Post-Neoadjuvant Treatment Strategies for Patients with Early Breast Cancer
Abstract
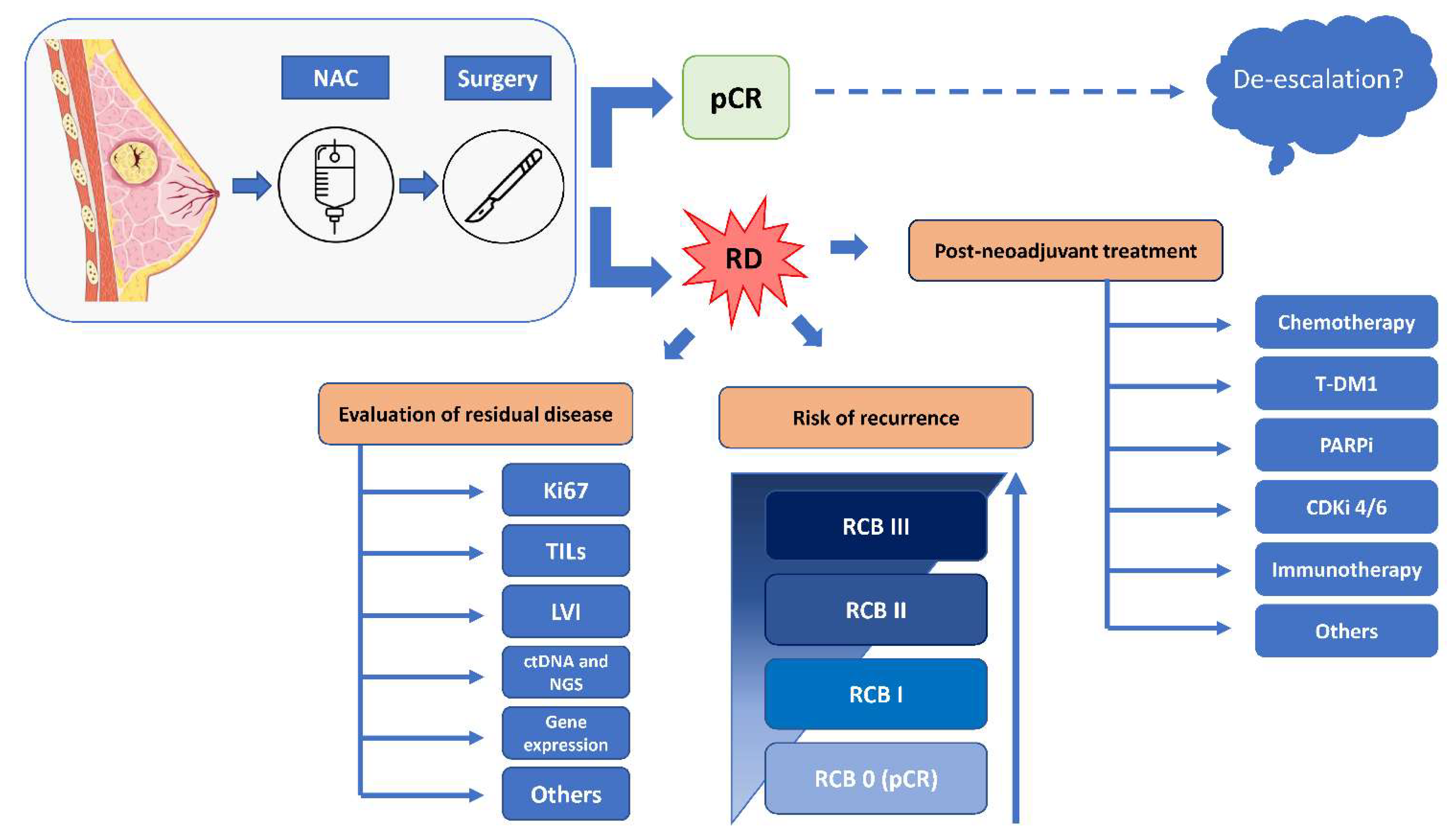
Simple Summary
Abstract
1. Introduction
2. Triple-Negative Breast Cancer
3. HER2-Positive Breast Cancer
4. Hormone Receptor-Positive Breast Cancer
5. Post-Neoadjuvant Locoregional Treatment
6. Pathological Evaluation of Residual Disease
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dafni, U.; Tsourti, Z.; Alatsathianos, I. Breast Cancer Statistics in the European Union: Incidence and Survival across European Countries. Breast Care 2019, 14, 344–353. [Google Scholar] [CrossRef] [PubMed]
- European Network of Cancer Registries. Available online: Https://www.encr.eu (accessed on 1 August 2022).
- Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Zambelli, A.; Sgarra, R.; De Sanctis, R.; Agostinetto, E.; Santoro, A.; Manfioletti, G. Heterogeneity of triple-negative breast cancer: Understanding the Daedalian labyrinth and how it could reveal new drug targets. Expert. Opin. Ther. Targets 2022, 26, 557–573. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. Available online: https://pubmed.ncbi.nlm.nih.gov/32101663/ (accessed on 1 August 2022). [CrossRef]
- Houssami, N.; Macaskill, P.; von Minckwitz, G.; Marinovich, M.L.; Mamounas, E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur. J. Cancer 2012, 48, 3342–3354. [Google Scholar] [CrossRef]
- Thomssen, C.; Balic, M.; Harbeck, N.; Gnant, M. St. Gallen/Vienna 2021: A Brief Summary of the Consensus Discussion on Customizing Therapies for Women with Early Breast Cancer. Breast Care 2021, 16, 135–143. [Google Scholar] [CrossRef]
- Gradishar, W.; Moran, M.; Abraham, J. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Available online: https://www.nccn.org/ (accessed on 15 January 2022).
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Geber, R.D.; de Azamuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, R.D.; Gelber, K.-A.; Eisen, A.; Johannsson, O.T. Prespecified Event-Driven Analysis of Overall Survival in the Olympia Phase III Trial of Adjuvant Olaparib in Germline Brca1/2 Mutation Associated Breast Cancer; ESMO Virtual Plenary Abstr VP1-2022; ESMO Congress: Madrid, Spain, 2022. [Google Scholar]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 tri. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Mayer, I.A.; Zhao, F.; Arteaga, C.L.; Symmans, W.F.; Park, B.H.; Burnette, B.L.; Tevaarwek, A.; Garcia, S.F.; Smith, K.L.; Makower, D.F.; et al. Randomized Phase III Postoperative Trial of Platinum-Based Chemotherapy Versus Capecitabine in Patients with Residual Triple-Negative Breast Cancer Following Neoadjuvant Chemotherapy: ECOG-ACRIN EA1131. J. Clin. Oncol. 2021, 39, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Marmé, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.-B.; Bear, H.; McCarthy, N.; Olivé, M.M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer—The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 282–293. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Rodriguez, J.L.M.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3987. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Rastogi, P.; Harbeck, N.; Toi, M.; Hegg, R.; Sohn, J.; Guarneri, V.; Cortes, J.; Hamilton, E.; Wei, R.; et al. VP8-2021: Adjuvant abemaciclib combined with endocrine therapy (ET): Updated results from monarchE. Ann. Oncol. 2021, 32, 1646–1649. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Chan, A.; Moy, B.; Mansi, J.; Ejlertsen, B.; Holmes, F.A.; Chia, S.; Iwata, H.; Gnant, M.; Loibl, S.; Barrios, C.H.; et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer from the Phase III ExteNET Trial. Clin. Breast Cancer 2021, 21, 80–91.e7. Available online: https://www.sciencedirect.com/science/article/pii/S1526820920302585 (accessed on 1 August 2022). [CrossRef]
- Schneider, B.P.; Jiang, G.; Ballinger, T.J.; Shen, F.; Chitambar, C.; Nanda, R.; Falkson, C.; Lynce, F.C.; Gallagher, C.; Isaacs, C.; et al. BRE12-158: A Postneoadjuvant, Randomized Phase II Trial of Personalized Therapy Versus Treatment of Physician’s Choice for Patients with Residual Triple-Negative Breast Cancer. J. Clin. Oncol. 2021, 40, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Schneeweiss, A.; Huober, J.B.; Braun, M.; Rey, J.; Blohmer, J.U.; Furlanetto, J.; Zahm, D.M.; Hanusch, C.; Tomalia, J.; et al. Durvalumab improves long-term outcome in TNBC: Results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J. Clin. Oncol. 2021, 39 (Suppl. S15), 506. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. VP7-2021: KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann. Oncol. 2021, 32, 1198–1200. [Google Scholar] [CrossRef]
- Agostinetto, E.; Losurdo, A.; Nader-Marta, G.; Santoro, A.; Punie, K.; Barroso, R.; Popovic, L.; Solinas, C.; Kok, M.; de Azambuja, E.; et al. Progress and pitfalls in the use of immunotherapy for patients with triple negative breast cancer. Expert. Opin. Investig. Drugs 2022, 31, 567–591. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- de Azambuja, E.; Holmes, A.P.; Piccart-Gebhart, M.; Holmes, E.; Di Cosimo, S.; Swaby, R.F.; Untch, M.; Jackisch, C.; Lang, I.; Smith, I.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014, 15, 1137–1146. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Waldron-Lynch, M.; Eric-Wong, J.; Kirk, S.; Cortés, J. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive e. Eur. J. Cancer 2018, 89, 27–35. [Google Scholar] [CrossRef]
- Gnant, M.; Martin, M.; Holmes, F.-A.; Jackisch, C.; Chia, S.K.; Iwata, H.; Moy, B.; Martinez, N.; Mansi, J.; Morales, S.; et al. Abstract P2-13-01: Efficacy of neratinib in hormone receptor-positive patients who initiated treatment within 1 year of completing trastuzumab-based adjuvant therapy in HER2+ early-stage breast cancer: Subgroup analyses from the phase III ExteNET trial. Cancer Res. 2019, 79 (Suppl. S4), P2-13-01. [Google Scholar] [CrossRef]
- Agostinetto, E.; Montemurro, F.; Puglisi, F.; Criscitiello, C.; Bianchini, G.; Del Mastro, L.; Introna, M.; Tondini, C.; Santoro, A.; Zambelli, A. Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives. Cancers 2022, 14, 2136. [Google Scholar] [CrossRef]
- Cortés, J. Trastuzumab Deruxtecan (T-DXd) vs Trastuzumab Emtansine (T-DM1) in Patients (Pts) with HER2+ Metastatic Breast Cancer (mBC): Results of the Randomized Phase III DESTINYBreast03 Study; ESMO Congress: Madrid, Spain, 2021. [Google Scholar]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.-H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, J.M.; Gebhart, G.; Ruiz Borrego, M.; Stradella, A.; Bermejo, B.; Schmid, P.; Marmé, F.; Escrivá-de-Romani, S.; Calvo, L.; Ribelles, N.; et al. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021, 22, 858–871. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Jeruss, J.S.; Tucker, S.L.; Kolli, A.; Newman, L.A.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Cheng, J.; Prowell, T.M.; Bloomquist, E.; Tang, S.; Wedam, S.B.; Royce, M.; Krol, D.; Osgood, C.; Ison, G.; et al. Overall survival in patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer treated with a cyclin-dependent kinase 4/6 inhibitor plus fulvestrant: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2021, 22, 1573–1581. [Google Scholar] [CrossRef]
- Mayer, E.L.; Dueck, A.C.; Martin, M.; Rubovszky, G.; Burstein, H.J.; Bellet-Ezquerra, M.; Miller, K.D.; Zdenkowski, N.; Winer, E.P.; Pfeiler, G.; et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021, 22, 212–222. [Google Scholar] [CrossRef]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.M.; Shao, Z.M.; Fasching, P.A.; Huang, C.S.; Jaliffe, G.G.; Tryakin, A.; Goetz, M.P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1571–1581. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Dubsky, P.; Pinker, K.; Cardoso, F.; Montagna, G.; Ritter, M.; Denkert, C.; Rubio, I.T.; de Azambuja, E.; Curigliano, G.; Gentilini, O.; et al. Breast conservation and axillary management after primary systemic therapy in patients with early-stage breast cancer: The Lucerne toolbox. Lancet Oncol. 2021, 22, e18–e28. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Macaskill, P.; Irwig, L.; Sardanelli, F.; Mamounas, E.; von Minckwitz, G.; Guarneri, V.; Partridge, S.C.; Wright, F.C.; Choi, J.H.; et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: Individual patient data meta-analysis. BMC Cancer 2015, 15, 662. [Google Scholar] [CrossRef]
- Samiei, S.; de Mooij, C.M.; Lobbes, M.B.I.; Keymeulen, K.B.M.I.; van Nijnatten, T.J.A.; Smidt, M.L. Diagnostic Performance of Noninvasive Imaging for Assessment of Axillary Response After Neoadjuvant Systemic Therapy in Clinically Node-positive Breast Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2021, 273, 694–700. [Google Scholar] [CrossRef]
- Vriens, B.E.P.J.; de Vries, B.; Lobbes, M.B.I.; van Gastel, S.M.; van den Berkmortel, F.W.P.J.; Smilde, T.J.; van Warmerdam, S.M.; de Boer, M.; van Spronsen, D.J.; Smidt, M.L.; et al. Ultrasound is at least as good as magnetic resonance imaging in predicting tumour size post-neoadjuvant chemotherapy in breast cancer. Eur. J. Cancer 2016, 52, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chagpar, A.B.; Middleton, L.P.; Sahin, A.A.; Dempsey, P.; Buzdar, A.U.; Mirza, A.N.; Ames, F.C.; Barbiera, G.V.; Feig, B.W.; Hunt, K.K.; et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann. Surg. 2006, 243, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Geng, C.; Chen, X.; Pan, X.; Li, J. The Feasibility and Accuracy of Sentinel Lymph Node Biopsy in Initially Clinically Node-Negative Breast Cancer after Neoadjuvant Chemotherapy: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0162605. [Google Scholar] [CrossRef]
- El Hage Chehade, H.; Headon, H.; El Tokhy, O.; Heeney, J.; Kasem, A.; Mokbel, K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3,398 patients. Am. J. Surg. 2016, 212, 969–981. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- de Wild, S.R.; de Munck, L.; Simons, J.M.; Verloop, J.; van Dalen, T.; Elkhuizen, P.H.M.; Houben, R.M.A.; van Leeuwen, A.E.; Linn, S.C.; Pijnappel, R.M.; et al. De-escalation of radiotherapy after primary chemotherapy in cT1–2N1 breast cancer (RAPCHEM. BOOG 2010–03): 5-year follow-up results of a Dutch, prospective, registry study. Lancet Oncol. 2022, 23, 1201–1210. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinbenbij, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. Available online: https://www.sciencedirect.com/science/article/pii/S1470204514704607 (accessed on 1 August 2022). [CrossRef]
- Ashok, A.; Sude, N.S.; Rakesh, B.A.; Karanam, V.P.K. Prospective Evaluation of Response Outcomes of Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Cureus 2022, 14, e21831. [Google Scholar]
- Beddok, A.; Cottu, P.; Fourquet, A.; Kirova, Y. Combination of Modern Radiotherapy and New Targeted Treatments for Breast Cancer Management. Cancers 2021, 13, 6358. [Google Scholar] [CrossRef]
- Viale, G.; Fusco, N. Pathology after neoadjuvant treatment—How to assess residual disease. Breast 2022, 62 (Suppl. S1), S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Jung, W.-H.; Koo, J.S. Pathologic Evaluation of Breast Cancer after Neoadjuvant Therapy. J. Pathol. Transl. Med. 2016, 50, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Zhaveri, S.; Valente, C.; Pisapati, K.; Pickholz, E.; Weltz, S.; Nayak, A.; Oza, T.; Corben, A.; Weltz, C.; et al. Response in breast vs axilla after neoadjuvant treatment and implications for nonoperative management of invasive breast cancer. Breast J. 2021, 27, 120–125. [Google Scholar] [CrossRef]
- Sejben, A.; Kószó, R.; Kahán, Z.; Cserni, G.; Zombori, T. Examination of Tumor Regression Grading Systems in Breast Cancer Patients Who Received Neoadjuvant Therapy. Pathol. Oncol. Res. 2020, 26, 2747–2754. [Google Scholar] [CrossRef]
- Baker, G.M.; King, T.A.; Schnitt, S.J. Evaluation of breast and axillary lymph node specimens in breast cancer patients treated with neoadjuvant systemic therapy. Adv. Anat. Pathol. 2019, 26, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Veys, I.; Pop, C.F.; Barbieux, R.; Moreau, M.; Noterman, D.; De Neubourg, F.; Nogaret, H.M.; Liberale, G.; Larsimont, D.; Bourgeois, P. ICG fluorescence imaging as a new tool for optimization of pathological evaluation in breast cancer tumors after neoadjuvant chemotherapy. PLoS ONE 2018, 13, e0197857. [Google Scholar] [CrossRef] [PubMed]
- Lanjewar, S.; Patil, P.; Fineberg, S. Pathologic reporting practices for breast cancer specimens after neoadjuvant chemotherapy—A survey of pathologists in academic institutions across the United States. Mod. Pathol. 2020, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef]

| Study | Population (N ) | Intervention | Comparator | Results | |
|---|---|---|---|---|---|
| HER2-negative/TNBC | CREATE-X [12] | HER2-negative with residual disease after NAC (910) | Capecitabine | No adjuvant therapy | 5y DFS: 74.1% vs. 67.6%, HR 0.70 5y OS: 89.2% vs. 83.6%, HR 0.59 |
| TNBC (286) | 5y DFS: 69.8% vs. 56.1%, HR 0.58 5y OS: 78.8% vs. 70.3%, HR 0.52 | ||||
| OlympiA [13,14] | Overall (1836) TNBC (1509) | Olaparib | Placebo | 4y iDFS: 82.7% vs. 75.4%, HR 0.63 3y DDFS: 86.5% vs. 79.1%, HR 0.61 4y OS: 89.8% vs. 86.4%, HR 0.68 | |
| KEYNOTE-522 [8,15] | 1174 | NAC + pembrolizumab and adjuvant pembrolizumab | NAC and adjuvant placebo | pCR: 64.8% vs. 51.2%3y EFS: 84.5% vs. 76.8%, HR 0.63 | |
| Impassion-031 [16] | 455 | NAC + atezolizumab | NAC | pCR: 58% vs. 41% | |
| ECOG-ACRIN EA1131 [17] | 410 | Platinum | Capecitabine | 3y iDFS: 42.8% vs. 53.5%, HR 1.16 | |
| Hormone receptor positive | PENELOPE-B [18] | 1250 | Palbociclib | Placebo | 3y iDFS: 81.2% vs. 77.7%, HR 0.93 |
| PALLAS [19] | 5796 | Palbociclib + ET | ET | 4y iDFS: 84.2% vs. 84.5%, HR 0.96 | |
| MonarchE [20,21] | Overall (5637) | Abemaciclib + ET | ET | 3y iDFS: 88.8% vs. 83.4%, HR 0.69 3y DRFS: 90.3% vs. 86.1%, HR 0.68 | |
| Prior NAC (2087) | 3y iDFS: HR 0.69 | ||||
| OlympiA [12] | Hormone receptor positive (325) | Olaparib | Placebo | 3y iDFS: 83.5% vs. 77.2%, HR 0.70 | |
| HER2 positive | KATHERINE [22] | 1486 | T-DM1 | Trastuzumab | 3y iDFS: 88.3% vs. 77.0%, HR 0.50 |
| ExteNET [23] | Overall (2840) Hormone receptor positive ≤ 1y trastuzumab (1334) | Neratinib | Placebo | 5y iDFS: 90.8% vs. 85.7%, HR 0.58 8y OS: 91.5% vs. 89.4%, HR 0.79 |
| Trial | Design | Status | Population | Treatment | Endpoint |
|---|---|---|---|---|---|
| HER2-negative/TNBC | |||||
| SASCIA NCT04595565 | III | Recruiting | RD after NAC | Arm A: Sacituzumab govitecan Arm B: TPC (capecitabine or platinum-based) | iDFS |
| SWOG S1418/BR006 NCT02954874 | III | Active, not recruiting | High-risk after NAC | Arm A: Observation Arm B: Pembrolizumab | iDFS |
| A-Brave NCT02926196 | III | Active, not recruiting | High risk after NAC | Arm A: Avelumab Arm B: Observation | DFS |
| ASPRIA trial NCT04434040 | II | Recruiting | RD and ctDNA after NAC | Sacituzumab + atezolizumab | Rate of undetectable ctDNA-6 Cycles |
| BreastImmune03 NCT03818685 | II | Active, not recruiting | RD after NAC | Arm A: RT + Nivolumab and Ipilimumab Arm B: RT + Capecitabine | DFS |
| PERSEVERE NCT04849364 | II | Recruiting | RD after NAC based on plasma ctDNA positivity and genomic marker | ctDNA positive with a genomic target: Arm 1a: DNA Repair pathway (talazoparib + capecitabine) Arm 1b: Immunotherapy pathway (atezolizumab + capecitabine) Arm 1c: PI3K Pathway (inavolisib + capecitabine) Arm 1d: DNA Repair + Immunotherapy (talazoparib + atezolizumab + capecitabine) ctDNA positive without a genomic target: Arm 2: Capecitabine or TPC ctDNA negative: Arm 3: observation, capecitabine or TPC | 2y DFS |
| PHOENIX DDR/Anti-PD-L1 Trial NCT03740893 | IIa | Recruiting | RD after NAC | Arm A: Standard of care Arm B: AZD6738 (selective ATR kinase inhibitor) Arm C: Olaparib Arm D: Durvalumab | Change in mean proliferation index (Ki67) |
| NCT03872388 | II | Recruiting | RD after NAC | Arm A: Atorvastatin +/− capecitabine Arm B: Observation +/− capecitabine | Proportion of patients with undetectable CTCs at 6 months |
| NCT04197687 | II | Recruiting | RD after NAC | Arm A: T-DM1 + TPIV100 and Sargramostim Arm B: T-DM1 + Placebo | iDFS |
| NCT04437160 | II | Recruiting | RD after NAC | Arm A: Epirubicin or Pirarubicin Arm B: Cyclophosphamide | RFS |
| NCT02445391 | II | Recruiting | RD after NAC | Arm A: Platinum based CT Arm B: Capecitabine | iDFS |
| APOLLO NCT04501523 | II | Recruiting | High risk identified with ctDNA after NAC | Arm A: ctDNA positive, non-pCR: Tislelizumab (anti-PD1) + capecitabine Arm B: ctDNA positive, non-pCR: capecitabine Arm C: ctDNA positive, pCR: capecitabine Arm D: Follow up | 5y iDFS |
| OXEL NCT03487666 | II | Active, not recruiting | RD after NAC | Arm A: Nivolumab Arm B: Capecitabine Arm C: Nivolumab + capecitabine | PIS at week 6 |
| NCT04677816 | II | Recruiting | Vitamin D deficient in pre and post neoadjuvant setting | Arm A: Vitamin D Supplementation Arm B: Observation | pCR in vit D group |
| ZEST NCT04915755 | III | Recruiting | BRCA wild type with ctDNA after definitive therapy | Arm A: Niraparib Arm B: placebo | DFS |
| COGNITION-GUIDE NCT05332561 | II | Not yet recruiting | High-risk patients with RD after NAC | Genomics-guided targeted therapy (including ICI, PARPi, ADC, PI3Ki, AKTi, anti-HER2 therapy) | iDFS |
| MK-3475-522/KEYNOTE-522 NCT03036488 | III | Active, not recruiting | High-risk early stage pre and post neoadjuvant setting | Neoadjuvant → Adjuvant Arm A: CT + Pembrolizumab → Pembrolizumab Arm A: CT + Placebo → Placebo | pCR EFS |
| HER2-positive | |||||
| DESTINY-Breast05 NCT04622319 | III | Recruiting | RD after NAC | Arm A: T-DXd Arm B: T-DM1 | iDFS |
| DESTINY-Breast11 NCT05113251 | III | Recruiting | High-risk early stage pre and post neoadjuvant setting | A: neoadjuvant T-Dxd B: T-Dxd followed by taxane + trastuzumab pertuzumab C: AC followed by taxane + trastuzumab pertuzumab | pCR |
| CompassHER2-RD NCT04457596 | III | Recruiting | High risk patients with RD after NAC | Arm A: T-DM1 + Tucatinib Arm B: T-DM1 + Placebo | iDFS |
| CompassHER2-pCR NCT04266249 | II | Recruiting | pCR after NAC with taxane + trastuzumab pertuzumab | Arm A (pCR): trastuzumab pertuzumab Arm B (no pCR): T-DM1 | iDFS |
| Astefania NCT04873362 | III | Recruiting | RD after NAC | Arm A: T-DM1 + Atezolizumab Arm B: T-DM1 + Placebo | iDFS |
| DECRESCENDO NCT04675827 | II | Recruiting | De-escalation HER2 therapy after NAC | Neoadjuvant: taxane + pertuzumab and transtuzumab FDC SC Adjuvant: in pCR group (RCB = 0): Pertuzumab and trastuzumab FDC SC in RD (RCB = 1): T-DM1 in RD (RCB ≥2): anthracycline based CT → T-DM1 | 3y-RFS in HER2-enriched pCR |
| ATP NCT04254263 | III | Recruiting | RD after NAC | Arm A: Pyrotinib 2 years Arm B: Placebo 2 years | iDFS |
| NCT04973319 | III | Not yet recruiting | RD after NAC | Arm A: Adjuvant trastuzumab pertuzumab + Pyrotinib Arm B: Adjuvant trastuzumab pertuzumab | iDFS |
| PHERGAIN-2 NCT04733118 | II | Recruiting | CT free pCR guided strategy | Neoadjuvant: Trastuzumab and pertuzumab FDC SC +/− ET Adjuvant: Cohort A (pCR): Trastuzumab and pertuzumab FDC SC +/− ET Cohort B: T-DM1 +/− ET 10 cycles Cohort C: T-DM1 +/− ET 10 cycles (+/− TPC before T-DM1) | 3y RFI |
| COGNITION-GUIDE NCT05332561 | II | Not yet recruiting | High-risk patients with RD after NAC | Genomics-guided targeted therapy (including ICI, PARPi, ADC, PI3Ki, AKTi, anti-HER2 therapy) | iDFS |
| NCT04197687 | II | Recruiting | RD after NAC | Arm A no pCR: T-DM1 + TPIV100 ID and sargramostim Arm B no pCR: T-DM1 + placebo + sargmamostim pCR: trastuzumab and pertuzumab 1 year | iDFS |
| Hormone receptor positive, HER2-negative | |||||
| MK-3475-522/KEYNOTE-522 NCT03725059 | III | Active, not recruiting | High-risk early stage pre and post neoadjuvant setting | Neoadjuvant -> Adjuvant Arm A: CT + Pembrolizumab -> ET + Pembrolizumab Arm B: CT + Placebo -> ET + Placebo | pCR EFS |
| CheckMate 7FL NCT04109066 | III | Active, not recruiting | High-risk early stage pre and post neoadjuvant setting | Neoadjuvant -> Adjuvant Arm A: CT + Nivolumab -> ET + Nivolumab Arm B: CT + Placebo -> ET + Placebo | pCR EFS |
| ZEST NCT04915755 | III | Recruiting | BRCA-mutated patients with ctDNA after surgery or adjuvant therapy | Arm A: Niraparib Arm B: placebo | DFS |
| COGNITION-GUIDE NCT05332561 | II | Not yet recruiting | High-risk patients with RD after NAC | Genomics-guided targeted therapy (including ICI, PARPi, ADC, PI3Ki, AKTi, anti-HER2 therapy) | iDFS |
| RSBNAT NCT03638648 | II | Not yet recruiting | RD after NAC | Stratified according to multiple gene test-based recurrence risk level: Cohort A: High risk: capecitabine Cohort B: Low risk: control group | 2y DFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostinetto, E.; Jacobs, F.; Debien, V.; De Caluwé, A.; Pop, C.-F.; Catteau, X.; Aftimos, P.; de Azambuja, E.; Buisseret, L. Post-Neoadjuvant Treatment Strategies for Patients with Early Breast Cancer. Cancers 2022, 14, 5467. https://doi.org/10.3390/cancers14215467
Agostinetto E, Jacobs F, Debien V, De Caluwé A, Pop C-F, Catteau X, Aftimos P, de Azambuja E, Buisseret L. Post-Neoadjuvant Treatment Strategies for Patients with Early Breast Cancer. Cancers. 2022; 14(21):5467. https://doi.org/10.3390/cancers14215467
Chicago/Turabian StyleAgostinetto, Elisa, Flavia Jacobs, Véronique Debien, Alex De Caluwé, Catalin-Florin Pop, Xavier Catteau, Philippe Aftimos, Evandro de Azambuja, and Laurence Buisseret. 2022. "Post-Neoadjuvant Treatment Strategies for Patients with Early Breast Cancer" Cancers 14, no. 21: 5467. https://doi.org/10.3390/cancers14215467
APA StyleAgostinetto, E., Jacobs, F., Debien, V., De Caluwé, A., Pop, C.-F., Catteau, X., Aftimos, P., de Azambuja, E., & Buisseret, L. (2022). Post-Neoadjuvant Treatment Strategies for Patients with Early Breast Cancer. Cancers, 14(21), 5467. https://doi.org/10.3390/cancers14215467







