27] Retrospective study | FA | 4/M | - | RD | RIC | CY | Yes (N/S) | No | N/A | N/A | N/A | N/A | Yes | - | Oral cavity | - | - | 11 | Oral cavity | T3N0M0 | SCC | BSC | Dead |
| FA | 6/M | - | RD | RIC | CY | Yes (N/S) | No | N/A | N/A | N/A | N/A | Yes | - | Oral cavity | - | - | 5 | Oral cavity | T2NxM0 | SCC | SE | Dead | |
| FA | 6/F | - | RD | RIC | CY | Yes (N/S) | Yes | - | - | - | . | Yes | - | Oral cavity | - | - | 6 | Oral cavity | T2N0M0 | SCC | SE, RT | Dead | |
| FA | 7/M | - | RD | RIC | CY | Yes (N/S) | Yes | - | - | - | . | Yes | - | Oral cavity | - | - | 8 | Oral cavity | T3NxM0 | SCC | BSC | Dead | |
| FA | 10/M | - | RD | RIC | CY | Yes (N/S) | Yes | - | - | - | . | Yes | - | Oral cavity | - | - | 5 | Oral cavity | T1N0M0 | SCC | SE | Alive (follow-up: 5 years) | |
| Liu et al., 2020 [1] Case report | AML | 14/F | - | - | - | - | CS | No | N/A | N/A | N/A | N/A | Yes | - | Oral cavity, Skin | - | CS, MTX | 2 | Buccal mucosa | TisN0M0 | SCC | SE | Alive (follow-up:4 years) |
| Santarone et al., 2021 [6] Retrospective study | SAA | 15/M | - | - | RIC | CY | Yes (N/S) | Yes | - | - | III | Yes (N/S) | Yes | - | Oral Cavity | Extensive | CS, S, AZA | 32.8 | Lower lip | T1NxM0 | SCC | SE, RT | Dead after 14 months |
| TM | 14/M | - | - | MAC | BU CY | Yes (N/S) | Yes | - | - | I | Yes (N/S) | Yes | - | Oral Cavity | Extensive | CS, S | 11.8 | Tongue | T2NxM0 | SCC | SE, CH | Dead after 2 years | |
| TM | 13M | - | - | MAC | BU CY | Yes (N/S) | No | N/A | N/A | N/A | N/A | Yes | - | Oral Cavity | Extensive | CS, S | 21.1 | Tongue | T3N0M0 | SCC | SE, CH, RT | Alive (follow-up:4 years) | |
| ALL | 18/F | - | - | MAC | TBI TH FLU | Yes (N/S) | No | N/A | N/A | N/A | N/A | Yes | - | Oral Cavity | Extensive | CS, S, ECP | 9.8 | Buccal mucosa | T2N0M0 | SCC | SE | Alive (follow-up: 2.5 years) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantile, T.; Coppola, N.; Canfora, F.; Adamo, D.; Ruoppo, E.; Mignogna, M.D.; Leuci, S. Oral Cancer in HSCT Pediatric Patients Arising on GVHD: A Comprehensive Review. Cancers 2022, 14, 5775. https://doi.org/10.3390/cancers14235775
Cantile T, Coppola N, Canfora F, Adamo D, Ruoppo E, Mignogna MD, Leuci S. Oral Cancer in HSCT Pediatric Patients Arising on GVHD: A Comprehensive Review. Cancers. 2022; 14(23):5775. https://doi.org/10.3390/cancers14235775
Chicago/Turabian StyleCantile, Tiziana, Noemi Coppola, Federica Canfora, Daniela Adamo, Elvira Ruoppo, Michele Davide Mignogna, and Stefania Leuci. 2022. "Oral Cancer in HSCT Pediatric Patients Arising on GVHD: A Comprehensive Review" Cancers 14, no. 23: 5775. https://doi.org/10.3390/cancers14235775
APA StyleCantile, T., Coppola, N., Canfora, F., Adamo, D., Ruoppo, E., Mignogna, M. D., & Leuci, S. (2022). Oral Cancer in HSCT Pediatric Patients Arising on GVHD: A Comprehensive Review. Cancers, 14(23), 5775. https://doi.org/10.3390/cancers14235775









