Simple Summary
Colorectal cancer (CRC) is a highly prevalent form of cancer, and represents a serious, global, health threat. Available therapeutic approaches have failed to provide control over the increasing prevalence and incidence of CRC. In this context, CRC prevention may provide a fruitful strategy. Edible plants have the potential to alter numerous molecular pathways, which may fight against the pathogenesis of CRC, and the gut microbiota could represent this link between dietary factors and CRC incidence. Spices and their active principles are reported to alter the balance of gut microbial species by increasing eubiotic and decreasing dysbiotic strains. The present study is designed to highlight the cancer prevention potential of spices while focusing mainly on gut microbial modulation. Although several spices and their active components have shown CRC-preventing properties via gut microbial modulation, the literature is still very limited, and expanding the literature going forward is essential before any conclusion can be drawn.
Abstract
Colorectal cancer (CRC) is the second most frequent cause of cancer-related mortality among all types of malignancies. Sedentary lifestyles, obesity, smoking, red and processed meat, low-fiber diets, inflammatory bowel disease, and gut dysbiosis are the most important risk factors associated with CRC pathogenesis. Alterations in gut microbiota are positively correlated with colorectal carcinogenesis, as these can dysregulate the immune response, alter the gut’s metabolic profile, modify the molecular processes in colonocytes, and initiate mutagenesis. Changes in the daily diet, and the addition of plant-based nutraceuticals, have the ability to modulate the composition and functionality of the gut microbiota, maintaining gut homeostasis and regulating host immune and inflammatory responses. Spices are one of the fundamental components of the human diet that are used for their bioactive properties (i.e., antimicrobial, antioxidant, and anti-inflammatory effects) and these exert beneficial effects on health, improving digestion and showing anti-inflammatory, immunomodulatory, and glucose- and cholesterol-lowering activities, as well as possessing properties that affect cognition and mood. The anti-inflammatory and immunomodulatory properties of spices could be useful in the prevention of various types of cancers that affect the digestive system. This review is designed to summarize the reciprocal interactions between dietary spices and the gut microbiota, and highlight the impact of dietary spices and their bioactive compounds on colorectal carcinogenesis by targeting the gut microbiota.
1. Introduction
Colorectal cancer (CRC) is the most prevalent form of carcinoma, and represents a leading component of the global health burden. Advancements in treatment methods, colonoscopy, and avoidance of risk factors, such as smoking and red meat consumption, have contributed to a decline in CRC cases over the last three decades in the United States [1,2]. However, similar declines have only been observed in developed countries [3]. Despite innovative strategies of treatment and diagnosis, CRC remains the third most common cancer and the second leading cause of mortality across the globe. In the year 2018 alone, 1.8 million new CRC cases were recorded including 881,000 deaths [4]. CRC cases may rise to 2.5 million by the year 2035 [3]. The modifiable risk factors for CRC include obesity [5], cigarette smoking [6], heavy alcohol use [7], poor diet [8], and a sedentary lifestyle [9]. The genetic contribution towards CRC is in the range of 12–35% as demonstrated in twin and family studies [10,11]. While 60–65% of cases arise sporadically without any family history of CRC [12]. This sizeable sporadic contribution to the instigation of CRC shows the significance of environmental factors, which play a large role in causing CRC [13]. Among environmental factors, infectious agents are responsible for 15 percent of all cancers [14]. Colorectal carcinogenesis is a process involving years of development, possibly taking decades. In such scenarios, early life risk factors and lifestyle modification are pertinent contributors [15]. The current rise of CRC in the young adult population in the US is alarming [2], and this supports the concept that early life risk factors provide a major impact on CRC carcinogenesis [16].
The human microflora counts around thirty trillion bacteria without considering fungi and viruses. The microbiota is not only altered by the environment but also by the relationship between the host and the symbiotic organisms [17]. The total number of microbial cells is 10 times greater than that of human somatic cells [18,19,20,21,22] and these include over 1000 different species of bacteria populating our gut. Most of these belong to the Firmicutes and Bacteroides phyla and are linked to the protection of the host, as they can produce metabolites and bioproducts promoting a protective effect against different pathologies. The dietary compounds and vitamins produced by these bacteria are considered protective elements against the infiltration of gut pathogens and the development of pathologies [23,24,25]. The impairment of the microbiota could lead to dysbiosis, and several studies sustain this link between tumorigenesis and microbiome diversity, thanks to the combination of next-generation sequencing and computational analysis [26,27,28,29,30,31]. A well-regulated microbiome is essential for maintaining the homeostasis of the metabolism and immune response, in fact, several clinical studies underline how the immunotherapeutic response could be influenced by the gut microbiome, suggesting that treatments could be enhanced or depressed according to the gut microbiota status [31,32,33,34].
Another important role of the microbiome is the recognition of the conserved regions of Gram-negative pathogenic bacteria after the production of immunoglobin G antibodies [35,36]. However, the composition and the alteration of the microbiota are also related to different host life stages and diets [37,38,39,40,41]. It is calculated that 20% of all cancers are related to dysbiosis, and with this perspective, probiotics could be used as therapeutic agents to re-establish the normal microbial environment, enhancing the immune response to counteract tumor growth. Literature data have shown that gut microbiota may provide the missing link between dietary factors and CRC incidence [42]. Some dietary components, such as saturated fats, processed carbohydrates, red meat, and ultra-processed food can affect the gut microbiota and lead to inflammation [43], and inflammation is a known factor for 20–30% of CRC cases and is acknowledged as the principal driver of tumorigenesis [44,45,46].
While chemotherapy and radiotherapy are the key approaches employed for the treatment of patients with cancer, both are associated with serious adverse events that may outweigh their therapeutic benefits in certain cases. Drug resistance is another concern that is very common for anticancer therapies and may result in failure of the treatment [47]. Nature has provided a range of preventive and therapeutic agents with the potential to fight against the most devastating chronic disorders including cancer [48,49]. Edible plants containing phytochemicals are known to alter numerous molecular pathways that may impact anticancer effects (i.e., oxidative stress, inflammatory cascade, apoptosis, epigenetic regulation, p53 signaling pathway, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) pathway, mitogen-activated protein kinases (MAPKs), proteasome pathway, insulin-like growth factor-I mediated signal transduction pathway, matrix metalloproteinases (MMPs), vascular endothelial growth factor, Hippo signaling pathway, phosphoinositide 3-kinase–protein kinase B–mammalian target of a rapamycin signaling pathway (PI3K/Akt/mTOR), cyclooxygenase-2, and the Janus kinase–signal transducer and activator of transcription signaling pathway) [50,51,52,53,54,55,56,57].
Some spices such as turmeric, black cumin, ginger, ginseng, garlic, saffron, and black pepper, are potential sources of cancer prevention owing to their natural bioactive compounds (curcumin, thymoquinone, piperine, and capsaicin) [58,59,60]. About 80% of the world population is currently relying on phytomedicine for their primary healthcare [61], in fact, these natural products are commonly considered a safer alternative for patients, if compared to systematic chemotherapeutic drugs although their scientific validity and efficacy are currently under analysis [62,63]. These spices and herbs have been used for thousands of years in small amounts thanks to their beneficial effects. In particular, curcuma, ginger, garlic, clove, chili pepper, saffron, and flaxseed seem to inhibit CRC growth thanks to their chemotherapeutic roles [58,64,65,66]. CRC development is sustained by cancer stem cells (CSC), which are self-renewal and pluripotent stem cells able to promote carcinogenesis and the formation of heterogeneous tumors [67]. Increasing evidence sustains the link between microbiota alterations and mature tumor formation. In particular, their metabolome [68] can promote pro or anti-carcinogenic actions. The preservation of the CSC is essential and mediated by several phytochemicals such as curcumin, quercetin, lycopene, cinnamic acid, resveratrol, sibilin, and epigallocatechin-3-gallate [EGCG] [69]. The main pathways involved in the regulation of the CSC phenotype are Hedgehog, Notch, and Wnt/β-catenin [70], which are modulated thanks to the colonic microbiota transformation of phytochemicals. At the same time, these substances can modify the microbiota population. Thus, the diet can change the colonic bacteria and vice versa in a triangular rapport where is involved CRC formation.
The previous similar reviews [71,72] focused their attention on the molecular basis of CRC linking the antioxidant/anti-inflammatory activities of these spices or other diet-derived phytochemicals and CRC pathogenesis. In some cases, recent articles also considered the relationship between the dietary compounds and the gut microbiota-derived metabolites without considering that these two aspects are essential for CRC prevention. In fact, year after year, it is clear how new discoveries on CRC lead to the hypothesis that the anti-inflammatory and antioxidant activities of herbs and spices, normally consumed in the diet, are not the only mechanism of action that intervenes in CRC prevention. The role of microbiota, in fact, seems to be crucial in the modulation of the microbiome and control of the CSC population. The aim of this review is to focus on spice-derived bioactive compounds influencing gut microbiota strains, with special reference to CRC prevention.
2. Gut Dysbiosis and Carcinogenesis
The maintenance of healthy gut microbiota during an individual’s lifespan, and any potential loss of diversity, is strictly connected with their diet. The progression of a disease could also involve the long-term depletion of specific groups of bacteria, which could be induced by lifestyle changes and other societal factors [37,38]. Healthy conditions are completely different from those of patients affected by dysbiosis. In the first case, the immune system can easily recognize pathogenic microbes, promoting their consequent elimination [73], most gut bacteria are non-pathogenic, and they offer an important defense role in inhibiting colonization by pathogens. The immune cells (i.e., dendritic cells, macrophages, and phagocytes) are involved in the gut microbiome and are essential for the recognition of pathogenic bacteria [74]. Healthy individuals could suffer either mild or severe issues if bacteria translocate across the epithelial mucosa. Kupffer cells may be involved, after the production of endotoxins and viable or dead bacteria. However, in the case of dysbiosis, the commensal bacteria may also spread into extra-intestinal sites and tissues. Obviously, this event can promote septic shocks, sepsis, organ failure, and death [75] over short-term periods. The dysregulation of the microbiota is associated with various pathologies, and this could be also induced using antibiotics which are known to reduce microbiotal diversity. The state of the art sustains that diabetes types 1 and 2, obesity, arthritis, Crohn’s disease, arthritis, and celiac disease are linked with the deregulation of the microbiotal metabolism and inflammation, which promotes the incidence of these pathologies [75,76,77,78,79,80].
Obviously, the microbiota is strongly involved in the absorption and metabolization of nutrients, thanks to the expression of a great number of genes, which are not expressed in our own organism. The impairment and the downregulation of these processes can promote inflammation, which may also lead to cancer in the longer-term [81,82]. The increased incidence and prevalence of cancer over recent decades are mainly due to a higher exposure to cancer-causing molecules, but also to high-fat diets, which promote dysbiosis and the inflammation process [78]. The microbial alteration could be one of the main factors, which contribute to carcinogenesis [83], in fact, different studies have supported the importance of the relationship between carcinogenesis and lifestyle. The inflammation process remains a driving force in the progression of cancer, promoting its development through the production of inflammatory cytokines [84], with microbial dysbiosis leading to increased concentrations of interleukin (IL)-1, 6, 10, and tumor necrosis factor alpha (TNF-α). The production of IL-10 is essential for the body’s elimination of cancer, in fact it is considered the most effective anti-inflammatory cytokine involved in tumorigenesis [85,86,87]. Wnt signaling is involved with NF-ĸB and MAPKs, which together can lead to an increase in oxidative stress and inhibition of apoptosis [88,89]. Animal and human studies have shown that bacteria such as Fusobacteria, Alistipes, Porphyromonadaceae, Coriobacteridae, Staphylococcaceae, Akkermansia species and Methanobacteriales are predominantly increased in CRC, while Lactobacillus, Bifidobacterium, Faecalibacterium species, Treponema, Roseburia, and Ruminococcus are known to reduce [90].
The production of toxins can also influence the tumorigenesis process, with Helicobacter pylori, Escherichia coli, and Shigella flexneri, for example, inducing double-strand DNA cuts causing apoptosis or alteration of the cell cycle [91]. Starting from E. coli, colibactin and cytolethal distengin toxins induce genomic instability, promoting breaks in the host’s DNA and tumorigenesis [89]. S. Flexneri instead produces cysteine proteases, such as virulence gene A and the inisitol phosphate phosphatase D, with the final response, in this case, being necrosis, with the development of cancer and cell death due to the degradation of the p53 gene and host damage [92]. Fusobacterium nucleatum disrupts the junction of β-catenin through the effector adhesin A (FadA); moreover, it is responsible for the production of virulence factor (Fap2) but in this case, it is through the mediation of blocks of natural killer cells (NK cells) through the binding of the NK inhibitory receptor [92,93,94]. Bacteroides fragilis produces a toxin responsible for DNA damage after the production of reactive oxygen species and hydrogen peroxide [95], the same is the case for Enterococcus fecalis, which is responsible for the production of extracellular superoxide, able to trigger mutations in host DNA [55]. Finally, Lactobacillus casei is responsible for the production of the ferrichrome siderophore, which activates c-Jun N-terminal kinase (JNK) signaling and consequent apoptosis [96].
3. Gut Microbial Alteration, Chemotherapy, and Cancer Prevention
Our gut contains trillions of microorganisms interacting with the host, and it is important to underline their essential role in bodily function. Digestion, secretion of metabolites, and the intervention of the immune system as cited above, are strictly related to the microbiota. Bacteria-free mouse models underline how dysbiosis is related to immunoglobulin A, lymphadenitis, and the absence of mucus [97,98]. Cancers very often become resistant to the drugs most used for their treatment [99,100], and unfortunately in 90% of cases, this phenomenon is responsible for the patient’s death [101,102,103]. Obviously, this problem requires attention and time to promote the development of new treatments, and the gut microbiota in particular may also influence the efficacy of antitumor therapies [104].
The negative impact of the absence of a microbiota is becoming clearer year after year, with different studies on mice treated with antibiotics underlining the efficacy of chemotherapy and immunotherapy [105]. Moreover, it is possible that the efficacy of chemotherapy treatments may be heightened under normal conditions, promoting the destruction of cancer through the intervention of T-lymphocytes and myeloid cells. The antibiotic treatments applied in certain mice studies [106] can impair the presence of bacteria and the production of cytokines, however further clinical studies are required to confirm these preliminary findings. The combination of metabolomics and metagenomics underlines the importance of the gut-brain axis [107], which regulates the composition of the gut flora through the production of neuro-hormones and hormones. The case of cyclophosphamide is particularly interesting, a chemotherapeutic drug able to promote the T-cell immune response in the presence of commensal microbiota, which translocates from the spleen to the lymph nodes promoting their anticancer effect [94,108]. It appears that Bifidobacterium can enhance dendritic cells, promoting the activation of T CD8-positive cells and enhancing the efficiency of anti-programmed death ligand (PDL-1) therapy [109]. The five-year survival rate was found to have increased by 80% for 1000 sarcoma patients treated with killed microorganism activate (Serratia and Streptococcus) [110]. The T lymphocytes associated with antigen 4 (CTLA-4) seem to have anticancer effects, promoting the production of CTLA-4 inhibitors. In the absence of CTLA-4, germ-free mice registered a positive response against cancer following an exposure to Bacteroides [111] underlying the anticancer effects of these molecules.
Only a few studies to date appear to sustain the relationship between cancer prevention and the microbiota. The production of short-chain fatty acids (SCFAs) by microbiota (i.e., Propionibacteria such as P. freudenreichii) [112,113,114] has an anti-cancer effect [115], inhibiting the deacetylases of cancer cells. Indeed, a lower concentration of butyrate is registered in cancer patients. The production of SCFAs stimulates the production of IL-18, promoting the healing process in mucosal tissues [116]. Probiotic administration also exhibits interesting effects, as it seems to trigger the immune response with an antitumor effect. Gram-negative bacteria activate TLR4 and T-cells, with Salmonella enterica, for example, appearing to be very effective against cervical cancer [117]. Finally, L. casei stimulates apoptosis in cancer cells thanks to ferricrome production, through the activation of the JNK signaling pathway [89].
4. Spice-Derived Phytochemicals and CRC Prevention by Modulating Gut Bacteria for In Vivo Studies
Predominantly used as flavoring, coloring, and aromatic agents in beverages and foods, spices are gaining attention for their potential health benefits. The nutritional, antioxidant, anti-inflammatory, antimicrobial, and other medicinal uses of spices have paramount importance [118]. Numerous health benefits of these food adjuncts have been recognized by pioneering experimental studies involving both in vitro and in vivo studies over the past few decades, including their antioxidant and anti-inflammatory potential, digestive stimulant effects, hypolipidemic actions, anti-lithogenic properties, antidiabetic influence, antimutagenic, and anticarcinogenic potentials [119]. Studies have shown that spices and their bioactive compounds may inhibit or even activate pathways related to cell division, proliferation, and detoxification, in addition to immunomodulatory and anti-inflammatory effects [120]. The chemopreventive properties of spice-derived phytochemicals are mainly attributed to the regulation of B-cell leukemia/lymphoma 2 protein, K-ras, MMP pathways, apoptotic pathway, and caspase activation [71]. Considering the scope of the current review, a link between gut microbial modulation by spices and the prevention of CRC pathogenesis has been comprehensively discussed in the sections below. Table 1 summarizes these studies, highlighting the effects of spice-derived phytochemicals on gut microbiota and their ultimate effect on intestinal health. Figure 1 and Figure 2 illustrate the modulation of gut microbes with spices as part of CRC prevention.

Table 1.
Summary of anti-colon cancer effects of spice phytocompounds/phytocomplex by modulation of gut microbiota in in vivo studies.
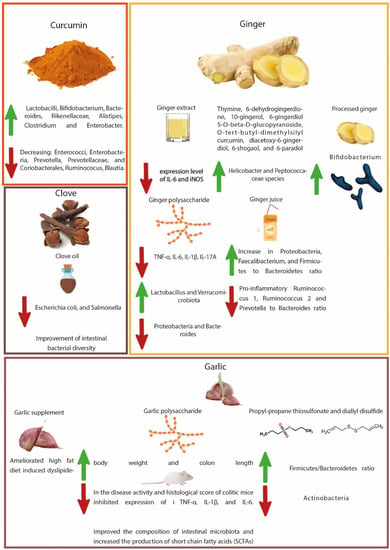
Figure 1.
Illustration of gut microbial modulation with curcumin, ginger, garlic, and clove regards to CRC prevention.
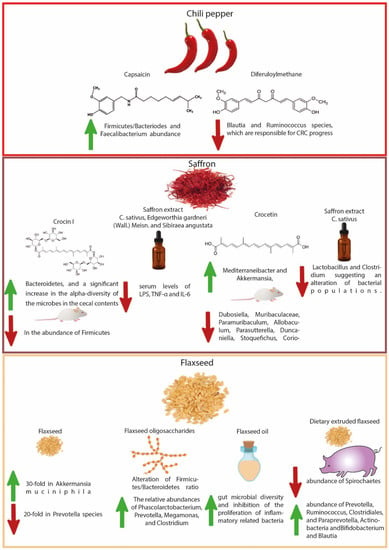
Figure 2.
Illustration of gut microbial modulation with chili pepper, saffron, and flaxseed regards to CRC prevention.
4.1. Turmeric-Derived Compounds
Curcumin, derived from the roots of the plant known as Curcuma longa L., is a natural product that has been extensively studied for the prevention and treatment of cancer [143,144]. Curcumin exerts its anticancer action via various mechanisms, e.g., by inducing apoptosis, thereby inhibiting cell proliferation of cancerous cells, activating caspase, and inducing the expression of anti-oncogenes such as p53 [145,146]. Interruptions in mucosal barrier function play a significant role in CRC. The persistent inflammation of, and oxidative stress within, intestinal epithelial cells are the most evident causes of colorectal carcinogenesis. Dysfunctions in the mucosal barrier further synergize with this vicious progression of carcinogenesis [147]. The circulating lipopolysaccharide (LPS), due to dysfunction in the gut microbiota, may be a possible cause for the development of chronic inflammatory disorders. The translocation of LPS into systemic circulation occurs due to a dysfunction in the intestinal barrier [148]. The western style diet has been reported to increase intestinal permeability and may be responsible for intestinal barrier dysfunction [149]. Many studies have demonstrated that pretreatment with curcumin attenuates LPS-induced inflammatory cytokines by modulating the p38 MAPK pathway. Curcumin exerts this action most likely on intestinal epithelial cells, thereby reducing intestinal barrier dysfunction [150,151].
The higher concentration of curcumin in the gastrointestinal tract after oral administration suggests that it may regulate the gut microbiota, resulting in various pharmacological actions despite its low systemic bioavailability [121,152]. The present data suggest that curcumin is metabolized by the gut microbiota into different metabolites through diverse pathways, including demethoxylation, hydroxylation, and demethylation. Moreover, these metabolites have been found to be more active compared to the parent molecule curcumin. The higher concentration of curcumin in the gastrointestinal tract after oral administration shows its preferential impact on gut microbiota composition. On the other hand, the processing of the parent molecule transforms it into its bioactive metabolites, resulting in its various therapeutic and pharmacological actions [153].
As evidenced in many studies, curcumin shows a direct influence on gut microbiota by increasing the ratio of beneficial bacteria compared to pathological ones [154,155,156]. An in vivo study has shown a significant effect of curcumin on numerous bacterial families in the gut including Prevotellaceae, Rikenellaceae, and Bacteroidaceae [155]. Curcumin administration considerably alters the ratios of beneficial and pathogenic intestinal microflora by enhancing the number and diversity of Lactobacilli and Bifidobacterium, and decreasing the bacterial load of Enterococci, Enterobacteria, Prevotellaceae, and Coriobacterales, thus explaining its immune modulation and anti-tumor effects in the colon [121]. Curcumin administration to mice significantly increased the number of Bacteroides, Rikenellaceae, Alistipes, and Bacteroidaceae while decreasing the number of Prevotella and Prevotellaceae [122]. The number of Prevotella has been observed to be higher in patients with CRC, compared to cancer-free patients [157].
In patients with CRC, increased levels of Ruminococus species of bacteria have been noticed in the gut microbiota [158,159]. Interestingly, a pilot study elucidated that curcumin, when used as a dietary supplement, reduced Ruminococus and Blautia bacterial species, and increased the population of Clostridium and Enterobacter in gut microbiota [123]. The suppressive activity of curcumin on gut microbiotal species shows its anticancer potential in preventing CRC. Through modulating gut microbiota, the administration of nanoparticles of curcumin in mice has demonstrated increased numbers of butyrate-producing bacteria, increased fecal butyrate levels, and suppressed NF-ĸB activation, in colonic epithelial cells. Moreover, it also downregulated the expression of mucosal mRNA in inflammatory mediators [124].
Supplementation of rats with curcumin showed improvements in fecal microbes (reduced Coriobacterales and increased Lactobacillales), resulting in the regulation of the host immune system, which in turn lowered oxidative and inflammatory stresses, and hyper-immune activation, which may lower the incidence of inflammatory gastrointestinal disorders such as inflammatory bowel disease (IBD) [125]. Literature also reported the eradication of H. pylori production with curcumin treatment and its attachment to the human gastric adenocarcinoma cell lines die to its anti-adhesion properties [160,161,162]. Treatment of animals (infected with Toxoplasma gondii) with curcumin not only reduced the number of pro-inflammatory Enterobacteria and Enterococci, but also increased the abundance of anti-inflammatory Lactobacilli and Bifidobacteria [126]. Oral supplementation of curcumin alleviated acute inflammation of the small intestine by downregulating the Th1-type immune response and preventing bacterial translocation by maintaining the intestinal-barrier function [149]. It inhibited mRNA expression on the mucosa on inflammatory mediators and activated NF-ĸB in colon epithelial cells accompanied by enhanced butyrate-producing bacteria and fecal butyrate levels.
4.2. Ginger-Derived Compounds
Ginger rhizome (Zingiber officinale Roscoe) belonging to the plant family Zingiberaceae, is extensively used as a hot dietary spice in foods and drinks because of its distinctive flavor [163]. Ginger rhizome has a rich chemistry, containing phenolic compounds, terpenes, polysaccharides, organic acids, and raw fibers [164]. The volatile oil components of ginger include sesquiterpenes, zingerberene, curcumene, farnesene, and 40 different monoterpenoid hydrocarbons [165] while the main non-volatile active compounds of ginger include geingerols, shogoals, paradols and zingerone [166,167]. The active constituents [6]-shogaol and [6]-gingerol have shown anti-proliferative activity against various forms of gastrointestinal cancer [167].
As evidenced by numerous studies, ginger extract has a protective activity against ulcerative colitis, a chronic IBD of unknown pathology [168,169,170]. Recently Guo et al. [127] identified the mechanism by which ginger ameliorates dextran sulfate sodium (DSS) induced ulcerative colitis. They found that oral administration of ginger extract modulates the gut microbiota, where it reduces the population of pathogenic bacteria such as Lactobacillus murinus, Lachnospiraceae bacterium 615, and Ruminiclostridium_sp. KB18. Moreover, the ginger extract also reduces the expression level of mRNA of inflammatory cytokines, such as IL-6 and inducible nitric oxide synthase. These studies show that ginger most likely modulates the gut microbiota to reduce inflammation, consequently preventing CRC.
An in vivo study demonstrated a decrease in susceptibility to DSS-induced colitis in mice with ginger extract (containing 16-compounds including thymine, 6-dehydrogingerdione, 10-gingerol, 6-gingerdiol 5-O-β-D-glucopyranoside, O-tert-butyl-dimethylsilyl curcumin, diacetoxy-6-gingerdiol, 6-shogaol, and 6-paradol) following antibiotic exposure in early life [128]. Supplementation to mice with ginger extract for 4-weeks ameliorated weight loss, colon shortening, inflammatory cascade, intestinal barrier dysfunction, and gut dysbiosis. It increased the bacterial diversity and altered the abundance of Helicobacter and Peptococcaceae species, modulating gut microbial structure and composition adversely affected by antibiotic exposure. A Japanese traditional herbal medicine (Daikenchuto), containing processed ginger, ginseng, and Chinese or Japanese pepper, significantly promoted the growth of Bifidobacterium adolescentis, but not that of E. coli and Fusobacterium nucleatum, in human fecal samples, suggesting an in vitro bifidogenic effect that may contribute to the beneficial effects on colon [129]. Ginger polysaccharides relieved DSS-induced ulcerative colitis in mice via gut microbial modulation, maintaining intestinal barrier integrity [130]. Ginger polysaccharides reduced the level of colonic pro-inflammatory mediators such as TNF-α, IL-6, IL-1β, IL-17A, and interferon (IFN)-γ. In addition, ginger polysaccharides restored gut barrier function, restrained apoptosis, and modulated gut microbiota (by balancing Firmicutes/Bacteroidetes ratio, increasing Lactobacillus and Verrucomicrobiota, and decreasing Proteobacteria and Bacteroides). An intervention with ginger juice in healthy adults decreased the relative abundance of pro-inflammatory Ruminococcus_1 and Ruminococcus_2 and Prevotella to Bacteroides ratio, with an increase in Proteobacteria, Faecalibacterium, and Firmicutes to Bacteroidetes ratio [131].
4.3. Garlic-Derived Compounds
Garlic (Allium sativum L.), belonging to the plant family Liliaceae, is a widespread dietary spice consumed around the globe [171,172]. Garlic consists of various bioactive compounds such as saponins, phenolic compounds, organosulfur compounds, and polysaccharides [173]. The presence of bioactive organosulfur compounds in garlic raises the possibility of anticancer activity [174,175,176,177]. Garlic has a paradoxical effect on the gut microbiota, however, whole garlic supplementation has revealed that it increases the α-diversity of the gut microbiota and as a result ameliorated high-fat diet-induced dyslipidemia [178]. Similarly, the GarGIC Trial results showed that the administration of Kyolic aged garlic extract lowered blood pressure in hypertensive patients by reducing arterial stiffness, inflammation and improving the gut microbiotal profile [179]. The anticancer action of garlic has been explored by its interaction with multiple pathways in carcinogenesis. More experimental and clinical trials are necessary to identify the role of garlic in cancer and particularly in CRC via gut microbiota modulation.
A. sativum polysaccharides (200 or 400 mg/kg/day) demonstrated anti-inflammatory activities via modulation of gut microbiota in an experimental model of DSS-induced colitis [132]. Garlic polysaccharides increased body weight and colon length with a decrease in disease activity and histological scores of colitic mice as well as inhibiting the expression of inflammatory mediators i.e., TNF-α, IL-1β, and IL-6. Moreover, they improved the composition of intestinal microbiota and increased the production of SCFAs. The key intestinal microbial strains associated with the inflammatory intestinal conditions identified were Muribaculaceae, Lachnospiraceae NK4A136 group, Lachnospiraceae, Helicobacter, Mucispirillum, Ruminococcus 1, and Ruminiclostridium 5. Propyl-propane thiosulfonate (one of the biologically active compounds present in A. sativum) modulated immune responses, contributing to anti-inflammatory effects in experimental colitis [133]. The immunomodulatory effects of propyl-propane thiosulfonate were supported by reducing the in vitro production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and downstream regulation of MAPK-signaling pathways (p44/42 ERK and p38), and in vivo by improving the intestinal epithelial barrier integrity, reducing the expression of pro-inflammatory mediators (TNF-α, IL-1β, IL-8, IL-17, and iNOS), and restoration of gut microbial alteration induced by DSS exposure (increased Firmicutes/Bacteroidetes ratio and decreased Actinobacteria). On the contrary, another study showed alteration of gut microbiota with diallyl disulfide and induction of fatty liver in the same fashion as caused by a high-fat diet [180].
4.4. Clove-Derived Compounds
Clove (Syzygium aromaticum (L.) Merr. and L.M.Perry) belongs to the Myrtaceae plant family, and is one of the oldest and most valuable dietary spices [181]. The major bioactive constituents of clove oil are eugenol (70–90%) eugenyl acetate, β-caryophyllene, and various sesquiterpenes [182]. Other phytochemicals from clove include bicornin, eugenitin, myricetin, gallic acid, methyl salicylate, methyl amayl ketone, vanillin, ellagic acid, kaempferol, stigmasterol, oleanolic acid, β-caryophyllene and crategolic acid [183]. Considering the broad phytochemistry and biological activities of clove, it has the therapeutic potential to prevent various types of cancers and other diseases [184].
Regarding the effect of clove against CRC, an active fraction of clove extract has demonstrated an anti-proliferative effect against CRC (HCT-116) cells. The active fraction of clove extract induced apoptosis in HCT-116 cell lines, autophagy and inhibited the phosphorylation of the PI3K/Akt/mTOR signaling pathway [185]. In another study, ethyl acetate extract of cloves demonstrated antitumor activity both in in vivo and in vitro models. The clove extract shows dose-dependent induction of apoptosis and has downregulated cell cycle proteins. The authors suggested that clove extract has the potential to be used as a therapeutic herb for treating CRC [186]. Similarly, eugenol has anti-inflammatory effects as observed in mice with DSS-induced colitis, where eugenol treatment has ameliorated the colonic inflammation and oxidative stress in the DSS group [187].
An intake of S. aromaticum oil 1.5 mL/kg in the diet administered to quails led to an improvement in body weight, activities of antioxidant enzymes, lipid profile, and intestinal bacterial diversity [134]. The coliforms, E. coli, and Salmonella species were found to be lowered in the ileal contents of quails supplemented with S. aromaticum oil, suggesting a reduction in intestinal pathogens, aiming to promote a healthy intestinal status.
4.5. Chili Pepper-Derived Compounds
Chili pepper belongs to the Capsicum genus, a member of the family Solanaceae. The use of chilies as complementary and alternative medicine in developing countries is rapidly increasing. Alkaloids are the most active compounds present in Capsicum, known as capsaicinoids, such as capsaicin, dihydrocapsaicin, nordihydrocapsaicin, norcapsaicin, nornordihydrocapsaicin, homocapsaicin and homodihydrocapsaicin [188]. To date, no clinical investigation has confirmed the effects of capsaicin in human colon cancer, and few studies are focused on the relationship between capsaicin consumption and microbiota alterations [189].
A recent study on 512,000 adults revealed that consumption of spices is associated with a lower risk of GI cancer after 5 years of consumption, however, capsaicin also seems to have a negative effect on human health, even if most studies underline that only high doses seem to be harmful. An inverse association was found between spicy food consumption and CRC risk for those who never/rarely consumed and consumed monthly, 1–2 days/week, 3–5 days/week, and 6–7 days/week [190]. This could be related to an increase in butyrogenic bacteria and a decrease in LPS-producing bacteria. Another study found that consumption of 5 mg/d or 10 mg/day capsaicin on a regular basis increased Firmicutes/Bacteriodes and Faecalibacterium abundance. This event leads to an increase in glucagon-like peptide 1 and gastric inhibitory polypeptide with a decrease in ghrelin [135]. Diferuloylmethane is another interesting compound, which can influence the progress of CRC by changing the gut microbiome. Different studies registered lower intestinal inflammation through a reduction in NF-ĸB in colonic epithelial cells. Another positive effect is the growth of T cells in CD4+ Foxp3+ DSS colitis models and the reduction of Blautia and Ruminococcus species, which are responsible for CRC progress [191].
4.6. Saffron-Derived Compounds
Saffron (Crocus sativus, L) belongs to the plant family Iridaceae, and has been used as a food additive for centuries [192,193]. The phytochemistry of saffron reveals more than 150 compounds, principally comprising flavonoids, apocarotenoids (picrocrocin, crocin, and crocetin), safranal, terpenes, aromatic hydrocarbons, alkaloids, and amino acids [194]. Saffron and its bioactive constituents have the potential to prevent and treat various types of cancer, as evidenced by multiple studies [195]. Crocin significantly prevented DSS and azoxymethane-induced colitis by reducing the level of mRNA expression, inflammatory cytokines, and NF-κB, in colorectal mucosa [196]. Similarly, in another in vivo study, crocin synergized the anti-proliferative action of 5-flurouracil via Wnt/PI3K pathway in CRC mice, associated with colitis [197].
In a recent study, saffron extract was administered to CRC cells for anti-proliferation and anti-motility progression by targeting MET transcriptional regulator (MACC1) expression [198]. This accumulating evidence shows the therapeutic potential of saffron in the prevention of CRC via gut microbiota modulation. Crocin-I ameliorated the disruption of gut dysbiosis in mice induced by chronic corticosterone administration. High-throughput sequencing of 16s rRNA demonstrated that crocin-I could mitigate gut dysbiosis through significant decreases in the abundance of Firmicutes and an increase of Bacteroidetes, and a significant increase in the α-diversity of the microbes in the cecal contents [136]. An herbal formula containing C. sativus, Edgeworthia gardneri (Wall.) Meisn., and Sibiraea angustata modulated gut microbiota with the regulation of gut-liver axis in Zucker diabetic fatty rats [199]. The formula modulated the dysbiosis of gut microbiota and maintained intestinal epithelial homeostasis, resulting in the reduction of serum levels of LPS, TNF-α, and IL-6.
Some pieces of recently published literature have also reported the negative effects of saffron and crocetin on gastrointestinal diseases such as colitis. While investigating the effects of crocetin on the regulation of intestinal barrier function and intestinal microbiota composition in mice, Feng et al. [137] observed prolonged recovery of colitis due to the promotion of inflammation with disturbed intestinal homeostasis under crocetin (10 mg/kg/day for 21-days) with an altered composition of gut microflora and its metabolic products compared to the DSS group. The 16s rDNA sequencing analysis of the feces samples showed a higher abundance of Mediterraneibacter and Akkermansia, and a lower abundance of Dubosiella, Muribaculaceae, Paramuribaculum, Allobaculum, Parasutterella, Duncaniella, Stoquefichus, Coriobacteriaceae UCG-002, and Candidatus. In addition, crocetin intake also reduced the levels of bile acids including 7-ketodeoxycholic acid, 12-ketodeoxycholic acid, 3-sulfodeoxycholic acid, chenodeoxycholate, 6-ethylchenodeoxycholic acid, glycochenodeoxycholate-7-sulfate, sulfolithocholic acid, and glycocholate in the colon. Another study showed disruption of the cecal microbiome and brush border membrane functionality with C. sativus flower water extract [138]. The C. sativus extract (1%, 2%, 5%, and 10%) was administered in the amnion of the Gallus gallus eggs and was allowed to be consumed by the developing embryo over the next few days. The hatchlings were euthanized, and blood, duodenum, and cecum were harvested for assessment, which showed a significant increase in Mucin 2 gene expression and Paneth cell number proportional to the increase in extract concentration, accompanied by a dose-dependent reduction of Lactobacillus and Clostridium suggesting an alteration of bacterial populations.
4.7. Flaxseed-Derived Compounds
Flaxseed (Linum usitatissimum L.) belonging to the Linaceae, is one of the richest dietary sources of omega-3 fatty acids. Other compounds identified in flaxseed include dietary fibers, lignans, and phenolics [200]. Flaxseed is already being extensively used in animal studies to treat cancers of different origins. Numerous studies have demonstrated the prevention of colon carcinogenesis in preclinical studies due to the consumption of flaxseed. Flaxseed possesses immunomodulatory effects, possibly due to prebiotic effects. It maintains the integrity of the intestinal epithelial barrier, inhibiting inflammatory responses and promoting the proliferation of beneficial phyla that may help in preventing CRC development and pathogenesis [201]. Dietary flaxseed supplementation in healthy C57Bl/6 male mice exhibited an alteration in fecal microbial community structure (i.e., a 30-fold decrease in Akkermansia muciniphila abundance and a 20-fold increase in Prevotella species) along with a significant increase in fecal branched-chain fatty acids, thus decreasing susceptibility to gut-associated diseases including inflammatory pathologies and cancer [139].
Flaxseed polysaccharides may reach the colon intact (without being degraded) where changes in carbohydrate contents, reducing sugars, and culture pH suggest that these polysaccharides may be broken down and used by gut microbiota. Zhou et al. [202] observed a modulation of the structure and composition of gut microbiota with flaxseed polysaccharides through the alteration of the Firmicutes/Bacteroidetes ratio, and enhanced relative abundances of Phascolarctobacterium, Prevotella, Megamonas, and Clostridium, which can degrade polysaccharides. Moreover, the fermentation of flaxseed polysaccharides increased the concentration of SCFAs, particularly propionate and butyrate. Flaxseed oligosaccharides alleviated DSS-induced colitis via the modulation of gut microbiota and repairing of the intestinal barrier in mice [140]. Flaxseed oligosaccharides (200 mg/kg/day) resulted in the improvement of colonic histology, downregulation of oxidative stress markers (malondialdehyde and myeloperoxidase), and suppressed pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) while increasing the levels of an anti-inflammatory cytokine (IL-10). The 16S rDNA gene high-throughput sequencing indicated an increase in gut microbial diversity and inhibition of the proliferation of inflammatory-related bacteria (Clostridiales). An increase in propionic and butyric acids was also observed in mice treated with flaxseed oligosaccharides.
Flaxseed oil supplementation in pigs with intrauterine growth retardation improved intestinal function and immunity (downregulated intestinal expression of MyD88, NF-κB, TNF-α, and IL-10 genes) associated with altered colonic microflora, by decreasing the abundance of Spirochaetes and increasing phylum Actinobacteria, and genera Bifidobacterium and Blautia [141]. Treatment of CEABAC10 transgenic mice (with Crohn’s disease) with dietary extruded flaxseed for 12 weeks ameliorated the adherent-invasive E. coli-induced intestinal inflammation [142]. Analysis of mucosa-associated microbiota showed a higher abundance of Prevotella, Ruminococcus, Clostridiales, and Paraprevotella, in addition to higher butyrate concentration in mice treated with flaxseed. Conversely, ground flaxseeds (rich in omega-3 fatty acids, lignans, and fibers) exacerbated Citrobacter rodentium-induced colitis in C57BL/6 mice despite the higher levels of omega-3 fatty acids and cecal SCFAs [203].
5. Conclusions
The available literature data suggest that spices and their phytochemicals could be one of the dietary factors that may prevent the risk of CRC development by affecting tumor behavior and targeting numerous molecular mechanisms. Many processes (i.e., oxidative stress, inflammatory cascade, apoptosis, and proliferation) can be influenced by one or more spice-derived phytochemicals. Studies on gut microbial modulation by spice-derived phytochemicals in CRC are still very limited, as spice-derived phytochemicals have been studied in this regard. Thus, the exploration of other spice-derived phytochemicals is essential to provide further insights into the interesting relationship between spice-derived phytochemicals and gut microbiota in CRC. Certain spice-derived phytochemicals have been found to exacerbate gut dysbiosis and intestinal inflammation, such as diallyl disulfide, saffron, crocetin, and ground flaxseeds. So, further confirmation is required on whether this phenomenon will affect the CRC-preventing activity of other spices such as garlic and flaxseed. Additionally, the data reviewed from the literature has mainly been based on preclinical studies, thus robust clinical trials are needed to determine who will benefit from an adequate intake of spice-derived phytochemicals, and what interactions (both positive and negative) may exist among spices with other dietary components or medications (that an individual with CRC may regularly consume). Moreover, the testing of phytochemicals, both in cell cultures and animal studies, at much higher doses than would be regularly ingested, represents pharmacological therapeutic intervention rather than a dietary preventive approach, and thus spice-derived phytochemicals must be tested within the range of dietary doses to assess the actual potential of dietary spices to prevent CRC via gut microbial modulation.
Author Contributions
Conceptualization, M.D. (Maria Daglia) and H.U.; writing original draft, M.D. (Marco Dacrema), A.A., H.U. and A.K.; writing—review and editing, H.U., A.A., A.K., A.D.M., J.X. and A.M.C.M.; supervision, M.D. (Maria Daglia). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CRC | colorectal cancer |
| CSC | cancer stem cells |
| CTLA-4 | T lymphocytes associated with antigen 4 |
| DSS | dextran sulfate sodium |
| IBD | inflammatory bowel disease |
| IL-1 | interleukin-1 |
| JNK pathway | c-Jun N-terminal kinase pathway |
| LPS | Lipopolysaccharide |
| MAPKs | mitogen activated protein kinases |
| MMPs | matrix metalloproteinases |
| NF-ĸB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cells | natural killer cells |
| SCFAs | short chain fatty acids |
| TNF-α | tumor necrosis factor alpha |
References
- Vogelaar, I.; van Ballegooijen, M.; Schrag, D.; Boer, R.; Winawer, S.J.; Habbema, J.D.F.; Zauber, A.G. How Much Can Current Interventions Reduce Colorectal Cancer Mortality in the US? Mortality Projections for Scenarios of Risk-factor Modification, Screening, and Treatment. Cancer 2006, 107, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 10207, 1467–1480. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, J.; Zhu, Y.; Luo, L.; He, T.; Hu, H.; Liu, H.; Zhang, Y.; Luo, D.; Xu, S.; et al. Abdominal Obesity and Colorectal Cancer Risk: Systematic Review and Meta-Analysis of Prospective Studies. Biosci. Rep. 2017, 37, BSR20170945. [Google Scholar] [CrossRef]
- Liang, P.S.; Chen, T.Y.; Giovannucci, E. Cigarette Smoking and Colorectal Cancer Incidence and Mortality: Systematic Review and Meta-analysis. Int. J. Cancer 2009, 124, 2406–2415. [Google Scholar] [CrossRef]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 Studies of the Association of Alcohol with Colorectal Cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef]
- Zheng, X.; Hur, J.; Nguyen, L.H.; Liu, J.; Song, M.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Willett, W.C.; Chan, A.T.; et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J. Natl. Cancer Inst. 2021, 113, 543–552. [Google Scholar] [CrossRef]
- de Rezende, L.F.M.; de Sá, T.H.; Markozannes, G.; Rey-López, J.P.; Lee, I.M.; Tsilidis, K.K.; Ioannidis, J.P.; Eluf-Neto, J. Physical Activity and Cancer: An Umbrella Review of the Literature Including 22 Major Anatomical Sites and 770,000 Cancer Cases. Br. J. Sports Med. 2018, 52, 826–833. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and Heritable Factors in the Causation of Cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Graff, R.E.; Möller, S.; Passarelli, M.N.; Witte, J.S.; Skytthe, A.; Christensen, K.; Tan, Q.; Adami, H.O.; Czene, K.; Harris, J.R.; et al. Familial Risk and Heritability of Colorectal Cancer in the Nordic Twin Study of Cancer. Clin. Gastroenterol. Hepatol. 2017, 15, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and Familial Colon Cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut Microbiota in Colorectal Cancer: Mechanisms of Action and Clinical Applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global Burden of Cancers Attributable to Infections in 2012: A Synthetic Analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Hughes, L.A.; van den Brandt, P.A.; Goldbohm, R.A.; de Goeij, A.F.; de Bruïne, A.P.; van Engeland, M.; Weijenberg, M.P. Childhood and Adolescent Energy Restriction and Subsequent Colorectal Cancer Risk: Results from the Netherlands Cohort Study. Int. J. Epidemiol. 2010, 39, 1333–1344. [Google Scholar] [CrossRef]
- Nimptsch, K.; Wu, K. Is Timing Important? The Role of Diet and Lifestyle during Early Life on Colorectal Neoplasia. Curr. Colorectal Cancer Rep. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Gao, Z.; Huang, L.; Qin, H. Gut Microbiota and Colorectal Cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Louca, P.; Gibson, R.; Menni, C.; Spector, T.D.; le Roy, C.I. The Complexities of the Diet-Microbiome Relationship: Advances and Perspectives. Genome Med. 2021, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth Cells Directly Sense Gut Commensals and Maintain Homeostasis at the Intestinal Host-Microbial Interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Naik, S. Compartmentalized and Systemic Control of Tissue Immunity by Commensals. Nat. Immunol. 2013, 14, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic Genome Assessment of B-Vitamin Biosynthesis Suggests Co-Operation among Gut Microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genomics Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 2017, 168, 928–943. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A Single-Cell Survey of the Small Intestinal Epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Korem, T.; Zeevi, D.; Suez, J.; Weinberger, A.; Avnit-Sagi, T.; Pompan-Lotan, M.; Matot, E.; Jona, G.; Harmelin, A.; Cohen, N.; et al. Growth Dynamics of Gut Microbiota in Health and Disease Inferred from Single Metagenomic Samples. Science (1979) 2015, 349, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Hazama, S. Novel Biomarkers for Personalized Cancer Immunotherapy. Cancers 2019, 11, 1223. [Google Scholar] [CrossRef] [PubMed]
- Picardo, S.L.; Coburn, B.; Hansen, A.R. The Microbiome and Cancer for Clinicians. Crit. Rev. Oncol. Hematol. 2019, 141, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.D.; Nicholson, J.K. Gut Microbiome Interactions with Drug Metabolism, Efficacy, and Toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Douek, D.C. Microbial Translocation across the GI Tract. Annu. Rev. Immunol. 2012, 30, 149. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Núñez, G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Xu, Z.; Knight, R. Dietary Effects on Human Gut Microbiome Diversity. Br. J. Nutr. 2015, 113, S1–S5. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The Role of the Microbiome in Cancer Development and Therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host Lifestyle Affects Human Microbiota on Daily Timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z. Gut Microbiota: An Important Link between Western Diet and Chronic Diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016, 70, 395. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.R.; Bakir, I.A.; Hart, A.L.; Graham, T.A. Clonal Evolution of Colorectal Cancer in IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory Networks Underlying Colorectal Cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef]
- Alam, W.; Ullah, H.; Santarcangelo, C.; di Minno, A.; Khan, H.; Daglia, M.; Arciola, C.R. Micronutrient Food Supplements in Patients with Gastro-Intestinal and Hepatic Cancers. Int. J. Mol. Sci. 2021, 22, 8014. [Google Scholar] [CrossRef]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.S.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA Targeting by Quercetin in Cancer Treatment and Chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary Phytochemicals as Epigenetic Modifiers in Cancer: Promise and Challenges. Semin. Cancer Biol. 2016, 40, 82–99. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids Nanoparticles in Cancer: Treatment, Prevention and Clinical Prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.J.; Xiao, J. Anti-Cancer Effects of Polyphenols via Targeting P53 Signaling Pathway: Updates and Future Directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT Signaling Pathway Using Phytocompounds for Cancer Prevention and Therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural Products Targeting the PI3K-Akt-MTOR Signaling Pathway in Cancer: A Novel Therapeutic Strategy. Semin. Cancer Biol. 2019, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Asghari, M.H.; Nabavi, S.F.; Gulei, D.; Berindan-Neagoe, I.; Bishayee, A.; Nabavi, S.M. Targeting Hippo Signaling Pathway by Phytochemicals in Cancer Therapy. Semin. Cancer Biol. 2020, 80, 183–194. [Google Scholar] [CrossRef]
- Tewari, D.; Priya, A.; Bishayee, A.; Bishayee, A. Targeting Transforming Growth Factor-β Signalling for Cancer Prevention and Intervention: Recent Advances in Developing Small Molecules of Natural Origin. Clin. Transl. Med. 2022, 12, e795. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-Modulating Phytochemicals in Cancer Therapeutics: Current Evidences and Future Perspectives. Semin. Cancer Biol. 2020, 80, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S.; et al. Targeting NF-ΚB Signaling Pathway in Cancer by Dietary Polyphenols. Crit. Rev. Food Sci. Nutr. 2019, 60, 2790–2800. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for Prevention and Treatment of Cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, H.; Fan, D.; Deng, J. The Anticancer Activity and Mechanisms of Ginsenosides: An Updated Review. eFood 2020, 1, 226–241. [Google Scholar] [CrossRef]
- Jing, H. Black Garlic: Processing, Composition Change, and Bioactivity. eFood 2020, 1, 242–246. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Urbi, Z. Effect of Naphthalene Acetic Acid on the Adventitious Rooting in Shoot Cuttings of Andrographis paniculata (Burm. f.) Wall. Ex Nees: An Important Therapeutical Herb. Int. J. Agron. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sharfaraz, A.; Dutta, A.; Ahsan, A.; Masud, M.A.; Ahmed, I.A.; Goh, B.H.; Urbi, Z.; Sarker, M.M.R.; Ming, L.C. A Review of Ethnobotany, Phytochemistry, Antimicrobial Pharmacology and Toxicology of Nigella sativa L. Biomed. Pharmacother. 2021, 143, 112182. [Google Scholar] [CrossRef]
- DeLuca, J.A.; Garcia-Villatoro, E.L.; Allred, C.D. Flaxseed Bioactive Compounds and Colorectal Cancer Prevention. Curr. Oncol. Rep. 2018, 20, 59. [Google Scholar] [CrossRef]
- Jaksevicius, A.; Carew, M.; Mistry, C.; Modjtahedi, H.; Opara, E.I. Inhibitory Effects of Culinary Herbs and Spices on the Growth of HCA-7 Colorectal Cancer Cells and Their COX-2 Expression. Nutrients 2017, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A. PI3K/Akt/MTOR Pathway as a Therapeutic Target for Colorectal Cancer: A Review of Preclinical and Clinical Evidence. Curr. Drug Targets 2019, 20, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Xin, L.; Liang, A.; Fu, Y. Cancer Stem Cell Hypothesis: A Brief Summary and Two Proposals. Cytotechnology 2013, 65, 505–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexander, J.L.; Scott, A.J.; Pouncey, A.L.; Marchesi, J.; Kinross, J.; Teare, J. Colorectal Carcinogenesis: An Archetype of Gut Microbiota–Host Interaction. Ecancermedicalscience 2018, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [PubMed]
- Koury, J.; Zhong, L.; Hao, J. Targeting Signaling Pathways in Cancer Stem Cells for Cancer Treatment. Stem Cells Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Kader, M.A.; Goh, K.W.; Islam, M.; Khan, M.S.; Harun-Ar, M.R.; Ooi, J.; Melo, H.C.; Al-Worafi, Y.M.; Moshawih, S.; et al. Herb and Spices in Colorectal Cancer Prevention and Treatment: A Narrative Review. Front. Pharmacol. 2022, 13, 865801. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-Derived Phytochemicals Targeting Colon Cancer Stem Cells and Microbiota in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3976. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.R. Gut Flora in Health and Disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Anwar, A.; Shah, M.R.; Siddiqui, R. Cytotoxic Effects of Benzodioxane, Naphthalene Diimide, Porphyrin and Acetamol Derivatives on HeLa Cells. SAGE Open Med. 2018, 6. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Arch. Med. Res. 2021, 52, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Emerging Roles of the Microbiome in Cancer. Carcinogenesis 2014, 35, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, B. New Therapeutic Targets for Cancer: The Interplay between Immune and Metabolic Checkpoints and Gut Microbiota. Clin. Transl. Med. 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS One 2012, 7, e39743. [Google Scholar] [CrossRef]
- Wu, N.; Yang, X.; Zhang, R.; Li, J.; Xiao, X.; Hu, Y.; Chen, Y.; Yang, F.; Lu, N.; Wang, Z.; et al. Dysbiosis Signature of Fecal Microbiota in Colorectal Cancer Patients. Microb. Ecol. 2013, 66, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, J.; Rai, R.P.; Prasad, K.N. Role of Helicobacter pylori in Gastric Cancer: Updates. World J. Gastroenterol. 2016, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Ushijima, T. Epigenetic Impact of Infection on Carcinogenesis: Mechanisms and Applications. Genome Med. 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science (1979) 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Nougayrède, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli Induces DNA Double-Strand Breaks in Eukaryotic Cells. Science (1979) 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Dennis, K.L.; Blatner, N.R.; Gounari, F.; Gounari, F. Current Status of Interleukin-10 and Regulatory T-Cells in Cancer. Curr. Opin. Oncol. 2013, 25, 637–645. [Google Scholar] [CrossRef]
- Al-Hebshi, N.N.; Borgnakke, W.S.; Johnson, N.W. The Microbiome of Oral Squamous Cell Carcinomas: A Functional Perspective. Curr. Oral Health Rep. 2019, 6, 145–160. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers (Basel) 2019, 11, 38. [Google Scholar] [CrossRef]
- Borges-Canha, M.; Portela-Cidade, J.P.; Dinis-Ribeiro, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Role of Colonic Microbiota in Colorectal Carcinogenesis: A Systematic Review. Rev. Esp. Enferm. Dig. 2015, 107, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Klampfer, L. Cytokines, Inflammation and Colon Cancer. Curr. Cancer Drug Targets 2011, 11, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Bergounioux, J.; Elisee, R.; Prunier, A.L.; Donnadieu, F.; Sperandio, B.; Sansonetti, P.; Arbibe, L. Calpain Activation by the Shigella flexneri Effector VirA Regulates Key Steps in the Formation and Life of the Bacterium’s Epithelial Niche. Cell Host Microbe 2012, 11, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via Its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The Role of Gut Microbiota in Cancer Treatment: Friend or Foe? Gut 2013, 69, 1867–1876. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Shields, C.E.D.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine Catabolism Contributes to Enterotoxigenic Bacteroides fragilis-Induced Colon Tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-Derived Ferrichrome Inhibits Colon Cancer Progression via JNK-Mediated Apoptosis. Nat. Commun. 2016, 7, 12365. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Jakobsson, H.E.; Holmén-Larsson, J.; Schütte, A.; Ermund, A.; Rodríguez-Piñeiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The Immune System Bridges the Gut Microbiota with Systemic Energy Homeostasis: Focus on TLRs, Mucosal Barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rao, Y.; Guo, X.; Liu, N.; Liu, S.; Wen, P.; Li, S.; Li, Y. Oral Microbiome in Patients with Oesophageal Squamous Cell Carcinoma. Sci. Rep. 2019, 9, 19055. [Google Scholar] [CrossRef]
- Nair, M.; Sandhu, S.S.; Sharma, A.K. Cancer Molecular Markers: A Guide to Cancer Detection and Management. Semin. Cancer Biol. 2018, 52, 39–55. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers (Basel) 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Rueff, J.; Rodrigues, A.S. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint. Methods Mol. Biol. 2016, 1395, 1–18. [Google Scholar] [PubMed]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to Cancer Chemotherapy: Failure in Drug Response from ADME to P-Gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Jobin, C. The Microbiome and Cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Daillère, R.; Roberti, M.P.; Routy, B.; Kroemer, G. Anticancer Effects of the Microbiome and Its Products. Nat. Rev. Microbiol. 2017, 15, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.; Smith, L.; Bouladoux, N.; Weingarten, R.; Molina, D.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science (1979) 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-Gut-Brain Axis: Enteroendocrine Cells and the Enteric Nervous System Form an Interface between the Microbiota and the Central Nervous System. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.; Pfirschke, C.; Engblom, C.; Pittet, M.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science (1979) 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science (1979) 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of Gut Microbiota in the Development and Treatment of Colorectal Cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.; Duong, C.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science (1979) 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.B.A.S.; Belzacq, A.S.; Haouzi, D.; Rouault, A.; Metivier, D.; Kroemer, G.; Brenner, C. Propionibacteria Induce Apoptosis of Colorectal Carcinoma Cells via Short-Chain Fatty Acids Acting on Mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Sun, W.; Yu, S.; Yang, Y.; Ai, L. Butyrate Production from High-Fiber Diet Protects against Lymphoma Tumor. Leuk. Lymphoma 2016, 57, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, Y.; Sun, J. Breast and Gut Microbiome in Health and Cancer. Genes Dis. 2021, 8, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers (Basel) 2020, 12, 1406. [Google Scholar] [CrossRef]
- Salcedo, R.; Worschech, A.; Cardone, M.; Jones, Y.; Gyulai, Z.; Dai, R.M.; Wang, E.; Ma, W.; Haines, D.; O’hUigin, C.; et al. MyD88-Mediated Signaling Prevents Development of Adenocarcinomas of the Colon: Role of Interleukin 18. J. Exp. Med. 2010, 207, 1625–1636. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.; Chow, S.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.; Skinner, S.; et al. Efficacy of Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Vaccine against Cervical Infection and Precancer Caused by Oncogenic HPV Types (PATRICIA): Final Analysis of a Double-Blind, Randomised Study in Young Women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Shylaja, M.R.; Peter, K.V. Spices in the Nutraceutical and Health Food Industry. In International Symposium on Medicinal and Nutraceutical Plants; International Society for Horticultural Science: Macon, GA, USA, 2007; pp. 369–378. [Google Scholar]
- Srinivasan, K. Role of Spices beyond Food Flavoring: Nutraceuticals with Multiple Health Effects. Food Rev. Int. 2005, 21, 167–188. [Google Scholar] [CrossRef]
- Kaefer, C.M.; Milner, J.A. The Role of Herbs and Spices in Cancer Prevention. J. Nutr. Biochem. 2008, 19, 347–361. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Intestinal Microbiota and Metabolic Diseases: Pharmacological Implications. Trends Pharmacol. Sci. 2016, 37, 169–171. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle Curcumin Ameliorates Experimental Colitis via Modulation of Gut Microbiota and Induction of Regulatory T Cells. PLoS One 2017, 12, e0185999. [Google Scholar] [CrossRef] [PubMed]
- McFadden, R.M.T.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The Role of Curcumin in Modulating Colonic Microbiota during Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-Inflammatory Effects of Resveratrol, Curcumin and Simvastatin in Acute Small Intestinal Inflammation. PLoS One 2010, 5, e15099. [Google Scholar] [CrossRef]
- Guo, S.; Geng, W.; Chen, S.; Wang, L.; Rong, X.; Wang, S.; Wang, T.; Xiong, L.; Huang, J.; Pang, X.; et al. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front. Pharmacol. 2021, 12, 632569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; He, Q.; Wang, M.; Lu, H.; You, Y.; Chen, L.; Cheng, J.; Li, F.; Fu, X.; et al. Ginger Extract Decreases Susceptibility to Dextran Sulfate Sodium-Induced Colitis in Mice Following Early Antibiotic Exposure. Front. Med. 2022, 8. [Google Scholar]
- Sasaki, K.; Sasaki, D.; Sasaki, K.; Nishidono, Y.; Yamamori, A.; Tanaka, K.; Kondo, A. Growth Stimulation of Bifidobacterium from Human Colon Using Daikenchuto in an In Vitro Model of Human Intestinal Microbiota. Sci. Rep. 2021, 22, 4580. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Chen, Z.; Yuan, Q.; Ma, M.; Gao, C.; Zhou, Y.; Zhou, H.; Wu, X.; Wu, D.; Farag, M.A.; et al. Ginger Polysaccharides Relieve Ulcerative Colitis via Maintaining Intestinal Barrier Integrity and Gut Microbiota Modulation. Int. J. Biol. Macromol. 2022, 219, 730–739. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Jiang, H.; Zhang, S.; Pang, X.; Gao, S.; Zhang, H.; Zhang, S.; Xiao, Q.; Chen, L.; et al. Gut Microbiota Variation with Short-Term Intake of Ginger Juice on Human Health. Front. Microbiol. 2021, 11, 576061. [Google Scholar] [CrossRef]
- Shao, X.; Sun, C.; Tang, X.; Zhang, X.; Han, D.; Liang, S.; Qu, R.; Hui, X.; Shan, Y.; Hu, L.; et al. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of Jinxiang Garlic (Allium sativum L.) Polysaccharides toward Dextran Sodium Sulfate-Induced Colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Banos, A.; Guillamón, E.; García, F.; Rodríguez-Nogales, A.; Gálvez, J. The Immunomodulatory Properties of Propyl-propane Thiosulfonate Contribute to Its Intestinal Anti-inflammatory Effect in Experimental Colitis. Mol. Nutr. Food Res. 2019, 63, 1800653. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abd El-Hack, M.E.; Mahgoub, S.A.; Saadeldin, I.M.; Swelum, A.A. Effects of Clove (Syzygium aromaticum) Oil on Quail Growth, Carcass Traits, Blood Components, Meat Quality, and Intestinal Microbiota. Poult. Sci. 2019, 98, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Fu, Z. Crocin-I Ameliorates the Disruption of Lipid Metabolism and Dysbiosis of the Gut Microbiota Induced by Chronic Corticosterone in Mice. Food Funct. 2019, 10, 6779–6791. [Google Scholar] [CrossRef]
- Feng, P.; Li, Q.; Liu, L.; Wang, S.; Wu, Z.; Tao, Y.; Huang, P.; Wang, P. Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability. Int. J. Mol. Sci. 2022, 23, 3832. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology in Vivo (Gallus gallus). Nutrients 2022, 14, 220. [Google Scholar] [CrossRef]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary Flaxseed Modulates the Colonic Microenvironment in Healthy C57Bl/6 Male Mice Which May Alter Susceptibility to Gut-Associated Diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, W.; Deng, Q.; Huang, Q.; Wang, X.; Yang, C.; Huang, F. Flaxseed Oligosaccharides Alleviate DSS-Induced Colitis through Modulation of Gut Microbiota and Repair of the Intestinal Barrier in Mice. Food Funct. 2020, 11, 8077–8088. [Google Scholar] [CrossRef]
- Che, L.; Zhou, Q.; Liu, Y.; Hu, L.; Peng, X.; Wu, C.; Zhang, R.; Tang, J.; Wu, F.; Fang, Z.; et al. Flaxseed Oil Supplementation Improves Intestinal Function and Immunity, Associated with Altered Intestinal Microbiome and Fatty Acid Profile in Pigs with Intrauterine Growth Retardation. Food Funct. 2019, 10, 8149–8160. [Google Scholar] [CrossRef]
- Plissonneau, C.; Sivignon, A.; Chassaing, B.; Capel, F.; Martin, V.; Etienne, M.; Wawrzyniak, I.; Chausse, P.; Dutheil, F.; Mairesse, G.; et al. Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn’s Disease. Int. J. Mol. Sci. 2022, 23, 5891. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Killian, P.H.; Melchart, D. The Role of Curcumin in Prevention and Management of Metastatic Disease. Int. J. Mol. Sci. 2018, 19, 1716. [Google Scholar] [CrossRef]
- Mirzaei, H.; Bagheri, H.; Ghasemi, F.; Khoi, J.M.; Pourhanifeh, M.H.; Heyden, Y.V.; Mortezapour, E.; Nikdasti, A.; Jeandet, P.; Khan, H.; et al. Anti-Cancer Activity of Curcumin on Multiple Myeloma. Anticancer Agents Med. Chem. 2021, 21, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and Therapeutic Effects of Curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Genua, F.; Raghunathan, V.; Jenab, M.; Gallagher, W.M.; Hughes, D.J. The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development. Front. Oncol. 2021, 11, 6349. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin Improves Intestinal Barrier Function: Modulation of Intracellular Signaling, and Organization of Tight Junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral Supplementation with Non-Absorbable Antibiotics or Curcumin Attenuates Western Diet-Induced Atherosclerosis and Glucose Intolerance in LDLR−/− Mice–Role of Intestinal Permeability and Macrophage Activation. PLoS One 2014, 9, e108577. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-Mediated Regulation of Intestinal Barrier Function: The Mechanism Underlying Its Beneficial Effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef]
- Wang, P.; Su, C.; Feng, H.; Chen, X.; Dong, Y.; Rao, Y.; Ren, Y.; Yang, J.; Shi, J.; Tian, J.; et al. Curcumin Regulates Insulin Pathways and Glucose Metabolism in the Brains of APPswe/PS1dE9 Mice. Int. J. Immunopathol. Pharmacol. 2017, 30, 25–43. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Bidirectional Interactions between Dietary Curcumin and Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 2896–2902. [Google Scholar] [CrossRef]
- Zhai, S.S.; Ruan, D.; Zhu, Y.W.; Li, M.C.; Ye, H.; Wang, W.C.; Yang, L. Protective Effect of Curcumin on Ochratoxin A–Induced Liver Oxidative Injury in Duck Is Mediated by Modulating Lipid Metabolism and the Intestinal Microbiota. Poult. Sci. 2020, 99, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Regulative Effects of Curcumin Spice Administration on Gut Microbiota and Its Pharmacological Implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of Gut Microbiota Contributes to Curcumin-Mediated Attenuation of Hepatic Steatosis in Rats. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.K.; Papineni, R.V.; Umar, S. Chemoprevention in Gastrointestinal Physiology and Disease. Natural Products and Microbiome. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1–G15. [Google Scholar] [CrossRef] [PubMed]
- Youssef, O.; Lahti, L.; Kokkola, A.; Karla, T.; Tikkanen, M.; Ehsan, H.; Carpelan-Holmström, M.; Koskensalo, S.; Böhling, T.; Rautelin, H.; et al. Stool Microbiota Composition Differs in Patients with Stomach, Colon, and Rectal Neoplasms. Dig. Dis. Sci. 2018, 63, 2950–2958. [Google Scholar] [CrossRef]
- Mori, G.; Orena, B.S.; Cultrera, I.; Barbieri, G.; Albertini, A.M.; Ranzani, G.N.; Carnevali, I.; Tibiletti, M.G.; Pasca, M.R. Gut Microbiota Analysis in Postoperative Lynch Syndrome Patients. Front. Microbiol. 2019, 10, 1746. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; De, R.; Pal, I.; Mukhopadhyay, A.K.; Saha, D.R.; Swarnakar, S. Curcumin Alleviates Matrix Metalloproteinase-3 and-9 Activities during Eradication of Helicobacter pylori Infection in Cultured Cells and Mice. PLoS One 2011, 6, e16306. [Google Scholar] [CrossRef]
- Haghi, A.; Azimi, H.; Rahimi, R. A Comprehensive Review on Pharmacotherapeutics of Three Phytochemicals, Curcumin, Quercetin, and Allicin, in the Treatment of Gastric Cancer. J. Gastrointest. Cancer 2017, 48, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; di Minno, A.; Santarcangelo, C.; Khan, H.; Xiao, J.; Arciola, C.R.; Daglia, M. Vegetable Extracts and Nutrients Useful in the Recovery from Helicobacter pylori Infection: A Systematic Review on Clinical Trials. Molecules 2021, 26, 2272. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Ginger Rhizomes (Zingiber officinale): A Spice with Multiple Health Beneficial Potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.S.; Connell, D.W. Ginger—Chemistry, Technology, and Quality Evaluation: Part 1. Crit. Rev. Food Sci. Nutr. 1983, 17, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the Chemopreventive Effects of Ginger and Its Phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Ginger and Its Constituents: Role in Prevention and Treatment of Gastrointestinal Cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef] [PubMed]
- El-Abhar, H.S.; Hammad, L.N.; Gawad, H.S.A. Modulating Effect of Ginger Extract on Rats with Ulcerative Colitis. J. Ethnopharmacol. 2008, 118, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral Delivery of Nanoparticles Loaded with Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohns Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; di Gioia, F.; Polyzos, N.; Tzortzakis, N. Natural Antioxidants, Health Effects and Bioactive Properties of Wild Allium Species. Curr. Pharm. Des. 2020, 26, 1816–1837. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical Composition and Bioactive Compounds of Garlic (Allium sativum L.) as Affected by Pre-and Post-Harvest Conditions: A Review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Miroddi, M.; Calapai, F.; Calapai, G. Potential Beneficial Effects of Garlic in Oncohematology. Mini Rev. Med. Chem. 2011, 11, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of Garlic: Promising Candidates for Cancer Therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Banerjee, S.; Bose, S.; Mazumder, S.; Haber, R.A.; Farzaei, M.H.; Bishayee, A. Garlic Constituents for Cancer Prevention and Therapy: From Phytochemistry to Novel Formulations. Pharmacol. Res. 2022, 175, 105837. [Google Scholar] [CrossRef] [PubMed]
- de Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer Potential of Garlic and Its Bioactive Constituents: A Systematic and Comprehensive Review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, M.A.; Hou, D.X. Preventive Effects and Mechanisms of Garlic on Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Sali, A. The Effect of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front. Nutr. 2018, 5, 122. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Huang, M.; Wu, H.; Yang, C.; Zhang, X.; Yang, L.; Chen, G.; Li, S.; Wang, Q.; et al. Fatty Liver and Alteration of the Gut Microbiome Induced by Diallyl Disulfide. Int. J. Mol. Med. 2019, 44, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—from the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The Chemical Composition and Biological Activity of Clove Essential Oil, Eugenia Caryophyllata (Syzigium aromaticum L. Myrtaceae): A Short Review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant Activity of Clove Oil–A Powerful Antioxidant Source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, G.; Zhang, D.; An, W.; Lai, H.; Li, X.; Cao, S.; Lin, X. Active Fraction of Clove Induces Apoptosis via PI3K/Akt/MTOR-Mediated Autophagy in Human Colorectal Cancer HCT-116 Cells. Int. J. Oncol. 2018, 53, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Schmitz, J.C.; Wei, J.; Cao, S.; Beumer, J.H.; Strychor, S.; Cheng, L.; Liu, M.; Wang, C.; Wu, N.; et al. Clove Extract Inhibits Tumor Growth and Promotes Cell Cycle Arrest and Apoptosis. Oncol. Res. 2014, 21, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, X.; Tang, S.; Yin, J.; Song, Z.; He, X.; Yin, Y. Eugenol Alleviates Dextran Sulfate Sodium-Induced Colitis Independent of Intestinal Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 10506–10514. [Google Scholar] [CrossRef] [PubMed]
- Saleh, B.K.; Omer, A.; Teweldemedhin, B. Medicinal Uses and Health Benefits of Chili Pepper (Capsicum Spp.): A Review. MOJ Food Process. Technol. 2018, 6, 325–328. [Google Scholar] [CrossRef]
- de Jong, P.R.; Takahashi, N.; Harris, A.R.; Lee, J.; Bertin, S.; Jeffries, J.; Jung, M.; Duong, J.; Triano, A.I.; Lee, J.; et al. Ion Channel TRPV1-Dependent Activation of PTP1B Suppresses EGFR-Associated Intestinal Tumorigenesis. J. Clin. Invest. 2014, 124, 3793–3806. [Google Scholar] [CrossRef]
- Chan, W.C.; Millwood, I.Y.; Kartsonaki, C.; Du, H.; Guo, Y.; Chen, Y.; Bian, Z.; Walters, R.G.; Lv, J.; He, P.; et al. Spicy Food Consumption and Risk of Gastrointestinal-Tract Cancers: Findings from the China Kadoorie Biobank. Int. J. Epidemiol. 2021, 50, 199–211. [Google Scholar] [CrossRef]
- Hayashi, K.; Shibata, C.; Nagao, M.; Sato, M.; Kakyo, M.; Kinouchi, M.; Saijo, F.; Miura, K.; Ogawa, H.; Sasaki, I. Intracolonic Capsaicin Stimulates Colonic Motility and Defecation in Conscious Dogs. Surgery 2010, 147, 789–797. [Google Scholar] [CrossRef]
- Ashktorab, H.; Soleimani, A.; Singh, G.; Amin, A.; Tabtabaei, S.; Latella, G.; Stein, U.; Akhondzadeh, S.; Solanki, N.; Gondré-Lewis, M.C.; et al. Saffron: The Golden Spice with Therapeutic Properties on Digestive Diseases. Nutrients 2019, 11, 943. [Google Scholar] [CrossRef]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and Biological Properties of the World’s Most Expensive Spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Xing, B.; Li, S.; Yang, J.; Lin, D.; Feng, Y.; Lu, J.; Shao, Q. Phytochemistry, Pharmacology, and Potential Clinical Applications of Saffron: A Review. J. Ethnopharmacol. 2021, 281, 114555. [Google Scholar] [CrossRef] [PubMed]
- Gezici, S. Comparative Anticancer Activity Analysis of Saffron Extracts and a Principle Component, Crocetin for Prevention and Treatment of Human Malignancies. J. Food Sci. Technol. 2019, 56, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Tung, N.H.; Shoyama, Y.; Sugie, S.; Mori, T.; Tanaka, T. Dietary Crocin Inhibits Colitis and Colitis-Associated Colorectal Carcinogenesis in Male ICR Mice. Evid. base Compl. Alternative Med. 2012, 2012, 820415. [Google Scholar] [CrossRef] [PubMed]
- Amerizadeh, F.; Rezaei, N.; Rahmani, F.; Hassanian, S.M.; Moradi-Marjaneh, R.; Fiuji, H.; Boroumand, N.; Nosrati-Tirkani, A.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Crocin Synergistically Enhances the Antiproliferative Activity of 5-flurouracil through Wnt/PI3K Pathway in a Mouse Model of Colitis-associated Colorectal Cancer. J. Cell. Biochem. 2018, 119, 10250–10261. [Google Scholar] [CrossRef] [PubMed]
- Güllü, N.; Kobelt, D.; Brim, H.; Rahman, S.; Timm, L.; Smith, J.; Soleimani, A.; di Marco, S.; Bisti, S.; Ashktorab, H.; et al. Saffron Crudes and Compounds Restrict MACC1-Dependent Cell Proliferation and Migration of Colorectal Cancer Cells. Cells 2020, 9, 1829. [Google Scholar] [CrossRef]
- Li, M.; Ding, L.; Hu, Y.L.; Qin, L.L.; Wu, Y.; Liu, W.; Wu, L.L.; Liu, T.H. Herbal Formula LLKL Ameliorates Hyperglycaemia, Modulates the Gut Microbiota and Regulates the Gut-liver Axis in Zucker Diabetic Fatty Rats. J. Cell. Mol. Med. 2021, 25, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J. Flaxseed (Linum usitatissimum L.) Bioactive Compounds and Peptide Nomenclature: A Review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Mendonça, L.A.; dos Santos Ferreira, R.; de Cássia Avellaneda Guimarães, R.; de Castro, A.P.; Franco, O.L.; Matias, R.; Carvalho, C.M. The Complex Puzzle of Interactions among Functional Food, Gut Microbiota, and Colorectal Cancer. Front. Oncol. 2018, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Z.; Huang, F.; Yang, C.; Huang, Q. In Vitro Digestion and Fermentation by Human Fecal Microbiota of Polysaccharides from Flaxseed. Molecules 2020, 25, 4354. [Google Scholar] [CrossRef] [PubMed]
- Määttänen, P.; Lurz, E.; Botts, S.R.; Wu, R.Y.; Yeung, C.W.; Li, B.; Abiff, S.; Johnson-Henry, K.C.; Lepp, D.; Power, K.A.; et al. Ground Flaxseed Reverses Protection of a Reduced-Fat Diet against Citrobacter rodentium-Induced Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G788–G798. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).