Testicular Neoplasms: Primary Tumour Size Is Closely Interrelated with Histology, Clinical Staging, and Tumour Marker Expression Rates—A Comprehensive Statistical Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment, Data Procurement
2.2. Statistical Analysis
3. Results
3.1. General Results
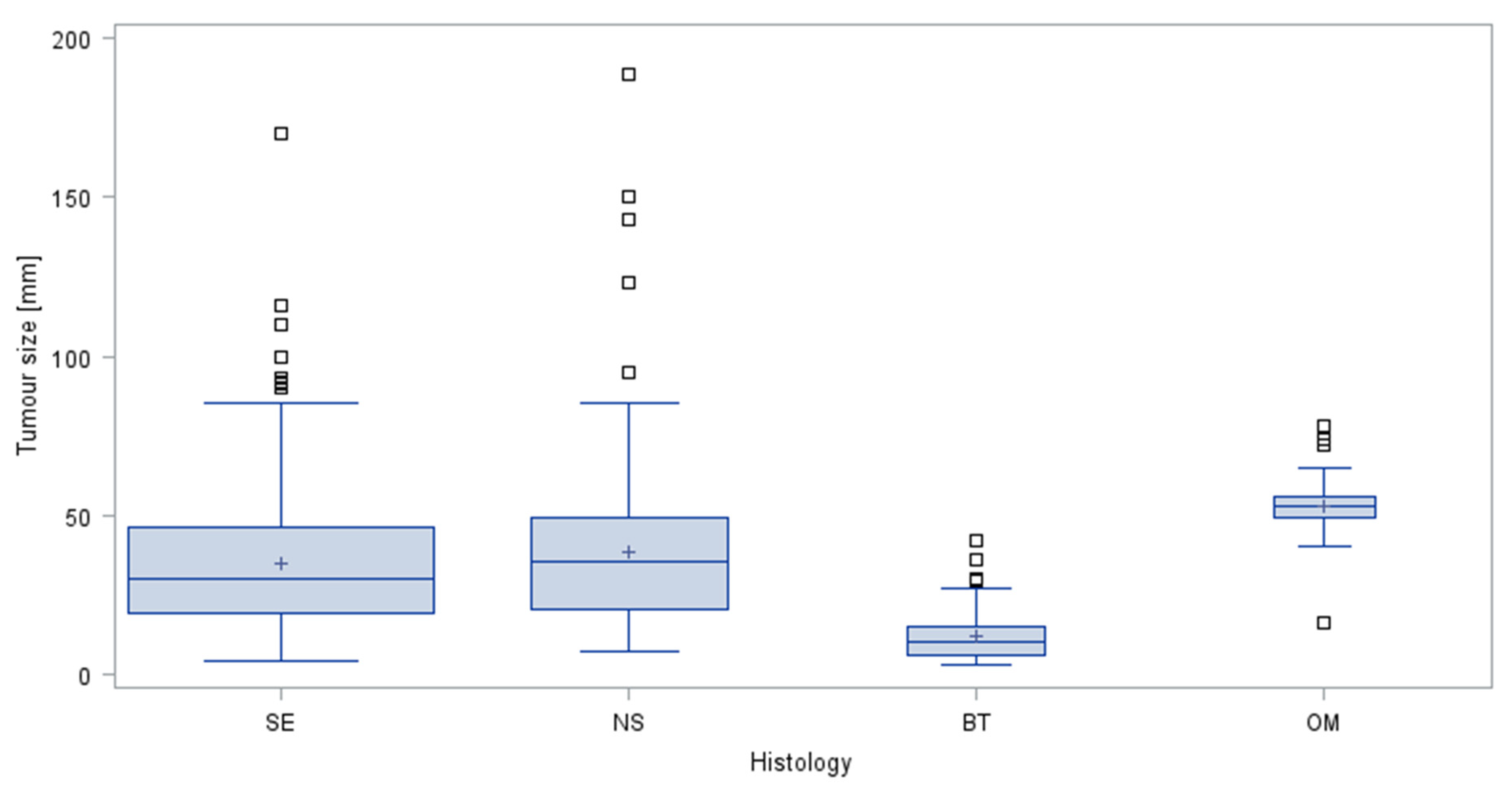
3.2. Assumption # 1 (Association of Tumour Size with Histology)
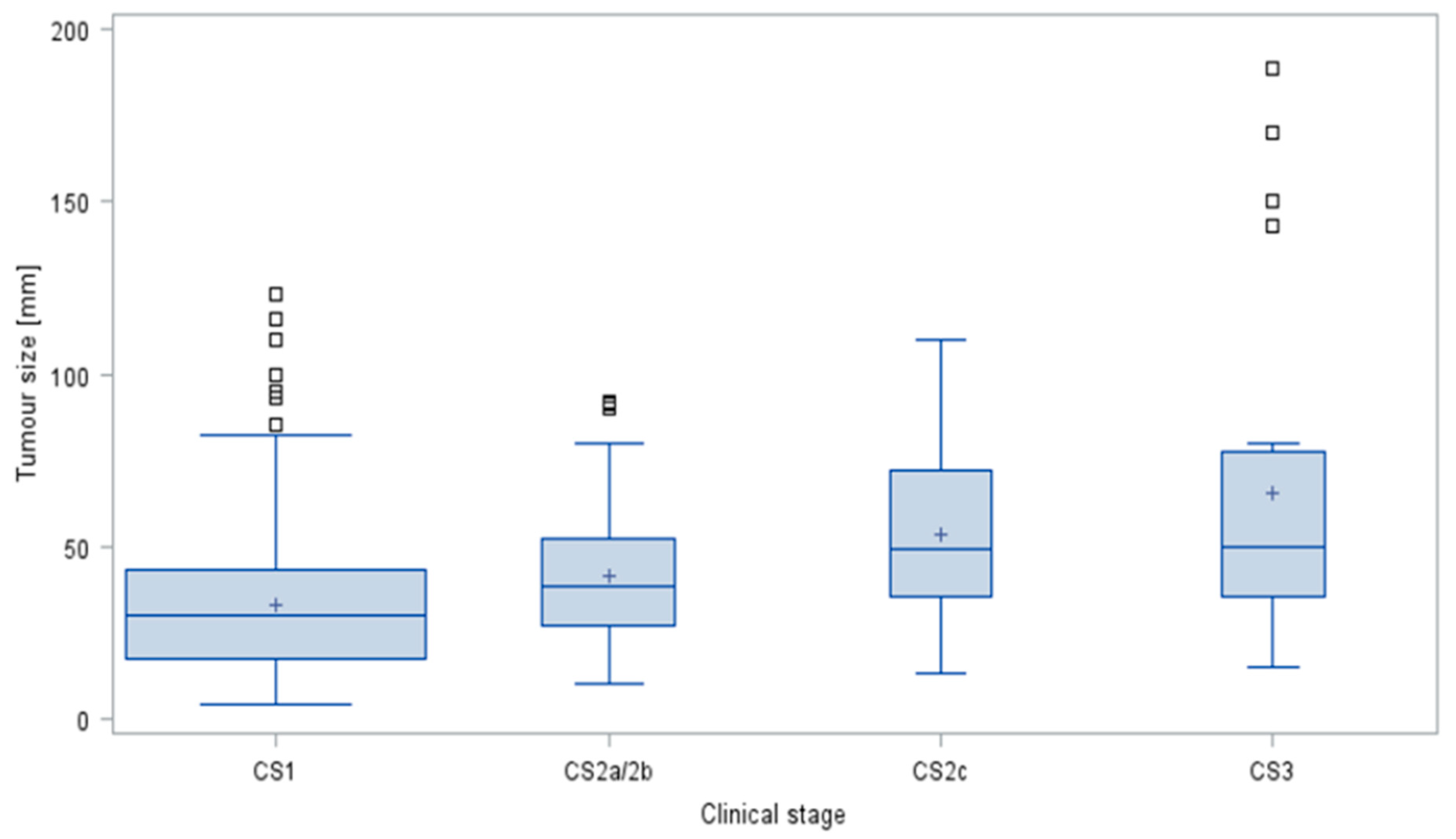
3.3. Assumption #2 (Association of Tumour Size and Clinical Staging in GCTs)
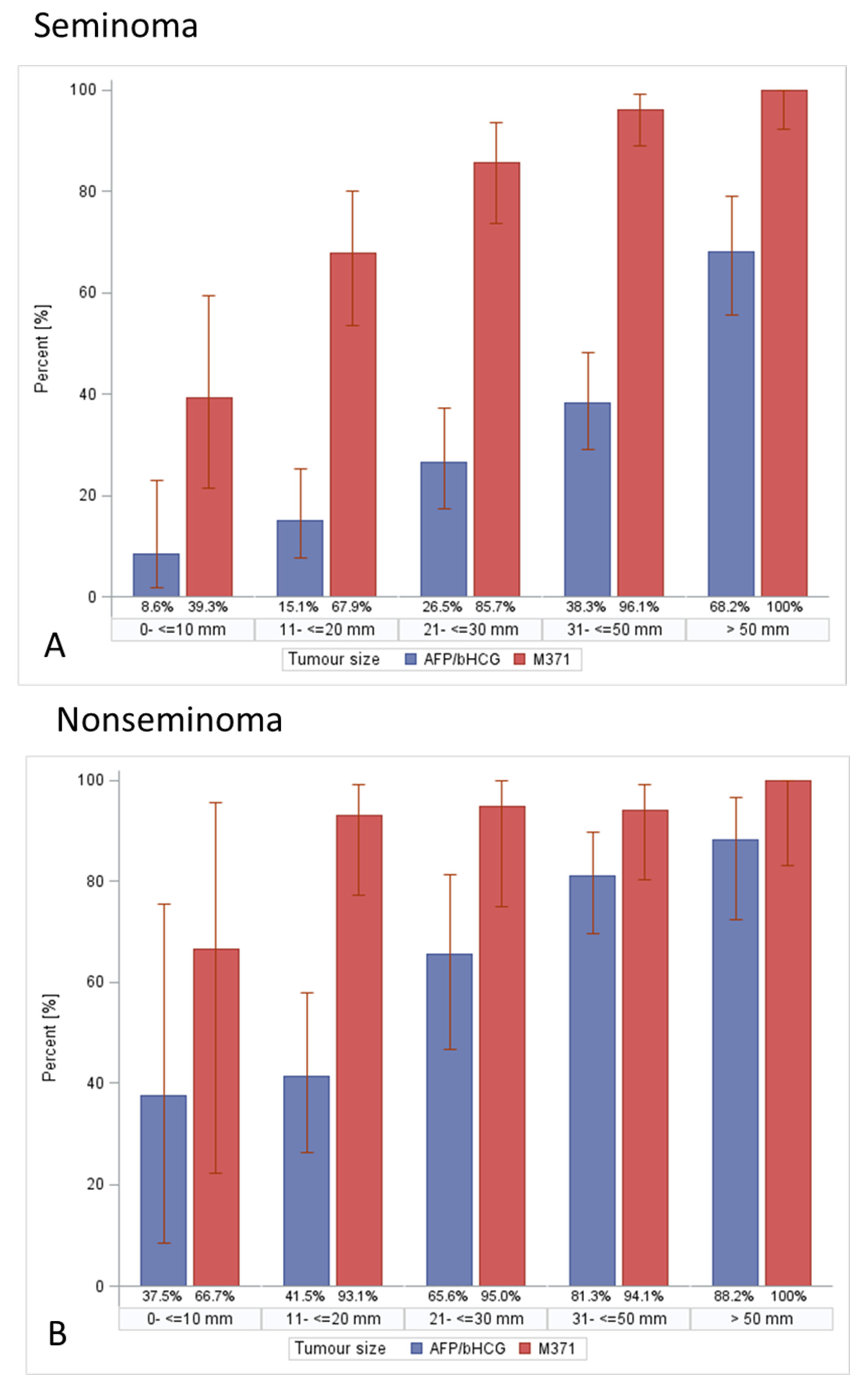
3.4. Assumption #3 (Tumour Size Is Associated with Frequencies of Tumour Marker Expression in GCTs)
3.5. Assumption #4 (Association of Patient Age with Tumour Size)
4. Discussion
4.1. Tumour Size—General Considerations
4.2. Association of Primary Tumour Size with Histology (Assumption #1)
4.3. The Impact of Tumour Size on Clinical Staging in Testicular Germ Cell Tumours (Assumption #2)
4.4. The Association of Tumour Size with Serum Tumour Marker Expression Rates in Germ Cell Tumours (Assumption #3)
4.5. No Association between Patient Age and Tumour Size (Assumption #4)
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha fetoprotein |
| AUC | area under the curve |
| bHCG | beta human chorionic gonadotropin |
| BT | benign tumours |
| CI | confidence interval |
| CS | clinical stage |
| GCT | germ cell tumour |
| IQR | inter quartile range |
| LDH | lactate dehydrogenase |
| M371 | microRNA-371a-3p |
| mm | milli meter |
| NS | nonseminoma |
| n | number |
| OM | other testicular malignancies |
| ROC | receiver operating characteristic |
| SE | seminoma |
| STM | serum tumour marker |
| TS | tumour size |
| ULN | upper limit of norm |
| Q1 | first quartile |
| Q3 | third quartile |
References
- Boden, G.; Gibb, R. Radiotherapy and testicular neoplasms. Lancet 1951, 2, 1195–1197. [Google Scholar] [CrossRef]
- Dixon, F.J.; Moore, R.A. Testicular tumors. A Clinicopathologic study. Cancer 1953, 6, 427–454. [Google Scholar] [CrossRef]
- Javadpour, N. The National Cancer Institute Experience with Testicular Cancer. J. Urol. 1978, 120, 651–659. [Google Scholar] [CrossRef]
- Collins, D.H.; Pugh, R.C.B. Classification and frequency of testicular tumours. Br. J. Urol. 1964, 36, 1–11. [Google Scholar]
- Fraley, E.E.; Lange, P.H.; Kennedy, B.J. Germ-cell testicular cancer in adults (first of two parts). N. Engl. J. Med. 1979, 301, 1370–1377. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Gentile, G.; Rizzo, M.; Bianchi, L.; Falcone, M.; Dente, D.; Cilletti, M.; Franceschelli, A.; Vagnoni, V.; Garofalo, M.; Schiavina, R.; et al. Testis Sparing Surgery for Small Testicular Masses: Retrospective Analysis of a Multi-Center Cohort. J. Urol. 2020, 203, 760–766. [Google Scholar] [CrossRef]
- Tsili, A.C.; Bougia, C.K.; Pappa, O.; Argyropoulou, M. Ultrasonography of the scrotum: Revisiting a classic technique. Eur. J. Radiol. 2021, 145, 110000. [Google Scholar] [CrossRef]
- Ager, M.; Donegan, S.; Boeri, L.; Mayor de Castro, J.; Donaldson, J.F.; Omar, M.I.; Dimitropoulos, K.; Tharakan, T.; Janisch, F.; Muilwijk, T.; et al. Radiological features characterising indeterminate testes masses; A systematic review and meta-analysis. BJU Int. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Bertolotto, M.; Muça, M.; Currò, F.; Bucci, S.; Rocher, L.; Cova, M.A. Multiparametric US for scrotal diseases. Abdom. Radiol. 2018, 43, 899–917. [Google Scholar] [CrossRef]
- Zengerling, F.; Kunath, F.; Jensen, K.; Ruf, C.; Schmidt, S.; Spek, A. Prognostic factors for tumor recurrence in patients with clinical stage I seminoma undergoing surveillance-A systematic review. Urol. Oncol. 2018, 36, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.P.; Simonsen-Richter, H.; Kulejewski, M.; Anheuser, P.; Zecha, H.; Isbarn, H.; Pichlmeier, U. Serum Tumour Markers in Testicular Germ Cell Tumours: Frequencies of Elevated Levels and Extents of Marker Elevation Are Significantly Associated with Clinical Parameters and with Response to Treatment. BioMed Res. Int. 2019, 2019, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Leão, R.; Albersen, M.; Looijenga, L.H.J.; Tandstad, T.; Kollmannsberger, C.; Murray, M.J.; Culine, S.; Coleman, N.; Belge, G.; Hamilton, R.J.; et al. Circulating MicroRNAs, the Next-Generation Serum Biomarkers in Testicular Germ Cell Tumours: A Systematic Review. Eur. Urol. 2021, 80, 456–466. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Radtke, A.; Geczi, L.; Matthies, C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.; Pichler, R.; et al. Serum levels of microRNA-371a-3p (M371 Test) as a new biomarker of testicular germ cell-tumors: Results of a prospective multicentric study. J. Clin. Oncol. 2019, 37, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Obeidat, S.; Hickerton, B.; Long, R. Has increasing public health awareness influenced the size of testicular tumours among adult populations over the last 40 years? J. Public Health 2016, 39, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Scandura, G.; Verrill, C.; Protheroe, A.; Joseph, J.; Ansell, W.; Sahdev, A.; Shamash, J.; Berney, D.M. Incidentally detected testicular lesions <10 mm in diameter: Can orchidectomy be avoided? BJU Int. 2017, 121, 575–582. [Google Scholar] [CrossRef]
- Heinzelbecker, J.; Katzmarzik, M.; Weiss, C.; Trojan, L.; Michel, M.S.; Haecker, A. Changes of Stage, Predictive Factors and Adjuvant Treatment Modalities in Seminomatous Testicular Cancer from 1987 to 2007 and Their Impact on the Status of Metastasis, Recurrence-Free and Overall Survival: A Single-Center Analysis. Urol. Int. 2011, 87, 282–287. [Google Scholar] [CrossRef]
- Song, G.; Xiong, G.Y.; Fan, Y.; Huang, C.; Kang, Y.M.; Ji, G.J.; Chen, J.C.; Xin, Z.C.; Zhou, L.Q. The role of tumor size, ultrasonographic findings, and serum tumor markers in predicting the likelihood of malignant testicular histology. Asian J. Androl. 2019, 21, 196–200. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Richter-Simonsen, H.; Kulejewski, M.; Ikogho, R.; Zecha, H.; Anheuser, P.; Pichlmeier, U. Testicular germ-cell tumours: A descriptive analysis of clinical characteristics at first presentation. Urol. Int. 2018, 100, 409–419. [Google Scholar] [CrossRef]
- Kliesch, S.; Schmidt, S.; Wilborn, D.; Aigner, C.; Albrecht, W.; Bedke, J.; Beintker, M.; Beyersdorff, D.; Bokemeyer, C.; Busch, J.; et al. Management of Germ Cell Tumours of the Testis in Adult Patients. German Clinical Practice Guideline Part I: Epidemiology, Classification, Diagnosis, Prognosis, Fertility Preservation, and Treatment Recommendations for Localized Stages. Urol. Int. 2021, 105, 169–180. [Google Scholar] [CrossRef]
- Rothermundt, C.; Thurneysen, C.; Cathomas, R.; Müller, B.; Mingrone, W.; Hirschi-Blickenstorfer, A.; Wehrhahn, T.; Ruf, C.; Rothschild, S.; Seifert, B.; et al. Baseline characteristics and patterns of care in testicular cancer patients: First data from the Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS). Swiss Med. Wkly. 2018, 148, w14640. [Google Scholar] [PubMed]
- Heinzelbecker, J.; Katzmarzik, M.; Weiss, C.; Trojan, L.; Haecker, A. During twenty years of Cisplatin-based therapy the face of nonseminomatous testicular germ cell tumors is still changing: An evaluation of presentation, management, predictive factors and survival. Int. Braz. J. Urol. 2013, 39, 10–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paffenholz, P.; Held, L.; Loosen, S.H.; Pfister, D.; Heidenreich, A. Testis-sparing surgery for benign testicular masses–diagnostics and therapeutic approaches. J. Urol. 2018, 200, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Woldu, S.L.; Aydin, A.M.; Rao, A.V.; Hutchinson, R.C.; Singla, N.; Clinton, T.N.; Krabbe, L.M.; Passoni, N.M.; Raj, G.V.; Miller, D.S.; et al. Differences at Presentation and Treatment of Testicular Cancer in Hispanic Men: Institutional and National Hospital-Based Analysis. Urology 2018, 112, 103–111. [Google Scholar] [CrossRef]
- Bhardwa, J.M.; Powles, T.; Berney, D.; Baithun, S.; Nargund, V.H.; Oliver, R.T. Assessing the size and stage of testicular germ cell tumours: 1984-2003. BJU Int. 2005, 96, 819–821. [Google Scholar] [CrossRef]
- Agnarsson, B.A.; Gudbjartsson, T.; Einarsson, G.V.; Magnusson, K.; Thoroddsen, A.; Bergthorsson, J.T.; Amundadottir, L.; Barkardottir, R.B.; Björnsson, J. Testicular germ cell tumours in Iceland: A nationwide clinicopathological study. APMIS 2006, 114, 779–783. [Google Scholar] [CrossRef]
- Kobayashi, K.; Saito, T.; Kitamura, Y.; Nobushita, T.; Kawasaki, T.; Hara, N.; Takahashi, K. Effect of the time from the presentation of symptoms to medical consultation on primary tumor size and survival in patients with testicular cancer: Shift in the last 2 decades. Urol. Oncol. 2014, 32, 43.e17–43.e22. [Google Scholar] [CrossRef]
- MacKay, E.N.; Sellers, A.H. A statistical study of malignant testicular tumours based on the experience of the Ontario cancer Foundation Clinics, 1938-1961. Canad. Med. Ass. J. 1966, 94, 889–899. [Google Scholar]
- Krag Jacobsen, G.; Barlebo, H.; Olsen, J.; Schultz, H.P.; Starklint, H.; Sögaaard, H.; Vaeth, M.; Dateca Study Group. Testicular germ cell tumours in Denmark 1976-1980. Acta Radiol. Oncol. 1984, 23, 239–247. [Google Scholar]
- Egan, J.; Cheaib, J.G.; Biles, M.J.; Huang, M.M.; Metcalf, M.; Matoso, A.; Pierorazio, P. Testis-Sparing Surgery: A Single Institution Experience. Urology 2020, 147, 192–198. [Google Scholar] [CrossRef]
- Schwen, Z.R.; Liu, J.L.; Gabrielson, A.T.; Patel, H.D.; Gupta, M.; Rowe, S.P.; Herati, A.S.; Pierorazio, P.M. Testicular ultrasound underestimates the size of small testicular masses: A radiologic-pathologic correlation study. World J. Urol. 2021, 39, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Keske, M.; Canda, A.E.; Yalcin, S.; Kilicarslan, A.; Kibar, Y.; Tuygun, C.; Onder, E.; Atmaca, A.F.; Yildirim, A.; Ozkanli, S.S.; et al. Is testis-sparing surgery safe in small testicular masses? Results of a multicentre study. Can. Urol. Assoc. J. 2017, 11, E100–E104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pratsinis, M.; Fankhauser, C.; Pratsinis, K.; Beyer, J.; Bührer, E.; Cathomas, R.; Fischer, N.; Hermanns, T.; Hirschi-Blickenstorfer, A.; Kamradt, J.; et al. Metastatic Potential of Small Testicular Germ Cell Tumors: Implications for Surveillance of Small Testicular Masses. Eur. Urol. Open Sci. 2022, 40, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Belfield, J.; Findlay-Line, C. Testicular Germ Cell Tumours-The Role of Conventional Ultrasound. Cancers 2022, 14, 3882. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, A.; Freeman, S.; Jędrzejczyk, M.; Dobruch, J.; Dong, Y.; Bertolotto, M.; Dietrich, C.F. Incidental Findings and How to Manage Them: Testis- A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2787–2802. [Google Scholar] [CrossRef]
- Wardak, S.; Pang, K.H.; Castiglione, F.; Lindsay, J.; Walkden, M.; Heffernan Ho, D.; Kirkham, A.; Hadway, P.; Nigam, R.; Rees, R.; et al. Management of small testicular masses: Outcomes from a single centre multidisciplinary team. BJU Int. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Shilo, Y.; Zisman, A.; Lindner, A.; Raz, O.; Strauss, S.; Siegel, Y.I.; Segal, M.; Sandbank, J.; Leibovici, D. The predominance of benign histology in small testicular masses. Urol. Oncol. 2012, 30, 719–722. [Google Scholar] [CrossRef]
- Galosi, A.B.; Fulvi, P.; Fabiani, A.; Servi, L.; Filosa, A.; Leone, L.; Marronaro, A.; Caraceni, E.; Montironi, R. Testicular sparing surgery in small testis masses: A multinstitutional experience. Arch. Ital. Urol. Androl. 2016, 88, 320–324. [Google Scholar] [CrossRef][Green Version]
- Lagabrielle, S.; Durand, X.; Droupy, S.; Izard, V.; Marcelli, F.; Huyghe, E.; Ferriere, J.M.; Ferretti, L. Testicular tumours discovered during infertility workup are predominantly benign and could initially be managed by sparing surgery. J. Surg. Oncol. 2018, 118, 630–635. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Frey, U.; Lock, G. Contemporary diagnostic work up of testicular germ cell tumours. Nat. Rev. Urol. 2013, 10, 703–712. [Google Scholar] [CrossRef]
- Gobbo, A.; Negri, L.; Casale, P.; Fasulo, V.; Lughezzani, G.; Saitta, C.; Benaglia, R.; Buffi, N.M.; Levi Setti, P. Is testis sparing surgery safe in patients with incidental small testicular lesions referring to a fertility center? A retrospective analysis reporting factors correlated to malignancy and long-term oncological outcomes. Urol. Oncol. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Narayan, Y.; Brown, D.; Ivaz, S.; Das, K.; Moussa, M.; Tsampoukas, G.; Papatsoris, A.; Buchholz, N. Incidental testicular masses and the role of organ-sparing approach. Arch. Ital. Urol. Androl. 2021, 93, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Corrie, D.; Mueller, E.J.; Thompson, I.M. Management of ultrasonically detected nonpalpable testis masses. Urology 1991, 38, 429–431. [Google Scholar] [CrossRef]
- Staudacher, N.; Tulchiner, G.; Bates, K.; Ladurner, M.; Kafka, M.; Aigner, F.; Pichler, R.; Horninger, W. Organ-Sparing Surgery in Testicular Tumor: Is This the Right Approach for Lesions ≤ 20 mm? J. Clin. Med. 2020, 9, 2911. [Google Scholar] [CrossRef]
- Del Real, O.J.; Calvo de la Barra, C.I.; Jiménez, J.A.; Sepulveda, F.; Domínguez, J. Predicting malignancy in small testicular lesions. Cent. Eur. J. Urol. 2022, 75, 47–51. [Google Scholar] [CrossRef]
- Ates, F.; Malkoc, E.; Zor, M.; Demirer, Z.; Alp, B.F.; Basal, S.; Guragac, A.; Yildirim, I. Testis-Sparing Surgery in Small Testicular Masses Not Suspected to Be Malignant. Clin. Genitourin. Cancer 2016, 14, e49–e53. [Google Scholar] [CrossRef]
- Hindley, R.G.; Chandra, A.; Saunders, A.; O’Brien, T.S. Impalpable testis cancer. BJU Int. 2003, 92, 572–574. [Google Scholar] [CrossRef]
- Niemczyk, G.; Zapała, Ł.; Borkowski, T.; Szabłoński, W.; Radziszewski, P.; Cudnoch-Jędrzejewska, A. Feasibility of active surveillance in small testicular mass: A mini review. Cent. Eur. J. Urol. 2021, 74, 10–13. [Google Scholar]
- Bieniek, J.M.; Juvet, T.; Margolis, M.; Grober, E.D.; Lo, K.C.; Jarvi, K.A. Prevalence and management of incidental small testicular masses discovered on ultrasonographic evaluation of male infertility. J. Urol. 2018, 199, 481–486. [Google Scholar] [CrossRef]
- Eifler, J.B.J.; King, P.; Schlegel, P.N. Incidental Testicular Lesions Found During Infertility Evaluation are Usually Benign and May be Managed Conservatively. J. Urol. 2008, 180, 261–265. [Google Scholar] [CrossRef]
- Beck, S.D.; Foster, R.S.; Bihrle, R.; Donohue, J.P. Significance of primary tumor size and preorchiectomy serum tumor marker level in predicting pathologic stage at retroperitoneal lymph node dissection in clinical Stage A nonseminomatous germ cell tumors. Urology 2007, 69, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Warde, P.; Specht, L.; Horwich, A.; Oliver, T.; Panzarella, T.; Gospodarowicz, M.; Von Der Maase, H. Prognostic factors for relapse in stage I seminoma managed by surveillance: A pooled analysis. J. Clin. Oncol. 2002, 20, 4448–4452. [Google Scholar] [CrossRef] [PubMed]
- Boormans, J.L.; Mayor de Castro, J.; Marconi, L.; Yuan, Y.; Laguna Pes, M.P.; Bokemeyer, C.; Nicolai, N.; Algaba, F.; Oldenburg, J.; Albers, P. Testicular Tumour Size and Rete Testis Invasion as Prognostic Factors for the Risk of Relapse of Clinical Stage I Seminoma Testis Patients Under Surveillance: A Systematic Review by the Testicular Cancer Guidelines Panel. Eur. Urol. 2018, 73, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.; Eggener, S.E.; Bass, E.B.; Chelnick, D.M.; Daneshmand, S.; Feldman, D.; Gilligan, T.; Karam, J.A.; Leibovich, B.; Liauw, S.L.; et al. Diagnosis and Treatment of Early Stage Testicular Cancer: AUA Guideline. J. Urol. 2019, 202, 272–281. [Google Scholar] [CrossRef]
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leão, R.; et al. Testicular seminoma and non-seminoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 362–375. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Madsen, E.L.; Blaabjerg, O.; Petersen, P.H.; von der Maase, H.; Jacobsen, G.K.; Rorth, M. Serum lactate dehydrogenase isoenzyme 1 and relapse in patients with nonseminomatous testicular germ cell tumors clinical stage I. Acta Oncol. 2002, 40, 536–540. [Google Scholar] [CrossRef]
- Hartmann, M.; Pottek, T.; Bussar-Maatz, R.; Weissbach, L. Elevated human chorionic gonadotropin concentrations in the testicular vein and in peripheral venous blood in seminoma patients. An analysis of various parameters. Eur. Urol. 1997, 31, 408–413. [Google Scholar]
- Huertas Mora, R.A.; Larrodera López, L.; Gómez Matobella, I.; Cortes Funes, H. Human B-HCG and AFP as biological markers in germinal testicular tumors. Article in Spanish. Rev. Esp. Oncol. 1984, 31, 321–330. [Google Scholar]
- Myklebust, M.P.; Thor, A.; Rosenlund, B.; Gjengstø, P.; Karlsdottir, Á.; Brydøy, M.; Bercea, B.S.; Olsen, C.; Johnson, I.; Berg, M.I.; et al. Serum miR371 in testicular germ cell cancer before and after orchiectomy, assessed by digital-droplet PCR in a prospective study. Sci. Rep. 2021, 11, 15582. [Google Scholar] [CrossRef]
- Nason, G.J.; Aditya, I.; Leao, R.; Anson-Cartwright, L.; Jewett, M.A.S.; O’Malley, M.; Sweet, J.; Hamilton, R.J. Partial orchiectomy: The Princess Margaret cancer centre experience. Urol. Oncol. 2020, 38, 605.e19–605.e24. [Google Scholar] [CrossRef]

| n (%) | Age (Years) | |||||
|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | ||
| Total | 641 (100%) | 17 | 31 | 38 | 47 | 98 |
| Seminoma (SE) | 365 (56.94%) | 17 | 33 | 40 | 48 | 78 |
| Nonseminoma (NS) | 179 (27.93%) | 17 | 26 | 31 | 37 | 74 |
| Benign tumours (BT) | 79 (12.32%) | 19 | 32 | 41 | 50 | 68 |
| Other malignant tumours (OM) | 18 (2.81%) | 52 | 68 | 72.5 | 78 | 98 |
| GCT (mm) | Seminomas (mm) | Nonseminomas (mm) | Benign Tumours (mm) | Other Malignant Tumours (mm) | |
|---|---|---|---|---|---|
| Mean | 35.9 | 34.8 | 38.2 | 11.8 | 53.0 |
| Std Dev | 23.2 | 22.2 | 25.7 | 7.8 | 14.3 |
| Min | 3 | 4 | 7 | 3 | 16 |
| Q1 | 15 | 19 | 20 | 6 | 49 |
| Median | 30 | 30 | 35 | 10 | 53 |
| Q3 | 46 | 46 | 49 | 15 | 56 |
| Max | 189 | 170 | 189 | 42 | 78 |
| n | 544 | 365 | 179 | 79 | 18 |
| Histologic Groups | p * |
|---|---|
| SE vs. NS | 0.1255 |
| (SE + NS) vs. (BT + OM) | <0.0001 |
| (SE + NS) vs. BT | <0.0001 |
| (SE + NS) vs. OM | <0.0001 |
| BT vs. OM | <0.0001 |
| Size Categories | SE | NS | BT | OM | Total | p * |
|---|---|---|---|---|---|---|
| ≤10 mm (n = 87) | 35 (40.23%) | 8 (9.20%) | 44 (50.57%) | 0 (0.00%) | 100% | <0.0001 |
| >10 mm (n = 554) | 330 (59.57%) | 171 (30.87%) | 35 (6.32%) | 18 (3.25%) | 100% |
| Size Categories | SE | NS | BT | OM | Total |
|---|---|---|---|---|---|
| ≤10 mm (n = 87) | 35 (40.23%) | 8 (9.20%) | 44 (50.57%) | 0 (0.00%) | 100% |
| 11–20 (n = 141) | 73 (51.77%) | 41 (29.08%) | 26 (18.44%) | 1 (0.71%) | 100% |
| 21–30 (n = 123) | 84 (68.29%) | 32 (26.02%) | 7 (5.69%) | 0 (0.00%) | 100% |
| >30 mm (n = 290) | 173 (59.66%) | 98 (33.79%) | 2 (0.69%) | 17 (5.86%) | 100% |
| Size Categories | GCT + OM | BT | p * |
|---|---|---|---|
| ≤10 (n = 87) | 43 (49.43%) | 44 (50.57%) | <0.0001 |
| 11–20 (n = 141) | 115 (81.56%) | 26 (18.44%) | |
| 21–30 (n = 123) | 116 (94.31%) | 7 (5.69%) | |
| >30 mm (n = 290) | 288 (99.31%) | 2 (0.69%) |
| CS1 (n) | CS2a/2b (n) | CS2c (n) | CS3 (n) | |
|---|---|---|---|---|
| ≤10 mm | 42 (97.67%) | 1 (2.33%) | 0 (0.00%) | 0 (0.00%) |
| 11–20 mm | 98 (85.96%) | 11 (9.65%) | 3 (2.63%) | 2 (1.75%) |
| 21–30 mm | 94 (81.03%) | 19 (16.38%) | 0 (0.00%) | 3 (2.59%) |
| >30 mm | 187 (69.00%) | 53 (19.56%) | 12 (4.43%) | 19 (7.01%) |
| CS1 (n) | >CS1 (n) | p * | |
|---|---|---|---|
| ≤10 mm | 42 (97.67%) | 1 (2.33%) | <0.0001 |
| 11–20 mm | 98 (85.96%) | 16 (14.04%) | |
| 21–30 mm | 94 (81.03%) | 22 (18.97%) | |
| >30 mm | 187 (69.00%) | 84 (31.00%) |
| CS1 (mm) | CS2a,b (mm) | CS2c (mm) | CS3 (mm) | |
|---|---|---|---|---|
| Mean | 32.6 | 41.2 | 53.2 | 65.5 |
| Std Dev | 20 | 19.5 | 30.1 | 48.3 |
| Min | 4 | 10 | 13 | 15 |
| Q1 | 17 | 27 | 35 | 35.5 |
| Median | 30 | 38 | 49 | 50 |
| Q3 | 43 | 52 | 72 | 77.5 |
| Max | 123 | 92 | 110 | 189 |
| n | 421 | 84 | 15 | 24 |
| bHCG | AFP | LDH | AFP/bHCG | M371 | |
|---|---|---|---|---|---|
| n/N | n/N | n/N | n/N | n/N | |
| ≤10 mm | 6/87 (6.90%) | 2/87 (2.30%) | 3/87 (3.45%) | 8/87 (9.20%) | 16/75 (21.33%) |
| >10 mm | 202/553 (36.53%) | 116/553 (20.98%) | 122/546 (22.34%) | 241/553 (43.58%) | 309/375 (82.40%) |
| p-value * | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| bHCG | AFP | LDH | AFP/bHCG | M371 | |
|---|---|---|---|---|---|
| n/N | n/N | n/N | n/N | n/N | |
| ≤10 mm | 5/43 (11.63%) | 1/43 (2.33%) | 3/43 (6.98%) | 6/43 (13.95%) | 15/34 (44.12%) |
| >10 mm | 299/500 (59.80%) | 115/500 (23.00%) | 119/493 (24.14%) | 239/500 (47.80%) | 302/335 (90.15%) |
| p-value * | 0.0002 | 0.0015 | 0.0101 | <0.0001 | <0.0001 |
| bHCG | AFP | LDH | AFP/ bHCG | M371 | |
|---|---|---|---|---|---|
| n/N | n/N | n/N | n/N | n/N | |
| ≤10 mm | 4/42 (9.52%) | 1/42 (2.38%) | 3/42 (7.14%) | 5/42 (11.90%) | 15/34 (44.12%) |
| >10 mm | 132/378 (34.92%) | 70/378 (18.52%) | 63/375 (16.80%) | 158/378 (41.80%) | 240/273 (87.91%) |
| p-value * | 0.0008 | 0.0081 | 0.1039 | 0.0002 | <0.0001 |
| bHCG | AFP | LDH | AFP/bHCG | M371 | |
|---|---|---|---|---|---|
| n/N | n/N | n/N | n/N | n/N | |
| Total population | |||||
| ≤10 mm (n = 87) | 6/87 (6.90%) | 2/87 (2.30%) | 3/87 (3.45%) | 8/87 (9.20%) | 16/75 (21.33%) |
| 11–20 mm (n = 141) | 23/141 (16.31%) | 14 /141 (9.93%) | 12/140 (8.57%) | 30/141 (21.28%) | 66/107 (61.68%) |
| 21–30 mm (n = 123) | 47/122 (30.33%) | 21/122 (17.21%) | 15/121 (12.39%) | 43/122 (35.25%) | 67/81 (82.72%) |
| 31–50 mm (n = 179) | 76/179 (42.46%) | 52 /179 (29.05%) | 45/176 (25.57%) | 93/179 (51.96%) | 107/114 (93.86%) |
| >50 mm (n = 111) | 66/111 (59.46%) | 29/111 (26.13%) | 50/109 (45.87%) | 75/111 (67.57%) | 69/73 (94.52%) |
| p-value * | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Seminoma | |||||
| ≤10 mm | 2/35 (5.71%) | 1/35 (2.86%) | 3/35 (8.57%) | 3/35 (8.57%) | 11/28 (39.29%) |
| 11–20 mm (n = 73) | 8/73 (10.96%) | 3/73 (4.11%) | 8/72 (11.11%) | 11/73 (15.07%) | 36/53 (67.92%) |
| 21–30 mm (n = 84) | 18/83 (21.69%) | 5/83 (6.02%) | 9/83 (10.84%) | 22/83 (26.51%) | 48/56 (85.71%) |
| 31–50 mm (n = 107) | 35/107 (32.71%) | 8/107 (7.48%) | 27/106 (25.47%) | 41/107 (38.32%) | 74/77 (96.10%) |
| >50 mm (n = 66) | 43/66 (65.15%) | 2/66 (3.03%) | 35/66 (53.03%) | 45/66 (68.18%) | 46/46 (100%) |
| p-value * | <0.0001 | 0.9133 | <0.0001 | <0.0001 | <0.0001 |
| Nonseminoma | |||||
| ≤10 mm (n = 8) | 3/8/37.50%) | 0/8 (0.00%) | 0/8 (0.00%) | 3/8 (37.50%) | 4/6 (66.67%) |
| 11–20 mm (n = 41) | 14/41 (34.15%) | 10/41 (24.39%) | 3/41 (7.32%) | 17/41 (41.46%) | 27/29 (93.10%) |
| 21–30 mm (n = 32) | 19/32 (59.38%) | 16/32 (50.00%) | 6/31 (19.35%) | 21/32 (65.63%) | 19/20 (95.00%) |
| 31–50 mm (n = 64) | 41/64 (64.06%) | 44/64 (68.75%) | 17/62 (27.42%) | 52/64 (81.25%) | 32/34 (94.12%) |
| >50 mm (n = 34) | 23/34 (67.65%) | 27/34 (79.41%) | 14/32 (43.75%) | 30/34 (88.24%) | 20/20 (100%) |
| p-value * | 0.0022 | <0.0001 | <0.0001 | <0.0001 | 0.0745 |
| Age Categories (years) | Tumour Size (mm) | p * | ||||
|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | ||
| ≤30 | 3 | 18 | 32 | 50 | 189 | 0.0117 |
| 31–40 | 4 | 15 | 28 | 43 | 95 | |
| 41–50 | 4 | 14 | 24 | 40 | 110 | |
| >50 | 3 | 15 | 35 | 53 | 170 | |
| Age Categories (years) | Tumour Size (mm) | p * | ||||
|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | ||
| ≤30 | 6 | 24 | 35 | 50 | 189 | 0.0161 |
| 31–40 | 4 | 20 | 30 | 45 | 95 | |
| 41–50 | 4 | 15 | 27 | 42 | 110 | |
| >50 | 7 | 19 | 36.5 | 53 | 170 | |
| Patients | Cutoff | AUC | 95% CI | Sensitivity | Specificity | ||
|---|---|---|---|---|---|---|---|
| First Author [#] | Year | (n) | (mm) | (%) | (%) | ||
| Shilo [37] | 2012 | 11 | 18.5 | 0.902 | 87 | 83 | |
| Paffenholz [23] | 2018 | 28 | 14 | 0.896 | 83 | 89 | |
| Gentile [7] | 2019 | 108 | 8.5 | 0.75 | 0.63; 0.86 | 81 | 58 |
| Staudacher [44] | 2020 | 60 | 13.5 | 0.726 | 0.623; 0.828 | 55 | 85 |
| Schwen [31] | 2021 | 22 | 10 | 0.60 | 31.8 | 88.7 | |
| Gobbo [41] | 2022 | 56 | 10 | 0.59 | 0.43; 0.75 | 25 | 92.9 |
| Del Real [45] | 2022 | 22 | 18.3 | 0.753 | 83 | 74 | |
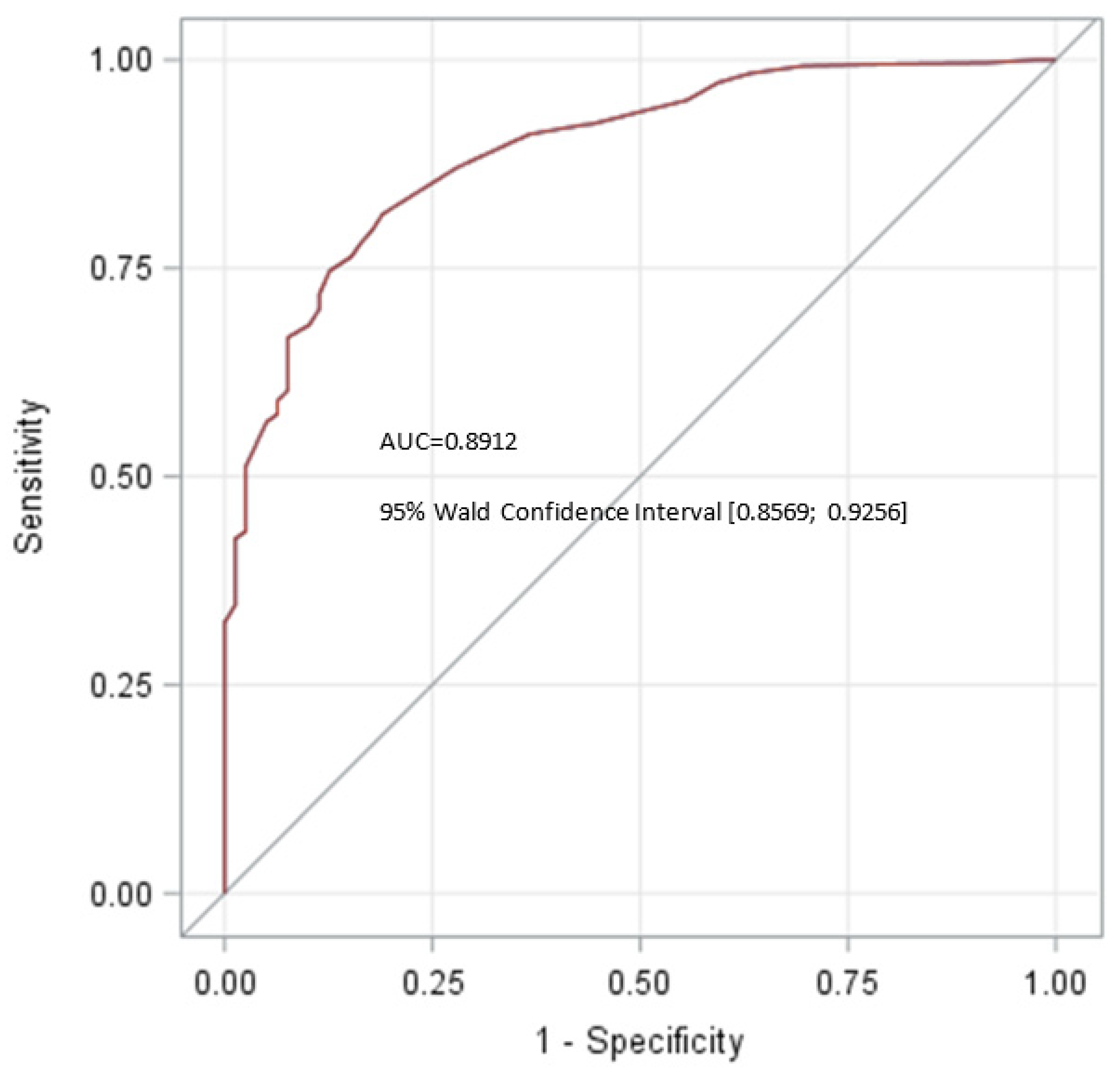
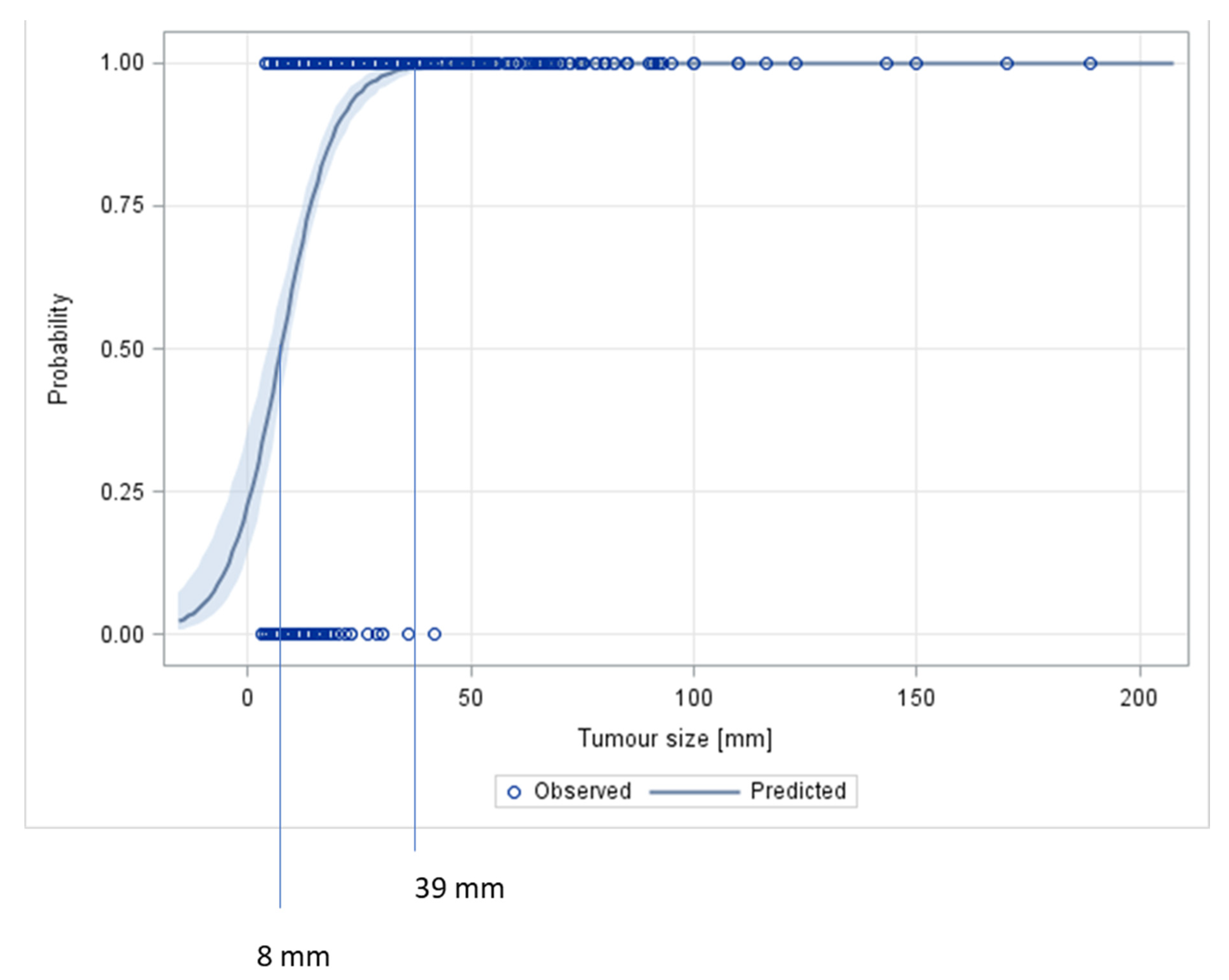
| Present study | 2022 | 79 | 16 | 0.8912 | 0.8569; 0.9256 | 81.5 | 81.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieckmann, K.-P.; Isbarn, H.; Grobelny, F.; Dumlupinar, C.; Utschig, J.; Wülfing, C.; Pichlmeier, U.; Belge, G. Testicular Neoplasms: Primary Tumour Size Is Closely Interrelated with Histology, Clinical Staging, and Tumour Marker Expression Rates—A Comprehensive Statistical Analysis. Cancers 2022, 14, 5447. https://doi.org/10.3390/cancers14215447
Dieckmann K-P, Isbarn H, Grobelny F, Dumlupinar C, Utschig J, Wülfing C, Pichlmeier U, Belge G. Testicular Neoplasms: Primary Tumour Size Is Closely Interrelated with Histology, Clinical Staging, and Tumour Marker Expression Rates—A Comprehensive Statistical Analysis. Cancers. 2022; 14(21):5447. https://doi.org/10.3390/cancers14215447
Chicago/Turabian StyleDieckmann, Klaus-Peter, Hendrik Isbarn, Francesca Grobelny, Cansu Dumlupinar, Julia Utschig, Christian Wülfing, Uwe Pichlmeier, and Gazanfer Belge. 2022. "Testicular Neoplasms: Primary Tumour Size Is Closely Interrelated with Histology, Clinical Staging, and Tumour Marker Expression Rates—A Comprehensive Statistical Analysis" Cancers 14, no. 21: 5447. https://doi.org/10.3390/cancers14215447
APA StyleDieckmann, K.-P., Isbarn, H., Grobelny, F., Dumlupinar, C., Utschig, J., Wülfing, C., Pichlmeier, U., & Belge, G. (2022). Testicular Neoplasms: Primary Tumour Size Is Closely Interrelated with Histology, Clinical Staging, and Tumour Marker Expression Rates—A Comprehensive Statistical Analysis. Cancers, 14(21), 5447. https://doi.org/10.3390/cancers14215447






