Mayo Clinic Clival Chordoma Case Series: Impact of Endoscopic Training on Clinical Care
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Pathological Information
3.2. Surgical Complications
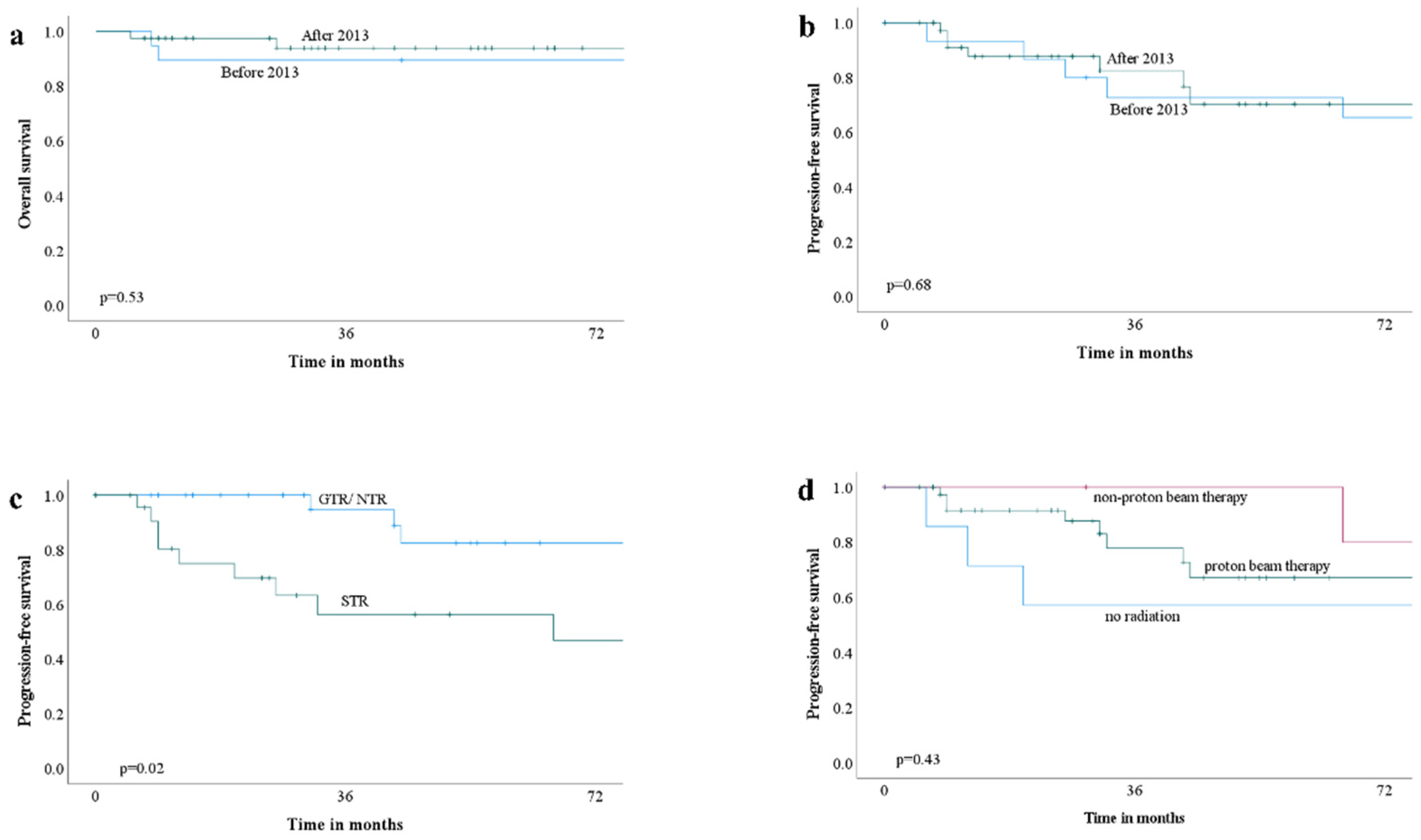
3.3. Adjuvant Radiation Therapy
3.4. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMaster, M.L.; Goldstein, A.M.; Bromley, C.M.; Ishibe, N.; Parry, D.M. Chordoma: Incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 2001, 12, 1–11. [Google Scholar] [CrossRef]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma-Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef]
- Stefàno, P.L.; Barletta, G.; Andrei, V.; Cerillo, A.G.; Nesi, G.; Pilato, G.; Pradella, S.; Santi, R.; Del Bene, M.R. Cardiac Metastasis of Sacral Chordoma. Ann. Thorac. Surg. 2021, 111, e319–e321. [Google Scholar] [CrossRef]
- Chambers, P.W.; Schwinn, C.P. Chordoma. A clinicopathologic study of metastasis. Am. J. Clin. Pathol. 1979, 72, 765–776. [Google Scholar] [CrossRef]
- Zweckberger, K.; Giese, H.; Haenig, B.; Federspil, P.A.; Baumann, I.; Albrecht, T.; Uhl, M.; Unterberg, A. Clivus chordomas: Heterogeneous tumor extension requires adapted surgical approaches. Clin. Neurol. Neurosurg. 2020, 199, 106305. [Google Scholar] [CrossRef]
- Cavallo, L.M.; Mazzatenta, D.; d’Avella, E.; Catapano, D.; Fontanella, M.M.; Locatelli, D.; Luglietto, D.; Milani, D.; Solari, D.; Vindigni, M.; et al. The management of clival chordomas: An Italian multicentric study. J. Neurosurg. 2020, 135, 93–102. [Google Scholar] [CrossRef]
- Zoli, M.; Milanese, L.; Bonfatti, R.; Faustini-Fustini, M.; Marucci, G.; Tallini, G.; Zenesini, C.; Sturiale, C.; Frank, G.; Pasquini, E.; et al. Clival chordomas: Considerations after 16 years of endoscopic endonasal surgery. J. Neurosurg. 2018, 128, 329–338. [Google Scholar] [CrossRef]
- Fernandez-Miranda, J.C.; Gardner, P.A.; Snyderman, C.H.; Devaney, K.O.; Mendenhall, W.M.; Suárez, C.; Rinaldo, A.; Ferlito, A. Clival chordomas: A pathological, surgical, and radiotherapeutic review. Head Neck 2014, 36, 892–906. [Google Scholar] [CrossRef]
- Fletcher, C.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours; World Health Organization: Geneva, Switzerland, 2013; Volume 5. [Google Scholar]
- Garcia-Navarro, V.; Anand, V.K.; Schwartz, T.H. Gasket seal closure for extended endonasal endoscopic skull base surgery: Efficacy in a large case series. World Neurosurg. 2013, 80, 563–568. [Google Scholar] [CrossRef]
- Graffeo, C.S.; Dietrich, A.R.; Grobelny, B.; Zhang, M.; Goldberg, J.D.; Golfinos, J.G.; Lebowitz, R.; Kleinberg, D.; Placantonakis, D.G. A panoramic view of the skull base: Systematic review of open and endoscopic endonasal approaches to four tumors. Pituitary 2014, 17, 349–356. [Google Scholar] [CrossRef]
- Rahme, R.J.; Arnaout, O.M.; Sanusi, O.R.; Kesavabhotla, K.; Chandler, J.P. Endoscopic Approach to Clival Chordomas: The Northwestern Experience. World Neurosurg. 2018, 110, e231–e238. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Huang, L. Factors Influencing Cerebrospinal Fluid Leaking following Pituitary Adenoma Transsphenoidal Surgery: A Meta-Analysis and Comprehensive Review. Biomed. Res. Int. 2022, 2022, 5213744. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Tian, K.; Wang, K.; Li, D.; Ma, J.; Jia, G.; Zhang, L.; Zhang, J. Clinical features and surgical outcomes of patients with skull base chordoma: A retrospective analysis of 238 patients. J. Neurosurg. 2017, 127, 1257–1267. [Google Scholar] [CrossRef]
- Cannizzaro, D.; Tropeano, M.P.; Milani, D.; Spaggiari, R.; Zaed, I.; Mancarella, C.; Lasio, G.B.; Fornari, M.; Servadei, F.; Cardia, A. Microsurgical versus endoscopic trans-sphenoidal approaches for clivus chordoma: A pooled and meta-analysis. Neurosurg. Rev. 2021, 44, 1217–1225. [Google Scholar] [CrossRef]
- Chibbaro, S.; Cornelius, J.F.; Froelich, S.; Tigan, L.; Kehrli, P.; Debry, C.; Romano, A.; Herman, P.; George, B.; Bresson, D. Endoscopic endonasal approach in the management of skull base chordomas—Clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg. Rev. 2014, 37, 217–225. [Google Scholar] [CrossRef]
- Stippler, M.; Gardner, P.A.; Snyderman, C.H.; Carrau, R.L.; Prevedello, D.M.; Kassam, A.B. Endoscopic endonasal approach for clival chordomas. Neurosurgery 2009, 64, 268–277. [Google Scholar] [CrossRef]
- Komotar, R.J.; Starke, R.M.; Raper, D.M.S.; Anand, V.K.; Schwartz, T.H. The Endoscope-Assisted Ventral Approach Compared with Open Microscope-Assisted Surgery for Clival Chordomas. World Neurosurg. 2011, 76, 318–327. [Google Scholar] [CrossRef]
- Zou, M.X.; Lv, G.H.; Zhang, Q.S.; Wang, S.F.; Li, J.; Wang, X.B. Prognostic Factors in Skull Base Chordoma: A Systematic Literature Review and Meta-Analysis. World Neurosurg. 2018, 109, 307–327. [Google Scholar] [CrossRef]
- Yin, L.X.; Low, C.M.; Puccinelli, C.L.; O’Brien, E.K.; Stokken, J.K.; Van Abel, K.M.; Janus, J.R.; Link, M.J.; Van Gompel, J.J.; Choby, G. Olfactory outcomes after endoscopic skull base surgery: A systematic review and meta-analysis. Laryngoscope 2019, 129, 1998–2007. [Google Scholar] [CrossRef]
- Khalafallah, A.M.; Rakovec, M.; Burapachaisri, K.; Fung, S.; Kozachik, S.L.; Valappil, B.; Abou-Al-Shaar, H.; Wang, E.W.; Snyderman, C.H.; Zenonos, G.A.; et al. The Suprasellar Meningioma Patient-Reported Outcome Survey: A disease-specific patient-reported outcome measure for resection of suprasellar meningioma. J. Neurosurg. 2022, 136, 1551–1559. [Google Scholar] [CrossRef]
- Shukla, A.; Ahmed, O.G.; Orlov, C.P.; Price, C.; Mukherjee, D.; Choby, G.; Rowan, N.R. Quality-of-life instruments in endoscopic endonasal skull base surgery-A practical systematic review. Int. Forum. Allergy Rhinol. 2021, 11, 1264–1268. [Google Scholar] [CrossRef]
- Riva, G.; Zenga, F.; Motatto, G.M.; Di Perna, G.; Castelli, M.; Tavassoli, M.; Baldassarre, B.M.; Caria, M.; Pecorari, G. Quality of Life After Endoscopic Skull Base Surgery: Validation and Reliability of the Italian Version of the Sino-Nasal Outcome Test for Neurosurgery (SNOT-NC). World Neurosurg. 2022, 163, e426–e434. [Google Scholar] [CrossRef]
- Jozaghi, Y.; Phan, J.; Hanna, E.Y.; Kupferman, M.E.; Su, S.Y. Functional Outcomes and Quality of Life in Patients with Sinonasal, Nasopharyngeal, and Anterior Skull Base Tumors. Curr. Oncol. Rep. 2022, 24, 775–781. [Google Scholar] [CrossRef]
- Jägersberg, M.; El Rahal, A.; Dammann, P.; Merkler, D.; Weber, D.C.; Schaller, K. Clival chordoma: A single-centre outcome analysis. Acta Neurochir. 2017, 159, 1815–1823. [Google Scholar] [CrossRef]
- Wu, X.; Lin, X.; Chen, Y.; Kong, W.; Xu, J.; Yu, Z. Response of Metastatic Chordoma to the Immune Checkpoint Inhibitor Pembrolizumab: A Case Report. Front. Oncol. 2020, 10, 565945. [Google Scholar] [CrossRef]
- Hindi, N.; Casali, P.G.; Morosi, C.; Messina, A.; Palassini, E.; Pilotti, S.; Tamborini, E.; Radaelli, S.; Gronchi, A.; Stacchiotti, S. Imatinib in advanced chordoma: A retrospective case series analysis. Eur. J. Cancer 2015, 51, 2609–2614. [Google Scholar] [CrossRef]
- Migliorini, D.; Mach, N.; Aguiar, D.; Vernet, R.; Landis, B.N.; Becker, M.; McKee, T.; Dutoit, V.; Dietrich, P.Y. First report of clinical responses to immunotherapy in 3 relapsing cases of chordoma after failure of standard therapies. Oncoimmunology 2017, 6, e1338235. [Google Scholar] [CrossRef]
- Baig Mirza, A.; Ravindran, V.; Okasha, M.; Boardman, T.M.; Maratos, E.; Sinan, B.; Thomas, N. Systematic Review Comparing Open versus Endoscopic Surgery in Clival Chordomas and a 10-Year Single-Center Experience. J. Neurol. Surg. B Skull Base 2022, 83, e113–e125. [Google Scholar] [CrossRef]
- La Corte, E.; Broggi, M.; Bosio, L.; Danesi, G.; Ferroli, P. Tailored surgical strategy in clival chordomas: An extraordinary selection bias that limits approach comparison. J. Neurosurg. Sci. 2018, 62, 519–521. [Google Scholar] [CrossRef]
- Park, H.H.; Park, J.Y.; Chin, D.K.; Lee, K.S.; Hong, C.K. The timing of fusion surgery for clival chordoma with occipito-cervical joint instability: Before or after tumor resection? Neurosurg. Rev. 2020, 43, 119–129. [Google Scholar] [CrossRef]
- Hsu, W.; Kosztowski, T.A.; Zaidi, H.A.; Gokaslan, Z.L.; Wolinsky, J.-P. Image-guided, endoscopic, transcervical resection of cervical chordoma: Technical note. J. Neurosurg. Spine SPI 2010, 12, 431–435. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Sommer, J. Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Li, L.; Li, Y.; Li, P.; Lv, G. Radioresistance of chordoma cells is associated with the ATM/ATR pathway, in which RAD51 serves as an important downstream effector. Exp. Ther. Med. 2017, 14, 2171–2179. [Google Scholar] [CrossRef]
- Ares, C.; Hug, E.B.; Lomax, A.J.; Bolsi, A.; Timmermann, B.; Rutz, H.P.; Schuller, J.C.; Pedroni, E.; Goitein, G. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: First long-term report. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1111–1118. [Google Scholar] [CrossRef]
- Bugoci, D.M.; Girvigian, M.R.; Chen, J.C.; Miller, M.M.; Rahimian, J. Photon-based fractionated stereotactic radiotherapy for postoperative treatment of skull base chordomas. Am. J. Clin. Oncol. 2013, 36, 404–410. [Google Scholar] [CrossRef]
- Chen, K.; Mo, J.; Zhou, M.; Wang, G.; Wu, G.; Chen, H.; Zhang, K.; Yang, H. Expression of PTEN and mTOR in sacral chordoma and association with poor prognosis. Med. Oncol. 2014, 31, 886. [Google Scholar] [CrossRef]
- Yakkioui, Y.; Temel, Y.; Creytens, D.; Jahanshahi, A.; Fleischeuer, R.; Santegoeds, R.G.; Van Overbeeke, J.J. A comparison of cell-cycle markers in skull base and sacral chordomas. World Neurosurg. 2014, 82, e311–e318. [Google Scholar] [CrossRef]
- Kitamura, Y.; Sasaki, H.; Kimura, T.; Miwa, T.; Takahashi, S.; Kawase, T.; Yoshida, K. Molecular and clinical risk factors for recurrence of skull base chordomas: Gain on chromosome 2p, expression of brachyury, and lack of irradiation negatively correlate with patient prognosis. J. Neuropathol. Exp. Neurol. 2013, 72, 816–823. [Google Scholar] [CrossRef]
- Zenonos, G.A.; Fernandez-Miranda, J.C.; Mukherjee, D.; Chang, Y.F.; Panayidou, K.; Snyderman, C.H.; Wang, E.W.; Seethala, R.R.; Gardner, P.A. Prospective validation of a molecular prognostication panel for clival chordoma. J. Neurosurg. 2019, 130, 1528–1537. [Google Scholar] [CrossRef]
- Förander, P.; Bartek, J., Jr.; Fagerlund, M.; Benmaklouf, H.; Dodoo, E.; Shamikh, A.; Stjärne, P.; Mathiesen, T. Multidisciplinary management of clival chordomas; long-term clinical outcome in a single-institution consecutive series. Acta Neurochir. 2017, 159, 1857–1868. [Google Scholar] [CrossRef]
- La Corte, E.; Broggi, M.; Raggi, A.; Schiavolin, S.; Acerbi, F.; Danesi, G.; Farinotti, M.; Felisati, G.; Maccari, A.; Pollo, B.; et al. Peri-operative prognostic factors for primary skull base chordomas: Results from a single-center cohort. Acta Neurochir. 2021, 163, 689–697. [Google Scholar] [CrossRef]
- Hasselblatt, M.; Thomas, C.; Hovestadt, V.; Schrimpf, D.; Johann, P.; Bens, S.; Oyen, F.; Peetz-Dienhart, S.; Crede, Y.; Wefers, A.; et al. Poorly differentiated chordoma with SMARCB1/INI1 loss: A distinct molecular entity with dismal prognosis. Acta Neuropathol. 2016, 132, 149–151. [Google Scholar] [CrossRef]

| Total | Before 2013 | After 2013 | p | |
|---|---|---|---|---|
| n | 58 | 19 | 39 | - |
| Age | 45 ± 17 | 42 ± 18 | 46 ± 17 | 0.63 |
| Male | 31 (53%) | 9 (47%) | 22 (56%) | 0.52 |
| Body mass index | 30 ± 6 | 27 ± 6.2 | 31 ± 5.9 | 0.78 |
| Referred after a biopsy at an outside hospital | 3 | 2 | 1 | 0.25 |
| Previous treatment at an outside hospital | 11 | 0 | 11 | 0.01 |
| Revision surgery | 10 | 0 | 10 | 0.02 |
| Previous radiation at an outside hospital | 0 | 0 | 1 | 1 |
| Recurrent case | 5 | 0 | 5 | 0.16 |
| Size (cm) | 3.7 ± 1.6 | 3.7 ± 1.5 | 3.6 ± 1.6 | 0.92 |
| Location | - | |||
| Upper clivus | 25 (43%) | 9 | 16 | |
| Middle clivus | 14 | 4 | 10 | |
| Lower clivus | 7 (12%) | 3 | 4 | |
| Holoclivus | 12 | 3 | 9 | |
| Operative approach | 36 | - | ||
| EEA | 1 | 6 (32%) | 30 (77%) | |
| Endoscopic transcervical | 4 | 0 | 1 | |
| MicroEA | 10 | 4 | 0 | |
| Open craniotomy | 2 | 7 | 3 | |
| EEA, open craniotomy | 2 | 0 | 2 | |
| EEA, OC fusion | 12 | 0 | 2 | |
| EEA, OC fusion, endoscopic transcervical | 0 | 1 | ||
| Midline mandibular osteotomy | 2 | 0 | ||
| Degree of resection | 0.002 | |||
| GTR or NTR | 28 | 3 (16%) | 25 (64%) | |
| STR | 30 | 16 | 14 | |
| The location of residual tumor after STR | - | |||
| EEA | ||||
| Posterior to paraclival ICA, petrous apex, cavernous sinus | 8 | 3 | 5 | |
| Dorsum sella, cavernous sinus | 2 | 1 | 1 | |
| Prepontine cistern (attached to the pons) | 1 | - | 1 | |
| Sella, sphenoid sinus, clivus | 2 | 1 | 1 | |
| Endoscopic transcervical | ||||
| Peri-odontoid space | 1 | - | 1 | |
| MicroEA | ||||
| Posterior to paraclival ICA | 1 | 1 | - | |
| Image unavailable | 3 | 3 | - | |
| Open craniotomy | ||||
| Various sites (refer to the manuscript) | 6 | 3 | 3 | |
| Image unavailable | 3 | 3 | - | |
| EEA, open craniotomy | ||||
| Posterior suprasellar region | 1 | - | 1 | |
| EEA, OC fusion, endoscopic transcervical | ||||
| Prevertebral space of the craniovertebral junction | 1 | - | 1 | |
| Midline mandibular osteotomy | ||||
| Image unavailable | 1 | 1 | - | |
| New cranial nerve deficits | 7 | 6 (32%) | 1 (2.6%) | 0.004 |
| CSF leak | 4 | 0 | 4 | 0.29 |
| Additional surgery before radiation at an outside hospital | 3 | 1 | 2 | 1 |
| Adjuvant radiation | 0.03 | |||
| Yes | 47 (81%) | 12 | 35 | |
| No | 11 | 7 | 4 | |
| Adjuvant radiation | <0.001 | |||
| Proton beam therapy | 40 | 5 | 35 | |
| GKS and/or EBRT | 7 | 7 | 0 | |
| Recurrence | 17 | 10 | 7 | 0.005 |
| Death | 11 | 9 | 2 | <0.001 |
| Progression-free survival | 31 (IQR 13–64) | 88 ± 67 | 29 (IQR 11–51) | - |
| Overall survival | 54 (IQR 26–102) | 142 ± 77 | 35 (IQR 14–57) | - |
| Before 2013 | After 2013 | |||
|---|---|---|---|---|
| Clival Region | Approach | Degree of Resection | Approach | Degree of Resection |
| Upper | EEA (4), craniotomy (3), microEA (2) | GTR (1), STR (8) | EEA (14), EEA + craniotomy (1), craniotomy (1) | GTR (10), STR (6) |
| Middle | Craniotomy (3), microEA (1) | GTR (1), STR (3) | EEA (10) | GTR/NTR (8), STR (2) |
| Lower | Midline mandibular osteotomy (2), craniotomy (1) | GTR (1), STR (2) | EEA (1), EEA + craniotomy (1), EEA + OC fusion (1), EEA + OC fusion + endoscopic transcervical (1) | GTR/NTR (2), STR (2) |
| Holo | EEA (2), microEA (1) | STR (3) | EEA (5), craniotomy (2), EEA + OC fusion (1), endoscopic transcervical (1) | GTR/NTR (5), STR (4) |
| Cranial Nerve Deficits | |
|---|---|
| Time | |
| After 2013 | Reference |
| Before 2013 | 17 (1.9–160), 0.001 |
| Age | 0.99 (0.94–1.0), 0.59 |
| Sex | |
| Male | Reference |
| Female | 0.84 (0.17–4.2), 0.84 |
| Body mass index | 0.85 (0.71–1.0), 0.059 |
| Revision surgery | |
| No | Reference |
| Yes | 0.0 (0.0- ), 1 |
| Recurrent case | |
| No | Reference |
| Yes | 0.0 (0.0-), 1 |
| Size | 1.4 (0.86–2.1), 0.19 |
| Location | |
| Upper | Reference |
| Middle | 0.56 (0.053–6.0), 0.64 |
| Lower | 2.9 (0.38–22), 0.30 |
| Whole | 0.67 (0.062–7.2), 0.74 |
| Degree of resection | |
| GTR/NTR | Reference |
| STR | 2.8 (0.50–16), 0.24 |
| Univariate | Multivariate | |
|---|---|---|
| Time | ||
| After 2013 | Reference | Reference |
| Before 2013 | 5.5 (1.6–19), 0.007 | 3.8 (0.71–21), 0.12 |
| Age | 1.0 (0.98–1.0), 0.46 | - |
| Sex | - | |
| Male | Reference | |
| Female | 0.52 (0.16–1.7), 0.27 | |
| Body mass index | 0.94 (0.85–1.0), 0.20 | - |
| Previous treatment | 0.52 (0.097–2.7), 0.44 | - |
| Revision surgery | - | |
| No | Reference | |
| Yes | 0.61 (0.11–3.3), 0.57 | |
| Recurrent case | - | |
| No | Reference | |
| Yes | 0.55 (0.057–5.3), 0.60 | |
| Size | 1.2 (0.83–1.7), 0.36 | - |
| Location | - | |
| Upper | Reference | |
| Middle | 1.6 (0.39–6.6), 0.51 | |
| Lower | 1.9 (0.34–11), 0.46 | |
| Whole | 0.57 (0.098–3.3), 0.53 | |
| Degree of resection | ||
| GTR/NTR | Reference | Reference |
| STR | 3.8 (1.1–13), 0.03 | 2.2 (0.53–8.8), 0.28 |
| Adjuvant radiation | ||
| None | Reference | Reference |
| Proton beam therapy | 0.13 (0.026–0.61), 0.01 | 0.30 (0.048–1.9), 0.20 |
| GKS and/or EBRT | 0.38 (0.049–2.9), 0.35 | 0.21 (0.022–1.9), 0.17 |
| Factor | Hazard Ratio (95% Confidence Interval), p |
|---|---|
| Time | |
| After 2013 | Reference |
| Before 2013 | 1.3 (0.42–3.8), 0.68 |
| Age | 1.0 (0.99–1.1), 0.15 |
| Sex | |
| Male | Reference |
| Female | 0.84 (0.30–2.3), 0.73 |
| Body mass index | 0.98 (0.90–1.1), 0.73 |
| Previous treatment | 2.0 (0.43–9.6), 0.38 |
| Revision surgery | 2.5 (0.53–12), 0.25 |
| Size | 1.2 (0.94–1.6), 0.14 |
| Location | |
| Upper | Reference |
| Middle | 1.2 (0.34–4.0), 0.80 |
| Lower | 2.1 (0.53–8.6), 0.29 |
| Whole | 0.63 (0.13–3.0), 0.57 |
| Degree of resection | |
| GTR/NTR | Reference |
| STR | 3.2 (1.1–9.4), 0.033 |
| Adjuvant radiation | |
| None | Reference |
| Proton beam therapy | 0.51 (0.16–1.7), 0.27 |
| GKS and/or EBRT | 0.40 (0.094–1.7), 0.21 |
| Adjuvant radiation | |
| Yes | Reference |
| No | 2.1 (0.73–6.3), 0.17 |
| 1-Year | 3-Year | 5-Year | 10-Year | |||||
| PFS | OS | PFS | OS | PFS | OS | PFS | OS | |
| Before 2013 | 94% 15/16 | 89% 17/19 | 73% 11/15 | 89% 17/19 | 73% 11/15 | 89% 16/18 | 40% 6/15 | 72% 13/18 |
| After 2013 | 87% 27/31 | 97% 31/32 | 74% 14/19 | 90% 19/21 | 36% 4/11 | 83% 10/12 | - | - |
| Overall | 89% 42/47 | 94% 48/51 | 74% 25/34 | 90% 36/40 | 58% 15/26 | 87% 26/30 | - | - |
| Authors, Year | N | GTR/NTR | Postop CND | CSF Leak (%) | Adjuvant RT; PBT | Recurrence (%) | PFS/OS (%) | Follow-Up (Months) |
| Chibbaro et al., 2014 [16] | 54 patients (58 EEA) | 44 (81%) | ND | 4 (7%) | 54 (100%); 54 (100%) | 4 (11%) | ND/ND | Mean, 34 |
| Forander et al., 2017 [41] | 22 (EEA 11) | 3 (14%) | 4 (19%) | 2 (10%) | ND; 3 (14%) | ND | 5y: ND/82 | Median, 80 |
| Jagersberg et al., 2017 [25] | 13 (TSS 7) | 2 (15%) | 4 (31%) | 2 (15%) | 13 (100%); 11 (85%) | 7 (54%) | 5y: 53/83 | Mean, 78; median, 64 |
| Wang et al., 2017 [14] | 238 (TSS 51) | 157 (66%) | ND | 9 (4%) | ND | 82 (46%) | 5y: 45/76 | Mean, 44 |
| Zoli et al., 2018 [7] | 65 patients (EEA 80 cases) | 47 (59%) | 7 (9%) | 2 (3%) | 63 (97%); 60 (92%) | 24 (37%) | 5y: ND/77 | Mean, 52; median, 48 |
| La Corte et al., 2021 [42] | 59 patients (EEA 26) | 12 (20%) * | 7 (12%) | 9 (15%) | 44 (75%); 19 (32%) | 37 (63%) | Mean: 65 months/83 months | Mean, 83 |
| Zweckberger et al., 2020 [5] | 50 patients; 70 cases (TSS 24) | 12 (24%) | ND | 9 (13%) | 35 (50%); 2 (3%) | ND | 5y: 45/ND | ND |
| Cavallo et al., 2020 [6] | 167 patients; 182 cases (EEA 151) | 93 (51%) | ND | 9 (5%) | 141 (78%); 115 (63%) | 49 (27%) | 5y: 62/74 | Mean, 62 |
| Our study | 58 patients; 58 cases (EEA 36) | 28 (48%) | 7 (12%) | 4 (7%) | 47 (81%); 40 (69%) | 17 (29%) | 5y: 58/87 | Mean, 75; median, 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Laack, N.; Mahajan, A.; O’Brien, E.K.; Stokken, J.K.; Janus, J.R.; Choby, G.; Van Gompel, J.J. Mayo Clinic Clival Chordoma Case Series: Impact of Endoscopic Training on Clinical Care. Cancers 2022, 14, 5104. https://doi.org/10.3390/cancers14205104
Hong S, Laack N, Mahajan A, O’Brien EK, Stokken JK, Janus JR, Choby G, Van Gompel JJ. Mayo Clinic Clival Chordoma Case Series: Impact of Endoscopic Training on Clinical Care. Cancers. 2022; 14(20):5104. https://doi.org/10.3390/cancers14205104
Chicago/Turabian StyleHong, Sukwoo, Nadia Laack, Anita Mahajan, Erin K. O’Brien, Janalee K. Stokken, Jeffrey R. Janus, Garret Choby, and Jamie J. Van Gompel. 2022. "Mayo Clinic Clival Chordoma Case Series: Impact of Endoscopic Training on Clinical Care" Cancers 14, no. 20: 5104. https://doi.org/10.3390/cancers14205104
APA StyleHong, S., Laack, N., Mahajan, A., O’Brien, E. K., Stokken, J. K., Janus, J. R., Choby, G., & Van Gompel, J. J. (2022). Mayo Clinic Clival Chordoma Case Series: Impact of Endoscopic Training on Clinical Care. Cancers, 14(20), 5104. https://doi.org/10.3390/cancers14205104






