Simple Summary
Cancer in the head and neck region (HNSCC) is exponentially increasing due to human papillomavirus (HPV) infections. This paper helps us to understand the complexity of the inflammatory networks and the mechanisms of immune evasion in HPV+ HNSCC to open up new avenues and drive the discovery of useful tools to be translated clinically in the screening and treatment of these cases, especially to overcome resistance and improve patients’ quality of life.
Abstract
Head and neck squamous cell carcinomas (HNSCC) are a heterogeneous group of malignancies which have shown exponential incidence in the last two decades especially due to human papillomavirus (HPV) infection. The HPV family comprises more than 100 types of viruses with HPV16 and HPV18 being the most prevalent strains in HNSCC. Literature data reveal that the mutation profile as well as the response to chemotherapy and radiotherapy are distinct among HPV+ versus HPV-negative tumors. Furthermore, the presence of the virus induces activation of an immune response, in particular the recruitment of specific antiviral T lymphocytes to tumor sites. These T cells when activated produce soluble factors including cytokines and chemokines capable of modifying the local immune tumor microenvironment and impact on tumor response to the treatment. In this comprehensive review we investigated current knowledge on how the presence of an HPV can modify the inflammatory response systemically and within the tumor microenvironment’s immunological responses, thereby impacting on disease prognosis and survival. We highlighted the research gaps and emerging approaches necessary to discover novel immunotherapeutic targets for HPV-associated HNSCC.
1. Head and Neck Squamous Cell Carcinoma (HNSCC)
Head and neck squamous cell carcinoma (HNSCC) represents the sixth most frequent cancer worldwide [1]. HNSCC is etiologically associated to exposure to extrinsic carcinogens such as smoking and alcohol consumption [2,3]. Since the late 1990s, there has been an exponential rise in HNSCC incidence, especially oropharyngeal tumors (OPSCC) in countries with the highest median income, which is related to human papillomavirus (HPV) infection [4,5]. HPV has over 200 serotypes, with HPV16 and HPV18 strains being primarily responsible for HPV-related HNSCC [6]. HPV+ OPSCC is most common in healthier, younger, and non-smoking patients [5]. Significant progress has been achieved over the past few decades in the molecular profile and characterization of both HPV- and HPV+ HNSCC. These cooperative efforts established the impact of mutations in TP53 (84%), CDKN2A (58%), CCND1 (31%), and the overexpression of PI3K pathways (30%) in HPV-negative cases associated with tobacco, but fewer genomic alterations were observed in HPV+ HNSCC [4].
HPV+ OPSCC presents distinctive differences from HPV- tumors in the infiltrating immune cell population profile, underlining a unique biology of this malignancy [7,8,9]. The HPV infection is an early event and most of the HNSCC arises from deep lingual tonsils and palatine crypts. Reticulated crypt epithelium in the oropharynx is unique to this anatomical location in the head and neck, and may explain why HPV is estimated to be five times higher in the oropharynx when compared to the oral cavity, larynx, or hypopharynx [10]. During the course of the HNSCC’s development and progression, the tumor cells and the surrounding microenvironment (TME) are in constant communication and continuously evolve together [10,11]. Tumor cells can adapt several mechanisms to escape immune surveillance, favor tumor growth, proliferation, survival, and promote invasion [6,12,13]. In this scenario, the understanding of the inflammatory networks and the mechanisms of immune evasion in HPV+ HNSCC could help us to drive the discovery of useful tools to be translated clinically in the screening and treatment of these cases, especially to overcome resistance and improve patients’ quality of life.
2. Inflammatory Response in HNSCC
Inflammation in cancer has been described to initiate genetic instability in tumor cells [14]. During HPV infection, infiltrating immune cells interact with the virus to induce and/or activate epithelial cell differentiation [15]. The TME of HNSCC is composed of a heterogeneous cell population integrated in a complex extracellular matrix (ECM) [16]. The main cellular components of the TME are tumor-infiltrating lymphocytes (a.k.a.: TILs; or B and T lymphocytes), tumor-associated macrophages (TAMs), natural killer cells (NKs), tumor-associated neutrophils (TANs), dendritic cells (DCs), and cancer-associated fibroblasts (CAFs) [17,18] (Figure 1). In an initial stage, the tumor development can be enriched by cytotoxic innate lymphocytes (e.g., NKs) and adaptive immune cells (e.g., B and T lymphocytes); however, progressive cancer cells can regulate different signaling mechanisms that mimic immune tolerance in order to evade the tumoricidal attack and eventually lead to tumor metastasis [19].
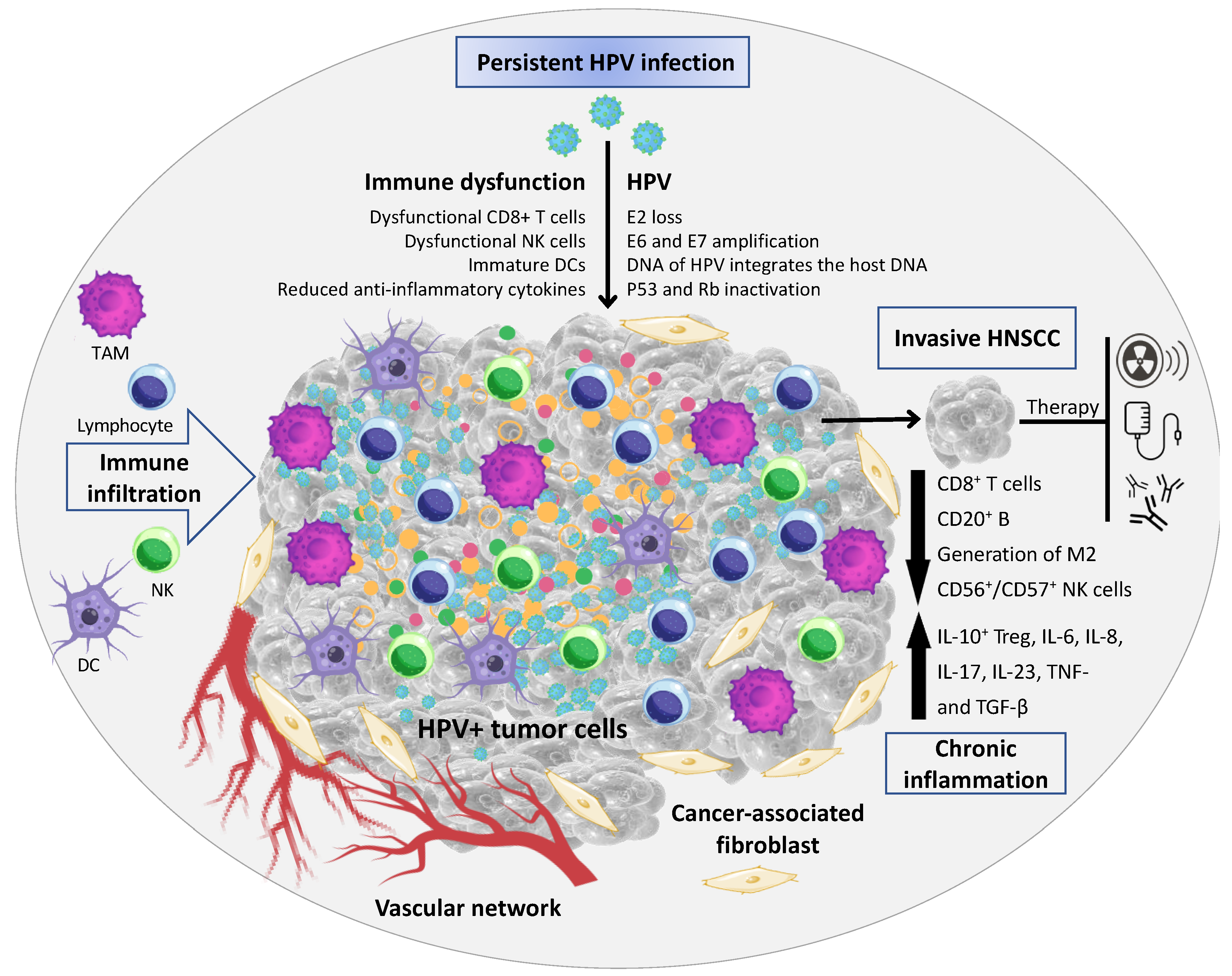
Figure 1.
Tumor microenvironment (TME) associated with HPV+ HNSCC. The TME comprises malignant epithelial cells, and a heterogeneous cell population integrated in a complex extracellular matrix (ECM) 16. The main cellular components of the TME are tumor-infiltrating lymphocytes (a.k.a.: TILs; or B and T lymphocytes), tumor-associated macrophages (TAMs), natural killer cells (NKs), tumor-associated neutrophils (TANs), dendritic cells (DCs), and cancer-associated fibroblasts (CAFs). In HPV+ HNSCC the virus has a key role in the immune dysfunction by the recruitment and activation of cytokines- and chemokines-regulating cells associated with tumor growth and dissemination (Image created using Canva Pro Software at https://www.canva.com/pro/, accessed on 28 September 2022).
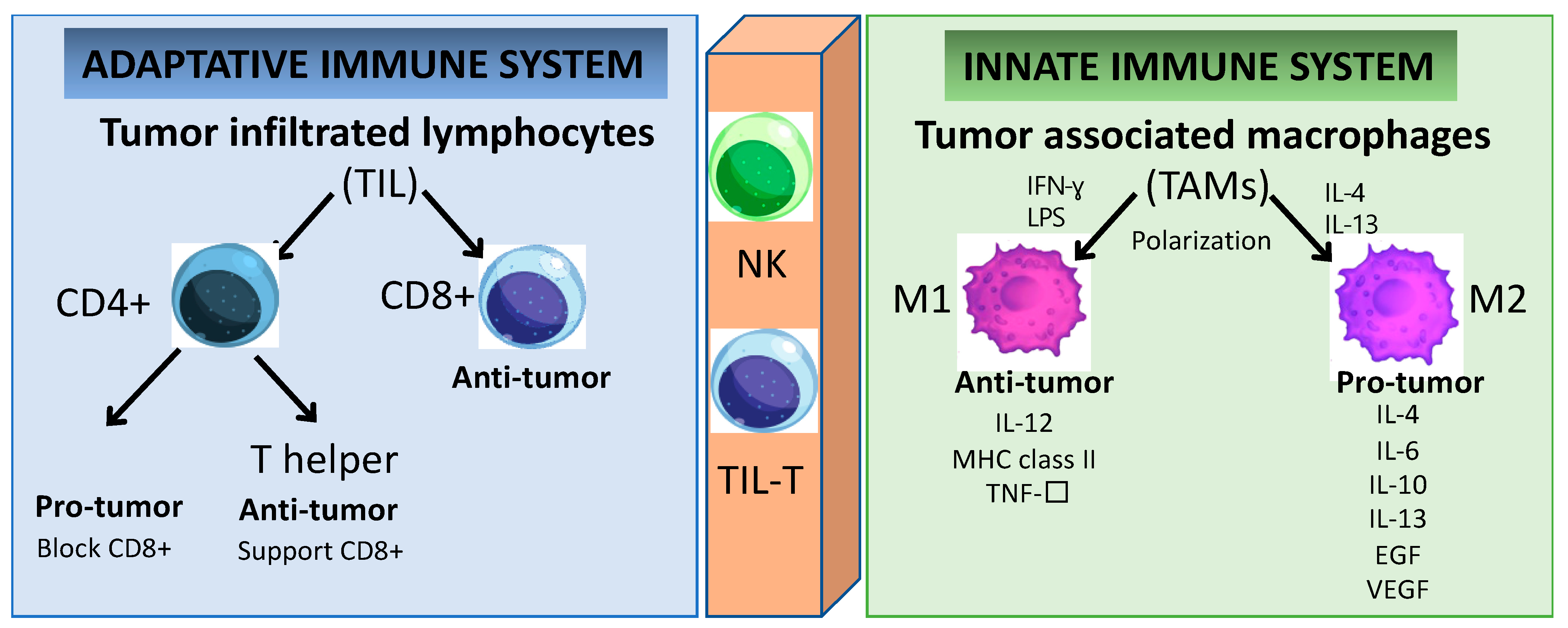
The better prognosis of HPV+ HNSCC when compared to HPV- HNSCC has been associated with the higher number of TILs [20,21,22]. The TIL population can be classified into two major subsets: CD4+ and CD8+ T lymphocytes (Figure 2). Furthermore, the effector CD4+ T lymphocytes are subdivided into two groups with distinctive characteristics: regulatory T (Treg) and helper T (Th) cells [23]. In HNSCC, the CD8+ T lymphocyte infiltration has anti-tumoral activity and its presence is related to a favorable outcome [24]. In HPV+ HNSCC, the tumor antigen tolerance has been attributed to the presence of abundant levels of activated Treg lymphocytes within the TME [25,26,27]. However, effector T cells can polarize into exhausted T cells leading to cancer immune evasion [28]. T-cell exhaustion is a hyporesponsive state of T cells characterized by increased inhibitory receptors (such as: cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), the T-cell immunoglobulin domain and mucin domain protein 3 (TIM-3), the lymphocyte activation gene 3 protein (LAG-3), the band T lymphocyte attenuator (BTLA), the T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT)), as well as the decreased effector cytokines (such as: interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), granzyme B (GzmB)), which impair the cytotoxicity leading to an inability to eliminate cancer cells [27,29,30,31,32,33,34]. Therefore, reversing the T-cell exhaustion status might represent a potential strategy to treat cancer. It is known that the secretion of immunesuppressive cytokines (such as the transforming growth factor-β (TGF-β) and interleukin-10 (IL-10)) is a major contributor to immune tolerance by regulatory T cells (Tregs) [35]. They are able to enhance tumor cell proliferation, survival, and metastasis by regulating anti-tumor immunity [36,37]. However, the therapeutic application of inhibitory cytokines remains a challenge to exploit their anti-tumor activity while keeping a low level of toxicity [38]. In the same way, multiple reports demonstrated the essential role of chemokines receptors (CCR4, CCR5, CXCR3, CXCR4, CCR6, and CCR7) in the regulation of Tregs for trafficking and homing for inflammatory sites in oral cell lines [39,40,41,42,43]. Chemokines are a superfamily of proteins that act as mediators not only affecting immune-cell infiltration into tumor sites, but also having a great impact on cancer progression by inducing ECM degradation via matrix metalloproteinase (MMP), and promoting neovascularization [44,45]. Chemokines are potentially dual-functional (homeostatic and inflammatory) during tumor development. The same chemokines can be either favorable or unfavorable prognostic indicators depending on the type and/or stage of the malignancies [45]. The contribution of chemokines to tumor progression depends on the balance between tumor-promoting and tumor-inhibiting factors [46]. Cytokines and chemokines may become formidable partners in synergistic therapeutic strategies combined with gene and/or cell therapy and monoclonal antibody-based therapies; however, further studies need to be carried out to confirm the safety and benefit over the current therapeutic strategy.
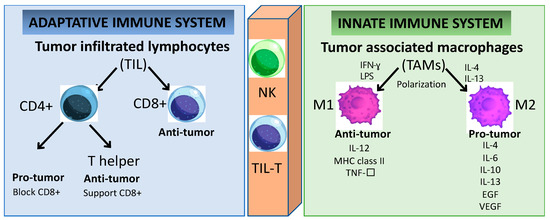
Figure 2.
Immune cells play a key role in tumor cell growth and dissemination. Cells of the innate immunity branch provide a rapid response to non-self-antigens. In contrast, cells of the adaptive immunity branch provide a slower but specific response. Several cell subsets, including TIL and NK cells, connect both branches of immunity because they express receptors similar to those in conventional B and T cells. Even though the specificity of these receptors is limited, the response to specific non-self-antigens is prompt.
The major components in the TME are the tumor-associated macrophages (TAMs) and they are highly dynamic and heterogeneous and are tamed by tumor cells to promote tumor growth and progression [47]. TAMs can express cytokines that stimulate tumor cell proliferation and survival by regulating the transforming growth factor (TGF-β), the epithelial growth factor (EGF) as well as the EGF ligants and receptor (EGFR), the hepatocyte growth factor (HGF), the platelet-derived growth factor (PDGF), and the fibroblast growth factor (FGF) [48]. These cells also play a crucial role in the reorganization of the TME by promoting tumor cell motility via ECM degradation and the initiation of angiogenesis in hypoxic areas with a poor blood supply [49]. TAMs involve multiple phenotypes associated with a wide range of functions under distinctive pathological conditions. For instance, macrophages can be classified into groups depending on their activity and polarized status, as classically (M1) or alternatively activated (M2) [50]. The hypoxic tumor area contains chemokines and immunomodulatory proteins (such as CSF1, TGF-β, CCL2, FTL and FTH) which promote the polarization of TAMs into M2 macrophages [49]. In conventional cell-mediated immune responses, M1 macrophages have pro-inflammatory functions activated through IFN-γ, Th1 cytokines, and lipopolysaccharides in response to the presence of pathogens [51]. M1 TAM promotes the destruction of cancer cells and the inhibition of angiogenesis, concomitant with the activation of an inflammatory reaction [52]. Conversely, the anti-inflammatory and immunosuppressive cytokine-chemokine TME is responsible for dampening the macrophage activation by inducing its polarization towards the anti-tumor M2 profile [18,53]. M2 TAMs secrete pro-tumor factors (such as IL-4, IL-6, IL-10, IL-13, EGF, and VEGF), while the tumor suppressor M1 TAMs expresses anti-tumor factors (such as IL-12, the major histocompatibility complex (MHC) class II, and TNF-α) [53]. Furthermore, M2 macrophages may be responsible for inefficient tumor antigen presentation via MHC class I to cytotoxic cells such as NKs and CD8+ T lymphocytes [18,43]. The overexpression of polarized M2 TAMs and Tregs in HNSCC is associated with poor overall survival in HNSCC [11,17,25,26]. The unbalanced immune response at the tumor site interferes with the recruitment and activation of effector cells to support (or not) an efficient immune response to the tumor. The circulating Tregs’ and TAMs’ polarization status leading to increased expression of immune-suppressive cytokines is a fundamental mechanism by which the tumors can escape the immune surveillance, and they are also a promising target for further investigations as anti-cancer therapies. However, the conditions within the TME endow the immune cells with plasticity and versatility, and this dynamic interaction will define the specific functions and how the tumor will progress.
3. HPV Infection and the Impact on the Immune System in HNSCC
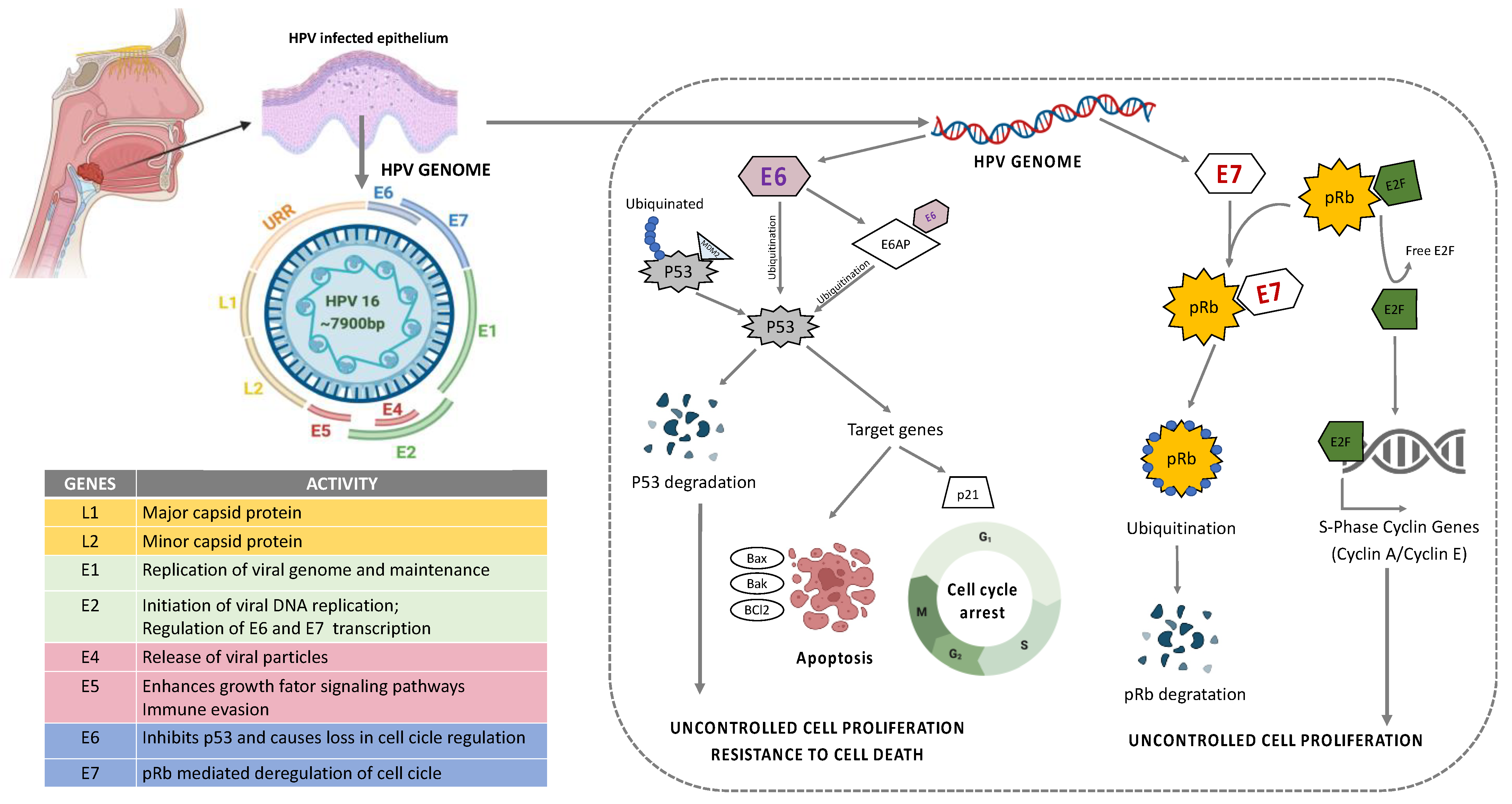
HPV is a group containing more than 100 different types of viruses with pathogenic behavior to humans [6]. They are a circular, non-enveloped, double-stranded DNA (dsDNA) virus approximately 8kb in size that infects basal keratinocytes [54]. Their genome can be divided into an early region (E1, E2, E4, E5, E6, and E7, responsible for virus replication and generation of oncoproteins), a late region (L1 and L2 are the major and minor capsid or coat proteins responsible for structural components of the virus), the virus-like particle (VLP), and long control region (LCR, responsible for the virus transcription and the epithelial tropism) [55,56] (Figure 3). The stratified epithelium of the oral cavity is the target site for HPV to initiate infection. HPV entry is achieved via complex interactions of the viral capsid with cellular proteins leading to conformational changes within the capsid via proteases and chaperones, and interaction of the capsid proteins with different cell receptors. The key mechanisms in HPV entry and trafficking are still under scientific debate and studies on HPV infection mechanisms produce diverse and contradictory results. The discrepancies may partially be attributable to the different virus genotypes, cell lines, and methods of virus production that have been used for the experimental setups and to different observations from in vivo and in vitro models [57,58,59,60,61].
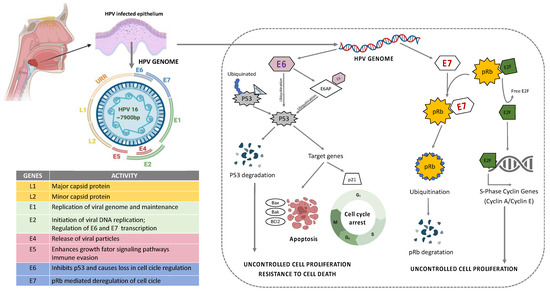
Figure 3.
Structure and organization of HPV16 genome. E6 mediated p53 manipulation and E7 mediated inhibition of pRb protein leading to sustained cell proliferation and resistance to apoptotic barrier.
Among HPV types, HPV16 and HPV18 are considered as high risk (HR) HPV and they are detected in 90% of the HPV+ HNSCC patients [62,63,64]. A higher frequency of oral sex and casual sexual activity involving multiple partners are associated with the elevated risk of HPV-related cancer in HNSCC [65,66]. Following infection, the virus can remain in its episomal form, or become integrated into the host genome [67]. In the majority of cases, HPV infection is transient and it is solved spontaneously; however, in certain individuals, the viral clearance does not occur and the infection becomes persistent resulting in lesions that may eventually progress to cancer. The mechanisms of the clearance of HPV infection in some individuals remain unknown, but persistent infection with HR-HPV is necessary for tumor development [68]. Most often, the integration of HPV DNA hijacks the host cell genome to initiate viral DNA replication and amplification of their own genome [10,11]. This may result in genetic rearrangements, chromosomal inversions and translocations, gene deletions, the activation of proto-oncogenes and loss of heterozygosity, which generates genomic instability and increases the risk of neoplastic cell transformation through uncontrolled cell proliferation and resistance to death (Figure 3) [68].
The HPV persistent infection also requires a tolerant TME-supporting virus evasion and/or the suppression of the immunological responses [69]. The intratumor immune dynamic in HPV+ HNSCC is different from HPV-negative HNSCC [25,26,27,69,70,71], mainly due to the activity of viral proteins constantly stimulating the immune cell repertoire [72]. HPV oncoproteins interact with the host cells leading to (i) the integration of the virus into the host genome [73]; (ii) the induction of cell proliferation and differentiation; (iii) the host-cell immortalization [74]; (iv) the inhibition of apoptosis [75,76,77,78,79,80]; and (v) the immune evasion [81]. Several mechanisms are involved in promoting these events and initiate tumorigenesis. Molecular analysis revealed that specific regions of the virus are able to directly interact with the host transcription factor binding sites orchestrating regulatory regions used by tumor cells to control immune response [82]. Regarding their immunomodulatory nature, these events impair the activation of neutrophils, NKs, and TILs cells by dampening the expression of IFN- and IFN-related proteins in both innate and adaptive immunological responses, that include the activation of non-canonical signaling pathways such as the mitogen-activated protein kinase (MAPK) [83], phosphatidylinositol 3-kinases (PI3K) [84], nuclear factor kappa B (NF-κB) pathways [85], as well as the signal transducer and activator of transcription 3 (STAT3) [86], that prolongs the expression of a subset of interferon-stimulated immune regulatory genes.
In HPV+ HNSCC, a higher M1/M2 TAM ratio can be observed [87,88]. M1 macrophages are associated with a better prognosis and survival rate, whereas M2 phenotype is one of the key determinants of tumor progression and treatment failure [89]. This is partially explained because HPV can modulate MHC class I on the cell surface of antigen-presenting cells (APCs), including TAMs, which would impair the viral protein presentation to cytotoxic cells [22]. Furthermore, tissue resident dendritic cells (DCs) are essential for immune surveillance and act as qualified APCs to the effector cells [90,91]. Due to their plasticity and the presence of multiple receptors on their surface, DCs crosstalk with all cells in the immune system, and are critical for the initiation of anti-viral and antigen-specific immune responses [92]. However, to the best of our knowledge, DCs have not been considered a valid prognostic factor in HPV+ HNSCC.
In general, HPV+ HNSCC show significantly higher levels of TILs, especially CD8+ T cells [11]. Circulating T cells are constantly recruited to the TME in response to inflammatory signals after the recognition of antigen epitopes presented by APCs cells [93]. CD8+ T cells are detectable in 64–75% of HPV+ HNSCC samples [94,95,96]. These TILs produce pro-inflammatory cytokines (i.e., IFNγ and IL-17) with anti-tumoral activity that is related with the favorable prognosis in HPV+ HNSCC [97,98,99]. Studies have demonstrated that the quantity and quality of the immune infiltrate is a valid predictive tool that may improve the stratification of HNSCC patients [26]. Both HPV+ and HPV-negative HNSCC are infiltrated with Treg cells and NK cells overexpressing CD56dim [100,101]. It was observed that different NK subsets are detected in HPV+ and HPV-negative tumors [102,103]. A common mechanism used by the virus to evade the host immune system is the reduction of MHC type I expression to escape a cytotoxic reaction. However, the specific role of NK cell-controlling HPV+ HNSCC is still under investigation [104]. This landscape provides a rationale for the investigation of agents targeting modulators of Tregs (e.g., CTLA-4, GITR, ICOS, IDO, and VEGFA) and NK cells (e.g., KIR, TIGIT, and 4-1BB) as adjuncts to anti–PD-1 in the treatment of advanced HNSCC [103].
4. The Impact of Therapeutic Schemes on the Immune Status of HNSCC
The main treatment for HNSCC includes surgery or radiation for the early-stage disease [105]. For recurrent/metastatic diseases, cytotoxic-based chemotherapy remains the standard therapeutic option and the median survival of HNSCC patients treated with palliative chemotherapy alone ranges from 6 to 10 months [106,107,108]. The combination of immunotherapeutic strategies represents a challenging approach, with a view to enhance anti-tumor immunity by targeting several aspects of the immune response [109]. The majority of HPV+ HNSCC patients have a favorable prognosis, and this raises the discussion about a less intensive treatment in order to decrease the side effects and improve patients’ quality of life. To date, several clinical trials have been proposed; however, few of them consider the HPV status for a personalized approach to target the TME dynamics (Table 1).
Furthermore, radiotherapy alone is known to induce substantial changes in the immune microenvironment in solid tumors [110]. Radiotherapy can control tumor growth by inducing cell death via direct DNA damage or generating reactive oxygen species (ROS) [110,111,112]. Although these mechanisms are important to kill cancer cells, radiation leads to the death of adjacent normal tissues causing severe adverse effects [110,113,114,115]. The radiotherapy influences the regulation of macrophage polarization [116], DCs phagocytosis [117], antigen intracellular processing and presentation to effector T cells [117,118,119], NK cell activation [120], as well as the cytokine and chemokine release [121]. The response of immune cells to radiation can determine the outcome of tumor therapy [122]. Recently, a large number of experimental and clinical trial studies have been conducted to manipulate the immune system, aiming to enhance the therapeutic efficiency of radiotherapy [122]. The clinical benefit can be observed in a substantial fraction of HNSCCs treated with immune checkpoint inhibitors, but the majority of tumors remain treatment-resistant [102]. Deciphering the basic mechanisms of upfront treatment resistance will require a detailed understanding of the immune infiltrative landscape of these tumors.
Standard treatment for locally advanced HNSCC consists mainly of chemoradiation using docetaxel, cisplatin, and/or fluorouracil in an attempt to eradicate potential microscopic residual cancer cells and ultimately improve loco-regional control and survival [123]. However, the intensification of therapy for patients who did not respond to the treatment is not able to overcome biologically aggressive HNSCC [123]. Checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 antibodies were shown to significantly improve disease-free survival and overall survival after the failure of platinum-based chemotherapy [124]. With the introduction of immune checkpoint inhibitors to the clinic, a new set of toxicities, specifically, immune-related adverse events, have emerged. The side effects range from minimal to lethal and require a completely different management approach. Ipilimumab, in particular, is associated with grade 3–5 toxicity in 10–45% of HNSCC patients, depending on the dose, and whether it was given as a single agent or in combination with other immune therapies, chemotherapies, or molecularly targeted therapies [125]. Several clinical trials are in progress to evaluate the utility of checkpoint inhibitors in different treatment settings (Table 1). A cost-benefit and quality-of-life analysis may address the true contribution of the chemoradiotherapy associated with checkpoint inhibitors as an advantage strategy to treat patients with advanced HNSCC.

Table 1.
Clinical trials for the treatment of HNSCC patients targeting the immune system.
Table 1.
Clinical trials for the treatment of HNSCC patients targeting the immune system.
| Target | NCT Number | Status | Interventions | Phases | Enrolled Patients, n | Period (Start Date–Completion Date) | URL Access | Related Articles with Results | HPV Status | Immune Dynamics Evaluation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intratumor Microenvironment | Peripheral Blood Cells | ||||||||||
| Inflammatory cell subsets | NCT00210470 | Completed | IRX-2 (multiple cytokines) Cyclophosphamide Indomethacin Zinc Omeprazole | 2 | 27 | 2005/07–2012/03 | https://ClinicalTrials.gov/show/NCT00210470 (accessed on 28 September 2022) | [126,127,128,129] | n.d. ** | Increased infiltration of TILs (CD3+, CD4+, CD8+ and CD20+ B cells) and CD68+ macrophages in tumor microenvironment. Peritumoral accumulation of CD4+ T cells. Predominance of intratumor CD8+ over CD4+ T cells. Higher CD20+ cells were associated with decreased tumor size. Increased survival rates associated with intratumor CD3+ and CD20+ cells. | Decreased levels of naïve T cells (CD3+CD45RA+CCR7+), central memory T cells (CD3+CD45RA−CCR7+CD27+), B lymphocytes (CD19+CD3−CD14−) and NKT cells (CD3+CD16+CD56+). |
| p-53- expressing tumor cells | NCT00496860 | Completed | ALT-801 (humanized soluble T-cell receptor directed against the p53-derived antigen fused to IL-2) | 1 | 26 | 2007/05–2009/10 | https://ClinicalTrials.gov/show/NCT00496860 (accessed on 28 September 2022) | [130] | n.d. | n.d. ** | Increased number of IFN-γ+ cells. Elevated serum IFN-γ levels. |
| Phosphodiesterase type-5 | NCT00843635 | Completed | Tadalafil (phosphodiesterase 5 (PDE5) inhibitor) | n/a * | 35 | 2008/09–2015/04 | https://ClinicalTrials.gov/show/NCT00843635 (accessed on 28 September 2022) | [131] | n.d. | n.d. | Decrease in m-MDSC and Treg cells numbers. Significant downregulation of MDSCs and nFoxp3:cFoxp3 ratio. Increased CD8+ cell activation. |
| Intratumor reactive T-cells and endothelial cells | NCT00953849 | Completed | Celecoxib (cyclooxygenase 2 inhibitor) Calcitriol (Vitamin D) | 1|2 | 21 | 2009/11–2015/12 | https://ClinicalTrials.gov/show/NCT00953849 (accessed on 28 September 2022) | none | n.d. | Intra-tumor increased IL-2, IFN-γ, and GM-CSF and decreased IL-6 staining. | n.d. |
| Tumor cells | NCT01302834 | Unknown | cetuximab (anti-EGFR mAb) cisplatin (apoptosis-inducer via DNA crosslinking) MRT | 3 | 987 | 2011/02– | https://ClinicalTrials.gov/show/NCT01302834 (accessed on 28 September 2022) | [132] | Yes | n.d. | n.d. |
| Interleukin-6 | NCT01403064 | Terminated | ALD518 (humanized anti-IL-6 antibody) | 2 | 76 | 2011/07–2014/03 | https://ClinicalTrials.gov/show/NCT01403064 (accessed on 28 September 2022) | none | n.d. | n.d. | n.d. |
| Anti-tumor cellular immunity | NCT01468896 | Active, not recruiting | Cetuximab (anti-EGFR mAb) Edodekin alfa (recombinant IL-12) | 1|2 | 23 | 2011/11– | https://ClinicalTrials.gov/show/NCT01468896 (accessed on 28 September 2022) | none | n.d. | n.d. | n.d. |
| HPV-infected cells and tumor cells | NCT01585428 | Completed | Fludarabine (inhibitor of DNA synthesis) Cyclophosphamide (inhibitor of protein synthesis) Young TIL (Tumor Infiltrating Lymphocytes) Aldesleukin (recombinant IL-2) | 2 | 29 | 2012/04–2016/08 | https://ClinicalTrials.gov/show/NCT01585428 (accessed on 28 September 2022) | [133] | Yes | n.d. | n.d. |
| Phosphodiesterase type-5 | NCT01697800 | Completed | Tadalafil (phosphodiesterase 5 (PDE5) inhibitor) | 2 | 40 | 2012/09–2014/07 | https://ClinicalTrials.gov/show/NCT01697800 (accessed on 28 September 2022) | none | n.d. | n.d. | n.d. |
| Inflammation and pain | NCT01883908 | Terminated | Acupuncture Usual medical care for pain relief | n/a | 4 | 2012/12–2015/02 | https://ClinicalTrials.gov/show/NCT01883908 (accessed on 28 September 2022) | none | n.d. | n.d. | n.d. |
| Innate and adaptive immunity crosstalk | NCT01984892 | Terminated | Poly-ICLC (TLR3-ligand) | 2 | 8 | 2013/11–2014/08 | https://ClinicalTrials.gov/show/NCT01984892 (accessed on 28 September 2022) | [134] | n.d. | n.d. | n.d. |
| HPV-specific T cell repertoire | NCT02002182 | Active, not recruiting | ADXS11-001/ADXS-HPV (immunobiological product from Listeria monocytogenes) | 2 | 15 | 2013/12–2023/08 | https://ClinicalTrials.gov/show/NCT02002182 (accessed on 28 September 2022) | none | Yes | n.d. | No difference between treatment and control groups on HPV-specific T cell response rate. |
| HPV-specific T and B cell repertoires | NCT02163057 | Completed | INO-3112 (plasmids encoding HPV oncoproteins delivered by electroporation system) | 1|2 | 22 | 2014/08–2017/01 | https://ClinicalTrials.gov/show/NCT02163057 (accessed on 28 September 2022) | none | Yes | Suggestive modulation of CD8+, perforin+ and FoxP3+ TILs. | n.d. |
| HPV-infected cells and tumor cells | NCT02280811 | Completed | Fludarabine (inhibitor of DNA synthesis) Cyclophosphamide (inhibitor of protein synthesis) E6 TCR (T cells genetically engineered with a TCR targeting HPV-16 E6 oncoprotein) Aldesleukin (recombinant IL-2) | 1|2 | 12 | 2014/10–2016/06 | https://ClinicalTrials.gov/show/NCT02280811 (accessed on 28 September 2022) | none | Yes | n.d. | Inconclusive results. |
| Anti-tumor cellular immunity | NCT02315066 | Completed | PF-04518600 (OX40 agonist) PF-05082566 (4-1BB agonist) | 1 | 174 | 2015/04–2020/11 | https://ClinicalTrials.gov/show/NCT02315066 (accessed on 28 September 2022) | [135] | n.d. | Upregulation of gene sets associated with anti-tumor immune response, mainly IFN-γ-related pathways. | Increased CD4+ and CD8+ T-cell clonal expansion. |
| Anti-tumor cellular immunity | NCT02521870 | Terminated | SD-101 (synthetic CpG oligonucleotide acting as TLR9 ligand) Pembrolizumab (programmed death receptor-1 (PD-1)-blocking antibody) | 1|2 | 241 | 2015/09–2020/04 | https://ClinicalTrials.gov/show/NCT02521870 (accessed on 28 September 2022) | [136] | n.d. | n.d. | n.d. |
| Lymph system | NCT03332160 | Completed | Flexitouch (pneumatic compression device) | n/a | 49 | 2018/01–2019/07 | https://ClinicalTrials.gov/show/NCT03332160 (accessed on 28 September 2022) | none | n.d. | n.d. | A slight decrease in IL-6 levels. |
| Intratumor reactive T-cells | NCT03463161 | Terminated | Pembrolizumab (programmed death receptor-1 (PD-1)-blocking antibody) Epacadostat (selective inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1) | 2 | 2 | 2018/03–2018/12 | https://ClinicalTrials.gov/show/NCT03463161 (accessed on 28 September 2022) | none | Yes | n.d. | n.d. |
| Intratumor reactive T-cells | NCT03938337 | Terminated | Pembrolizumab (programmed death receptor-1 (PD-1)-blocking antibody) Abemaciclib (inhibitor of cyclin-dependent kinases (CDK)) | 2 | 1 | 2019/10–2020/04 | https://ClinicalTrials.gov/show/NCT03938337 (accessed on 28 September 2022) | none | n.d. | n.d. | n.d. |
| HPV-infected cells and tumor cells | NCT04015336 | Terminated | E7 TCR (T cells genetically engineered with a TCR targeting HPV-16 E7 oncoprotein) | 2 | 1 | 2020/06–2020/07 | https://ClinicalTrials.gov/show/NCT04015336 (accessed on 28 September 2022) | none | Yes | n.d. | n.d. |
| Intratumor reactive T-cells and NK cells | NCT04099277 | Terminated | LY3435151 (anti-CD226) Pembrolizumab (programmed death receptor-1 (PD-1)-blocking antibody) | 1 | 2 | 2019/10–2020/03 | https://ClinicalTrials.gov/show/NCT04099277 (accessed on 28 September 2022) | none | n.d. | n.d. | n.d. |
| Intratumor reactive T-cells | NCT01848834 | Completed | Pembrolizumab (programmed death receptor-1 (PD-1)-blocking antibody) | 1 | 297 | 2013/05–2020/06 | https://ClinicalTrials.gov/show/NCT01848834 (accessed on 28 September 2022) | [137,138,139,140,141] | Yes | n.d. | n.d. |
| Intratumor reactive T-cells | NCT03083873 | Completed | LN-145 (autologous TIL-mediated adoptive cell transfer therapy) recombinant IL-2 non-myeloablative (NMA) lymphodepletion | 2 | 112 | 2017/01–2022/03 | https://clinicaltrials.gov/ct2/show/NCT03083873 (accessed on 28 September 2022) | none | Yes | n.d. | n.d. |
Data source: adapted from ClinicalTrials.gov. * n/a = not applicable. ** n.d. = not described.
5. Treatment Strategy and Vaccine for Patients with HPV+ HNSCC
The determination of the HPV status may guide clinicians in their prognostic assessment and treatment decision-making in the HNSCC population [142]. HPV+ HNSCC has better outcomes and is more sensitive to radiotherapy and chemotherapy compared with HPV-negative HNSCC, which may be due to the effective immune responses to viral and abundant numbers of infiltrating immune cells. The fact that HPV+ HNSCC has a good prognosis provides the rationale for several clinical trials with de-intensified treatment or alternative therapeutic approaches. De-intensification strategies involve less invasive surgery, such as transoral robotic surgery (TORS), which utilizes miniaturized instruments to perform the resection of selected cancer areas, as well as a reduction in the dose of chemotherapy and/or radiotherapy [143].
The adoption of anti-viral strategies to combat HPV infections, including anti-HPV vaccines, might also modulate the TME and influence the tumor response. HPV vaccines have a clear role in preventing cervical cancer and conditions related to HPV infection [66,144]. The prophylactic vaccines recommended by the FDA are bivalent (HPV16 and HPV18), quadrivalent (HPV6, HPV11, HPV16, and HPV18), or nine-valent (HPV6, HPV11, HPV16, HPV18, HPV31, HPV33, HPV45, HPV52, and HPV58) vaccines. Recently, the FDA and Health Canada approved an expanded indication for the HPV nine-valent vaccine for the prevention of HNSCC [144]. There is hope that preventive HPV vaccinations can also reduce the occurrence of HPV+ HNSCC. Several long-term trials are underway to evaluate their effectiveness and to understand how these vaccines modulate the anti-viral immunity. The goal of cancer vaccination is to obtain anti-cancer effects by activating or increasing an effective CD4+/CD8+ antigen-specific T cell response [145].
HPV vaccines commercially available to date (Gardasil, Gardasil 9, and Cervarix) are able to induce immune response by blocking the viral fusion and entry into the host cell [146]. Specifically, all of these vaccines are designed with VLPs from the HPV structural protein L1 [55], which stimulates naive B cells and increases antibody production [146,147]. These vaccines are now in clinical trials for HPV-driven cancers including HNSCC. However, the prophylactic HPV against L1 proteins appears to be ineffective in the treatment of HPV-induced cancers [148]. New HPV vaccines have been designed to target the oncoproteins E6 and E7 because they are constantly necessary and exclusively produced in cancer cells [146,149]. The challenge for therapeutic oncological vaccines is to stimulate an immune T cell response to endogenous antigens that is sufficiently potent to induce cytotoxic activity and broad enough to take tumors and TME heterogeneity into account.
6. Conclusions
In this review we discussed how the presence of HPV interferes with the local and systemic immune response that may lead to a complete response or resistance to the treatment and consequently impact on the prognosis and survival of patients with HNSCC. Understanding the immunological dynamic associated with tumor cell behavior is the key to developing novel immunotherapeutic targets and strategies to treat HNSCC.
Author Contributions
Manuscript drafting, tables, and literature review by L.R.C.C., S.B.S.C.C., P.R.F.B. and S.D.d.S. Figures by S.B.S.C.C. and S.D.d.S. Manuscript editing and review by P.R.F.B., M.H., M.A.A.-J. and S.D.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) (Finance Code 001), CAPES-Print Fellowships (LRCC and SBSCC), Federal University of Paraíba, Brazil, CBIE-FMP FRQ-S/RSBO#35376 (258000 and 258259), CIHR-NCOHR (New Frontier Seed Grant 2020-2022) and CIHR project grant (2022–2027). The authors acknowledge all the valuable support from the Head and Neck Foundation (Jewish General Hospital—Faculty of Medicine—McGill University) and the Marvin Carsley Research Fund.
Conflicts of Interest
The authors have no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Marziliano, A.; Teckie, S.; Diefenbach, M.A. Alcohol-related head and neck cancer: Summary of the literature. Head Neck 2020, 42, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin. Oncol. 2004, 31, 744–754. [Google Scholar] [CrossRef]
- Pytynia, K.B.; Dahlstrom, K.R.; Sturgis, E.M. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Zeng, Q.; Guo, X.-J.; Wang, H.; Liu, H.-H.; Dong, Z.-Y. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 2019, 9, 13404. [Google Scholar] [CrossRef] [PubMed]
- Kürten, C.H.L.; Kulkarni, A.; Cillo, A.R.; Santos, P.M.; Roble, A.K.; Onkar, S.; Reeder, C.; Lang, S.; Chen, X.; Duvvuri, U.; et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat. Commun. 2021, 12, 7338. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Liu, H.; Wan, Y.; Zhu, Y.; Zhang, M.; Tao, Y.; Zhou, H.; Liu, X.; Hou, J.; et al. The prognostic role of tumour-infiltrating lymphocytes in oral squamous cell carcinoma: A meta-analysis. J. Oral Pathol. Med. 2019, 48, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Faraji, F.; Zaidi, M.; Fakhry, C.; Gaykalova, D.A. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 2017, 19, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Fialová, A.; Koucký, V.; Hajdušková, M.; Hladíková, K.; Špíšek, R. Immunological Network in Head and Neck Squamous Cell Carcinoma—A Prognostic Tool Beyond HPV Status. Front. Oncol. 2020, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, X.; Ma, D.; Liu, X.; Chen, Y.; Wang, Y.; Lui, V.W.Y.; Xia, J.; Cheng, B.; Wang, Z. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer 2017, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Kowalski, L.P.; Coletta, R.D.; Salo, T.; Maschietto, M.; Chojniak, R.; Lima, J.M.; Mlynarek, A.; Hier, M.P.; Alaoui-Jamali, M.A.; Silva, S.D. Head and neck cancer: Emerging concepts in biomarker discovery and opportunities for clinical translation. Clin. Transl. Med. 2020, 10, e209. [Google Scholar] [CrossRef]
- Wang, H.C.; Chan, L.P.; Cho, S.F. Targeting the Immune Microenvironment in the Treatment of Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 1084. [Google Scholar] [CrossRef]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef]
- da Silva, S.D.; Marchi, F.A.; Su, J.; Yang, L.; Valverde, L.; Hier, J.; Bijian, K.; Hier, M.; Mlynarek, A.; Kowalski, L.P.; et al. Co-Overexpression of TWIST1-CSF1 Is a Common Event in Metastatic Oral Cancer and Drives Biologically Aggressive Phenotype. Cancers 2021, 13, 153. [Google Scholar] [CrossRef]
- Wolf, G.T.; Chepeha, D.B.; Bellile, E.; Nguyen, A.; Thomas, D.; McHugh, J.; University of Michigan Head and Neck SPORE Program. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015, 51, 90–95. [Google Scholar] [CrossRef]
- Sanchez-Canteli, M.; Granda-Díaz, R.; Del Rio-Ibisate, N.; Allonca, E.; López-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montuenga, L.M.; García-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2020, 69, 2089–2100. [Google Scholar] [CrossRef]
- Qureshi, H.A.; Zhu, X.; Yang, G.H.; Steadele, M.; Pierce, R.H.; Futran, N.D.; Lee, S.M.; Méndez, E.; Houghton, A.M.G. Impact of HPV status on immune responses in head and neck squamous cell carcinoma. Oral Oncol. 2022, 127, 105774. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Paroni, M.; Maglie, S.; Alfen, J.S.; Kastirr, I.; Gruarin, P.; De Simone, M.; Pagani, M.; Abrignani, S. Plasticity of Human CD4 T Cell Subsets. Front. Immunol. 2014, 5, 630. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Hiratsuka, H.; Koike, K.; Tsuchihashi, K.; Sonoda, T.; Ogi, K.; Miyakawa, A.; Kobayashi, J.; Kaneko, T.; Igarashi, T.; et al. Tumor-infiltrating CD8+ T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Med. 2019, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Matlung, S.E.; Wilhelmina Van Kempen, P.M.; Bovenschen, N.; Van Baarle, D.; Willems, S.M. Differences in T-cell infiltrates and survival between HPV+ and HPV- oropharyngeal squamous cell carcinoma. Futur. Sci. OA 2016, 2, FSO88. [Google Scholar] [CrossRef]
- Partlová, S.; Bouček, J.; Kloudová, K.; Lukešová, E.; Zábrodský, M.; Grega, M.; Fučíková, J.; Truxová, I.; Tachezy, R.; Špíšek, R.; et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015, 4, 965570. [Google Scholar] [CrossRef]
- Lechner, A.; Schlößer, H.A.; Thelen, M.; Wennhold, K.; Rothschild, S.I.; Gilles, R.; Quaas, A.; Siefer, O.G.; Huebbers, C.U.; Cukuroglu, E.; et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology 2019, 8, 1535293. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Jin, H.-T.; Anderson, A.C.; Tan, W.G.; West, E.E.; Ha, S.-J.; Araki, K.; Freeman, G.J.; Kuchroo, V.K.; Ahmed, R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 14733–14738. [Google Scholar] [CrossRef]
- Crawford, A.; Wherry, E.J. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr. Opin. Immunol. 2009, 21, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Olive, D.; Kuchroo, V.; Zarour, H.M. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012, 72, 887–896. [Google Scholar] [CrossRef]
- Komai, T.; Inoue, M.; Okamura, T.; Morita, K.; Iwasaki, Y.; Sumitomo, S.; Shoda, H.; Yamamoto, K.; Fujio, K. Transforming Growth Factor-β and Interleukin-10 Synergistically Regulate Humoral Immunity via Modulating Metabolic Signals. Front. Immunol. 2018, 9, 1364. [Google Scholar] [CrossRef]
- Hassuneh, M.R.; Nagarkatti, M.; Nagarkatti, P.S. Role of interleukin-10 in the regulation of tumorigenicity of a T cell lymphoma. Leuk. Lymphoma 2013, 54, 827–834. [Google Scholar] [CrossRef][Green Version]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 205031212110690. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, J.; Bromberg, J.S. Regulatory T cell migration during an immune response. Trends Immunol. 2012, 33, 174–180. [Google Scholar] [CrossRef]
- Huehn, J.; Hamann, A. Homing to suppress: Address codes for Treg migration. Trends Immunol. 2005, 26, 632–636. [Google Scholar] [CrossRef]
- MacDonald, K.G.; Orban, P.C.; Levings, M.K. T regulatory cell therapy in transplantation: Stability, localization and functional specialization. Curr. Opin. Organ Transplant. 2012, 17, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Y.; Yang, W.H.; Chen, H.T.; Huang, C.Y.; Tan, T.W.; Lin, Y.T.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J. Cell. Physiol. 2009, 220, 418–426. [Google Scholar] [CrossRef]
- Nisar, S.; Yousuf, P.; Masoodi, T.; Wani, N.A.; Hashem, S.; Singh, M.; Sageena, G.; Mishra, D.; Kumar, R.; Haris, M.; et al. Chemokine-cytokine networks in the head and neck tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 4584. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The ccl5/ccr5 axis in cancer progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Richmond, A. Chemokins modulate immune surveillance in tumorignesis, metastatsis, and response to immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front. Immunol. 2021, 12, 741305. [Google Scholar] [CrossRef]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Chen, C.; Song, Y.; Cai, Q.; Li, J.; Tang, Y.; Han, X.; Qu, W.; Chen, A.; Wang, H.; et al. Hypoxia modifies the polarization of macrophages and their inflammatory microenvironment, and inhibits malignant behavior in cancer cells. Oncol. Lett. 2019, 18, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Chen, Z.; Burk, R.D. Human papillomavirus genomics: Past, present and future. Curr. Probl. Dermatol. 2014, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef] [PubMed]
- Hagensee, M.E.; Yaegashi, N.; Galloway, D.A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 1993, 67, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.G.; Tung, J.S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef] [PubMed]
- Evander, M.; Frazer, I.H.; Payne, E.; Qi, Y.M.; Hengst, K.; McMillan, N.A. Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J. Virol. 1997, 71, 2449–2456. [Google Scholar] [CrossRef]
- McMillan, N.A.J.; Payne, E.; Frazer, I.H.; Evander, M. Expression of the α6 integrin confers papillomavirus binding upon receptor-negative B-cells. Virology 1999, 261, 271–279. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, K.D.; Park, S.N.; Cheong, S.W. α6 integrin is the main receptor of human papillomavirus type 16 VLP. Biochem. Biophys. Res. Commun. 2001, 283, 668–673. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Velders, M.P.; Nieland, J.D.; Schiller, J.T.; Nickoloff, B.J.; Kast, W.M. Physical interaction of human papillomavirus virus-like particles with immune cells. Int. Immunol. 2001, 13, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Rautava, J.; Syrjänen, S. Biology of Human Papillomavirus Infections in Head and Neck Carcinogenesis. Head Neck Pathol. 2012, 6, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Taberna, M.; Mena, M.; Pavón, M.A.; Alemany, L.; Gillison, M.L.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kotsantis, I.; Psyrri, A. Special Issue about Head and Neck Cancers: HPV Positive Cancers. Int. J. Mol. Sci. 2020, 21, 3388. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Dempsey, A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev. Med. (Baltim) 2011, 53, 5–11. [Google Scholar] [CrossRef]
- Kamal, M.; Lameiras, S.; Deloger, M.; Morel, A.; Vacher, S.; Lecerf, C.; Bièche, I. Human papilloma virus (HPV) integration signature in Cervical Cancer: Identification of MACROD2 gene as HPV hot spot integration site. Br. J. Cancer 2021, 124, 777–785. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Fernandes, T.A.A.D.M.; De Azevedo, J.C.V.; Cobucci, R.N.O.; De Carvalho, M.G.F.; Andrade, V.S.; De Araujo, J.M.G. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Dong, H.; Shu, X.; Xu, Q.; Zhu, C.; Kaufmann, A.M.; Zheng, Z.M.; Albers, A.E.; Qian, X. Current Status of Human Papillomavirus-Related Head and Neck Cancer: From Viral Genome to Patient Care. Virol. Sin. 2021, 36, 1284–1302. [Google Scholar] [CrossRef]
- Al-Sahaf, S.; Hunter, K.D.; Bolt, R.; Ottewell, P.D.; Murdoch, C. The IL-1/IL-1R axis induces greater fibroblast-derived chemokine release in human papillomavirus-negative compared to positive oropharyngeal cancer. Int. J. Cancer 2019, 144, 334–344. [Google Scholar] [CrossRef]
- Bloy, N.; Garcia, P.; Laumont, C.M.; Pitt, J.M.; Sistigu, A.; Stoll, G.; Yamazaki, T.; Bonneil, E.; Buqué, A.; Humeau, J.; et al. Immunogenic stress and death of cancer cells: Contribution of antigenicity vs adjuvanticity to immunosurveillance. Immunol. Rev. 2017, 280, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S.; Strati, K.; Shin, M.K.; Pitot, H.C.; Lambert, P.F. Human papillomavirus type 16 E6 and E7 oncoproteins act synergistically to cause head and neck cancer in mice. Virology 2010, 407, 60–67. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef] [PubMed]
- Katzenellenbogen, R. Telomerase induction in HPV infection and oncogenesis. Viruses 2017, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Kim, S.H.; Cho, E.A.; Song, Y.S.; Kim, W.H.; Juhnn, Y.S. Human papillomavirus type 16 E5 protein inhibits hydrogen peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis 2010, 31, 402–410. [Google Scholar] [CrossRef]
- Kabsch, K.; Mossadegh, N.; Kohl, A.; Komposch, G.; Schenkel, J.; Alonso, A.; Tomakidi, P. The HPV-16 E5 protein inhibits TRAIL- and FasL-mediated apoptosis in human keratinocyte raft cultures. Intervirology 2004, 47, 48–56. [Google Scholar] [CrossRef]
- Thomas, M.; Banks, L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J. Gen. Virol. 1999, 80, 1513–1517. [Google Scholar] [CrossRef]
- Filippova, M.; Song, H.; Connolly, J.L.; Dermody, T.S.; Duerksen-Hughes, P.J. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. J. Biol. Chem. 2002, 277, 21730–21739. [Google Scholar] [CrossRef]
- Filippova, M.; Parkhurst, L.; Duerksen-Hughes, P.J. The human papillomavirus 16 E6 protein binds to Fas-associated death domain and protects cells from Fas-triggered apoptosis. J. Biol. Chem. 2004, 279, 25729–25744. [Google Scholar] [CrossRef]
- Garnett, T.O.; Filippova, M.; Duerksen-Hughes, P.J. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006, 13, 1915–1926. [Google Scholar] [CrossRef]
- Bottley, G.; Watherston, O.G.; Hiew, Y.L.; Norrild, B.; Cook, G.P.; Blair, G.E. High-risk human papillomavirus E7 expression reduces cell-surface MHC class I molecules and increases susceptibility to natural killer cells. Oncogene 2008, 27, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Yadav, J.; Thakur, K.; Bibban, R.; Chhokar, A.; Tripathi, T.; Bhat, A.; Singh, T.; Jadli, M.; Singh, U.; et al. Human Papillomavirus Infection in Head and Neck Squamous Cell Carcinomas: Transcriptional Triggers and Changed Disease Patterns. Front. Cell. Infect. Microbiol. 2020, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, I.; Del-Castillo-Falconi, V.; Mitre-Aguilar, I.B.; Amador-Molina, A.; Carrillo-García, A.; Langley, E.; Zentella-Dehesa, A.; Soto-Reyes, E.; García-Carrancá, A.; Herrera, L.A.; et al. SOX2 as a new regulator of HPV16 transcription. Viruses 2017, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Sichero, L.; Sobrinho, J.S.; Villa, L.L. Identification of novel cellular transcription factors that regulate early promoters of human papillomavirus types 18 and 16. J. Infect. Dis. 2012, 206, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, V.; Van Der Meijden, E.; De Graaf, J.; Ter Schegget, J.; Struyk, L. A functional NF-κB binding site in the human papillomavirus type 16 long control region. Virology 2000, 272, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Grattendick, K.G.; Tyring, S.K. Interleukin-10 induces transcription of the early promoter of human papillomavirus type 16 (HPV16) through the 5′-segment of the upstream regulatory region (URR). Antivir. Res. 2002, 55, 331–339. [Google Scholar] [CrossRef]
- Chen, X.; Yan, B.; Lou, H.; Shen, Z.; Tong, F.; Zhai, A.; Wei, L.; Zhang, F. Immunological network analysis in HPV associated head and neck squamous cancer and implications for disease prognosis. Mol. Immunol. 2018, 96, 28–36. [Google Scholar] [CrossRef]
- Gameiro, S.F.; Ghasemi, F.; Barrett, J.W.; Koropatnick, J.; Nichols, A.C.; Mymryk, J.S.; Maleki Vareki, S. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 2018, 7, e1498439. [Google Scholar] [CrossRef]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journe, F.; Saussez, S. HPV involvement in the tumor microenvironment and immune treatment in head and neck squamous cell carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Harlé, G.; Kowalski, C.; Garnier, L.; Hugues, S. Lymph node stromal cells: Mapmakers of t cell immunity. Int. J. Mol. Sci. 2020, 21, 7785. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.R.; de Melo, C.M.L.; Barros, M.L.C.M.G.R.; de Cássia Pereira de Lima, R.; de Freitas, A.C.; Venuti, A. Activities of stromal and immune cells in HPV-related cancers. J. Exp. Clin. Cancer Res. 2018, 37, 137. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.L.; Acton, S.E.; Knoblich, K. Lymph node fibroblastic reticular cells in health and disease. Nat. Rev. Immunol. 2015, 15, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, M.; Goedemans, R.; Briet, R.J.P.; Gelderblom, H.; Nortier, J.W.R.; Gorter, A.; Smit, V.T.H.B.M.; Langeveld, A.P.M.; Jansen, J.C.; van der Burg, S.H. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int. J. Cancer 2012, 131, E74–E85. [Google Scholar] [CrossRef]
- Welters, M.J.P.; Ma, W.; Santegoets, S.J.A.M.; Goedemans, R.; Ehsan, I.; Jordanova, E.S.; Van Ham, V.J.; Van Unen, V.; Koning, F.; Van Egmond, S.I.; et al. Intratumoral HPV16-specific T cells constitute a type I–oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin. Cancer Res. 2018, 24, 634–647. [Google Scholar] [CrossRef]
- Hladíková, K.; Partlová, S.; Koucký, V.; Bouček, J.; Fonteneau, J.F.; Zábrodský, M.; Tachezy, R.; Grega, M.; Špíšek, R.; Fialová, A. Dysfunction of HPV16-specific CD8+ T cells derived from oropharyngeal tumors is related to the expression of Tim-3 but not PD-1. Oral Oncol. 2018, 82, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lukesova, E.; Boucek, J.; Rotnaglova, E.; Salakova, M.; Koslabova, E.; Grega, M.; Eckschlager, T.; Rihova, B.; Prochazka, B.; Klozar, J.; et al. High level of tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. Biomed Res. Int. 2014, 2014, 303929. [Google Scholar] [CrossRef]
- Solomon, B.; Young, R.J.; Bressel, M.; Urban, D.; Hendry, S.; Thai, A.; Angel, C.; Haddad, A.; Kowanetz, M.; Fua, T.; et al. Prognostic significance of PD-L1þ and CD8þ immune cells in HPVþ Oropharyngeal squamous cell carcinoma. Cancer Immunol. Res. 2018, 6, 295–304. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Charap, A.J.; Enokida, T.; Brody, R.; Sfakianos, J.; Miles, B.; Bhardwaj, N.; Horowitz, A. Landscape of natural killer cell activity in head and neck squamous cell carcinoma. J. Immunother. Cancer 2020, 8, e001523. [Google Scholar] [CrossRef]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Wittekindt, C.; Reuschenbach, M.; Hennig, B.; Thevarajah, M.; Würdemann, N.; Prigge, E.S.; Von Knebel Doeberitz, M.; Dreyer, T.; Gattenlöhner, S.; et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer 2016, 138, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Welsh, R.; Szomolanyi-Tsuda, E. NK cells and virus-related cancers. Crit. Rev. Oncog. 2014, 19, 107–119. [Google Scholar] [CrossRef]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): An open-label, phase 2, randomised trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Baxi, S.; Fury, M.; Ganly, I.; Rao, S.; Pfister, D.G. Ten years of progress in head and neck cancers. J. Natl. Compr. Cancer Netw. 2012, 10, 806–810. [Google Scholar] [CrossRef]
- Morand, G.B.; Madana, J.; Da Silva, S.D.; Roskies, M.; Sultanem, K.; Black, M.J.; Mlynarek, A.M.; Hier, M.P. Survival and quality of life in oropharyngeal cancer patients treated with primary chemoradiation after salivary gland transfer. J. Laryngol. Otol. 2016, 130, 755–762. [Google Scholar] [CrossRef]
- Roden, D.F.; Schreiber, D.; Givi, B. Triple-modality treatment in patients with advanced stage tonsil cancer. Cancer 2017, 123, 3269–3276. [Google Scholar] [CrossRef]
- Shibata, H.; Saito, S.; Uppaluri, R. Immunotherapy for Head and Neck Cancer: A Paradigm Shift From Induction Chemotherapy to Neoadjuvant Immunotherapy. Front. Oncol. 2021, 11, 3533. [Google Scholar] [CrossRef]
- Carvalho, H.d.A.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics 2018, 73, e557s. [Google Scholar] [CrossRef]
- Cohen-Jonathan, E.; Bernhard, E.J.; McKenna, W.G. How does radiation kill cells? Curr. Opin. Chem. Biol. 1999, 3, 77–83. [Google Scholar] [CrossRef]
- Galluzzi, L.; Maiuri, M.C.; Vitale, I.; Zischka, H.; Castedo, M.; Zitvogel, L.; Kroemer, G. Cell death modalities: Classification and pathophysiological implications. Cell Death Differ. 2007, 14, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.J.; Minn, A.J.; Vonderheide, R.H.; Wherry, E.J.; Hahn, S.M.; Maity, A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett. 2015, 368, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Apetoh, L. Radiotherapy and Immunogenic Cell Death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef]
- Rödel, F.; Frey, B.; Multhoff, G.; Gaipl, U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015, 356, 105–113. [Google Scholar] [CrossRef]
- Hildebrandt, G.; Seed, M.P.; Freemantle, C.N.; Alam, C.A.S.; Colville-Nash, P.R.; Trott, K.R. Mechanisms of the anti-inflammatory activity of low-dose radiation therapy. Int. J. Radiat. Biol. 1998, 74, 367–378. [Google Scholar] [CrossRef]
- Merrick, A.; Errington, F.; Milward, K.; O’Donnell, D.; Harrington, K.; Bateman, A.; Pandha, H.; Vile, R.; Morrison, E.; Selby, P.; et al. Immunosuppressive effects of radiation on human dendritic cells: Reduced IL-12 production on activation and impairment of naïve T-cell priming. Br. J. Cancer 2005, 92, 1450–1458. [Google Scholar] [CrossRef]
- Chiriva-Internati, M.; Grizzi, F.; Pinkston, J.; Morrow, K.J.; D’Cunha, N.; Frezza, E.E.; Muzzio, P.C.; Kast, W.M.; Cobos, E. Gamma-Radiation Upregulates MHC Class I/II and ICAM-I Molecules in Multiple Myeloma Cell Lines and Primary Tumors. Vitr. Cell. Dev. Biol.–Anim. 2006, 42, 89. [Google Scholar] [CrossRef]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518. [Google Scholar] [CrossRef]
- Rosental, B.Y.; Appel, M.; Yossef, R.; Hadad, U.; Brusilovsky, M.; Porgador, A. The Effect of Chemotherapy/Radiotherapy on Cancerous Pattern Recognition by NK Cells. Curr. Med. Chem. 2012, 19, 1780–1791. [Google Scholar] [CrossRef]
- Lugade, A.A.; Sorensen, E.W.; Gerber, S.A.; Moran, J.P.; Frelinger, J.G.; Lord, E.M. Radiation-Induced IFN-γ Production within the Tumor Microenvironment Influences Antitumor Immunity. J. Immunol. 2008, 180, 3132–3139. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Kong, L.; Meng, X.; Yang, J.; Yu, J. Radiotherapy combined with immune checkpoint blockade immunotherapy: Achievements and challenges. Cancer Lett. 2015, 365, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; O’Neill, A.; Rabinowits, G.; Tishler, R.; Khuri, F.; Adkins, D.; Clark, J.; Sarlis, N.; Lorch, J.; Beitler, J.J.; et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 257–264. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Gil, Z.; Billan, S. Precision medicine in head and neck cancer. Drug Resist. Updat. 2018, 40, 13–16. [Google Scholar] [CrossRef]
- Wargo, J.A.; Reuben, A.; Cooper, Z.A.; Oh, K.S.; Sullivan, R.J. Immune Effects of Chemorapy, Radiation, and Targeted rapy and Opportunities for Combination with Immunorapy. Semin. Oncol. 2015, 42, 601–616. [Google Scholar] [CrossRef]
- Freeman, S.M.; Franco, J.L.B.; Kenady, D.E.; Baltzer, L.; Roth, Z.; Brandwein, H.J.; Hadden, J.W. A phase 1 safety study of an IRX-2 regimen in patients with squamous cell carcinoma of the head and neck. Am. J. Clin. Oncol. Cancer Clin. Trials 2011, 34, 173–178. [Google Scholar] [CrossRef]
- Wolf, G.T.; Fee, W.E.; Dolan, R.W.; Moyer, J.S.; Kaplan, M.J.; Spring, P.M.; Suen, J.; Kenady, D.E.; Newman, J.G.; Carroll, W.R.; et al. Novel neoadjuvant immunotherapy regimen safety and survival in head and neck squamous cell cancer. Head Neck 2011, 33, 1666–1674. [Google Scholar] [CrossRef]
- Berinstein, N.L.; Wolf, G.T.; Naylor, P.H.; Baltzer, L.; Egan, J.E.; Brandwein, H.J.; Whiteside, T.L.; Goldstein, L.C.; El-Naggar, A.; Badoual, C.; et al. Increased lymphocyte infiltration in patients with head and neck cancer treated with the IRX-2 immunotherapy regimen. Cancer Immunol. Immunother. 2012, 61, 771–782. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Butterfield, L.H.; Naylor, P.H.; Egan, J.E.; Hadden, J.W.; Baltzer, L.; Wolf, G.T.; Berinstein, N.L. A short course of neoadjuvant IRX-2 induces changes in peripheral blood lymphocyte subsets of patients with head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2012, 61, 783–788. [Google Scholar] [CrossRef][Green Version]
- Fishman, M.N.; Thompson, J.A.; Pennock, G.K.; Gonzalez, R.; Diez, L.M.; Daud, A.I.; Weber, J.S.; Huang, B.Y.; Tang, S.; Rhode, P.R.; et al. Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin. Cancer Res. 2011, 17, 7765–7775. [Google Scholar] [CrossRef]
- Weed, D.T.; Vella, J.L.; Reis, I.M.; De La Fuente, A.C.; Gomez, C.; Sargi, Z.; Nazarian, R.; Califano, J.; Borrello, I.; Serafini, P. Tadalafil reduces myeloid-derived suppressor cells and regulatory t cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 39–48. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Sturgis, E.M.; Burtness, B.; Ridge, J.A.; Ringash, J.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Erlich, R.B.; Mark, A.; Bhardwaj, N.; Herberman, R.B. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: Case report, hypothesis, and clinical trial. Cancer Immunol. Res. 2014, 2, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Hamid, O.; Thompson, J.A.; Ros, W.; Eskens, F.A.L.M.; Doi, T.; Hu-Lieskovan, S.; Klempner, S.J.; Ganguly, B.; Fleener, C.; et al. A Phase I, Open-Label, Dose-Escalation Study of the OX40 Agonist Ivuxolimab in Patients with Locally Advanced or Metastatic Cancers. Clin. Cancer Res. 2022, 28, 71–83. [Google Scholar] [CrossRef]
- Ribas, A.; Medina, T.; Kummar, S.; Amin, A.; Kalbasi, A.; Drabick, J.J.; Barve, M.; Daniels, G.A.; Wong, D.J.; Schmidt, E.V.; et al. Sd-101 in combination with pembrolizumab in advanced melanoma: Results of a phase ib, multicenter study. Cancer Discov. 2018, 8, 1250–1257. [Google Scholar] [CrossRef]
- Muro, K.; Chung, H.C.; Shankaran, V.; Geva, R.; Catenacci, D.; Gupta, S.; Eder, J.P.; Golan, T.; Le, D.T.; Burtness, B.; et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016, 17, 717–726. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib keynote-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.M.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’Donnell, P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.H.; et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, Y.-F.; Lian, C.-L.; Wang, J.; Zhuo, R.-G.; Wu, S.-G. Survival Outcomes and Treatment Decision by Human Papillomavirus Status Among Patients With Stage IVC Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 668066. [Google Scholar] [CrossRef]
- Kelly, J.R.; Husain, Z.A.; Burtness, B. Treatment de-intensification strategies for head and neck cancer. Eur. J. Cancer 2016, 68, 125–133. [Google Scholar] [CrossRef]
- Wang, C.; Dickie, J.; Sutavani, R.V.; Pointer, C.; Thomas, G.J.; Savelyeva, N. Targeting head and neck cancer by vaccination. Front. Immunol. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Zhou, L.; Xu, N.; Egloff, A.M.; Uppaluri, R. Personalized cancer vaccination in head and neck cancer. Cancer Sci. 2021, 112, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.T.; Castellsagué, X.; Garland, S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012, 30, F123–F138. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Beyaert, S.; Machiels, J.-P.; Schmitz, S. Vaccine-Based Immunotherapy for Head and Neck Cancers. Cancers 2021, 13, 6041. [Google Scholar] [CrossRef]
- Yang, A.; Farmer, E.; Lin, J.; Wu, T.-C.; Hung, C.-F. The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus Res. 2017, 231, 148–165. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).