Ultrasound-Guided Needle Aspiration Biopsy of Superficial Metastasis of Lung Cancer with and without Rapid On-Site Evaluation: A Randomized Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
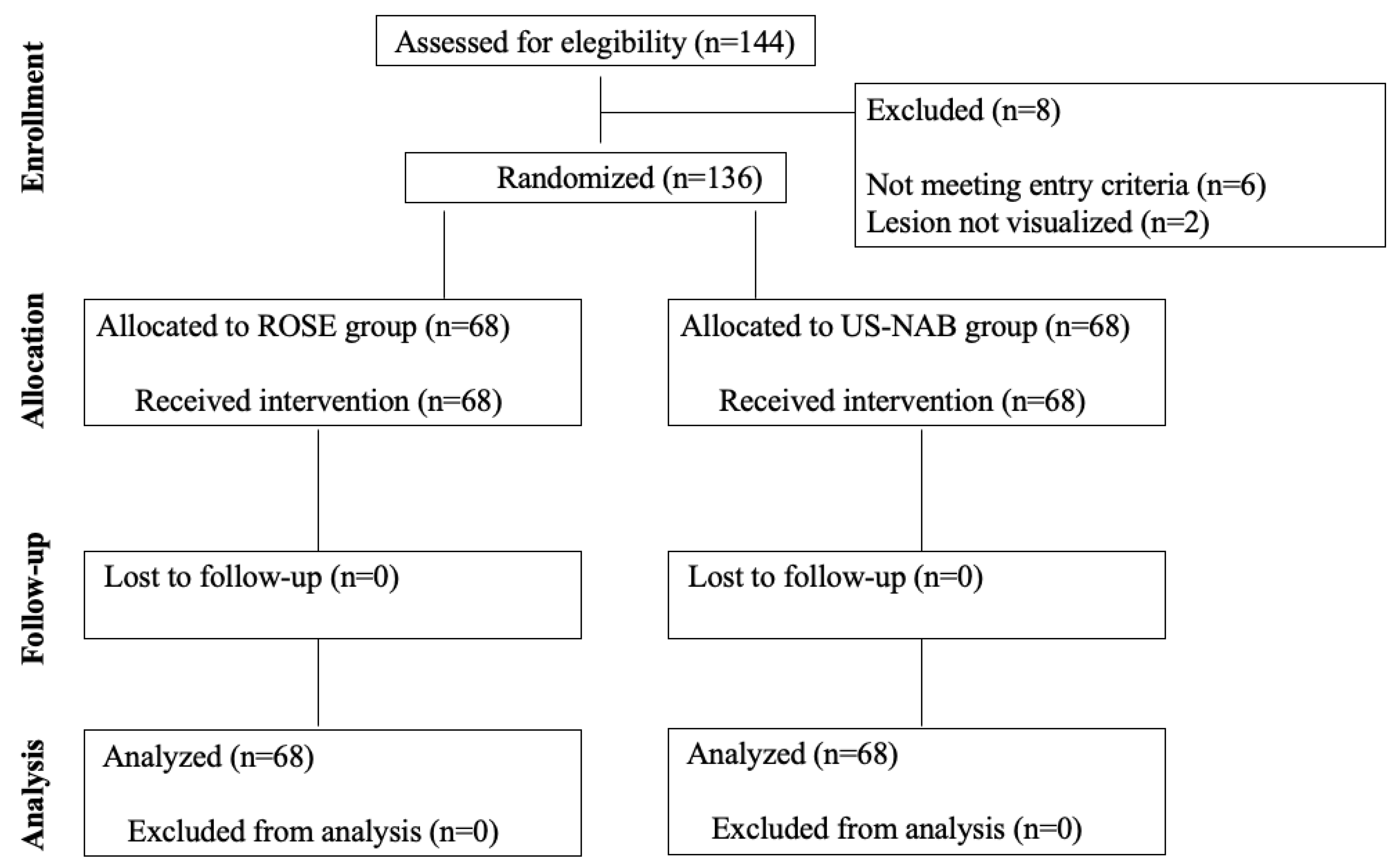
2.1. Study Design, Setting, and Participants
2.2. Randomization and Interventions
2.3. Outcomes
2.4. Sample Size Calculation and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, D.R.; Masters, G.A. Precision medicine in lung cancer treatment. Surg. Oncol. Clin. 2020, 29, 15–21. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Smeltzer, M.P.; Wynes, M.W.; Lantuejoul, S.; Soo, R.; Ramalingam, S.S.; Varella-Garcia, M.; Taylor, M.M.; Richeimer, K.; Wood, K.; Howell, K.E.; et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J. Thorac. Oncol. 2020, 15, 1434–1448. [Google Scholar] [CrossRef]
- Ahn, M.-J. Molecular testing in lung cancer: Still big gap in implementation for real-world use. J. Thorac. Oncol. 2020, 15, 1399–1400. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid biopbsy for advanced non-small cell lung cancer (NSCLC): A statement paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Gondos, A.; Paz-Ares, L.G.; Saldana, D.; Thomas, M.; Mascaux, C.; Bubendorf, L.; Barlesi, F. Genomic testing among patients with newly diagnosed advanced non-small cell lung cancer in the United States: A contemporary clinical practice patterns study. Lung Cancer 2022, 167, 41–48. [Google Scholar] [CrossRef]
- Hess, K.M.; Krein, P.M.; Haldane, D.; Han, Y.; Sireci, A.N. Biomarker testing for patients with advanced/metastatic non-squamous non-small cell lung cancer (NSCLC) in the USA, 2015-2021. JTO Clin. Res. Rep. 2022, 3, 100336. [Google Scholar]
- Stigt, J.A.; Oostdijk, A.d.H.; Boers, J.E.; van den Berg, J.W.K.; Groen, H.J.M. Percutaneous ultrasound-guided biopsies in the evaluation of thoracic tumors after PET-CT: A prospective diagnostic study. Respiration 2012, 83, 45–52. [Google Scholar] [CrossRef]
- Laursen, C.B.; Naur, T.M.; Bodtger, U.; Colella, S.; Naqibullah, M.; Minddal, V.; Konge, L.; Davidsen, J.R.; Hansen, N.C.; Graumann, O.; et al. Ultrasound-guided lung biopsy in the hands of respiratory physicians. Diagnostic yield and complications in 215 consecutive patients in 3 centers. J. Bronchol. Intervent. Pulmonol. 2016, 23, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Livi, V.; Paioli, D.; Cancellieri, A.; Betti, S.; Natali, F.; Ferrari, M.; Fiorentino, M.; Trisolini, R. Diagnosis and molecular profiling of lung cancer by percutaneous ultrasound-guided biopsy of superficial metastatic sites is safe and highly effective. Respiration 2021, 100, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; Paioli, D.; Scudeller, L.; Casadei, G.P.; Parri, S.F.; Livi, V.; Bondi, A.; Boaron, M.; et al. Rapid on-site evaluation of transbronchial aspirates for the diagnosis of hilar and mediastinal adenopathy. A randomized trial. CHEST 2011, 139, 395–401. [Google Scholar] [CrossRef]
- Yarmus, L.; Van der Kloot, T.; Lechtzin, N.; Napier, M.; Dressel, D.; Feller-Kopman, D. A randomized prospective trial of the utility of rapid on-site evaluation of transbronchial needle aspirate specimens. J. Bronchol. Intervent. Pulmonol. 2011, 18, 121–127. [Google Scholar] [CrossRef]
- Oki, M.; Saka, H.; Kitagawa, C.; Kogure, Y.; Murata, N.; Adachi, T.; Ando, M. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing of lung cancer: A randomized trial. Respiration 2013, 85, 486–492. [Google Scholar] [CrossRef]
- Kappelle, W.F.W.; Van Leerdam, M.E.; Schwartz, M.P.; Bülbül, M.; Buikhuisen, W.A.; Brink, M.A.; Sie-Go, D.M.D.S.; Pullens, H.J.M.; Nikolakopoulos, S.; Van Diest, P.J.; et al. Rapid on-site evaluation during endoscopic ultrasound-guided fine-needle aspiration of lymph nodes does not increase diagnostic yield: A randomized multicenter trial. Am. J. Gastroenterol. 2018, 113, 677–685. [Google Scholar] [CrossRef]
- Davenport, R.D. Rapid on-site evaluation of transbronchial aspirates. CHEST 1990, 98, 59–61. [Google Scholar] [CrossRef]
- Diette, G.B.; White, P.; Terry, P.; Jenckes, M.; Rosenthal, D.; Rubin, H.R. Utility of rapid on-site cytopathology assessment for bronchoscopic evaluation of lung masses and adenopathy. CHEST 2000, 117, 1186–1190. [Google Scholar] [CrossRef]
- Chin R, McCain TW, Lucia MA, Cappellari JO, Adair NE, Lovato JF; et al. Transbronchial needle aspiration in diagnosing and staging lung cancer. How many aspirates are needed? Am. J. Respir. Crit. Care Med. 2002, 166, 377–381. [Google Scholar] [CrossRef]
- Kramer, T.; Annema, J.T. Advanced bronchoscpic techniques for the diagnosis and treatment of peripheral lung lesions. Lung Cancer 2021, 161, 152–162. [Google Scholar] [CrossRef]
- Dhooria, S.; Aggarwal, A.N.; Gupta, D.; Behera, D.; Agarwal, R. Utility and safety of ultrasound with bronchoscope-guided fine-needle aspiration in mediastinal lymph node sampling: Systematic review and meta-analysis. Respir. Care 2015, 60, 1040–1050. [Google Scholar] [CrossRef]
- Takeshita, J.; Masago, K.; Kato, R.; Hata, A.; Kaji, R.; Fujita, S.; Katakami, N. CT-guided fine needle aspiration and core needle biopsies of pulmonary lesions: A single center experience with 750 biopsies in Japan. Am. J. Roentgenol. 2015, 204, 29–34. [Google Scholar] [CrossRef]
- Asano, F.; Aoe, M.; Ohsaki, Y.; Okada, Y.; Sasada, S.; Sato, S.; Suzuki, E.; Semba, H.; Fukuoka, K.; Fujino, S.; et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: A nationwide survey by the Japan Society for Respiratory Endoscopy. Respir. Res. 2013, 14, 50. [Google Scholar] [CrossRef]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: One-year results of the prospective, multicenter NAVIGATE study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef]
- Ost, D.E.; Ernst, A.; Lei, X.; Kovitz, K.L.; Benzaquen, S.; Diaz-Mendoza, J.; Greenhill, S.; Toth, J.; Feller-Kopman, D.; Puchalski, J.; et al. Diagnostic yield and complications for bronchoscopy for peripheral lung lesions. Am. J. Respir. Crit. Care Med. 2016, 193, 68–77. [Google Scholar] [CrossRef]
- Najafi, A.; Al Ahmar, M.; Bonnet, B.; Delpla, A.; Kobe, A.; Madani, K.; Roux, C.; Deschamps, F.; de Baère, T.; Tselikas, L. The PEARL approach for CT-guided lung biopsy: Assessment of complication rates. Radiology 2022, 302, 473–480. [Google Scholar] [CrossRef]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.M.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef]
- Koegelenburg, C.F.N.; von Groote-Bidlingmaier, F.; Bolliger, C.T. Transthoracic ultrasonography for the respiratory physician. Respiration 2012, 84, 337–350. [Google Scholar] [CrossRef]
- Stigt, J.A.; Groen, H.J.M. Percutaneous ultrasonography as imaging modality and sampling guide for Pulmonologists. Respiration 2014, 87, 441–451. [Google Scholar] [CrossRef]
- Diacon, A.H.; Shuurmans, N.N.; Theron, J.; Shuubert, P.T.; Wright, C.A.; Bolliger, C.T. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration 2004, 71, 519–522. [Google Scholar] [CrossRef]
- Ahmed, M.; Daneshvar, C.; Breen, D. Routine neck ultrasound by respiratory physicians in the diagnosis and staging of patients with lung cancer and mediastinal lymphadenopathy: A prospective pilot study. ERJ Open Res. 2020, 6, 00180–02019. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.T.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e142S–e165S. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Pilotto, S.; Facchinetti, F.; Bertolaccini, L.; Del Re, M.; Ferrara, R.; Franchina, T.; Malapelle, U.; Menis, J.; Passaro, A.; et al. Treatment of advanced non-small cell lung cancer: The 2019 AIOM (Italian Association of Medical Oncology) clinical practice guidelines. Crit. Rev. Oncol. Hematol. 2019, 146, 102858. [Google Scholar] [CrossRef]
- Singh, V.M.; Salunga, R.C.; Huang, V.J.; Tran, Y.; Erlander, M.; Plumlee, P.; Peterson, M.R. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Ann. Diagn. Pathol. 2013, 17, 322–326. [Google Scholar] [CrossRef]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; De Biase, D.; Valentini, I.; Casadei, G.; Paioli, D.; Ferrari, F.; Gordini, G.; Patelli, M.; et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration with and without rapid on-site evaluation for lung cancer genotyping. CHEST 2015, 148, 1430–1437. [Google Scholar] [CrossRef]
- Natali, F.; Cancellieri, A.; Tinelli, C.; De Silvestri, A.; Livi, V.; Ferrari, M.; Romagnoli, M.; Paioli, D.; Trisolini, R. A trained pulmonologist may reliably assess endosonography-derived lymph node samples during rapid on-site evaluation. Respiration 2019, 97, 540–547. [Google Scholar] [CrossRef]
- Natali, F.; Cancellieri, A.; Giunchi, F.; De Silvestri, A.; Livi, V.; Ferrari, M.; Paioli, D.; Betti, S.; Fiorentino, M.; Trisolini, R. Interobserver agreement between pathologist, pulmonologist and molecular pathologist to estimate the tumor burden in rose smears from endosonography and guided-bronchoscopy. Cytopathology 2020, 31, 303–309. [Google Scholar] [CrossRef]
| ROSE Group | US-NAB Group | |
|---|---|---|
| Mean (SD) age, years | 67.6 (10.3) | 67.3 (8.5) |
| Gender, n. (%) | ||
| Female | 30 (44) | 28 (41) |
| Male | 38 (56) | 40 (59) |
| Smoking history, n. (%) | ||
| Current | 19 (30) | 21 (31) |
| Former | 35 (51.5) | 36 (53) |
| Never | 14 (20.5) | 11 (16) |
| Lesions sampled, n. | 72 | 72 |
| Median (IQR) lesion size, mm | ||
| Short axis | 13.2 (9.2–18.3) | 12.4 (9–16.1) |
| Long axis | 18.2 (12.4–25.1) | 16.5 (12.8–26.6) |
| Metastatic site, n. (%) | ||
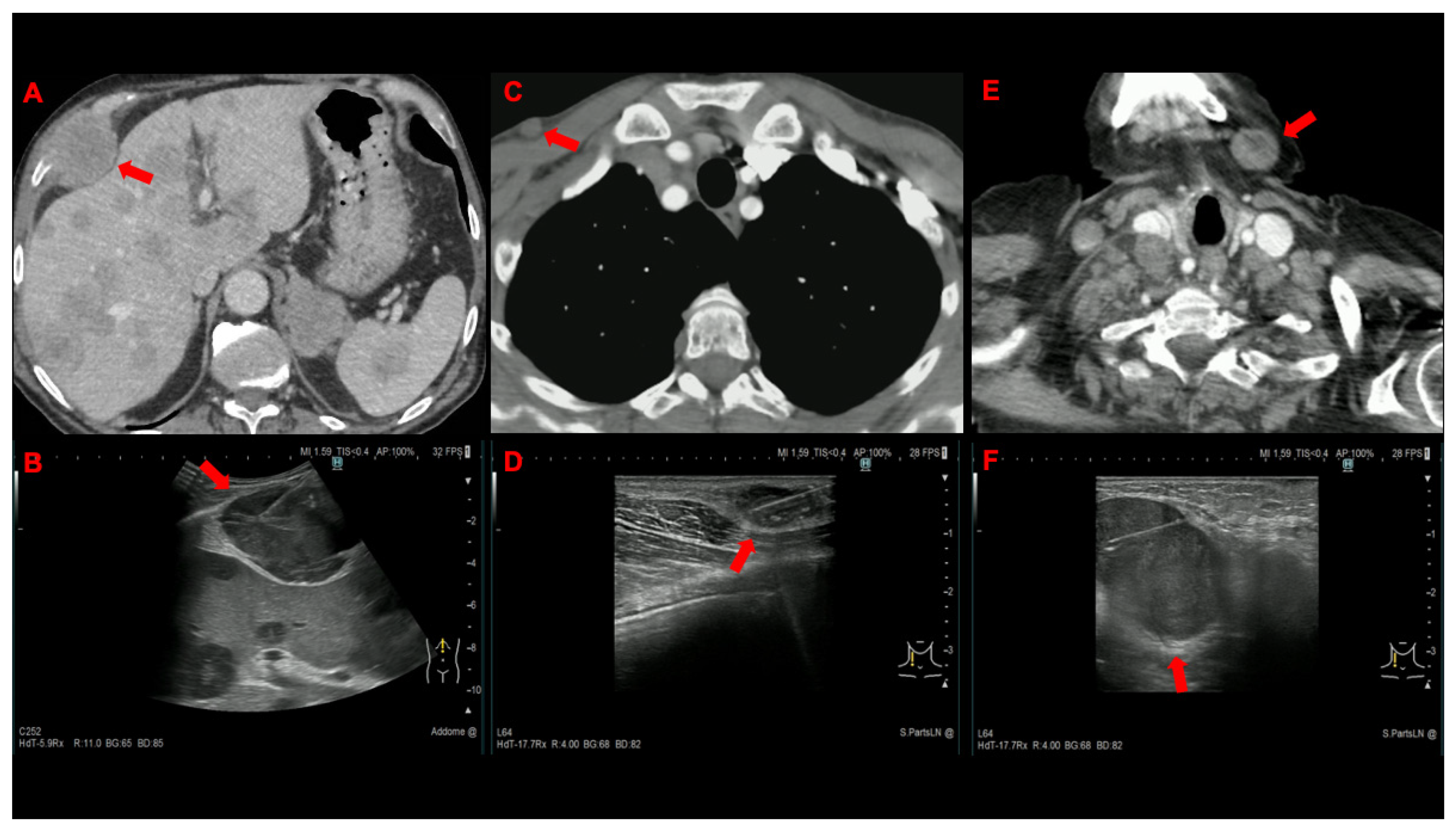
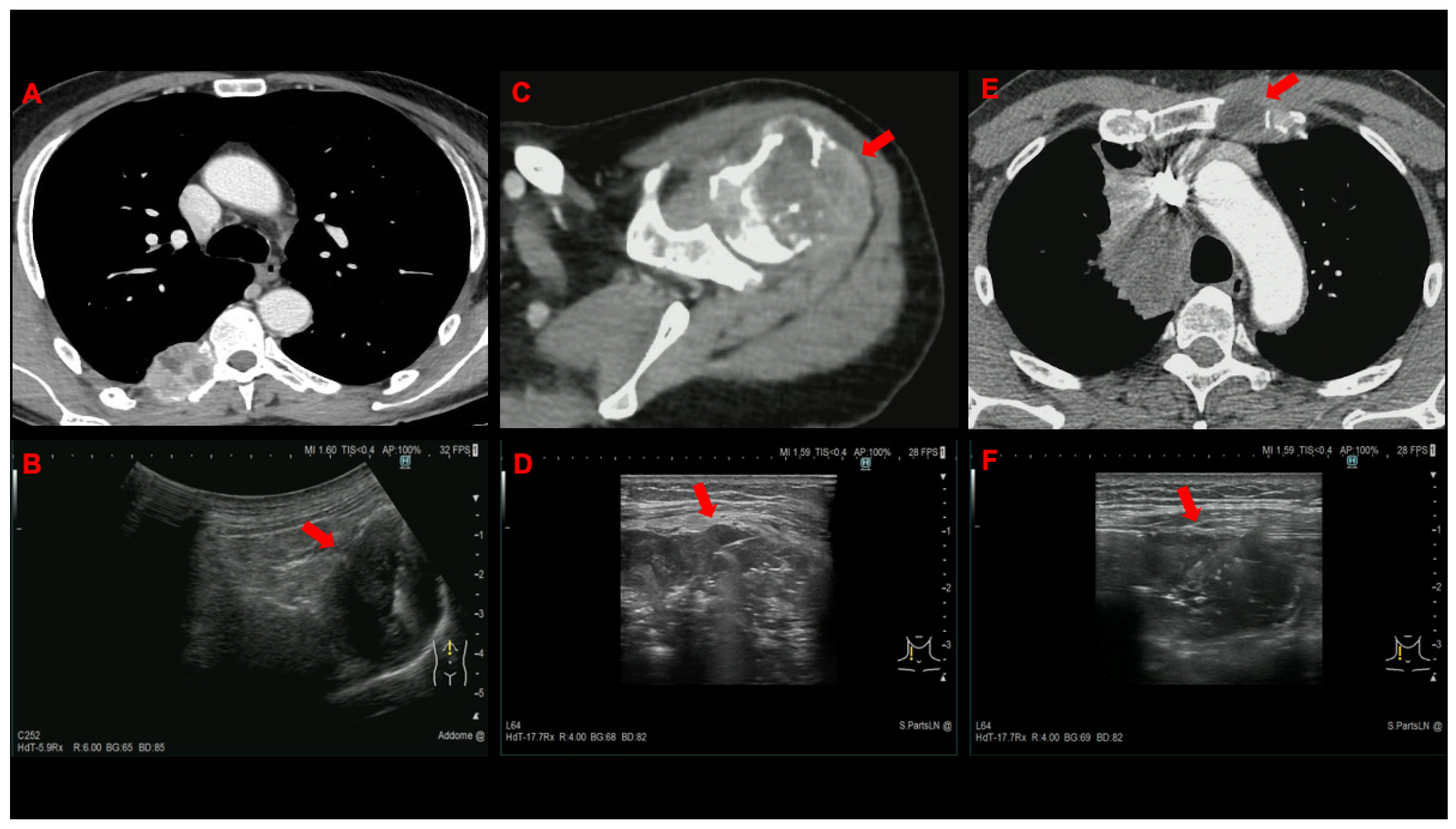
| Lymph node | 54 (75) | 49 (68) |
| Supraclavicular | 44 | 40 |
| Cervical | 8 | 7 |
| Axillary | 2 | 1 |
| Mammary | 0 | 1 |
| Bone | 7 (9.7) | 11 (15.3) |
| Subcutaneous tissue | 6 (8.3) | 6 (8.3) |
| Thoracic wall | 3 (4.2) | 3 (4.2) |
| Muscle | 2 (2.8) | 1 (1.4) |
| Pleura | 0 (0) | 2 (2.8) |
| Needle gauge, n. (%) | ||
| 22G | 4 (5.9) | 2 (3) |
| 22G + 18G | 53 (77.9) | 60 (88.2) |
| 22G + 16G | 11 (16.2) | 6 (8.8) |
| Tissue core retrieval, n. (%) | 64 (94.1) | 66 (97) |
| Final diagnosis per patient, n. (%) | ||
| Adenocarcinoma | 41 (60.3) | 49 (72) |
| Squamous cell carcinoma | 4 (5.9) | 1 (1.5) |
| Small-cell lung cancer | 11 (16) | 4 (5.9) |
| Large cell neuroendocrine carcinoma | 1 (1.5) | 0 (0) |
| Carcinoid tumor | 1 (1.5) | 1 (1.5) |
| NSCLC NOS | 4 (5.9) | 4 (5.9) |
| Metastasis from extrapulmonary tumor | 3 (4.5) | 5 (7.4) |
| Lymphoprolipherative disorder | 2 (2.9) | 2 (2.9) |
| Tuberculosis | 0 (0) | 2 (2.9) |
| Reactive lymphadenopathy | 1 (1.5) | 0 (0) |
| Outcomes | ROSE Group (No. 68) | US-NAB Group (No. 68) | p-Value |
|---|---|---|---|
| Primary outcome | |||
| Diagnostic yield for tissue diagnosis | 64/68 (94.1%) | 66/68 (97%) | 0.68 |
| Secondary outcome | |||
| Diagnostic yield for cancer genotyping ^ | 37/42 (88%) | 45/49 (91.8%) | 0.56 |
| Diagnostic yield for PD-L1 testing ^ | 43/46 (93.5%) | 48/53 (90.6%) | 0.60 |
| Complications | 1/68 (1.5%) | 0/68 (0.0%) | 1.00 |
| Study Group | Age/Sex | Lesion Location | No. Lesions Sampled | Needle Used | Tissue Core Retrieved | Final Diagnosis | |
|---|---|---|---|---|---|---|---|
| #1 | ROSE | 77/M | LN | 1 | 18G | No | NSCLC NOS |
| #2 | ROSE | 73/M | LN | 1 | 22G | No | SQCC |
| #3 | ROSE | 66/F | LN | 2 | 18G | Yes | Adenocarcinoma |
| #4 | ROSE | 48/M | LN | 2 | 22G | No | Adenocarcinoma |
| #5 | US-NAB | 66/F | LN | 1 | 18G | Yes | Adenocarcinoma |
| #6 | US-NAB | 86/M | Bone | 1 | 18G | Yes | Adenocarcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livi, V.; Sotgiu, G.; Cancellieri, A.; Paioli, D.; Leoncini, F.; Magnini, D.; Trisolini, R. Ultrasound-Guided Needle Aspiration Biopsy of Superficial Metastasis of Lung Cancer with and without Rapid On-Site Evaluation: A Randomized Trial. Cancers 2022, 14, 5156. https://doi.org/10.3390/cancers14205156
Livi V, Sotgiu G, Cancellieri A, Paioli D, Leoncini F, Magnini D, Trisolini R. Ultrasound-Guided Needle Aspiration Biopsy of Superficial Metastasis of Lung Cancer with and without Rapid On-Site Evaluation: A Randomized Trial. Cancers. 2022; 14(20):5156. https://doi.org/10.3390/cancers14205156
Chicago/Turabian StyleLivi, Vanina, Giovanni Sotgiu, Alessandra Cancellieri, Daniela Paioli, Fausto Leoncini, Daniele Magnini, and Rocco Trisolini. 2022. "Ultrasound-Guided Needle Aspiration Biopsy of Superficial Metastasis of Lung Cancer with and without Rapid On-Site Evaluation: A Randomized Trial" Cancers 14, no. 20: 5156. https://doi.org/10.3390/cancers14205156
APA StyleLivi, V., Sotgiu, G., Cancellieri, A., Paioli, D., Leoncini, F., Magnini, D., & Trisolini, R. (2022). Ultrasound-Guided Needle Aspiration Biopsy of Superficial Metastasis of Lung Cancer with and without Rapid On-Site Evaluation: A Randomized Trial. Cancers, 14(20), 5156. https://doi.org/10.3390/cancers14205156








