Afatinib and Dacomitinib Efficacy, Safety, Progression Patterns, and Resistance Mechanisms in Patients with Non-Small Cell Lung Cancer Carrying Uncommon EGFR Mutations: A Comparative Cohort Study in China (AFANDA Study)
Simple Summary
Abstract
1. Background
2. Methods
2.1. Study Design and Data Resources
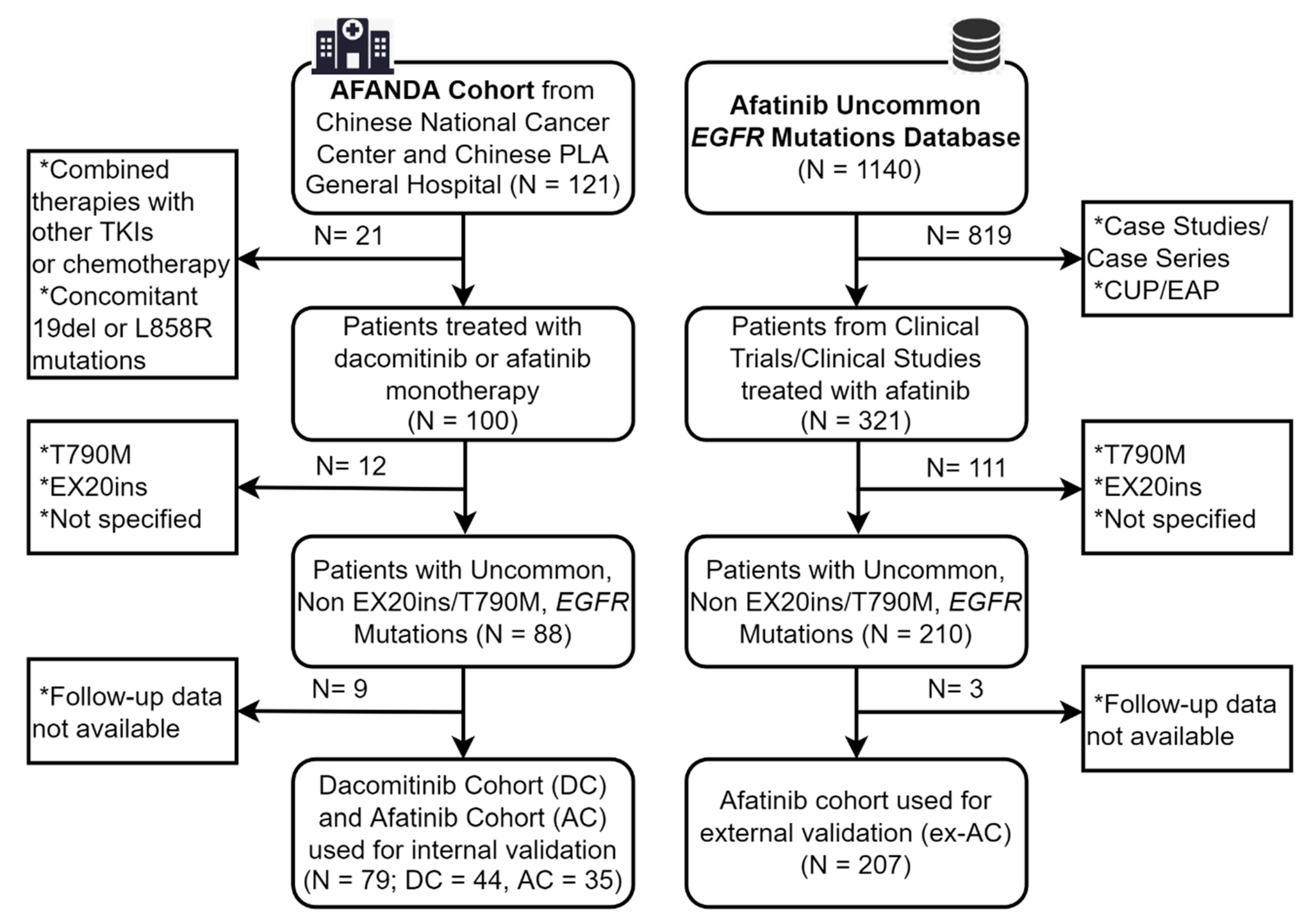
2.2. Patient Selection Criteria
2.3. Treatment and Efficacy/Toxicity Evaluation
2.4. Exploration of Resistance Mechanisms
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics in the DC and the AC
3.2. Efficacy Evaluation, Survival Analysis, and Subgroup Analysis in the DC and the AC
3.3. Univariate and Multivariate Analyses in the Pooled DC and AC
3.4. Toxicity Analysis in the DC and the AC
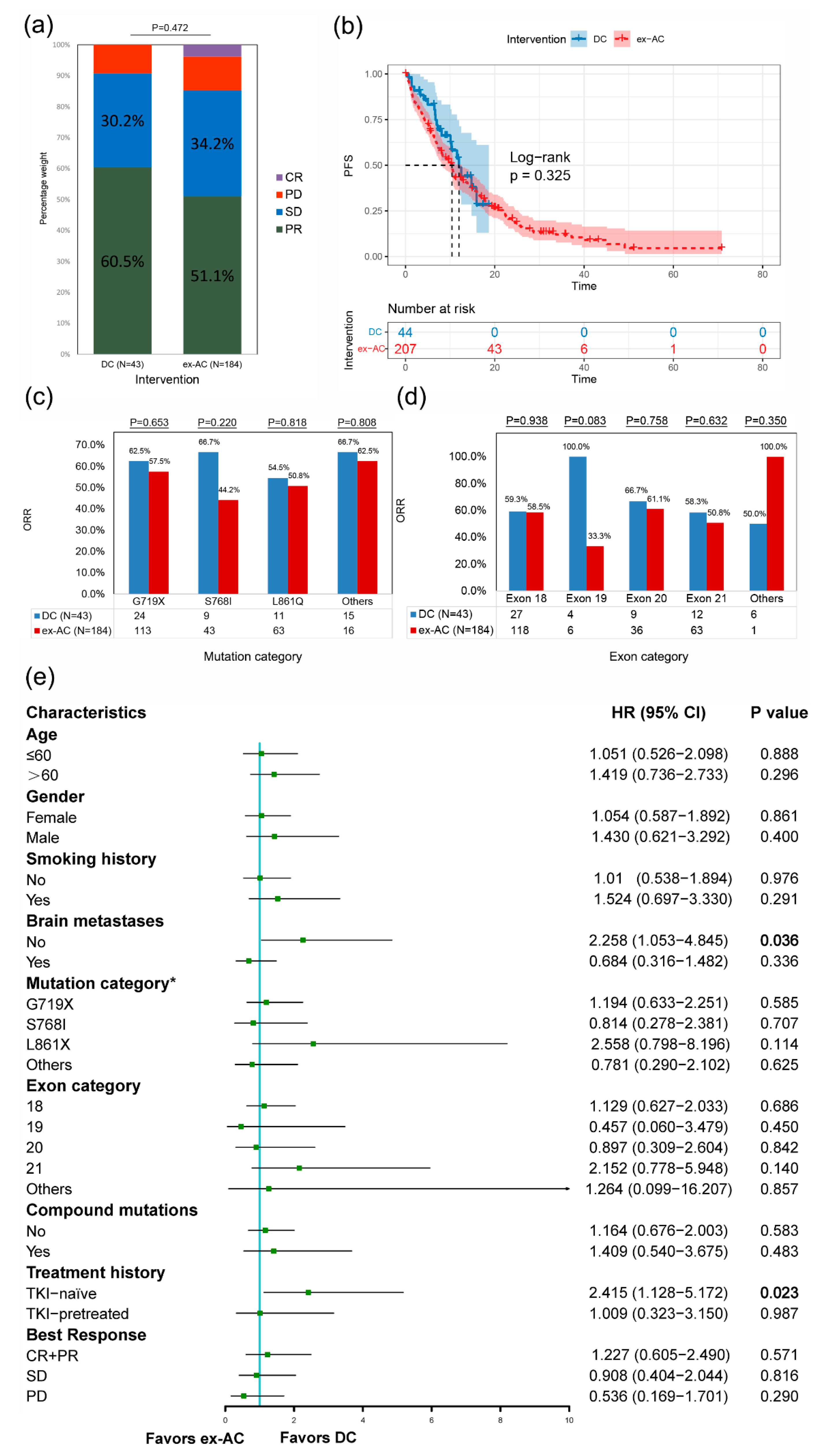
3.5. External Validation in the DC and the ex-AC
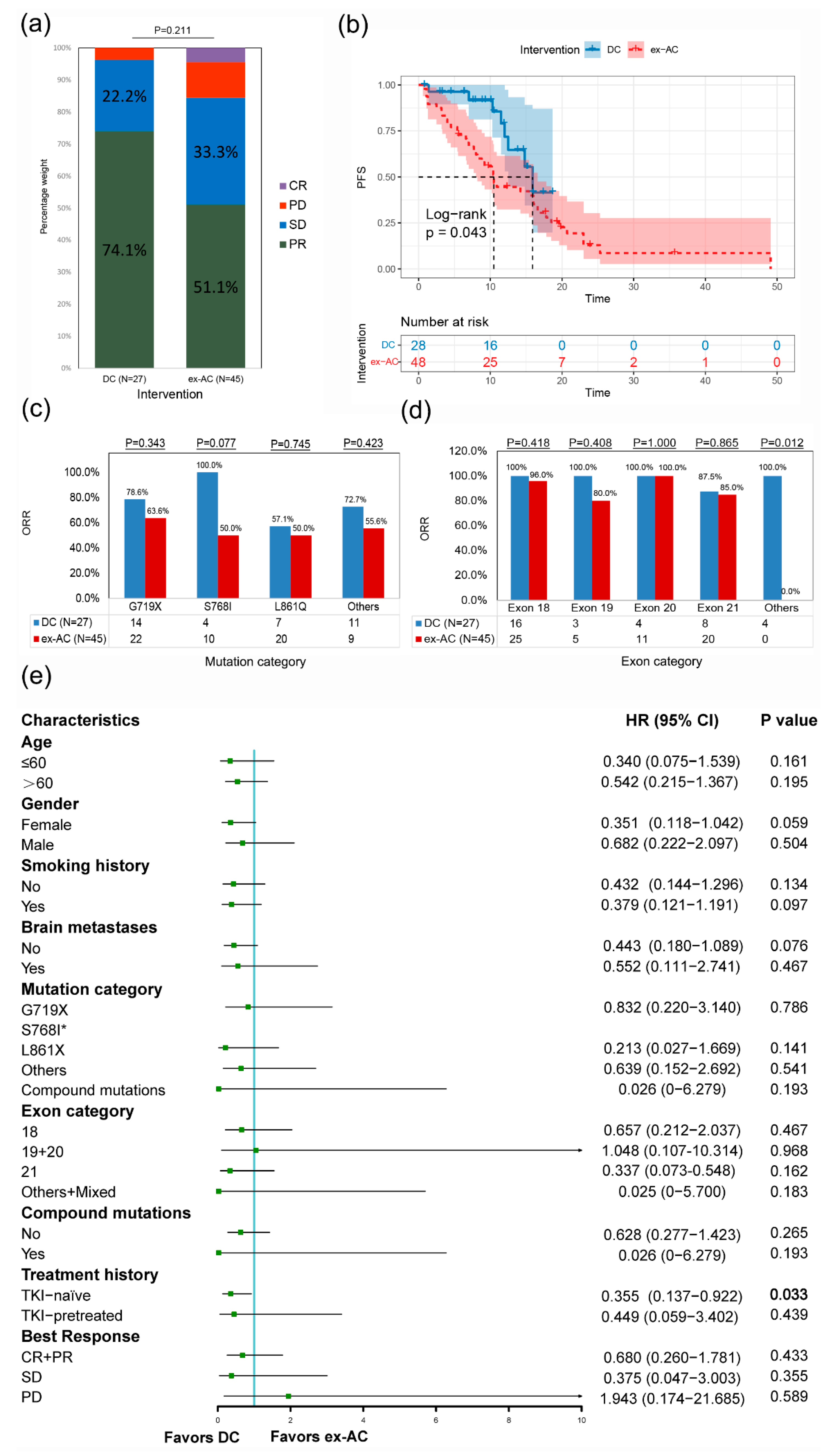
3.6. External Validation in the DC and the ex-AC after PSM
3.7. Progression Patterns, Resistance Mechanisms, and Subsequent Therapies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Deligiorgi, M.V.; Trafalis, D.T. Repurposing denosumab in lung cancer beyond counteracting the skeletal related events: An intriguing perspective. Expert Opin. Biol. Ther. 2020, 20, 1331–1346. [Google Scholar] [CrossRef]
- Remon, J.; Hendriks, L.E.L.; Cardona, A.F.; Besse, B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat. Rev. 2020, 90, 102105. [Google Scholar] [CrossRef]
- Huang, C.H.; Ju, J.S.; Chiu, T.H.; Huang, A.C.; Tung, P.H.; Wang, C.C.; Liu, C.Y.; Chung, F.T.; Fang, Y.F.; Guo, Y.K.; et al. Afatinib treatment in a large real-world cohort of non-small cell lung cancer patients with common and uncommon epidermal growth factor receptor mutation. Int. J. Cancer 2021, 150, 626–635. [Google Scholar] [CrossRef]
- Wu, J.Y.; Yu, C.J.; Chang, Y.C.; Yang, C.H.; Shih, J.Y.; Yang, P.C. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 3812–3821. [Google Scholar] [CrossRef]
- Baek, J.H.; Sun, J.M.; Min, Y.J.; Cho, E.K.; Cho, B.C.; Kim, J.H.; Ahn, M.J.; Park, K. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer except both exon 19 deletion and exon 21 L858R: A retrospective analysis in Korea. Lung Cancer 2015, 87, 148–154. [Google Scholar] [CrossRef]
- Kuiper, J.L.; Hashemi, S.M.; Thunnissen, E.; Snijders, P.J.; Grünberg, K.; Bloemena, E.; Sie, D.; Postmus, P.E.; Heideman, D.A.; Smit, E.F. Non-classic EGFR mutations in a cohort of Dutch EGFR-mutated NSCLC patients and outcomes following EGFR-TKI treatment. Br. J. Cancer 2016, 115, 1504–1512. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Yang, C.-T.; Shih, J.-Y.; Huang, M.-S.; Su, W.-C.; Lai, R.-S.; Wang, C.-C.; Hsiao, S.-H.; Lin, Y.-C.; Ho, C.-L.; et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J. Thorac. Oncol. 2015, 10, 793–799. [Google Scholar] [CrossRef]
- Yang, J.C.; Sequist, L.V.; Geater, S.L.; Tsai, C.M.; Mok, T.S.; Schuler, M.; Yamamoto, N.; Yu, C.J.; Ou, S.H.; Zhou, C.; et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef]
- Yang, J.C.H.; Schuler, M.; Popat, S.; Miura, S.; Heeke, S.; Park, K.; Märten, A.; Kim, E.S. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J. Thorac. Oncol. 2020, 15, 803–815. [Google Scholar] [CrossRef]
- Wu, Y.L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Jänne, P.A.; Boss, D.S.; Camidge, D.R.; Britten, C.D.; Engelman, J.A.; Garon, E.B.; Guo, F.; Wong, S.; Liang, J.; Letrent, S.; et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Reckamp, K.L.; Giaccone, G.; Camidge, D.R.; Gadgeel, S.M.; Khuri, F.R.; Engelman, J.A.; Koczywas, M.; Rajan, A.; Campbell, A.K.; Gernhardt, D.; et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer 2014, 120, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Suda, K.; Kobayashi, Y.; Ohara, S.; Fujino, T.; Koga, T.; Chiba, M.; Shimoji, M.; Tomizawa, K.; Takemoto, T.; et al. Effects of secondary EGFR mutations on resistance against upfront osimertinib in cells with EGFR-activating mutations in vitro. Lung Cancer 2018, 126, 149–155. [Google Scholar] [CrossRef]
- Kris, M.G.; Camidge, D.R.; Giaccone, G.; Hida, T.; Li, B.T.; O’Connell, J.; Taylor, I.; Zhang, H.; Arcila, M.E.; Goldberg, Z.; et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann. Oncol. 2015, 26, 1421–1427. [Google Scholar] [CrossRef]
- Li, H.S.; Zhang, J.Y.; Yan, X.; Xu, H.Y.; Hao, X.Z.; Xing, P.Y.; Wang, Y. A real-world study of dacomitinib in later-line settings for advanced non-small cell lung cancer patients harboring EGFR mutations. Cancer Med. 2022, 11, 1026–1036. [Google Scholar] [CrossRef]
- Li, H.-S.; Yang, G.-J.; Cai, Y.; Li, J.-L.; Xu, H.-Y.; Zhang, T.; Zhou, L.-Q.; Wang, Y.-Y.; Wang, J.-L.; Hu, X.-S.; et al. Dacomitinib for Advanced Non-small Cell Lung Cancer Patients Harboring Major Uncommon EGFR Alterations: A Dual-Center, Single-Arm, Ambispective Cohort Study in China. Front. Pharmacol. 2022, 13, 919652. [Google Scholar] [CrossRef]
- Li, H.-S.; Li, J.-L.; Yan, X.; Xu, H.-Y.; Zhou, L.-Q.; Hu, X.-S.; Wang, Y.-Y.; Lei, S.-Y.; Wang, Y. Efficacy of dacomitinib in patients with non-small cell lung cancer carrying complex EGFR mutations: A real-world study. J. Thorac. Dis. 2022, 14, 1428–1440. [Google Scholar] [CrossRef]
- Popat, S.; Hsia, T.C.; Hung, J.Y.; Jung, H.A.; Shih, J.Y.; Park, C.K.; Lee, S.H.; Okamoto, T.; Ahn, H.K.; Lee, Y.C.; et al. Tyrosine Kinase Inhibitor Activity in Patients with NSCLC Harboring Uncommon EGFR Mutations: A Retrospective International Cohort Study (UpSwinG). Oncologist 2022, 27, 255–265. [Google Scholar] [CrossRef]
- Janning, M.; Süptitz, J.; Albers-Leischner, C.; Delpy, P.; Tufman, A.; Velthaus-Rusik, J.L.; Reck, M.; Jung, A.; Kauffmann-Guerrero, D.; Bonzheim, I.; et al. Treatment outcome of atypical EGFR mutations in the German National Network Genomic Medicine Lung Cancer (nNGM). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 602–615. [Google Scholar] [CrossRef]
- Van de Stadt, E.A.; Yaqub, M.; Lammertsma, A.A.; Poot, A.J.; Schuit, R.C.; Remmelzwaal, S.; Schwarte, L.A.; Smit, E.F.; Hendrikse, H.; Bahce, I. Identifying advanced stage NSCLC patients who benefit from afatinib therapy using (18)F-afatinib PET/CT imaging. Lung Cancer 2021, 155, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; de Marinis, F.; Tu, H.Y.; Laktionov, K.K.; Feng, J.; Poltoratskiy, A.; Zhao, J.; Tan, E.H.; Gottfried, M.; Lee, V.; et al. Afatinib in EGFR TKI-Naïve Patients with Locally Advanced or Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Pooled Analysis of Three Phase IIIb Studies. Front. Oncol. 2021, 11, 709877. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.S.; Kim, J.H.; Kim, Y.C.; Kim, H.G.; Cho, E.K.; Jin, J.Y.; Kim, M.; Märten, A.; Kang, J.H. An open-label expanded access program of afatinib in EGFR tyrosine kinase inhibitor-naïve patients with locally advanced or metastatic non-small cell lung cancer harboring EGFR mutations. BMC Cancer 2021, 21, 802. [Google Scholar] [CrossRef]
- De Marinis, F.; Laktionov, K.K.; Poltoratskiy, A.; Egorova, I.; Hochmair, M.; Passaro, A.; Migliorino, M.R.; Metro, G.; Gottfried, M.; Tsoi, D.; et al. Afatinib in EGFR TKI-naïve patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer: Interim analysis of a Phase 3b study. Lung Cancer 2021, 152, 127–134. [Google Scholar] [CrossRef]
- Brückl, W.M.; Reck, M.; Griesinger, F.; Schäfer, H.; Kortsik, C.; Gaska, T.; Rawluk, J.; Krüger, S.; Kokowski, K.; Budweiser, S.; et al. Afatinib as first-line treatment in patients with EGFR-mutated non-small cell lung cancer in routine clinical practice. Ther. Adv. Med. Oncol. 2021, 13, 17588359211012361. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, A.; Tamiya, M.; Nishihara, T.; Shiroyama, T.; Nakao, K.; Tsuji, T.; Takeuchi, N.; Isa, S.I.; Omachi, N.; Okamoto, N.; et al. Cerebrospinal Fluid Penetration Rate and Efficacy of Afatinib in Patients with EGFR Mutation-positive Non-small Cell Lung Cancer with Leptomeningeal Carcinomatosis: A Multicenter Prospective Study. Anticancer Res. 2017, 37, 4177–4182. [Google Scholar] [CrossRef]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Politi. Anal. 2007, 15, 199–236. [Google Scholar] [CrossRef]
- Hansen, B.B. Full Matching in an Observational Study of Coaching for the SAT. Publ. Am. Stat. Assoc. 2004, 99, 609–618. [Google Scholar] [CrossRef]
- Wu, S.G.; Yu, C.J.; Yang, J.C.; Shih, J.Y. The effectiveness of afatinib in patients with lung adenocarcinoma harboring complex epidermal growth factor receptor mutation. Ther. Adv. Med. Oncol. 2020, 12, 1758835920946156. [Google Scholar] [CrossRef]
- Cho, J.H.; Lim, S.H.; An, H.J.; Kim, K.H.; Park, K.U.; Kang, E.J.; Choi, Y.H.; Ahn, M.S.; Lee, M.H.; Sun, J.-M.; et al. Osimertinib for Patients With Non–Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J. Clin. Oncol. 2020, 38, 488–495. [Google Scholar] [CrossRef]
- Shen, Q.; Qu, J.; Chen, Z.; Zhou, J. Case Report: Dacomitinib Overcomes Osimertinib Resistance in NSCLC Patient Harboring L718Q Mutation: A Case Report. Front. Oncol. 2021, 11, 760097. [Google Scholar] [CrossRef]
- Chan, D. P76.87 Efficacy of Dacomitinib in EGFR TKI Refractory Metastatic Non-Small Cell Lung Cancer (EGFR Mutant) with Leptomeningeal Metastases. J. Thorac. Oncol. 2021, 16, S627. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.; Jung, H.A.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Park, K.; Choi, Y.L.; Sun, J.M. Repeat biopsy procedures and T790M rates after afatinib, gefitinib, or erlotinib therapy in patients with lung cancer. Lung Cancer 2019, 130, 87–92. [Google Scholar] [CrossRef]
- Wu, S.G.; Liu, Y.N.; Tsai, M.F.; Chang, Y.L.; Yu, C.J.; Yang, P.C.; Yang, J.C.; Wen, Y.F.; Shih, J.Y. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget 2016, 7, 12404–12413. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Chen, J.-S.; Liao, W.-Y.; Ho, C.-C.; Hsu, C.-L.; Yang, C.-Y.; Chen, K.-Y.; Lee, J.-H.; Lin, Z.-Z.; Shih, J.-Y.; et al. Clinical outcomes and secondary epidermal growth factor receptor (EGFR) T790M mutation among first-line gefitinib, erlotinib and afatinib-treated non-small cell lung cancer patients with activating EGFR mutations. Int. J. Cancer 2019, 144, 2887–2896. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liu, Z.; Wang, L.; Yao, Y.; Liu, Y.; Hao, X.Z.; Wang, J.; Xing, P.; Li, J. Efficacy of dacomitinib in patients with EGFR-mutated NSCLC and brain metastases. Thorac. Cancer 2021, 12, 3407–3415. [Google Scholar] [CrossRef]
- Peng, W.; Pu, X.; Jiang, M.; Wang, J.; Li, J.; Li, K.; Xu, Y.; Xu, F.; Chen, B.; Wang, Q.; et al. Dacomitinib induces objective responses in metastatic brain lesions of patients with EGFR-mutant non-small-cell lung cancer: A brief report. Lung Cancer 2021, 152, 66–70. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Hirsh, V.; Boyer, M.; Yang, J.C.; Mok, T.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-Lung 7 trial. Ann. Oncol. 2017, 28, 270–277. [Google Scholar] [CrossRef]
- Park, K.; Cho, B.C.; Kim, D.-W.; Ahn, M.-J.; Lee, S.-Y.; Gernhardt, D.; Taylor, I.; Campbell, A.K.; Zhang, H.; Giri, N.; et al. Safety and efficacy of dacomitinib in korean patients with KRAS wild-type advanced non-small-cell lung cancer refractory to chemotherapy and erlotinib or gefitinib: A phase I/II trial. J. Thorac. Oncol. 2014, 9, 1523–1531. [Google Scholar] [CrossRef]
- Morita, A.; Hosokawa, S.; Yamada, K.; Umeno, T.; Kano, H.; Kayatani, H.; Shiojiri, M.; Sakugawa, M.; Bessho, A. Dacomitinib as a retreatment for advanced non-small cell lung cancer patient with an uncommon EGFR mutation. Thorac. Cancer 2021, 12, 1248–1251. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Makhnin, A.; Tobi, Y.Y.; Daly, R.M.; Preeshagul, I.R.; Iqbal, A.N.; Ahn, L.S.; Hayes, S.A.; Heller, G.; Kris, M.G.; et al. Pilot Study of Dacomitinib for Patients With Metastatic EGFR-Mutant Lung Cancers With Disease Progression After Initial Treatment With Osimertinib. JCO Precis. Oncol. 2021, 5. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Ganguly, S.; Ghosh, J.; Roy, S.; Bakshi, R.; Dabkara, D. Real-world experience of dacomitinib in EGFR mutated advanced NSCLC: A single center experience from India. J. Clin. Oncol. 2021, 39, e21043. [Google Scholar] [CrossRef]
- Jänne, P.A.; Ou, S.I.; Kim, D.W.; Oxnard, G.R.; Martins, R.; Kris, M.G.; Dunphy, F.; Nishio, M.; O’Connell, J.; Paweletz, C.; et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2014, 15, 1433–1441. [Google Scholar] [CrossRef]


| Characteristic | DC (N = 44) | AC (N = 35) | p Value |
|---|---|---|---|
| Age | 0.859 | ||
| ≤60 | 21 (47.7) | 16 (45.7) | |
| >60 | 23 (52.3) | 19 (54.3) | |
| Sex | 0.367 | ||
| Female | 33 (75.0) | 23 (65.7) | |
| Male | 11 (25.0) | 12 (34.3) | |
| Smoking history | 0.802 | ||
| No | 29 (65.9) | 24 (68.6) | |
| Yes | 15 (34.1) | 11 (31.4) | |
| Histology | 1.000 | ||
| Adenocarcinoma | 43 (97.7) | 35 (100) | |
| Adenosquamous carcinoma | 1 (2.3) | 0 | |
| Stage | 0.286 | ||
| Relapsed | 12 (27.3) | 6 (17.1) | |
| IV | 32 (72.7) | 29 (82.9) | |
| Brain metastases | 0.125 | ||
| No | 24 (54.5) | 25 (71.4) | |
| Yes | 20 (45.5) | 10 (28.6) | |
| Total tumor burden | 0.758 | ||
| <3 metastatic organs | 34 (77.3) | 26 (74.3) | |
| ≥3 metastatic organs | 10 (22.7) | 9 (25.7) | |
| ECOG PS | 0.683 | ||
| 0 | 11 (25.0) | 6 (17.1) | |
| 1 | 28 (63.6) | 24 (68.6) | |
| 2 | 5 (11.4) | 5 (14.3) | |
| Application line | Median (range): 1 (1–6) | Median (range): 1 (1–5) | 0.475 |
| 1 | 27 (61.4) | 26 (74.3) | |
| 2 | 9 (20.5) | 5 (14.3) | |
| ≥3 | 8 (18.2) | 4 (11.4) | |
| Mutation category * | 0.396 | ||
| G719X | 24 (54.5) | 23 (65.7) | |
| G719X | 9 (20.5) | 10 (28.6) | |
| G719X + S768I | 9 (20.5) | 11 (31.4) | |
| G719X + L861X | 2 (4.5) | 0 | |
| G719X + others | 4 (9.1) | 2 (5.7) | |
| S768I | 9 (20.5) | 12 (34.3) | |
| S768I + G719X | 9 (20.5) | 11 (31.4) | |
| S768I + others | 0 (0) | 1 (2.9) | |
| L861X | 12 (27.3) | 5 (14.3) | |
| L861X | 9 (20.5) | 3 (8.6) | |
| L861X + G719X | 2 (4.5) | 1 (2.9) | |
| L861X + others | 1 (2.3) | 1 (2.9) | |
| Others | 15 (34.1) | 11 (31.4) | |
| Exon category * | 0.299 | ||
| 18 | 27 (61.4) | 25 (71.4) | |
| 19 | 4 (9.1) | 3 (8.6) | |
| 20 | 9 (20.5) | 13 (37.1) | |
| 21 | 13 (29.5) | 6 (17.1) | |
| Others | 6 (13.6) | 1 (2.9) | |
| Compound mutations | 0.274 | ||
| No | 28 (63.6) | 18 (51.4) | |
| Yes | 16 (36.4) | 17 (48.6) |
| Characteristic | N | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Median (Months) | p Value | HR | 95% CI | p Value | ||
| Intervention | 0.305 | 0.047 | ||||
| Dacomitinib/Afatinib | 44/35 | 12.0/10.0 | 1.909 | 1.009–3.610 | ||
| Smoking history | 0.914 | 0.184 | ||||
| No/Yes | 53/26 | 11.0/11.5 | 1.569 | 0.808–3.047 | ||
| Brain metastases | 0.004 | 0.096 | ||||
| No/Yes | 49/30 | 12.9/7.1 | 1.804 | 0.900–3.615 | ||
| Total tumor burden | <0.001 | 0.402 | ||||
| <3/≥3 metastatic organs | 60/19 | 12.4/6.0 | 1.351 | 0.668–2.733 | ||
| ECOG PS | <0.001 | <0.001 | ||||
| 0 | 17 | 28.6 | Reference | - | - | |
| 1 | 52 | 10.1 | 6.470 | 1.871–22.381 | 0.003 | |
| 2 | 10 | 4.3 | 30.327 | 6.915–133.005 | <0.001 | |
| Application line | 0.018 | 0.043 | ||||
| 1 | 53 | 12.9 | Reference | - | - | |
| 2 | 14 | 8.1 | 2.16 | 0.985–4.737 | 0.055 | |
| ≥3 | 12 | 6.5 | 2.55 | 1.073–6.059 | 0.034 | |
| Mutation category * | 0.619 | 0.129 | ||||
| G719X | 19 | 10.3 | Reference | - | - | |
| L861X | 12 | 10.0 | 3.725 | 1.193–11.627 | 0.024 | |
| Others | 16 | 12.4 | 1.958 | 0.756–5.071 | 0.166 | |
| Compound mutations | 32 | 11.0 | 1.796 | 0.840–3.838 | 0.131 | |
| AE | G1 | p Value | G2 | p Value | G3 | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| DC | AC | DC | AC | DC | AC | ||||
| Rash | 22 (50.0) | 15 (42.9) | 0.006 | 16 (36.4) | 3 (8.6) | 0.142 | 2 (4.5) | 0 | 0.036 |
| Diarrhea | 25 (56.8) | 6 (17.1) | 7 (15.9) | 8 (22.9) | 2 (4.5) | 8 (22.9) | |||
| Oral mucositis | 16 (36.4) | 12 (34.3) | 9 (20.5) | 8 (22.9) | 2 (4.5) | 0 | |||
| Paronychia | 7 (15.9) | 11 (31.4) | 6 (13.6) | 2 (5.7) | 0 | 0 | |||
| Dry skin | 12 (27.3) | 1 (2.9) | 5 (11.4) | 1 (2.9) | 1 (2.3) | 0 | |||
| Others | 4 (9.1) | 0 | 1 (2.3) | 0 | 0 | 0 | |||
| Characteristic | Before PSM | After PSM # | ||||
|---|---|---|---|---|---|---|
| DC (N = 44) | ex-AC (N = 207) | p Value | DC (N = 28) | ex-AC (N = 48) | p Value | |
| Age | 0.173 | 0.839 | ||||
| <60 | 21 (47.7) | 76 (36.7) | 11 (39.3) | 20 (41.7) | ||
| ≥60 | 23 (52.3) | 131 (63.3) | 17 (60.7) | 28 (58.3) | ||
| Sex | 0.005 | 0.333 | ||||
| Female | 33 (75.0) | 107 (51.7) | 20 (71.4) | 29 (60.4) | ||
| Male | 11 (25.0) | 100 (48.3) | 8 (28.6) | 19 (39.6) | ||
| Smoking history | <0.001 | 0.150 | ||||
| No | 29 (65.9) | 74 (35.7) | 18 (64.3) | 27 (56.3) | ||
| Yes | 15 (34.1) | 88 (42.5) | 10 (35.7) | 15 (31.3) | ||
| Unknown | 0 | 45 (21.7) | 0 | 6 (12.5) | ||
| Brain metastases | <0.001 | 0.951 | ||||
| No | 24 (54.5) | 186 (89.9) | 22 (78.6) | 38 (79.2) | ||
| Yes | 20 (45.5) | 21 (10.1) | 6 (21.4) | 10 (20.8) | ||
| Treatment history | <0.001 | 0.615 | ||||
| TKI-naïve | 27 (61.4) | 201 (97.1) | 24 (85.7) | 43 (89.6) | ||
| TKI-pretreated | 17 (38.6) | 6 (2.9) | 4 (14.3) | 5 (10.4) | ||
| Mutation category * | 0.001 | 0.535 | ||||
| G719X | 9 (20.5) | 82 (39.6) | 6 (21.4) | 7 (14.6) | ||
| L861X | 9 (20.5) | 57 (27.5) | 6 (21.4) | 13 (27.1) | ||
| S768I | 0 | 10 (4.8) | 0 | 3 (6.3) | ||
| Others | 10 (22.7) | 15 (7.2) | 7 (25.0) | 8 (16.7) | ||
| Compound mutations | 16 (36.4) | 43 (20.8) | 9 (32.1) | 17 (35.4) | ||
| Exon category * | <0.001 | 0.432 | ||||
| 18 | 12 (27.3) | 89 (43.0) | 8 (28.6) | 10 (20.8) | ||
| 19 | 4 (9.1) | 4 (1.9) | 3 (10.7) | 4 (8.3) | ||
| 20 | 0 | 13 (6.3) | 0 | 4 (8.3) | ||
| 21 | 11 (25) | 60 (29.0) | 8 (28.6) | 13 (27.1) | ||
| Others | 2 (4.5) | 0 | 1 (3.6) | 0 | ||
| Mixed | 15 (34.1) | 41 (19.8) | 8 (28.6) | 17 (35.4) | ||
| Compound mutations | 0.040 | 0.772 | ||||
| No | 28 (63.6) | 162 (78.3) | 19 (67.9) | 31 (64.6) | ||
| Yes | 16 (36.4) | 45 (21.7) | 9 (32.1) | 17 (35.4) | ||
| Characteristic | N | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Median (Months) | p Value | HR | 95% CI | p Value | ||
| Intervention | 0.325 | 0.029 | ||||
| Dacomitinib/Afatinib | 44/207 | 12.0/10.4 | 1.953 | 1.071–3.563 | ||
| Age | 0.569 | 0.553 | ||||
| <60/≥60 | 97/154 | 11.1/10.5 | 1.098 | 0.806–1.498 | ||
| Sex | 0.035 | 0.056 | ||||
| Female/Male | 140/111 | 12.0/8.2 | 1.397 | 0.991–1.97 | ||
| Smoking history | 0.528 | 0.946 | ||||
| No | 103 | 12.5 | Reference | - | - | |
| Yes | 103 | 8.4 | 1.054 | 0.726–1.529 | 0.782 | |
| Unknown | 45 | 10.5 | 1.063 | 0.691–1.635 | 0.781 | |
| Brain metastases | 0.180 | 0.272 | ||||
| No/Yes | 210/41 | 10.6/10.1 | 1.279 | 0.824–1.985 | ||
| Treatment history | 0.033 | 0.003 | ||||
| TKI-naïve/pretreated | 228/23 | 10.7/6.7 | 2.675 | 1.402–5.101 | ||
| Mutation category * | 0.059 | 0.040 | ||||
| G719X | 91 | 10.3 | Reference | - | - | |
| L861X | 66 | 7.4 | 1.495 | 1.041–2.147 | 0.029 | |
| S768I | 10 | 20.7 | 0.507 | 0.219–1.173 | 0.112 | |
| Others | 25 | 10.7 | 1.320 | 0.755–2.308 | 0.329 | |
| Compound mutations | 59 | 14.2 | 0.973 | 0.651–1.453 | 0.893 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-S.; Wang, S.-Z.; Xu, H.-Y.; Yan, X.; Zhang, J.-Y.; Lei, S.-Y.; Li, T.; Hao, X.-Z.; Zhang, T.; Yang, G.-J.; et al. Afatinib and Dacomitinib Efficacy, Safety, Progression Patterns, and Resistance Mechanisms in Patients with Non-Small Cell Lung Cancer Carrying Uncommon EGFR Mutations: A Comparative Cohort Study in China (AFANDA Study). Cancers 2022, 14, 5307. https://doi.org/10.3390/cancers14215307
Li H-S, Wang S-Z, Xu H-Y, Yan X, Zhang J-Y, Lei S-Y, Li T, Hao X-Z, Zhang T, Yang G-J, et al. Afatinib and Dacomitinib Efficacy, Safety, Progression Patterns, and Resistance Mechanisms in Patients with Non-Small Cell Lung Cancer Carrying Uncommon EGFR Mutations: A Comparative Cohort Study in China (AFANDA Study). Cancers. 2022; 14(21):5307. https://doi.org/10.3390/cancers14215307
Chicago/Turabian StyleLi, Hong-Shuai, Shou-Zheng Wang, Hai-Yan Xu, Xiang Yan, Jin-Yao Zhang, Si-Yu Lei, Teng Li, Xue-Zhi Hao, Tao Zhang, Guang-Jian Yang, and et al. 2022. "Afatinib and Dacomitinib Efficacy, Safety, Progression Patterns, and Resistance Mechanisms in Patients with Non-Small Cell Lung Cancer Carrying Uncommon EGFR Mutations: A Comparative Cohort Study in China (AFANDA Study)" Cancers 14, no. 21: 5307. https://doi.org/10.3390/cancers14215307
APA StyleLi, H.-S., Wang, S.-Z., Xu, H.-Y., Yan, X., Zhang, J.-Y., Lei, S.-Y., Li, T., Hao, X.-Z., Zhang, T., Yang, G.-J., Zhou, L.-Q., Liu, P., Wang, Y.-Y., Hu, X.-S., Xing, P.-Y., & Wang, Y. (2022). Afatinib and Dacomitinib Efficacy, Safety, Progression Patterns, and Resistance Mechanisms in Patients with Non-Small Cell Lung Cancer Carrying Uncommon EGFR Mutations: A Comparative Cohort Study in China (AFANDA Study). Cancers, 14(21), 5307. https://doi.org/10.3390/cancers14215307







