Oncological Outcomes for Patients Harboring Positive Surgical Margins Following Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Retrospective Multicentric Study on Behalf of the YAU Urothelial Group
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Selection and Collected Information
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
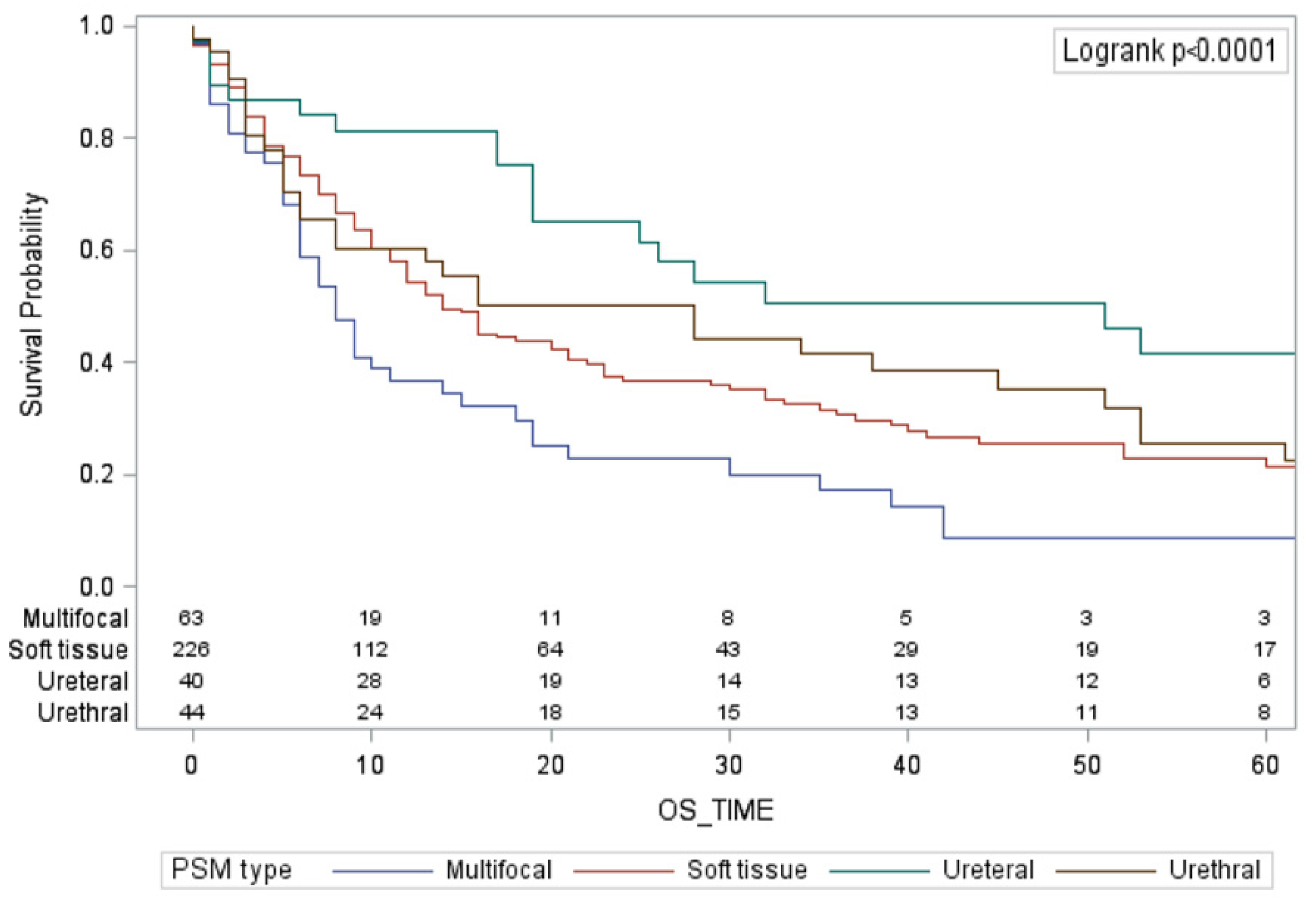
3.2. Survival Analyses
3.3. Predictive Factors for Survival in Uni- and Multivariable Analyses
4. Discussion
4.1. Future Perspectives
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xylinas, E.; Rink, M.; Novara, G.; Green, D.A.; Clozel, T.; Fritsche, H.-M.; Guillonneau, B.; Lotan, Y.; Kassouf, W.; Tilki, D.; et al. Predictors of Survival in Patients with Soft Tissue Surgical Margin Involvement at Radical Cystectomy. Ann. Surg. Oncol. 2013, 20, 1027–1034. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Soulie, M.; Larre, S.; Roupret, M.; Defortescu, G.; Murez, T.; Pignot, G.; Descazeaud, A.; Patard, J.-J.; Bigot, P.; et al. Positive Surgical Margins and Their Locations in Specimens Are Adverse Prognosis Features after Radical Cystectomy in Non-Metastatic Carcinoma Invading Bladder Muscle: Results from a Nationwide Case-Control Study: Radical Cystectomy Positive Surgical Margins. BJU Int. 2013, 111, 1253–1260. [Google Scholar] [CrossRef]
- Hong, X.; Li, T.; Ling, F.; Yang, D.; Hou, L.; Li, F.; Tan, W. Impact of Surgical Margin Status on the Outcome of Bladder Cancer Treated by Radical Cystectomy: A Meta-Analysis. Oncotarget 2017, 8, 17258–17269. [Google Scholar] [CrossRef] [PubMed]
- Dotan, Z.A.; Kavanagh, K.; Yossepowitch, O.; Kaag, M.; Olgac, S.; Donat, M.; Herr, H.W. Positive Surgical Margins in Soft Tissue Following Radical Cystectomy for Bladder Cancer and Cancer Specific Survival. J. Urol. 2007, 178, 2308–2312. [Google Scholar] [CrossRef]
- Novara, G.; Svatek, R.S.; Karakiewicz, P.I.; Skinner, E.; Ficarra, V.; Fradet, Y.; Lotan, Y.; Isbarn, H.; Capitanio, U.; Bastian, P.J.; et al. Soft Tissue Surgical Margin Status Is a Powerful Predictor of Outcomes after Radical Cystectomy: A Multicenter Study of More than 4400 Patients. J. Urol. 2010, 183, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Osman, Y.; El-Tabey, N.; Abdel-Latif, M.; Mosbah, A.; Moustafa, N.; Shaaban, A. The Value of Frozen-Section Analysis of Ureteric Margins on Surgical Decision-Making in Patients Undergoing Radical Cystectomy for Bladder Cancer. BJU Int. 2007, 99, 81–84. [Google Scholar] [CrossRef]
- Claps, F.; van de Kamp, M.W.; Mayr, R.; Bostrom, P.J.; Boormans, J.L.; Eckstein, M.; Mertens, L.S.; Boevé, E.R.; Neuzillet, Y.; Burger, M.; et al. Risk Factors Associated with Positive Surgical Margins’ Location at Radical Cystectomy and Their Impact on Bladder Cancer Survival. World J. Urol. 2021, 39, 4363–4371. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Research, C. For D.E and FDA-NCI Public Workshop: Defining Disease Recurrence and Harmonizing Conduct in Adjuvant Bladder and Kidney Cancer Trials; FDA: Silver Spring, MA, USA, 2019. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-nci-public-workshop-defining-disease-recurrence-and-harmonizing-conduct-adjuvant-bladder-and (accessed on 9 October 2022).
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant Atezolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma (IMvigor010): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Gschwend, J.E.; Heck, M.M.; Lehmann, J.; Rübben, H.; Albers, P.; Wolff, J.M.; Frohneberg, D.; de Geeter, P.; Heidenreich, A.; Kälble, T.; et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur. Urol. 2019, 75, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Lin, D.W.; Porter, M.P. The Association between Extent of Lymphadenectomy and Survival among Patients with Lymph Node Metastases Undergoing Radical Cystectomy. Cancer 2008, 112, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Koppie, T.M.; Vickers, A.J.; Vora, K.; Dalbagni, G.; Bochner, B.H. Standardization of Pelvic Lymphadenectomy Performed at Radical Cystectomy: Can We Establish a Minimum Number of Lymph Nodes That Should Be Removed? Cancer 2006, 107, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Faulkner, J.R.; Grossman, H.B.; Natale, R.B.; deVere White, R.; Sarosdy, M.F.; Crawford, E.D. Surgical Factors Influence Bladder Cancer Outcomes: A Cooperative Group Report. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 2781–2789. [Google Scholar] [CrossRef]
- Sargos, P.; Baumann, B.C.; Eapen, L.; Christodouleas, J.; Bahl, A.; Murthy, V.; Efstathiou, J.; Fonteyne, V.; Ballas, L.; Zaghloul, M.; et al. Risk Factors for Loco-Regional Recurrence after Radical Cystectomy of Muscle-Invasive Bladder Cancer: A Systematic-Review and Framework for Adjuvant Radiotherapy. Cancer Treat. Rev. 2018, 70, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Stadler, W.M.; Lerner, S.P.; Groshen, S.; Stein, J.P.; Shi, S.-R.; Raghavan, D.; Esrig, D.; Steinberg, G.; Wood, D.; Klotz, L.; et al. Phase III Study of Molecularly Targeted Adjuvant Therapy in Locally Advanced Urothelial Cancer of the Bladder Based on P53 Status. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3443–3449. [Google Scholar] [CrossRef]
- Cognetti, F.; Ruggeri, E.M.; Felici, A.; Gallucci, M.; Muto, G.; Pollera, C.F.; Massidda, B.; Rubagotti, A.; Giannarelli, D.; Boccardo, F.; et al. Adjuvant Chemotherapy with Cisplatin and Gemcitabine versus Chemotherapy at Relapse in Patients with Muscle-Invasive Bladder Cancer Submitted to Radical Cystectomy: An Italian, Multicenter, Randomized Phase III Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 695–700. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Skoneczna, I.; Kerst, J.M.; Albers, P.; Fossa, S.D.; Agerbaek, M.; Dumez, H.; de Santis, M.; Théodore, C.; Leahy, M.G.; et al. Immediate versus Deferred Chemotherapy after Radical Cystectomy in Patients with PT3–PT4 or N+ M0 Urothelial Carcinoma of the Bladder (EORTC 30994): An Intergroup, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2015, 16, 76–86. [Google Scholar] [CrossRef]
- Leow, J.J.; Martin-Doyle, W.; Rajagopal, P.S.; Patel, C.G.; Anderson, E.M.; Rothman, A.T.; Cote, R.J.; Urun, Y.; Chang, S.L.; Choueiri, T.K.; et al. Adjuvant Chemotherapy for Invasive Bladder Cancer: A 2013 Updated Systematic Review and Meta-Analysis of Randomized Trials. Eur. Urol. 2014, 66, 42–54. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Adjuvant Chemotherapy in Invasive Bladder Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data Advanced Bladder Cancer (ABC) Meta-Analysis Collaboration. Eur. Urol. 2005, 48, 189–199; discussion 199–201. [CrossRef]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaborators Group Adjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis of Individual Participant Data from Randomised Controlled Trials. Eur. Urol. 2022, 81, 50–61. [CrossRef] [PubMed]
- Fonteyne, V.; Dirix, P.; Van Praet, C.; Berghen, C.; Albersen, M.; Junius, S.; Liefhooghe, N.; Noé, L.; De Meerleer, G.; Ost, P.; et al. Adjuvant Radiotherapy After Radical Cystectomy for Patients with High-Risk Muscle-Invasive Bladder Cancer: Results of a Multicentric Phase II Trial. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, M.S.; Christodouleas, J.P.; Smith, A.; Abdallah, A.; William, H.; Khaled, H.M.; Hwang, W.-T.; Baumann, B.C. Adjuvant Sandwich Chemotherapy Plus Radiotherapy vs. Adjuvant Chemotherapy Alone for Locally Advanced Bladder Cancer after Radical Cystectomy: A Randomized Phase 2 Trial. JAMA Surg. 2018, 153, e174591. [Google Scholar] [CrossRef] [PubMed]
- di Meo, N.A.; Loizzo, D.; Pandolfo, S.D.; Autorino, R.; Ferro, M.; Porta, C.; Stella, A.; Bizzoca, C.; Vincenti, L.; Crocetto, F.; et al. Metabolomic Approaches for Detection and Identification of Biomarkers and Altered Pathways in Bladder Cancer. Int. J. Mol. Sci. 2022, 23, 4173. [Google Scholar] [CrossRef]
- Powles, T.; Assaf, Z.J.; Davarpanah, N.; Banchereau, R.; Szabados, B.E.; Yuen, K.C.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E.; et al. CtDNA Guiding Adjuvant Immunotherapy in Urothelial Carcinoma. Nature 2021, 595, 432–437. [Google Scholar] [CrossRef]
- Liu, W.; Tian, J.; Zhang, S.; Yang, E.; Shen, H.; Li, F.; Li, K.; Zhang, T.; Wang, H.; Svatek, R.S.; et al. The Utilization Status of Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Minerva Urol. Nephrol. 2021, 73, 144–153. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kwak, C.; Kim, H.H.; Ku, J.H. Prognostic Significance of Lymphovascular Invasion in Radical Cystectomy on Patients with Bladder Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e89259. [Google Scholar] [CrossRef]
- Murthy, V.; Bakshi, G.; Manjali, J.J.; Prakash, G.; Pal, M.; Joshi, A.; Dholakia, K.; Bhattacharjee, A.; Talole, S.; Puppalwar, A.; et al. Locoregional Recurrence after Cystectomy in Muscle Invasive Bladder Cancer: Implications for Adjuvant Radiotherapy. Urol. Oncol. 2021, 39, 496.e9–496.e15. [Google Scholar] [CrossRef]
| Total | ||
|---|---|---|
| N or Median | % or IQR | |
| N = 394 | ||
| Gender | ||
| Male | 319 | 81.0% |
| Female | 75 | 19.0% |
| Median age at diagnosis, years | 70 | 62–76 |
| Previous history of NMIBC | ||
| None | 270 | 68.5% |
| Yes | 116 | 29.4% |
| Missing | 8 | 2.0% |
| Tumor histology | ||
| UC | 369 | 93.7% |
| Squamous | 7 | 1.8% |
| Adenocarcinoma | 2 | 0.5% |
| Small cell | 3 | 0.8% |
| Micropapillary | 3 | 0.8% |
| Other | 10 | 2.5% |
| Clinical tumor stage | ||
| cT1 | 0 | 0% |
| cT2 | 290 | 73.6% |
| cT3 | 39 | 9.9% |
| cT4 | 32 | 8.1% |
| Missing | 33 | 8.4% |
| Clinical nodal stage | ||
| cN0 | 265 | 67.3% |
| cN1 | 28 | 7.1% |
| cN2 | 21 | 5.3% |
| cN3 | 3 | 0.8% |
| Missing | 77 | 19.5% |
| Neoadjuvant chemotherapy | ||
| No | 358 | 90.9% |
| Yes | 31 | 7.9% |
| Missing | 5 | 1.3% |
| Surgical approach | ||
| Open | 322 | 81.7% |
| Robotic | 30 | 7.6% |
| Laparoscopic | 19 | 4.8% |
| Missing | 23 | 5.9% |
| Urinary diversion | ||
| Neobladder | 38 | 9.6% |
| Ileal conduit | 195 | 49.5% |
| Continent cutaneous diversion | 8 | 2.0% |
| Cutaneous ureterostomy | 151 | 38.3% |
| Missing | 2 | 0.5% |
| Type of lymph nodes dissection | ||
| None | 70 | 17.8% |
| Standard | 248 | 62.9% |
| Extended | 69 | 17.5% |
| Missing | 7 | 1.8% |
| Median number of lymph nodes removed | 11 | (3–22) |
| Missing | 4.6% | |
| Pathologic staging at RC | ||
| pT0 | 11 | 2.8% |
| pT1 | 4 | 1.0% |
| pT2 | 42 | 10.7% |
| pT3 | 129 | 32.7% |
| pT4 | 204 | 51.8% |
| pTis | 4 | 1.0% |
| Concomitant carcinoma in situ at RC | ||
| No | 170 | 43.1% |
| Present | 103 | 26.1% |
| Missing | 121 | 30.7% |
| Pathologic lymph node staging at RC | ||
| pNx | 72 | 18.3% |
| pN0 | 167 | 42.4% |
| pN1 | 36 | 9.1% |
| pN2 | 87 | 22.1% |
| pN3 | 25 | 6.4% |
| Missing | 7 | 1.8% |
| Multifocal PSMs | ||
| No | 331 | 84.0% |
| Yes | 63 | 16.0% |
| Soft tissue PSMs | ||
| No | 105 | 26.6% |
| Yes | 283 | 71.8% |
| Missing | 6 | 1.5% |
| Urethral PSMs | ||
| No | 323 | 82.0% |
| Yes | 65 | 16.5% |
| Missing | 6 | 1.5% |
| Ureteral PSMs | ||
| No | 277 | 70.3% |
| Yes | 73 | 18.5% |
| Missing | 44 | 11.2% |
| Local-RFS | MFS | RFS | CSS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time from RC (Months) | % | (95% CI) | % | (95% CI) | % | (95% CI) | % | (95% CI) | % | (95% CI) |
| Overall Population | ||||||||||
| 12 | 52% | (46–58) | 50% | (44–55) | 44% | (38–49) | 64% | (58–69) | 55% | (49–60) |
| 24 | 38% | (32–44) | 38% | (32–43) | 31% | (26–37) | 49% | (43–55) | 40% | (34–45) |
| 36 | 31% | (26–38) | 30% | (24–36) | 24% | (19–30) | 42% | (35–48) | 33% | (27–38) |
| 48 | 28% | (22–34) | 27% | (21–33) | 22% | (16–27) | 36% | (29–42) | 28% | (22–33) |
| 60 | 24% | (19–30) | 24% | (19–30) | 20% | (14–24) | 30% | (24–37) | 23% | (18–29) |
| Median | 14 months | (11–17) | 12 months | (10–16) | 10 months | (8–12) | 23 months | (18–33) | 16 months | (12–19) |
| Multifocal PSMs | ||||||||||
| 12 | 37% | (23–50) | 29% | (17–42) | 23% | (12–36) | 47% | (32–60) | 38% | (24–50) |
| 24 | 19% | (8–33) | 35% | (19–51) | 15% | (6–27) | 31% | (17–46) | 23% | (12–36) |
| 36 | 15% | (5–29) | 21% | (11–34) | 7% | (2–20) | 23% | (11–39) | 17% | (10–33) |
| 48 | 11% | (3–25) | 6% | (1–17) | 7% | (2–20) | 12% | (8–35) | 9% | (2–20) |
| 60 | 7% | (1–20) | 6% | (1–17) | 3% | (0–15) | 8% | (1–21) | 6% | (1–16) |
| Median | 8 months | (5–14) | 7 months | (3–11) | 5 months | (3–10) | 9 months | (7–19) | 8 months | (6–11) |
| Soft Tissue PSMs | ||||||||||
| 12 | 47% | (40–54) | 43% | (37–50) | 38% | (32–44) | 58% | (51–64) | 50% | (43–56) |
| 24 | 32% | (25–38) | 29% | (23–36) | 25% | (19–31) | 40% | (33–47) | 33% | (26–39) |
| 36 | 27% | (20–33) | 23% | (16–30) | 19% | (13–25) | 35% | (27–42) | 28% | (21–34) |
| 48 | 23% | (17–30) | 20% | (14–27) | 18% | (12–24) | 28% | (20–36) | 22% | (16–28) |
| 60 | 20% | (13–28) | 19% | (13–26) | 16% | (11–23) | 25% | (18–33) | 18% | (12–25) |
| Median | 11 months | (9–14) | 11 months | (8–12) | 9 months | (6–11) | 16 months | (13–21) | 12 months | (10–16) |
| Urethral PSMs | ||||||||||
| 12 | 52% | (38–64) | 54% | (40–66) | 45% | (31–57) | 68% | (53–79) | 56% | (43–68) |
| 24 | 42% | (29–56) | 44% | (31–57) | 38% | (25–51) | 56% | (41–69) | 43% | (30–56) |
| 36 | 35% | (21–49) | 37% | (24–50) | 30% | (18–44) | 48% | (33–62) | 35% | (23–48) |
| 48 | 29% | (16–43) | 29% | (17–42) | 24% | (13–38) | 40% | (25–54) | 29% | (17–41) |
| 60 | 23% | (12–37) | 26% | (14–40) | 21% | (10–35) | 30% | (17–45) | 20% | (10–32) |
| Median | 13 months | (7–30) | 15 months | (5–37) | 9 months | (5–27) | 30 months | (14–53) | 15 months | (6–30) |
| Ureteric PSMs | ||||||||||
| 12 | 66% | (48–73) | 58% | (45–69) | 51% | (38–63) | 77% | (64–86) | 55% | (49–60) |
| 24 | 49% | (34–61) | 52% | (38–64) | 40% | (27–52) | 67% | (53–78) | 40% | (34–45) |
| 36 | 37% | (23–51) | 36% | (23–50) | 27% | (16–40) | 52% | (36–66) | 33% | (27–38) |
| 48 | 37% | (23–51) | 34% | (21–47) | 27% | (16–40) | 46% | (30–61) | 28% | (22–33) |
| 60 | 34% | (20–48) | 28% | (16–42) | 22% | (11–35) | 39% | (24–55) | 23% | (18–29) |
| Median | 23 months | (14–53) | 30 months | (8–36) | 14 months | (7–30) | 39 months | (26-NR) | 28 months | (18–51) |
| Loco Regional RFS | MFS | RFS | OS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | p | HR | 95% Confidence Interval | p | HR | 95% Confidence Interval | p | HR | 95% Confidence Interval | p | |||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |||||||||
| Age at Diagnosis | - | - | - | - | - | - | - | - | - | - | - | - | 1.01 | 1.01 | 1.03 | 0.015 |
| Number of lymph nodes removed | 0.98 | 0.97 | 0.99 | 0.022 | 0.98 | 0.97 | 0.99 | 0.007 | 0.98 | 0.97 | 0.99 | 0.007 | - | - | - | - |
| Pathologic staging at RC | ||||||||||||||||
| pT0-is-1–2 | Ref | Ref | Ref | Ref | ||||||||||||
| pT3–4 | 3.32 | 1.84 | 5.99 | <0.0001 | 2.80 | 1.66 | 4.70 | <0.0001 | 2.63 | 1.56 | 4.46 | 0.0003 | 3.37 | 1.93 | 5.87 | <0.0001 |
| Pathologic lymph node staging at RC | ||||||||||||||||
| pN0 | Ref | Ref | Ref | Ref | ||||||||||||
| pN1–2-3 | 2.14 | 1.49 | 3.08 | <0.0001 | 2.60 | 1.86 | 3.63 | <0.0001 | 2.43 | 1.72 | 3.44 | <0.0001 | 1.83 | 1.32 | 2.53 | 0.0003 |
| Type of positive surgical margin | ||||||||||||||||
| Soft tissue PSM | Ref | Ref | Ref | Ref | ||||||||||||
| Urethral PSM | 1.03 | 0.62 | 1.71 | 0.909 | 0.90 | 0.53 | 1.55 | 0.72 | 0.92 | 0.55 | 1.52 | 0.75 | 1.08 | 0.67 | 1.72 | 0.74 |
| Ureteral PSM | 0.85 | 0.48 | 1.50 | 0.587 | 0.73 | 0.42 | 1.28 | 0.27 | 0.87 | 0.52 | 1.45 | 0.59 | 0.65 | 0.37 | 1.14 | 0.13 |
| Multifocal PSMs | 1.44 | 0.98 | 2.11 | 0.056 | 1.51 | 1.04 | 2.18 | 0.02 | 1.47 | 1.02 | 2.12 | 0.03 | 1.50 | 1.06 | 2.13 | 0.02 |
| Adjuvant chemotherapy | ||||||||||||||||
| No | Ref | Ref | Ref | |||||||||||||
| Yes | 0.58 | 0.40 | 0.84 | 0.004 | 0.65 | 0.45 | 0.93 | 0.02 | 0.59 | 0.41 | 0.83 | 0.003 | 0.58 | 0.40 | 0.83 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcq, G.; Afferi, L.; Neuzillet, Y.; Nykopp, T.; Voskuilen, C.S.; Furrer, M.A.; Kassouf, W.; Aziz, A.; Bajeot, A.S.; Alvarez-Maestro, M.; et al. Oncological Outcomes for Patients Harboring Positive Surgical Margins Following Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Retrospective Multicentric Study on Behalf of the YAU Urothelial Group. Cancers 2022, 14, 5740. https://doi.org/10.3390/cancers14235740
Marcq G, Afferi L, Neuzillet Y, Nykopp T, Voskuilen CS, Furrer MA, Kassouf W, Aziz A, Bajeot AS, Alvarez-Maestro M, et al. Oncological Outcomes for Patients Harboring Positive Surgical Margins Following Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Retrospective Multicentric Study on Behalf of the YAU Urothelial Group. Cancers. 2022; 14(23):5740. https://doi.org/10.3390/cancers14235740
Chicago/Turabian StyleMarcq, Gautier, Luca Afferi, Yann Neuzillet, Timo Nykopp, Charlotte S. Voskuilen, Marc A. Furrer, Wassim Kassouf, Atiqullah Aziz, Anne Sophie Bajeot, Mario Alvarez-Maestro, and et al. 2022. "Oncological Outcomes for Patients Harboring Positive Surgical Margins Following Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Retrospective Multicentric Study on Behalf of the YAU Urothelial Group" Cancers 14, no. 23: 5740. https://doi.org/10.3390/cancers14235740
APA StyleMarcq, G., Afferi, L., Neuzillet, Y., Nykopp, T., Voskuilen, C. S., Furrer, M. A., Kassouf, W., Aziz, A., Bajeot, A. S., Alvarez-Maestro, M., Black, P., Roupret, M., Noon, A. P., Seiler, R., Hendricksen, K., Roumiguie, M., Pang, K. H., Laine-Caroff, P., Xylinas, E., ... Sargos, P., on behalf of the YAU Urothelial Group. (2022). Oncological Outcomes for Patients Harboring Positive Surgical Margins Following Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Retrospective Multicentric Study on Behalf of the YAU Urothelial Group. Cancers, 14(23), 5740. https://doi.org/10.3390/cancers14235740









