Trabectedin for Patients with Advanced Soft Tissue Sarcoma: A Non-Interventional, Prospective, Multicenter, Phase IV Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
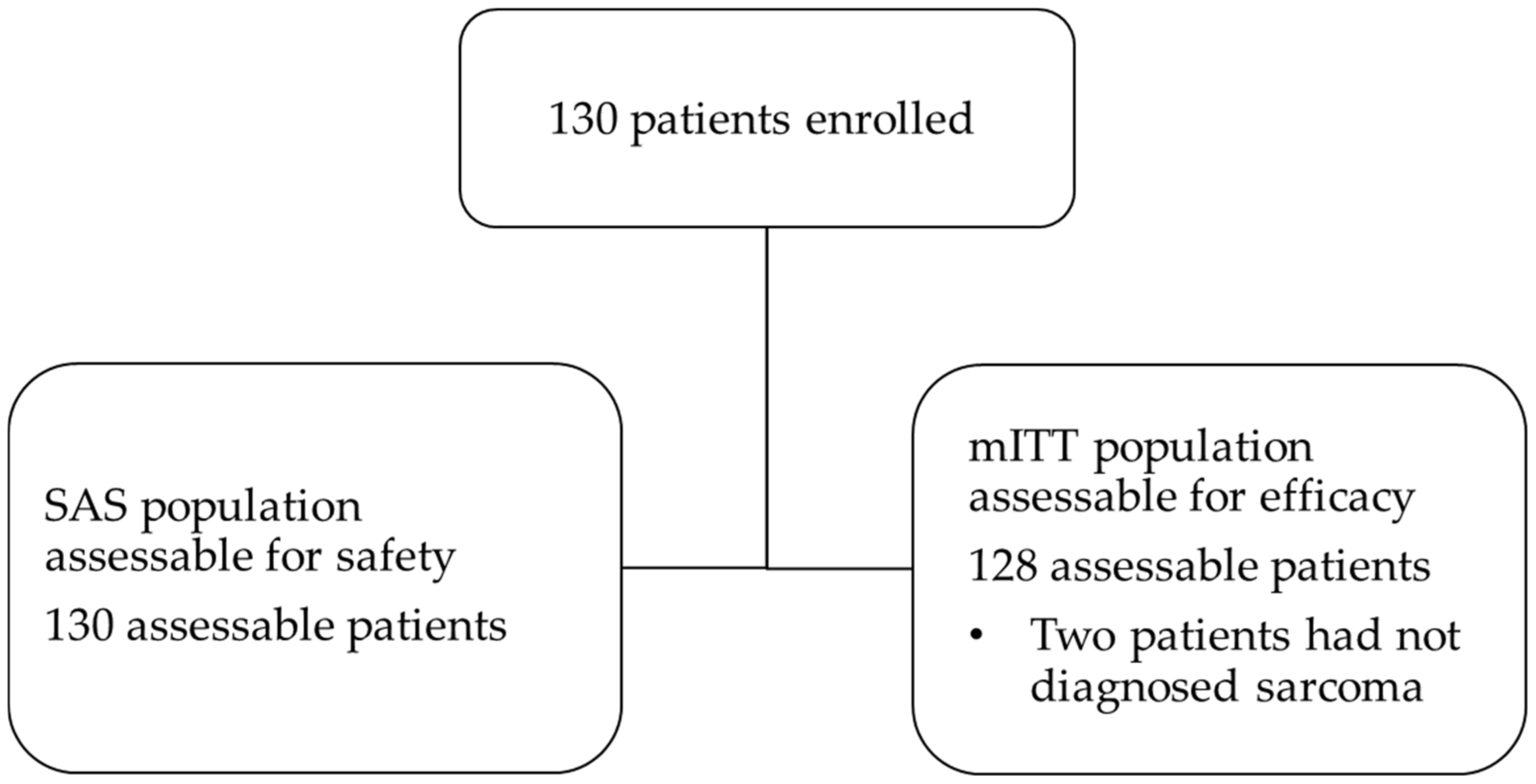
3.1. Patient Disposition and Characteristics
3.2. Extent of Exposure
3.3. Primary Efficacy Endpoint
3.4. Secondary Efficacy Endpoint
3.5. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma Epidemiology and Etiology: Potential Environmental and Genetic Factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Montella, L.; Altucci, L.; Sarno, F.; Buonerba, C.; De Simone, S.; Facchini, B.; Franzese, E.; De Vita, F.; Tafuto, S.; Berretta, M.; et al. Toward a Personalized Therapy in Soft-Tissue Sarcomas: State of the Art and Future Directions. Cancers 2021, 13, 2359. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treat-ment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv268–iv269. [Google Scholar] [CrossRef]
- Larsen, A.K.; Galmarini, C.M.; D’Incalci, M. Unique features of trabectedin mechanism of action. Cancer Chemother. Pharmacol. 2015, 77, 663–671. [Google Scholar] [CrossRef] [PubMed]
- D’Incalci, M. Trabectedin mechanism of action: What’s new? Future Oncol. 2013, 9 (Suppl. S12), 5–10. [Google Scholar] [CrossRef]
- D’Incalci, M.; Galmarini, C.M. A Review of Trabectedin (ET-743): A Unique Mechanism of Action. Mol. Cancer Ther. 2010, 9, 2157–2163. [Google Scholar] [CrossRef]
- Demetri, G.D.; Chawla, S.P.; von Mehren, M.; Ritch, P.; Baker, L.H.; Blay, J.Y.; Hande, K.R.; Keohan, M.L.; Samuels, B.L.; Schuetze, S.; et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyo-sarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J. Clin. Oncol. 2009, 27, 4188–4196. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.-Y. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii102–iii112. [Google Scholar]
- De Sanctis, R.; Marrari, A.; Marchetti, S.; Mussi, C.; Balzarini, L.; Lutman, F.R.; Daolio, P.; Bastoni, S.; Bertuzzi, A.F.; Quagliuolo, V.; et al. Efficacy of trabectedin in advanced soft tissue sarcoma: Beyond lipo- and leiomyosarcoma. Drug Des. Dev. Ther. 2015, 9, 5785–5791. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, P.; Grünwald, V.; Kasper, B.; Schuler, M.; Gelderblom, H. Efficacy of trabectedin in patients with some rare advanced soft tissue sarcoma subtypes other than liposarcoma and leiomyosarcoma. J. Med. Drug Rev. 2015, 5, 33–42. [Google Scholar]
- Serdà, P.C.; Terés, R.; Sebio, A.; Bagué, S.; Orellana, R.; Moreno, M.E.; Riba, M.; López-Pousa, A. Single-Center Experience with Trabectedin for the Treatment of Non-L-sarcomas. Adv. Ther. 2022, 39, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Araki, N.; Sugiura, H.; Ueda, T.; Yonemoto, T.; Takahashi, M.; Morioka, H.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015, 16, 406–416. [Google Scholar] [CrossRef]
- Le Cesne, A.; Blay, J.-Y.; Cupissol, D.; Italiano, A.; Delcambre, C.; Penel, N.; Isambert, N.; Chevreau, C.; Bompas, E.; Bertucci, F.; et al. A randomized phase III trial comparing trabectedin to best supportive care in patients with pre-treated soft tissue sarcoma: T-SAR, a French Sarcoma Group trial. Ann. Oncol. 2021, 32, 1034–1044. [Google Scholar] [CrossRef]
- Le Cesne, A.; Yovine, A.; Blay, J.-Y.; Delaloge, S.; Maki, R.G.; Misset, J.-L.; Frontelo, P.; Nieto, A.; Jiao, J.J.; Demetri, G.D. A retrospective pooled analysis of trabectedin safety in 1132 patients with solid tumors treated in phase II clinical trials. Investig. New Drugs 2012, 30, 1193–1202. [Google Scholar] [CrossRef]
- Buonadonna, A.; Benson, C.; Casanova, J.; Kasper, B.; López Pousa, A.; Mazzeo, F.; Thomas, B.; Nicolas, P. A noninterventional, multicenter, prospective phase IV study of trabectedin in patients with ad-vanced soft tissue sarcoma. Anticancer Drugs 2017, 28, 1157–1165. [Google Scholar] [CrossRef]
- Palmerini, E.; Sanfilippo, R.; Grignani, G.; Buonadonna, A.; Romanini, A.; Badalamenti, G.; Ferraresi, V.; Vincenzi, B.; Comandone, A.; Pizzolorusso, A.; et al. Trabectedin for Patients with Advanced Soft Tissue Sarcoma: A Non-Interventional, Retrospective, Multicenter Study of the Italian Sarcoma Group. Cancers 2021, 13, 1053. [Google Scholar] [CrossRef]
- Le Cesne, A.; Ray-Coquard, I.; Duffaud, F.; Chevreau, C.; Penel, N.; Nguyen, B.B.; Piperno-Neumann, S.; Delcambre, C.; Rios, M.; Chaigneau, L.; et al. Trabectedin in patients with advanced soft tissue sarcoma: A retrospective national analysis of the French Sarcoma Group. Eur. J. Cancer 2015, 51, 742–750. [Google Scholar] [CrossRef]
- Hoiczyk, M.; Grabellus, F.; Podleska, L.; Ahrens, M.; Schwindenhammer, B.; Taeger, G.; Pöttgen, C.; Schuler, M.; Bauer, S. Trabectedin in metastatic soft tissue sarcomas: Role of pretreatment and age. Int. J. Oncol. 2013, 43, 23–28. [Google Scholar] [CrossRef][Green Version]
- Grünwald, V.; Pink, D.; Egerer, G.; Schalk, E.; Augustin, M.; Deinzer, C.; Kob, V.; Reichert, D.; Kebenko, M.; Brandl, S.; et al. 1496P Trabectedin for patients with advanced soft tissue sarcoma: A non-interventional, prospective, multicenter study. Ann. Oncol. 2022, 33 (Suppl. S7), S1231. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gas-trointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Vora, J. Non interventional drug studies in oncology: Why we need them? Perspect. Clin. Res. 2010, 1, 128–133. [Google Scholar] [CrossRef]
- Van Glabbeke, M.; Van Oosterom, A.T.; Oosterhuis, J.W.; Mouridsen, H.; Crowther, D.; Somers, R.; Verweij, J.; Santoro, A.; Buesa, J.; Tursz, T. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens-a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J. Clin. Oncol. 1999, 17, 150–157. [Google Scholar]
- Kantidakis, G.; Litière, S.; Neven, A.; Vinches, M.; Judson, I.; Blay, J.Y.; Wardelmann, E.; Stacchiotti, S.; D’Ambrosio, L.; Marréaud, S.; et al. New benchmarks to design clinical trials with advanced or metastatic liposarcoma or synovial sar-coma patients: An EORTC-Soft Tissue and Bone Sarcoma Group (STBSG) meta-analysis based on a literature review for soft-tissue sarcomas. Eur. J. Cancer 2022, 174, 261–276. [Google Scholar] [CrossRef]
- Le Cesne, A.; Blay, J.Y.; Domont, J.; Tresch-Bruneel, E.; Chevreau, C.; Bertucci, F.; Delcambre, C.; Saada-Bouzid, E.; Piperno-Neumann, S.; Bay, J.-O.; et al. Interruption versus continuation of trabectedin in patients with soft-tissue sarcoma (T-DIS): A ran-domised phase 2 trial. Lancet Oncol. 2015, 16, 312–319. [Google Scholar] [CrossRef]
- Patel, S.; von Mehren, M.; Reed, D.R.; Kaiser, P.; Charlson, J.; Ryan, C.W.; Rushing, D.; Livingston, M.; Singh, A.; Seth, R.; et al. Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 2019, 125, 2610–2620. [Google Scholar] [CrossRef]
- Le Cesne, A. Making the Best of Available Options for Optimal Sarcoma Treatment. Oncology 2018, 95 (Suppl. S1), 11–20. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Italiano, A.; Ray-Coquard, I.; Le Cesne, A.; Duffaud, F.; Rios, M.; Collard, O.; Bertucci, F.; Bompas, E.; Isambert, N.; et al. Long-term outcome and effect of maintenance therapy in patients with advanced sarcoma treated with trabectedin: An analysis of 181 patients of the French ATU compassionate use program. BMC Cancer 2013, 13, 64. [Google Scholar] [CrossRef]
- Kotecki, N.; Le Cesne, A.; Tresch-Bruneel, E.; Mir, O.; Chevreau, C.; Bertucci, F.; Delcambre, C.; Saada-Bouzid, E.; Piperno-Neumann, S.; Bay, J.-O.; et al. Update of the T-DIS randomized phase II trial: Trabectedin rechallenge verus continuation in patients (pts) with advanced soft tissue sarcoma (ASTS). Ann. Oncol. 2016, 27 (Suppl. S6), vi486. [Google Scholar] [CrossRef]
- Yondelis Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/yondelis#product-information-section (accessed on 1 October 2022).

| Patients | Modified Intent-to-Treat Set (mITT) 1 n = 128 | |
|---|---|---|
| Age at study entry (years) | Median (range) | 58.5 (23–84) |
| ≤60 years | 70 (54.7%) | |
| >60 years | 58 (45.3%) | |
| ≤70 years | 99 (77.3%) | |
| >70 years | 29 (22.7%) | |
| Gender | Female | 65 (50.8%) |
| Male | 63 (49.2%) | |
| Histology | Leiomyosarcoma | 45 (35.2%) |
| Liposarcoma | 23 (18.0%) | |
| Pleomorphic undifferentiated sarcoma | 20 (15.6%) | |
| Synovial sarcoma | 8 (6.3%) | |
| Fibrosarcoma | 8 (6.3%) | |
| Angiosarcoma | 1 (0.8%) | |
| Other | 23 (18.0%) | |
| Site of primary tumor | Lower extremity | 23 (18.0%) |
| Abdomen (retroperitoneal) | 20 (15.6%) | |
| Upper extremity | 17 (13.3%) | |
| Uterus | 15 (11.7%) | |
| Abdomen (intraperitoneal) | 11 (8.6%) | |
| Other | 42 (32.8%) | |
| Eastern Cooperative Oncology Group (ECOG) performance status | 0 | 34 (26.6%) |
| 1 | 66 (51.6%) | |
| 2 | 10 (7.8%) | |
| 3 | 1 (0.8%) | |
| 4 | 1 (0.8%) | |
| Missing | 16 (12.5%) | |
| Tumor grade according to the French Federation of Cancer Centers Sarcoma Group grading systems (FNCLCC) 2 | Grade 1 | 13 (17.3%) |
| Grade 2 | 21 (28.0%) | |
| Grade 3 | 34 (45.3%) | |
| Grade X 3 | 6 (8.0%) | |
| Missing | 1 (1.3%) | |
| According to the Union for International Cancer Control (UICC) 2 | Grade 1 | 5 (9.3%) |
| Grade 2 | 9 (16.7%) | |
| Grade 3 | 23 (42.6%) | |
| Grade 4 | 3 (5.6%) | |
| Grade X 3 | 13 (24.1%) | |
| Missing | 1 (1.9%) | |
| Tumor stage according to the American Joint Committee on Cancer (AJCC) | Ia | 3 (2.3) |
| Ib | 7 (5.5) | |
| IIa | 4 (3.1) | |
| IIb | 5 (3.9) | |
| III | 15 (11.7) | |
| IV | 26 (20.3) | |
| Unknown | 68 (53.1) | |
| Time from first diagnosis to first treatment (months); n = 125 | Median (range) | 0.4 (0.0–149.7) |
| Time from diagnosis to last treatment before trabectedin (months); n = 125 | Median (range) | 15.9 (0.0–250.2) |
| Time from last progression to trabectedin treatment (months); n = 95 | Median (range) | 0.9 (0.0–2.5) |
| Patients | Modified Intent-to-Treat Set (mITT); n = 128 | |
|---|---|---|
| Prior treatments | Prior surgery | 111 (86.7%) |
| Prior radiotherapy | 64 (50.0%) | |
| Prior chemotherapy/ targeted treatments | 101 (78.9%) | |
| No. of lines of prior chemotherapy/targeted treatments, n = 128 | 0 lines | 27 (21.1%) |
| 1 line | 66 (51.6%) | |
| 2 lines | 25 (19.5%) | |
| ≥3 lines (3 to 6 lines) | 10 (7.8%) | |
| Types of prior chemotherapy/targeted treatments, n = 101 (≥4% of patients) | Doxorubicin | 87 (86.1%) |
| Ifosfamide | 63 (62.4%) | |
| Dacarbazine (DTIC) | 18 (17.8%) | |
| Trophosphamide | 16 (15.8%) | |
| Gemcitabine | 13 (12.9%) | |
| Docetaxel | 12 (11.9%) | |
| Epirubicin | 12 (11.9%) | |
| Olaratumab | 10 (9.9%) | |
| Pazopanib | 8 (7.9%) | |
| Best response to last prior chemotherapy/targeted treatments, n = 101 | Complete response (CR) | 2 (2.0%) |
| Partial response (PR) | 22 (21.8%) | |
| Stable disease (SD) | 39 (38.6%) | |
| Progressive disease (PD) | 22 (21.8%) | |
| Non evaluated (NE) | 16 (15.8%) | |
| Treatment Delivery | Modified Intent-to-Treat Set (mITT); n = 128 | |
|---|---|---|
| Number of cycles received per patient | Median (range) | 4 (1–44) |
| <6 cycles | 76 (59.4%) | |
| ≥6 cycles | 52 (40.6%) | |
| Dose reductions (per patient) | 0 cycle | 62 (48.4%) |
| 1 cycle | 17 (13.3%) | |
| 2 cycles | 21 (16.4%) | |
| >2 cycles | 18 (14.1%) | |
| Unknown 1 | 10 (7.8%) | |
| Cycle delays (per patient) | 0 cycle | 49 (38.3%) |
| 1 cycle | 22 (17.2%) | |
| 2 cycles | 8 (6.3%) | |
| >2 cycles | 44 (34.4%) | |
| Unknown 1 | 5 (3.9%) | |
| Best Response to Trabectedin per Patient (Unconfirmed) | Modified Intent-to-Treat Set (mITT); n = 128 | ||
|---|---|---|---|
| <6 Cycles n = 76 | ≥6 Cycles n = 52 | Total n = 128 | |
| Complete response (CR) | - | 1 (1.9%) | 1 (0.8%) |
| Partial response (PR) | 1 (1.3%) | 13 (25.0%) | 14 (10.9%) |
| Stable disease (SD) | 10 (13.2%) | 33 (63.5%) | 43 (33.6%) |
| Progressive disease (PD) | 35 (46.1%) | 3 (5.8%) | 38 (29.7%) |
| Not evaluable | 4 (5.3%) | 2 (3.9%) | 6 (4.7%) |
| Not done | 26 (34.2%) | - | 26 (20.3%) |
| Objective response rate (ORR; CR + PR) | 1 (1.3%) | 14 (26.9%) | 15 (11.7%) |
| Disease control rate (DCR; ORR + SD) | 11 (14.5%) | 47 (89.4%) | 58 (45.3%) |
| Fisher’s exact test (p-value) 1 | <0.0001 | - | |
| Treatment-Related ADR as per NCI-CTC, Worst Grade per Patient (≥3% of Patients) | Safety Analysis Set 1,2 n = 130 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 n = 79 | Grade 2 n = 73 | Grade 3 n = 67 | Grade 4 n = 23 | Grade 5 n = 2 | Total n = 105 | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| ALT increased | 9 | 6.9 | 11 | 8.5 | 14 | 10.8 | - | - | - | - | 34 | 26.2 |
| AP increased | 5 | 3.9 | 3 | 2.3 | 1 | 0.8 | - | - | - | - | 9 | 6.9 |
| Anemia | 10 | 7.7 | 16 | 12.3 | 12 | 9.2 | - | - | - | - | 38 | 29.2 |
| Anorexia | 15 | 11.5 | 5 | 3.9 | 3 | 2.3 | - | - | - | - | 23 | 17.7 |
| Arthralgia | 2 | 1.5 | 2 | 1.5 | - | - | - | - | 4 | 3.1 | ||
| AST increased | 9 | 6.9 | 9 | 6.9 | 5 | 3.9 | - | - | - | - | 23 | 17.7 |
| Leukopenia | 2 | 1.5 | 3 | 2.3 | 1 | 0.8 | - | - | - | - | 6 | 4.6 |
| Constipation | 16 | 12.3 | 2 | 1.5 | - | - | - | - | 18 | 13.9 | ||
| Diarrhea | 4 | 3.1 | 4 | 3.1 | 2 | 1.5 | - | - | - | - | 10 | 7.69 |
| Dry skin | 4 | 3.1 | - | - | - | - | - | - | - | - | 4 | 3.1 |
| Dysgeusia | 5 | 3.85 | 2 | 1.5 | - | - | - | - | - | - | 7 | 5.4 |
| Dyspnea | 3 | 2.3 | 2 | 1.5 | - | - | 1 | 0.8 | - | - | 6 | 4.6 |
| Edema limbs | 3 | 2.3 | - | - | 1 | 0.8 | - | - | - | - | 4 | 3.1 |
| Fatigue | 24 | 18.5 | 19 | 14.6 | 3 | 2.3 | - | - | - | - | 46 | 35.4 |
| Febrile neutropenia | - | - | - | - | 4 | 3.1 | 1 | 0.8 | - | - | 5 | 3.9 |
| Fever | 7 | 5.4 | 1 | 0.8 | - | - | 8 | 6.2 | ||||
| Night sweating | 4 | 3.1 | - | - | - | c | - | - | - | - | 4 | 3.1 |
| GGT increased | 7 | 5.4 | 3 | 2.3 | 11 | 8.5 | 1 | 0.8 | - | - | 22 | 16.9 |
| Headache | 5 | 3.9 | 1 | 0.8 | - | - | - | - | - | - | 6 | 4.6 |
| Hypoalbuminemia | 3 | 2.3 | 1 | 0.8 | - | - | - | - | - | - | 4 | 3.1 |
| Pneumonia 3 | - | - | - | - | 2 | 1.5 | 1 | 0.8 | 3 | 2.3 | ||
| Mucositis oral | 2 | 1.5 | 1 | 0.8 | 3 | 2.3 | - | - | - | - | 6 | 4.6 |
| Myalgia | 3 | 2.3 | 1 | 0.8 | 1 | 0.8 | - | - | - | - | 5 | 3.9 |
| Nausea | 33 | 25.4 | 16 | 12.3 | 5 | 3.9 | - | - | - | - | 54 | 41.5 |
| Neutrophil count decreased | 2 | 1.5 | 5 | 3.9 | 10 | 7.7 | 7 | 5.4 | - | - | 24 | 18.5 |
| Peripheral sensory neuropathy | 3 | 2.3 | 1 | 0.8 | 1 | 0.8 | - | - | 5 | 3.9 | ||
| Platelet count decreased | 14 | 10.8 | 5 | 3.9 | 13 | 10.0 | 8 | 6.2 | - | - | 40 | 30.8 |
| Sepsis 3 | - | - | - | - | - | - | 2 | 1.5 | 1 | 0.8 | 3 | 2.3 |
| Vomiting | 16 | 12.3 | 7 | 5.4 | 3 | 2.3 | - | - | 26 | 20.0 | ||
| White blood cell decreased | 7 | 5.4 | 12 | 9.2 | 27 | 20.8 | 8 | 6.2 | - | - | 54 | 41.4 |
| Median (95% CI) | Advanced Sarcoma | PFS (Months) | PFS-3/6 (%) | OS (Months) | ORR (%) | SD (%) | DCR (%) |
|---|---|---|---|---|---|---|---|
| Retrospective, Non-Interventional Studies | |||||||
| French RetrospectYon database Le Cesne et al., 2015 [19] | STS; n = 804 | 4.4 (3.9–4.9) | 59.0/40.0 | 12.2 (11.0–13.3) | 16.5 | 50.1 | 66.7 |
| L-sarcoma; n = 481 | 5.7 (4.9–6.5) | 64–69.0/NA | 15.0 (13.2–16.8) | 18.6 | 54.0 | 72.6 | |
| TrObs study Palmerini et al., 2021 [18] | STS; n = 512 | 5.1 (4.1–6.7) | NA/46.0 | 21.6 (19.3–25.0) | 13.7 (11.2–17.2) | 33.0 | 46.7 (43.2–51.9) |
| L-sarcoma; n = 348 | 8.3 (6–10.1) | NA/55.0 | 25.9 (22.4–33.4) | 16.6 | 37.4 | 53.4 | |
| non-L-sarcoma; n = 164 | 2.4 (1.8–3.4) | NA/26.0 | 11.3 (8.1–16.3) | 9.0 | 23.8 | 32.3 | |
| German retrospective study Hoiczyk M et al., 2013 [20] | STS; n = 101 | 2.1 | NA | NA | NA | NA | NA |
| L-sarcoma; n = 46 | 3.1 | 51/38 | NA | NA | NA | 55 | |
| non-L-sarcoma; n = 55 | 1.6 | 36/16 | NA | NA | NA | 34 | |
| Prospective, Non-Interventional Studies | |||||||
| Y-IMAGE study Buonadonna et al., 2017 [17] | STS; n = 218 | 5.9 (4.9–7.8) | 70.0/49.0 | 21.3 (18.8–24.3) | 26.6 (20.9–33) | 39.0 | 65.6 (58.9–71.9) |
| YON-SAR study Grünwald et al., 2022 | STS; n = 128 | 5.2 (3.3–6.7) | 60.7/44.5 | 15.2 (9.6–21.4) | 11.7 | 33.6 | 45.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grünwald, V.; Pink, D.; Egerer, G.; Schalk, E.; Augustin, M.; Deinzer, C.K.W.; Kob, V.; Reichert, D.; Kebenko, M.; Brandl, S.; et al. Trabectedin for Patients with Advanced Soft Tissue Sarcoma: A Non-Interventional, Prospective, Multicenter, Phase IV Trial. Cancers 2022, 14, 5234. https://doi.org/10.3390/cancers14215234
Grünwald V, Pink D, Egerer G, Schalk E, Augustin M, Deinzer CKW, Kob V, Reichert D, Kebenko M, Brandl S, et al. Trabectedin for Patients with Advanced Soft Tissue Sarcoma: A Non-Interventional, Prospective, Multicenter, Phase IV Trial. Cancers. 2022; 14(21):5234. https://doi.org/10.3390/cancers14215234
Chicago/Turabian StyleGrünwald, Viktor, Daniel Pink, Gerlinde Egerer, Enrico Schalk, Marinela Augustin, Christoph K. W. Deinzer, Viola Kob, Dietmar Reichert, Maxim Kebenko, Stephan Brandl, and et al. 2022. "Trabectedin for Patients with Advanced Soft Tissue Sarcoma: A Non-Interventional, Prospective, Multicenter, Phase IV Trial" Cancers 14, no. 21: 5234. https://doi.org/10.3390/cancers14215234
APA StyleGrünwald, V., Pink, D., Egerer, G., Schalk, E., Augustin, M., Deinzer, C. K. W., Kob, V., Reichert, D., Kebenko, M., Brandl, S., Hahn, D., Lindner, L. H., Hoiczyk, M., Ringsdorf, U., Hanker, L. C., Hempel, D., De Rivas, B., Wismann, T., & Ivanyi, P. (2022). Trabectedin for Patients with Advanced Soft Tissue Sarcoma: A Non-Interventional, Prospective, Multicenter, Phase IV Trial. Cancers, 14(21), 5234. https://doi.org/10.3390/cancers14215234








