CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer
Abstract
Simple Summary
Abstract
1. Introduction
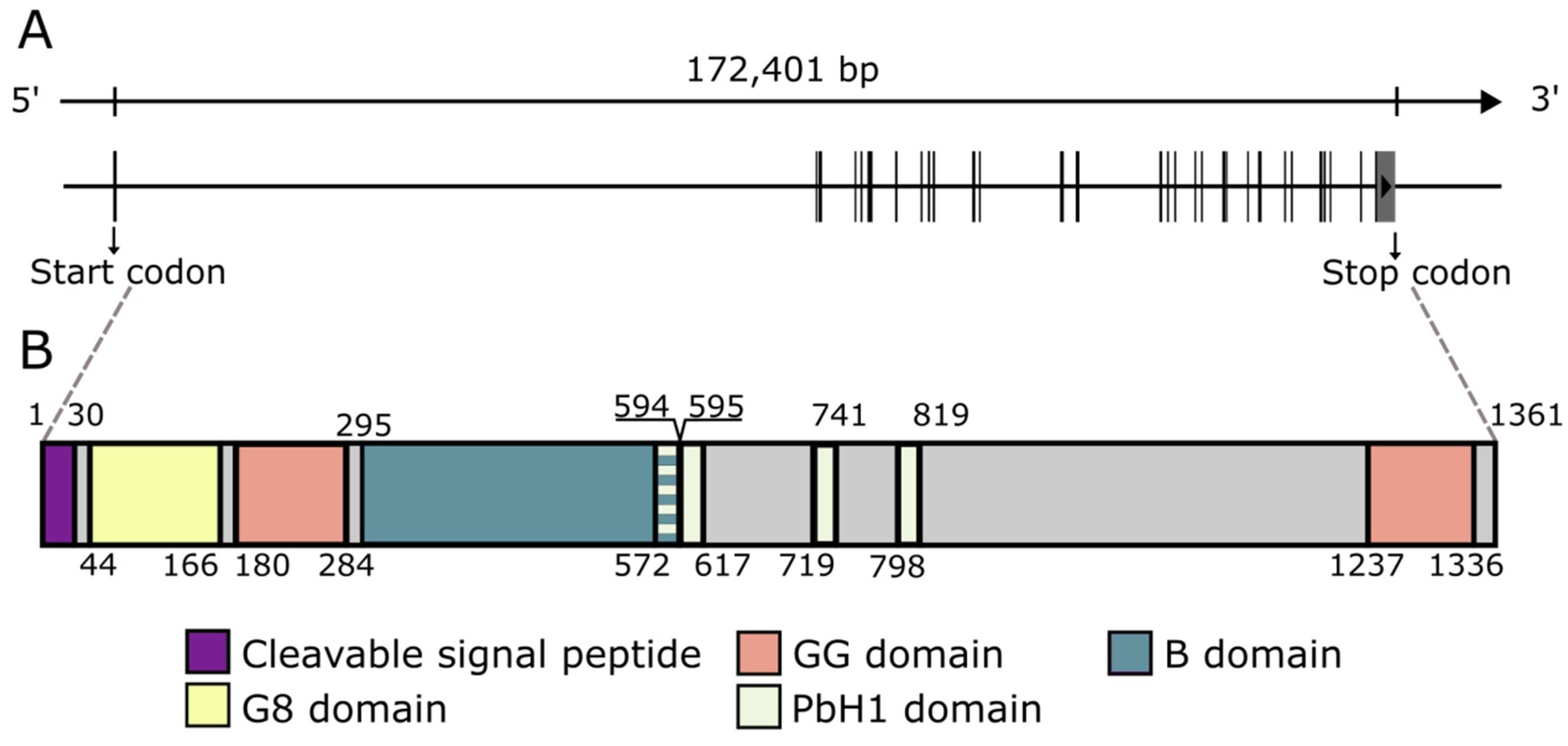
2. Regulation, Molecular Architecture, and Cellular Distribution of CEMIP
| Protein | Interaction with CEMIP Domain or Region | Evidence for Complex Formation with CEMIP | Proposed Function of CEMIP | Reference |
|---|---|---|---|---|
| Epidermal growth factor receptor (EGFR) | N-terminus (60 aa, including part of G8 domain) | Co-IP with full-length CEMIP, N- and C-terminal mutants | EGFR stability and signaling | [64] |
| Plexin A2 | N-terminus (100 aa, including part of G8 domain) | Co-IP with full-length CEMIP, N- and C-terminal mutants; PLA | Protection from Semaphorin 3A-Plexin A2-dependent cell death | [64] |
| Annexin A1 (ANXA1) | G8 | IP and MS; co-IP (also using G8-deleted CEMIP mutants) | Adherence to cell membranes and HA degradation | [60] |
| Binding immunoglobulin protein (BiP) | B domain (aa 295–591) | IP and MS; co-IP | ER retention and cell migration | [29] |
| O-GlcNAc transferase (OGT) | PbH1 domains (572–819 aa) | Co-IP; PLA | Elevated O-GlcNAcylation of β-catenin, glutamine metabolic reprogramming | [38] |
| Beta-catenin (β-catenin) | 820–1204 aa | Co-IP; PLA | Enhanced β-catenin nuclear translocation (via OGT-mediated O-GlcNAcylation), glutamine metabolic reprogramming | [38] |
| Coatomer protein complex α-subunit (COPA) | Second GG domain (1201–1361 aa) | MBP-tag pull down assay, MS, co-IP | Not addressed in the study (transport of CEMIP to ER?) | [65] |
| Glycogen phosphorylase kinase β-subunit (PHKB) | Second GG domain (1201–1361 aa) | MBP-tag pull down assay, MS, co-IP | Glycogen breakdown and cancer cell survival | [65] |
| PP2A | C-terminus (880–1362 aa) | IP and MS; co-IP | Enhancing phosphatase activity of PP2A leading to dephosphorylation of stathmin, microtubule destabilization, and enhanced cell motility | [37] |
| Clathrin heavy chain (CHC) | Not addressed | Co-IP | Clathrin-mediated endocytosis and HA degradation | [3] |
| Ephrin A2 (EPHA2) | Not addressed | IP and MS; co-IP | Not addressed in the study (migration/repulsion of cells?) | [59] |
| Inositol 1,4,5-triphosphate receptor 3 (ITPR3) | Not addressed | IP and MS; co-IP | Calcium ion transport into cytosol, signaling pathway activation, ferroptosis protection | [59,66] |
| Mitogen-activated protein kinase kinase 1 (MEK1) | Not addressed | Co-IP | Sustained MEK1-ERK1/2 activation | [47] |
| Protein tyrosine phosphatase 4A3 (PTP4A3) | Not addressed | IP and MS; co-IP | Activation of EGFR signaling | [23] |
| TGFBR1 and TGFBR2 | Not addressed | Co-IP | Promotion of TGFβ signal transduction | [18] |
| WW domain binding protein 11 (WBP11) | Not addressed | IP and MS; co-IP | Activation of FGFR expression and Wnt/β-catenin signaling | [23] |
3. CEMIP Functions and Their Relevance for Tumor Growth and Metastasis
3.1. Hyaluronan Depolymerization
3.2. Signaling
3.2.1. Reciprocal Regulation between Wnt/β-Catenin Signaling and CEMIP Expression
3.2.2. Regulation of EGFR Signaling by CEMIP
3.2.3. Regulation of BiP Expression by CEMIP and Its Role in Ca2+ Signaling
3.3. EMT
3.4. Reprogramming of Metabolism
4. Role of CEMIP in Shaping the Cancer Microenvironment
4.1. CEMIP-Containing Exosomes Promote Brain Metastasis
4.2. Putative Role for CEMIP in Regulating HA Metabolism in Cancer-Associated Fibroblasts (CAFs)
4.3. Regulation of Tumor- and Metastasis-Promoting Inflammation by CEMIP
5. Therapeutic Implications
6. Conclusions/Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Suyama, M.; Nagase, T.; Ohara, O. HUGE: A Database for Human Large Proteins Identified by Kazusa CDNA Sequencing Project. Nucleic Acids Res. 1999, 27, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Usami, S.I.; Nakamura, Y. Mutations in the Gene Encoding KIAA1199 Protein, an Inner-Ear Protein Expressed in Deiters’ Cells and the Fibrocytes, as the Cause of Nonsyndromic Hearing Loss. J. Hum. Genet. 2003, 48, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y.; et al. KIAA1199, a Deafness Gene of Unknown Function, Is a New Hyaluronan Binding Protein Involved in Hyaluronan Depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617. [Google Scholar] [CrossRef]
- Liu, J.; Yan, W.; Han, P.; Tian, D. The Emerging Role of KIAA1199 in Cancer Development and Therapy. Biomed. Pharmacother. 2021, 138, 111507. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhu, X.; Qiao, X.; Wang, Y.; Bu, J.; Zhang, X.; Ma, Q.; Liang, L.; Sun, L.; Liu, C. CEMIP as a Potential Biomarker and Therapeutic Target for Breast Cancer Patients. Int. J. Med. Sci. 2022, 19, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Sabates-Bellver, J.; Van Der Flier, L.G.; De Palo, M.; Cattaneo, E.; Maake, C.; Rehrauer, H.; Laczko, E.; Kurowski, M.A.; Bujnicki, J.M.; Menigatti, M.; et al. Transcriptome Profile of Human Colorectal Adenomas. Mol. Cancer Res. 2007, 5, 1263–1275. [Google Scholar] [CrossRef]
- Birkenkamp-Demtroder, K.; Maghnouj, A.; Mansilla, F.; Thorsen, K.; Andersen, C.L.; Øster, B.; Hahn, S.; Ørntoft, T.F. Repression of KIAA1199 Attenuates Wnt-Signalling and Decreases the Proliferation of Colon Cancer Cells. Br. J. Cancer 2011, 105, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, L.; Shen, Q.; Lv, Q.; Jin, M.; Ma, H.; Nie, X.; Zheng, X.; Huang, S.; Zhou, P.; et al. Down-Regulation of KIAA1199/CEMIP by MiR-216a Suppresses Tumor Invasion and Metastasis in Colorectal Cancer. Int. J. Cancer 2017, 140, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- LaPointe, L.C.; Pedersen, S.K.; Dunne, R.; Brown, G.S.; Pimlott, L.; Gaur, S.; McEvoy, A.; Thomas, M.; Wattchow, D.; Molloy, P.L.; et al. Discovery and Validation of Molecular Biomarkers for Colorectal Adenomas and Cancer with Application to Blood Testing. PLoS ONE 2012, 7, e29059. [Google Scholar]
- Dong, X.; Yang, Y.; Yuan, Q.; Hou, J.; Wu, G. High Expression of CEMIP Correlates Poor Prognosis and the Tumur Microenvironment in Breast Cancer as a Promisingly Prognostic Biomarker. Front. Genet. 2021, 12, 768140. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Wang, C.Y.; Tang, W.C.; Lee, Y.C.; Ta, H.D.K.; Lin, L.C.; Pan, S.R.; Ni, Y.C.; Anuraga, G.; Lee, K.H. Expression Profile and Prognostic Value of Wnt Signaling Pathway Molecules in Colorectal Cancer. Biomedicines 2021, 9, 1331. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.P.; Myeroff, L.L.; Kariv, R.; Platzer, P.; Xin, B.; Mikkola, D.; Lawrence, E.; Morris, N.; Nosrati, A.; Willson, J.K.V.; et al. Induction of KIAA1199/CEMIP Is Associated with Colon Cancer Phenotype and Poor Patient Survival. Oncotarget 2015, 6, 30500–30515. [Google Scholar] [CrossRef] [PubMed]
- Hartmans, E.; Orian-Rousseau, V.; Matzke-Ogi, A.; Karrenbeld, A.; de Groot, D.J.A.; de Jong, S.; van Dam, G.M.; Fehrmann, R.S.N.; Nagengast, W.B. Functional Genomic MRNA Profiling of Colorectal Adenomas: Identification and In Vivo Validation of CD44 and Splice Variant CD44v6 as Molecular Imaging Targets. Theranostics 2017, 7, 482–492. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Ma, Y.; Zeng, Y.; Dou, D.; Fan, H.; Song, J.; Yu, X.; Xin, D.; Du, G.; et al. Serum KIAA1199 Is an Advanced-Stage Prognostic Biomarker and Metastatic Oncogene in Cholangiocarcinoma. Aging 2020, 12, 23761–23777. [Google Scholar] [CrossRef]
- Lee, H.S.; Jang, C.Y.; Kim, S.A.; Park, S.B.; Jung, D.E.; Kim, B.O.; Kim, H.Y.; Chung, M.J.; Park, J.Y.; Bang, S.; et al. Combined Use of CEMIP and CA 19-9 Enhances Diagnostic Accuracy for Pancreatic Cancer. Sci. Rep. 2018, 8, 3383. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Wang, X.; Huang, J.; Zhu, H.; Hu, Z.; Wang, D. Association between KIAA1199 Overexpression and Tumor Invasion, TNM Stage, and Poor Prognosis in Colorectal Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 2909–2918. [Google Scholar] [PubMed]
- Evensen, N.A.; Li, Y.; Kuscu, C.; Liu, J.; Cathcart, J.; Banach, A.; Zhang, Q.; Li, E.; Joshi, S.; Yang, J.; et al. Hypoxia Promotes Colon Cancer Dissemination through Up-Regulation of Cell Migration-Inducing Protein (CEMIP). Oncotarget 2015, 6, 20723–20739. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, B.; Li, R.; Chen, J.; Xu, G.; Zhu, Y.; Li, J.; Liang, Q.; Hua, Q.; Wang, L.; et al. KIAA1199 Drives Immune Suppression to Promote Colorectal Cancer Liver Metastasis by Modulating Neutrophil Infiltration. Hepatology 2022, 76, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Ding, Y.; Shen, Q.; Zhang, C.; Li, J.; Nazar, M.; Wang, Y.; Zhou, X.; Huang, J. KIAA1199 Promotes Invasion and Migration in Non-Small-Cell Lung Cancer (NSCLC) via PI3K-Akt Mediated EMT. J. Mol. Med. 2019, 97, 127–140. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, J.; Li, J.; Du, W.; Zhang, Y.; Cai, T.; Liu, T.; Fu, Y.; Zeng, Y.; Liu, Z.; et al. Downregulation of KIAA1199 by MiR-486-5p Suppresses Tumorigenesis in Lung Cancer. Cancer Med. 2020, 9, 5570–5586. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Lei, J.; Zhang, X.; Huang, W.; Li, Y.; Wu, D. Overexpression of KIAA1199: An Independent Prognostic Marker in Nonsmall Cell Lung Cancer. J. Cancer Res. Ther. 2017, 13, 664–668. [Google Scholar] [PubMed]
- Jia, S.; Qu, T.; Wang, X.; Feng, M.; Yang, Y.; Feng, X.; Ma, R.; Li, W.; Hu, Y.; Feng, Y.; et al. KIAA1199 Promotes Migration and Invasion by Wnt/β-Catenin Pathway and MMPs Mediated EMT Progression and Serves as a Poor Prognosis Marker in Gastric Cancer. PLoS ONE 2017, 12, e0175058. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, T.; Li, W.; Li, M.; Zuo, Q.; Zou, Q.; Xiao, B. The MiR-29c-KIAA1199 Axis Regulates Gastric Cancer Migration by Binding with WBP11 and PTP4A3. Oncogene 2019, 38, 3134–3150. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Tanaka, F.; Mimori, K.; Tahara, K.; Inoue, H.; Mori, M. Clinicopathologic Significance of KIAA1199 Overexpression in Human Gastric Cancer. Ann. Surg. Oncol. 2009, 16, 2042–2051. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Liu, S.; Qin, T.; Zhao, Q.; Hou, X.; Ren, L. Prognostic Marker Identification Based on Weighted Gene Co-Expression Network Analysis and Associated In Vitro Confirmation in Gastric Cancer. Bioengineered 2021, 12, 4666–4680. [Google Scholar] [CrossRef]
- Koga, A.; Sato, N.; Kohi, S.; Yabuki, K.; Cheng, X.B.; Hisaoka, M.; Hirata, K. KIAA1199/CEMIP/HYBID Overexpression Predicts Poor Prognosis in Pancreatic Ductal Adenocarcinoma. Pancreatology 2017, 17, 115–122. [Google Scholar] [CrossRef]
- Rodrigues, G.; Hoshino, A.; Kenific, C.M.; Matei, I.R.; Steiner, L.; Freitas, D.; Kim, H.S.; Oxley, P.R.; Scandariato, I.; Casanova-Salas, I.; et al. Tumour Exosomal CEMIP Protein Promotes Cancer Cell Colonization in Brain Metastasis. Nat. Cell Biol. 2019, 21, 1403–1412. [Google Scholar] [CrossRef]
- Tsuji, S.; Nakamura, S.; Yamada, T.; de Vega, S.; Okada, Y.; Inoue, S.; Shimazawa, M.; Hara, H. HYBID Derived from Tumor Cells and Tumor-Associated Macrophages Contribute to the Glioblastoma Growth. Brain Res. 2021, 1764, 147490. [Google Scholar] [CrossRef]
- Evensen, N.A.; Kuscu, C.; Nguyen, H.L.; Zarrabi, K.; Dufour, A.; Kadam, P.; Hu, Y.J.; Pulkoski-Gross, A.; Bahou, W.F.; Zucker, S.; et al. Unraveling the Role of KIAA1199, a Novel Endoplasmic Reticulum Protein, in Cancer Cell Migration. J. Natl. Cancer Inst. 2013, 105, 1402–1416. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Zhang, J. Cell Migration Inducing Hyaluronidase 1 (CEMIP) Activates STAT3 Pathway to Facilitate Cell Proliferation and Migration in Breast Cancer. J. Recept. Signal Transduct. 2020, 41, 145–152. [Google Scholar] [CrossRef]
- Jiao, X.; Ye, J.; Wang, X.; Yin, X.; Zhang, G.; Cheng, X. KIAA1199, a Target of MicoRNA-486-5p, Promotes Papillary Thyroid Cancer Invasion by Influencing Epithelial-Mesenchymal Transition (EMT). Med. Sci. Monit. 2019, 25, 6788–6796. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, S.; Zhang, X.; Huang, L.; Zhao, H. Co-Expression of KIAA1199 and Hypoxia-Inducible Factor 1α Is a Biomarker for an Unfavorable Prognosis in Hepatocellular Carcinoma. Medicine 2020, 99, e23369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhai, X.; Shi, B.; Luo, D.; Jin, B. KIAA1199 Overexpression Is Associated with Abnormal Expression of EMT Markers and Is a Novel Independent Prognostic Biomarker for Hepatocellular Carcinoma. Onco. Targets. Ther. 2018, 11, 8341–8348. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liao, F.; Song, Y.; Zuo, G.; Tan, G.; Chu, L.; Wang, T. Overexpression of KIAA1199 Is an Independent Prognostic Marker in Laryngeal Squamous Cell Carcinoma. PeerJ 2020, 8, e9637. [Google Scholar] [CrossRef] [PubMed]
- Chanthammachat, P.; Promwikorn, W.; Pruegsanusak, K.; Roytrakul, S.; Srisomsap, C.; Chokchaichamnankit, D.; Svasti, J.; Boonyaphiphat, P.; Singkhamanan, K.; Thongsuksai, P. Comparative Proteomic Analysis of Oral Squamous Cell Carcinoma and Adjacent Non-Tumour Tissue from Thailand. Arch. Oral Biol. 2013, 58, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nishida, Y.; Ikuta, K.; Urakawa, H.; Koike, H.; Sakai, T.; Zhang, J.; Shimoyama, Y.; Imagama, S. Overexpression of KIAA1199, a Novel Strong Hyaluronidase, Is a Poor Prognostic Factor in Patients with Osteosarcoma. J. Orthop. Surg. Res. 2021, 16, 439. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, D.; Shen, Q.; Jin, M.; Lin, Z.; Ma, H.; Huang, S.; Zhou, P.; Wu, G.; Zhang, T. KIAA1199 Promotes Metastasis of Colorectal Cancer Cells via Microtubule Destabilization Regulated by a PP2A/Stathmin Pathway. Oncogene 2019, 38, 935–949. [Google Scholar] [CrossRef]
- Hua, Q.; Zhang, B.; Xu, G.; Wang, L.; Wang, H.; Lin, Z.; Yu, D.; Ren, J.; Zhang, D.; Zhao, L.; et al. CEMIP, a Novel Adaptor Protein of OGT, Promotes Colorectal Cancer Metastasis through Glutamine Metabolic Reprogramming via Reciprocal Regulation of β-Catenin. Oncogene 2021, 40, 6443–6455. [Google Scholar] [CrossRef]
- Jami, M.S.; Hou, J.; Liu, M.; Varney, M.L.; Hassan, H.; Dong, J.; Geng, L.; Wang, J.; Yu, F.; Huang, X.; et al. Functional Proteomic Analysis Reveals the Involvement of KIAA1199 in Breast Cancer Growth, Motility and Invasiveness. BMC Cancer 2014, 14, 194. [Google Scholar] [CrossRef]
- Liu, J.; Han, P.; Gong, J.; Wang, Y.; Chen, B.; Liao, J.; Tian, D. Knockdown of KIAA1199 Attenuates Growth and Metastasis of Hepatocellular Carcinoma. Cell Death Discov. 2018, 4, 102. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Li, M.; Wu, H.; Guo, Y.; Chen, J.; Shan, J.; Chen, X.; Shen, J.; Ma, Q.; et al. KIAA1199 Promotes Sorafenib Tolerance and the Metastasis of Hepatocellular Carcinoma by Activating the EGF/EGFR-Dependent Epithelial-Mesenchymal Transition Program. Cancer Lett. 2019, 454, 78–89. [Google Scholar] [CrossRef] [PubMed]
- CEMIP. Cell Migration Inducing Hyaluronidase 1 [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/57214 (accessed on 10 January 2021).
- Michishita, E.; Garcés, G.; Barrett, J.C.; Horikawa, I. Upregulation of the KIAA1199 Gene Is Associated with Cellular Mortality. Cancer Lett. 2006, 239, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kuscu, C.; Evensen, N.; Kim, D.; Hu, Y.J.; Zucker, S.; Cao, J. Transcriptional and Epigenetic Regulation of KIAA1199 Gene Expression in Human Breast Cancer. PLoS ONE 2012, 7, 44661. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Dong, P.; Chen, H.; Xu, L.; Liu, Y.; Ma, Y.; Zheng, Y.; Yang, J.; Zhou, Y.; Chen, L.; et al. Decreased Expression of ATF3, Orchestrated by β-Catenin/TCF3, MiR-17-5p and HOXA11-AS, Promoted Gastric Cancer Progression via Increased β-Catenin and CEMIP. Exp. Mol. Med. 2021, 53, 1706–1722. [Google Scholar] [CrossRef] [PubMed]
- Kwapiszewska, G.; Gungl, A.; Wilhelm, J.; Marsh, L.M.; Puthenparampil, H.T.; Sinn, K.; Didiasova, M.; Klepetko, W.; Kosanovic, D.; Schermuly, R.T.; et al. Transcriptome Profiling Reveals the Complexity of Pirfenidone Effects in Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2018, 52, 1800564. [Google Scholar] [CrossRef]
- Duong, H.Q.; Nemazanyy, I.; Rambow, F.; Tang, S.C.; Delaunay, S.; Tharun, L.; Florin, A.; Buttner, R.; Vandaele, D.; Close, P.; et al. The Endosomal Protein CEMIP Links WNT Signaling to MEK1–ERK1/2 Activation in Selumetinib-Resistant Intestinal Organoids. Cancer Res. 2018, 78, 4533–4548. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Liu, J.; Li, L.; Zhao, N.; Lin, B. Genome-Wide ChIP-Seq Analysis of TCF4 Binding Regions in Colorectal Cancer Cells. Int. J. Clin. Exp. Med. 2014, 7, 4253. [Google Scholar]
- Oba, T.; Sato, N.; Adachi, Y.; Amaike, T.; Kudo, Y.; Koga, A.; Kohi, S.; Hirata, K. Hypoxia Increases KIAA1199/CEMIP Expression and Enhances Cell Migration in Pancreatic Cancer. Sci. Rep. 2021, 11, 18193. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, L. Circ_001653 Silencing Promotes the Proliferation and ECM Synthesis of NPCs in IDD by Downregulating MiR-486-3p-Mediated CEMIP. Mol. Ther.-Nucleic Acids 2020, 20, 385–399. [Google Scholar] [CrossRef]
- Wang, X.D.; Lu, J.; Lin, Y.S.; Gao, C.; Qi, F. Functional Role of Long Non-Coding RNA CASC19/MiR-140-5p/CEMIP Axis in Colorectal Cancer Progression In Vitro. World J. Gastroenterol. 2019, 25, 1697–1714. [Google Scholar] [CrossRef]
- Hu, R.; Lu, Z. Long Non-Coding RNA HCP5 Promotes Prostate Cancer Cell Proliferation by Acting as the Sponge of MiR-4656 to Modulate CEMIP Expression. Oncol. Rep. 2020, 43, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, J.; Zheng, X.; Zhou, X.; Feng, Z.; Hu, W. MiR-148a-3p Targets CEMIP to Suppress the Genesis of Gastric Cancer Cells. Biochem. Biophys. Res. Commun. 2021, 575, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Zhang, D.; Li, Y.; Ren, M.; Ma, W.; Lu, G.; He, S. MiR-4677-3p Participates Proliferation and Metastases of Gastric Cancer Cell via CEMIP-PI3K/AKT Signaling Pathway. Cell Cycle 2021, 20, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mu, T. LncRNA LINC00958 Promotes Tumor Progression through MiR-4306/CEMIP Axis in Osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3182–3199. [Google Scholar] [PubMed]
- Yoshida, H.; Nagaoka, A.; Nakamura, S.; Tobiishi, M.; Sugiyama, Y.; Inoue, S. N-Terminal Signal Sequence Is Required for Cellular Trafficking and Hyaluronan-Depolymerization of KIAA1199. FEBS Lett. 2014, 588, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, H.; Zhao, S.; Yu, L. GG: A Domain Involved in Phage LTF Apparatus and Implicated in Human MEB and Non-Syndromic Hearing Loss Diseases. FEBS Lett. 2006, 580, 581–584. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Y.; Liu, X.H.; Li, Q.; Studholme, D.J.; Li, X.W.; Liang, S.P. G8: A Novel Domain Associated with Polycystic Kidney Disease and Non-Syndromic Hearing Loss. Bioinformatics 2006, 22, 2189–2191. [Google Scholar] [CrossRef]
- Tiwari, A.; Schneider, M.; Fiorino, A.; Haider, R.; Okoniewski, M.J.; Roschitzki, B.; Uzozie, A.; Menigatti, M.; Jiricny, J.; Marra, G. Early Insights into the Function of KIAA1199, a Markedly Overexpressed Protein in Human Colorectal Tumors. PLoS ONE 2013, 8, e69473. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, G.; Zhao, H.; Ling, H.; Xie, Z.; Xiao, C.; Chen, Y.; Lin, Y.; Jiang, T.; Jin, S.; et al. Secreted KIAA1199 Promotes the Progression of Rheumatoid Arthritis by Mediating Hyaluronic Acid Degradation in an ANXA1-Dependent Manner. Cell Death Dis. 2021, 12, 102. [Google Scholar] [CrossRef]
- Jurnak, F.; Yoder, M.D.; Pickergill, R.; Jenkins, J. Parallel β-Domains: A New Fold in Protein Structures. Curr. Opin. Struct. Biol. 1994, 4, 802–806. [Google Scholar] [CrossRef]
- CEMIP—Cell Migration-Inducing and Hyaluronan-Binding Protein Precursor—Homo Sapiens (Human)—CEMIP Gene & Protein. Available online: https://www.uniprot.org/uniprot/Q8WUJ3 (accessed on 31 March 2021).
- Usami, S.; Takumi, Y.; Suzuki, N.; Oguchi, T.; Oshima, A.; Suzuki, H.; Kitoh, R.; Abe, S.; Sasaki, A.; Matsubara, A. The Localization of Proteins Encoded by CRYM, KIAA1199, UBA52, COL9A3, and COL9A1, Genes Highly Expressed in the Cochlea. Neuroscience 2008, 154, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shostak, K.; Zhang, X.; Hubert, P.; Göktuna, S.I.; Jiang, Z.; Klevernic, I.; Hildebrand, J.; Roncarati, P.; Hennuy, B.; Ladang, A.; et al. NF-ΚB-Induced KIAA1199 Promotes Survival through EGFR Signalling. Nat. Commun. 2014, 5, 5232. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Fujita, Y.; Togashi, Y.; Sakai, K.; De Velasco, M.A.; Tomida, S.; Nishio, K. KIAA1199 Interacts with Glycogen Phosphorylase Kinase SS-Subunit (PHKB) to Promote Glycogen Breakdown and Cancer Cell Survival. Oncotarget 2014, 5, 7040–7050. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, X.; Wang, D.; Yu, Y.; Lu, D.; Chen, L.; Lv, F.; Li, Y.; Cheng, L.; Song, Y.; et al. CEMIP Promotes Extracellular Matrix-Detached Prostate Cancer Cell Survival by Inhibiting Ferroptosis. Cancer Sci. 2022, 113, 2056–2070. [Google Scholar] [CrossRef]
- Yoshino, Y.; Ishisaka, M.; Tsuruma, K.; Shimazawa, M.; Yoshida, H.; Inoue, S.; Shimoda, M.; Okada, Y.; Hara, H. Distribution and Function of Hyaluronan Binding Protein Involved in Hyaluronan Depolymerization (HYBID, KIAA1199) in the Mouse Central Nervous System. Neuroscience 2017, 347, 1–10. [Google Scholar] [CrossRef]
- Shimoda, M.; Yoshida, H.; Mizuno, S.; Hirozane, T.; Horiuchi, K.; Yoshino, Y.; Hara, H.; Kanai, Y.; Inoue, S.; Ishijima, M.; et al. Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization Controls Endochondral Ossification through Hyaluronan Metabolism. Am. J. Pathol. 2017, 187, 1162–1176. [Google Scholar] [CrossRef]
- Marella, M.; Jadin, L.; Keller, G.A.; Sugarman, B.J.; Frost, G.I.; Shepard, H.M. KIAA1199 Expression and Hyaluronan Degradation Colocalize in Multiple Sclerosis Lesions. Glycobiology 2018, 28, 958–967. [Google Scholar] [CrossRef]
- Liang, G.; Fang, X.; Yang, Y.; Song, Y. Knockdown of CEMIP Suppresses Proliferation and Induces Apoptosis in Colorectal Cancer Cells: Downregulation of GRP78 and Attenuation of Unfolded Protein Response. Biochem. Cell Biol. 2018, 96, 332–341. [Google Scholar] [CrossRef]
- Hetz, C. The Unfolded Protein Response: Controlling Cell Fate Decisions under ER Stress and Beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Oneyama, M.; Sakamoto, N.; Oue, N.; Kimura, Y.; Hiroshima, Y.; Hashimoto, I.; Hara, K.; Maezawa, Y.; Kano, K.; Aoyama, T.; et al. Clinical Significance of KIAA1199 as a Novel Target for Gastric Cancer Drug Therapy. Anticancer Res. 2019, 39, 6557–6573. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Fang, X.; Yang, Y.; Song, Y. Silencing of CEMIP Suppresses Wnt/β-Catenin/Snail Signaling Transduction and Inhibits EMT Program of Colorectal Cancer Cells. Acta Histochem. 2018, 120, 56–63. [Google Scholar] [CrossRef]
- Shen, F.; Zong, Z.H.; Liu, Y.; Chen, S.; Sheng, X.J.; Zhao, Y. CEMIP Promotes Ovarian Cancer Development and Progression via the PI3K/AKT Signaling Pathway. Biomed. Pharmacother. 2019, 114, 108787. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.; Stern, R. Chain Gangs: New Aspects of Hyaluronan Metabolism. Biochem. Res. Int. 2012, 2012, 893947. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Titze, J.; Shakibaei, M.; Schafflhuber, M.; Schulze-Tanzil, G.; Porst, M.; Schwind, K.H.; Dietsch, P.; Hilgers, K.F. Glycosaminoglycan Polymerization May Enable Osmotically Inactive Na+ Storage in the Skin. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H203–H208. [Google Scholar] [CrossRef]
- Sugár, D.; Agócs, R.; Tatár, E.; Tóth, G.; Horváth, P.; Sulyok, E.; Szabó, A.J. The Contribution of Skin Glycosaminoglycans to the Regulation of Sodium Homeostasis in Rats. Physiol. Res. 2018, 67, 777–785. [Google Scholar] [CrossRef]
- Milner, C.M.; Tongsoongnoen, W.; Rugg, M.S.; Day, A.J. The Molecular Basis of Inter-Alpha-Inhibitor Heavy Chain Transfer on to Hyaluronan. Biochem. Soc. Trans. 2007, 35, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Bauer, J.; Sleeman, J.P. Sugars in the Microenvironment: The Sticky Problem of HA Turnover in Tumors. Cancer Metastasis Rev. 2014, 33, 1059–1079. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Itano, N. Hyaluronan: A Modulator of the Tumor Microenvironment. Cancer Lett. 2016, 375, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan Activate Dendritic Cells via Toll-like Receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Voelcker, V.; Gebhardt, C.; Averbeck, M.; Saalbach, A.; Wolf, V.; Weih, F.; Sleeman, J.; Anderegg, U.; Simon, J. Hyaluronan Fragments Induce Cytokine and Metalloprotease Upregulation in Human Melanoma Cells in Part by Signalling via TLR4. Exp. Dermatol. 2008, 17, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, C.; Wang, X.; He, T.; Li, L.; Liang, X.; Wang, L.; Song, L.; Wei, Y.; Wu, Q.; et al. Hyaluronic Acid Oligosaccharides Improve Myocardial Function Reconstruction and Angiogenesis against Myocardial Infarction by Regulation of Macrophages. Theranostics 2019, 9, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Wong, G.; Earle, C.A.; Xia, W. Interaction of Low Molecular Weight Hyaluronan with CD44 and Toll-like Receptors Promotes the Actin Filament-Associated Protein 110-Actin Binding and MyD88-NFκB Signaling Leading to Proinflammatory Cytokine/Chemokine Production and Breast Tumor Invasion. Cytoskeleton 2011, 68, 671–693. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; He, Y.; Yang, C.; Wang, Y.; Shi, X.; Wei, G. Hyaluronan Oligosaccharides Promote Excisional Wound Healing through Enhanced Angiogenesis. Matrix Biol. 2010, 29, 107–116. [Google Scholar] [CrossRef]
- Bauer, J.; Rothley, M.; Schmaus, A.; Quagliata, L.; Ehret, M.; Biskup, M.; Orian-Rousseau, V.; Jackson, D.G.; Pettis, R.J.; Harvey, A.; et al. TGFβ Counteracts LYVE-1-Mediated Induction of Lymphangiogenesis by Small Hyaluronan Oligosaccharides. J. Mol. Med. 2018, 96, 199–209. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagaoka, A.; Nakamura, S.; Sugiyama, Y.; Okada, Y.; Inoue, S. Murine Homologue of the Human KIAA1199 Is Implicated in Hyaluronan Binding and Depolymerization. FEBS Open Bio 2013, 3, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, A.; Albeiroti, S.; West, G.A.; Willard, B.; Fiocchi, C.; de la Motte, C.A. Crohn’s Disease Fibroblasts Overproduce the Novel Protein KIAA1199 to Create Proinflammatory Hyaluronan Fragments. CMGH 2016, 2, 358–368.e4. [Google Scholar] [CrossRef] [PubMed]
- Kohi, S.; Sato, N.; Koga, A.; Matayoshi, N.; Hirata, K. KIAA1199 Is Induced by Inflammation and Enhances Malignant Phenotype in Pancreatic Cancer. Oncotarget 2017, 8, 17156–17163. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Frost, G.I.; Stern, R. The Six Hyaluronidase-like Genes in the Human and Mouse Genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Kohda, D.; Morton, C.J.; Parkar, A.A.; Hatanaka, H.; Inagaki, F.M.; Campbell, I.D.; Day, A.J. Solution Structure of the Link Module: A Hyaluronan-Binding Domain Involved in Extracellular Matrix Stability and Cell Migration. Cell 1996, 86, 767–775. [Google Scholar] [CrossRef]
- Yang, B.; Yang, B.L.; Savani, R.C.; Turley, E.A. Identification of a Common Hyaluronan Binding Motif in the Hyaluronan Binding Proteins RHAMM, CD44 and Link Protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Popolo, A.; Dal Piaz, F.; Vasaturo, M.; Picardi, P.; Parente, L.; Petrella, A. Role of Intracellular and Extracellular Annexin A1 in Migration and Invasion of Human Pancreatic Carcinoma Cells. BMC Cancer 2014, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Peng, Y.; Ye, L.; Wang, Y.; Qian, Z.; Chen, Y.; Wang, X.; Lin, Y.; Zhang, X.; Sun, X.; et al. Stimulation of TLR4 by LMW-HA Induces Metastasis in Human Papillary Thyroid Carcinoma through CXCR7. Clin. Dev. Immunol. 2013, 2013, 712561. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Murai, T.; Nishinakamura, H.; Kawashima, H.; Saya, H.; Miyasaka, M. Hyaluronan Oligosaccharides Induce CD44 Cleavage and Promote Cell Migration in CD44-Expressing Tumor Cells. J. Biol. Chem. 2003, 278, 32259–32265. [Google Scholar] [CrossRef] [PubMed]
- Hanabayashi, M.; Takahashi, N.; Sobue, Y.; Hirabara, S.; Ishiguro, N.; Kojima, T. Hyaluronan Oligosaccharides Induce MMP-1 and -3 via Transcriptional Activation of NF-ΚB and P38 MAPK in Rheumatoid Synovial Fibroblasts. PLoS ONE 2016, 11, e0161875. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.R.; Shapiro, S.; Bao, C.; Lowenstein, C.J.; Noble, P.W. Induction and Regulation of Macrophage Metalloelastase by Hyaluronan Fragments in Mouse Macrophages. J. Immunol. 1999, 162, 4171–4176. [Google Scholar] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of Matrix Metalloproteinases in Cancer Progression and Their Pharmacological Targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Klusmeier, S.; Rothley, M.; Dimmler, A.; Sipos, B.; Faller, G.; Thiele, W.; Allgayer, H.; Hohenberger, P.; Post, S.; et al. Accumulation of Small Hyaluronan Oligosaccharides in Tumour Interstitial Fluid Correlates with Lymphatic Invasion and Lymph Node Metastasis. Br. J. Cancer 2014, 111, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.H.; Qi, Y.Y.; Li, X.M.; Chen, W.P.; Wang, X.H.; Ji, X.W. Knockdown of KIAA1199 Suppresses IL-1β-Induced Cartilage Degradation and Inflammatory Responses in Human Chondrocytes through the Wnt/β-Catenin Signalling Pathway. Int. Immunopharmacol. 2019, 73, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, K.; Andersen, T.L.; Qiu, W.; Kassem, M. KIAA1199 Is a Secreted Molecule That Enhances Osteoblastic Stem Cell Migration and Recruitment. Cell Death Dis. 2019, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ke, G.M.; Lin, P.C.; Lin, K. Der Therapeutic DNA Vaccine Encoding CEMIP (KIAA1199) Ameliorates Kidney Fibrosis in Obesity through Inhibiting the Wnt/β-Catenin Pathway. Biochim. Biophys. Acta-Gen. Subj. 2021, 1865, 130019. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Wang, X.; Deng, X.; Huang, Y.; Tian, W. CEMIP Regulates the Proliferation and Migration of Vascular Smooth Muscle Cells in Atherosclerosis through the WNT-Beta-Catenin Signaling Pathway. Biochem. Cell Biol. 2020, 98, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, A.; Yoshida, H.; Nakamura, S.; Morikawa, T.; Kawabata, K.; Kobayashi, M.; Sakai, S.; Takahashi, Y.; Okada, Y.; Inoue, S. Regulation of Hyaluronan (HA) Metabolism Mediated by HYBID (Hyaluronan-Binding Protein Involved in HA Depolymerization, KIAA1199) and HA Synthases in Growth Factor-Stimulated Fibroblasts. J. Biol. Chem. 2015, 290, 30910–30923. [Google Scholar] [CrossRef] [PubMed]
- Boerboom, A.; Reusch, C.; Pieltain, A.; Chariot, A.; Franzen, R. KIAA1199: A Novel Regulator of MEK/ERK-Induced Schwann Cell Dedifferentiation. Glia 2017, 65, 1682–1696. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Hatipoglu, O.F.; Asano, K.; Inagaki, J.; Nishida, K.; Hirohata, S. Induction of Cemip in Chondrocytes by Inflammatory Cytokines: Underlying Mechanisms and Potential Involvement in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3140. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt Signaling in Cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Bienz, M.; Clevers, H. Linking Colorectal Cancer to Wnt Signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef]
- Olivier-Van Stichelen, S.; Dehennaut, V.; Buzy, A.; Zachayus, J.L.; Guinez, C.; Mir, A.M.; El Yazidi-Belkoura, I.; Copin, M.C.; Boureme, D.; Loyaux, D.; et al. O-GlcNAcylation Stabilizes β-Catenin through Direct Competition with Phosphorylation at Threonine 41. FASEB J. 2014, 28, 3325–3328. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Smith, D.; Latinkic, B.; Ewan, K.; Samuel, L.; Zollo, M.; Marino, N.; Tyas, L.; Jones, N.; Dale, T.C. A Functional Connectome: Regulation of Wnt/TCF-Dependent Transcription by Pairs of Pathway Activators. Mol. Cancer 2015, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Song, Y.; Sun, Y.; Li, X.; Chen, L.; Yang, L. AMPK/GSK3b/b-Catenin Cascade–Triggered Overexpression of CEMIP Promotes Migration and Invasion in Anoikis-Resistant Prostate Cancer Cells by Enhancing Metabolic Reprogramming. FASEB J. 2018, 32, 3924–3935. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR-Targeted Therapies in the Post-Genomic Era. Cancer Metastasis Rev. 2017, 36, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- El Bali, M.; Bakkach, J.; Bennani Mechita, M. Colorectal Cancer: From Genetic Landscape to Targeted Therapy. J. Oncol. 2021, 2021, 9918116. [Google Scholar] [CrossRef]
- Rizzolio, S.; Battistini, C.; Cagnoni, G.; Apicella, M.; Vella, V.; Giordano, S.; Tamagnone, L. Downregulating Neuropilin-2 Triggers a Novel Mechanism Enabling EGFR-Dependent Resistance to Oncogene-Targeted Therapies. Cancer Res. 2018, 78, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Al-Aidaroos, A.Q.O.; Yuen, H.F.; Guo, K.; Zhang, S.D.; Chung, T.H.; Chng, W.J.; Zeng, Q. Metastasis-Associated PRL-3 Induces EGFR Activation and Addiction in Cancer Cells. J. Clin. Investig. 2013, 123, 3459–3471. [Google Scholar] [CrossRef]
- Banach, A.; Jiang, Y.P.; Roth, E.; Kuscu, C.; Cao, J.; Lin, R.Z. CEMIP Upregulates BiP to Promote Breast Cancer Cell Survival in Hypoxia. Oncotarget 2019, 10, 4307–4320. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. Hypoxic Control of Metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, H.; Zhang, X.; Zhang, L.; Li, X.; Wang, C.; Sun, S. Cell Surface GRP78 Accelerated Breast Cancer Cell Proliferation and Migration by Activating STAT3. PLoS ONE 2015, 10, e0125634. [Google Scholar] [CrossRef] [PubMed]
- Fogh, B.S.; Multhaupt, H.A.B.; Couchman, J.R. Protein Kinase C, Focal Adhesions and the Regulation of Cell Migration. J. Histochem. Cytochem. 2014, 62, 172–184. [Google Scholar] [CrossRef]
- Zhan, B.O.; Kong, C.; Zhang, Z.; Dong, X.; Zhang, N. Inhibition of PKCα Reduces the Ability of Migration of Kidney Cancer Cells but Has No Impact on Cell Apoptosis. Exp. Ther. Med. 2017, 13, 2473–2479. [Google Scholar] [CrossRef][Green Version]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex Networks Orchestrate Epithelial-Mesenchymal Transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, A.; Wu, Y.; Yao, S.; Wang, M.; Niu, T.; Gao, C.; Li, Z.; Zhou, X.; Huo, Z.; et al. Analysis of Genomics and Immune Infiltration Patterns of Epithelial-Mesenchymal Transition Related to Metastatic Breast Cancer to Bone. Transl. Oncol. 2021, 14, 100993. [Google Scholar] [CrossRef] [PubMed]
- Fieber, C.; Baumann, P.; Vallon, R.; Termeer, C.; Simon, J.C.; Hofmann, M.; Angel, P.; Herrlich, P.; Sleeman, J.P. Hyaluronan-Oligosaccharide-Induced Transcription of Metalloproteases. J. Cell Sci. 2004, 117, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Orlichenko, L.S.; Radisky, D.C. Matrix Metalloproteinases Stimulate Epithelial-Mesenchymal Transition during Tumor Development. Clin. Exp. Metastasis 2008, 25, 593–600. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, D.; Li, G.; Ma, J.; Chen, P.; Yuan, W.; Hou, F.; Ge, J.; Zhong, M.; Tang, Y.; et al. EphA2 Promotes Epithelial-Mesenchymal Transition through the Wnt/β-Catenin Pathway in Gastric Cancer Cells. Oncogene 2014, 33, 2737–2747. [Google Scholar] [CrossRef]
- Nenkov, M.; Ma, Y.; Gaßler, N.; Chen, Y. Metabolic Reprogramming of Colorectal Cancer Cells and the Microenvironment: Implication for Therapy. Int. J. Mol. Sci. 2021, 22, 6262. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Fendt, S.M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- Brushia, R.J.; Walsh, D.A. Phosphorylase Kinase: The Complexity of Its Regulation Is Reflected in the Complexity of Its Structure. Front. Biosci. 1999, 4, 618–641. [Google Scholar] [CrossRef]
- Kwa, M.Q.; Herum, K.M.; Brakebusch, C. Cancer-Associated Fibroblasts: How Do They Contribute to Metastasis? Clin. Exp. Metastasis 2019, 36, 71–86. [Google Scholar] [CrossRef]
- McCarthy, J.B.; El-Ashry, D.; Turley, E.A. Hyaluronan, Cancer-Associated Fibroblasts and the Tumor Microenvironment in Malignant Progression. Front. Cell Dev. Biol. 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, J.; de Vega, S.; Cilek, M.Z.; Yoshinaga, C.; Nakamura, T.; Kasamatsu, S.; Yoshida, H.; Kaneko, H.; Ishijima, M.; Kaneko, K.; et al. Implication of HYBID (Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization) in Hyaluronan Degradation by Synovial Fibroblasts in Patients with Knee Osteoarthritis. Am. J. Pathol. 2020, 190, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Aoki, M.; Komiya, A.; Endo, Y.; Kawabata, K.; Nakamura, T.; Sakai, S.; Sayo, T.; Okada, Y.; Takahashi, Y. HYBID (Alias KIAA1199/CEMIP) and Hyaluronan Synthase Coordinately Regulate Hyaluronan Metabolism in Histamine-Stimulated Skin Fibroblasts. J. Biol. Chem. 2020, 295, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Erreni, M.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011, 4, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, O.S.; Spagnuolo, L.; De Visser, K.E. Immune Regulation of Metastasis: Mechanistic Insights and Therapeutic Opportunities. DMM Dis. Model. Mech. 2018, 11, dmm036236. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune Cell Promotion of Metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, Inflammation, and Cancer: An Eternal Fight between Good and Evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Deroyer, C.; Charlier, E.; Neuville, S.; Malaise, O.; Gillet, P.; Kurth, W.; Chariot, A.; Malaise, M.; de Seny, D. CEMIP (KIAA1199) Induces a Fibrosis-like Process in Osteoarthritic Chondrocytes. Cell Death Dis. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Shimoda, M.; Mochizuki, S.; Miyamae, Y.; Abe, H.; Chijiiwa, M.; Yoshida, H.; Shiozawa, J.; Ishijima, M.; Kaneko, K.; et al. Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization Is Up-Regulated and Involved in Hyaluronan Degradation in Human Osteoarthritic Cartilage. Am. J. Pathol. 2018, 188, 2109–2119. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, P.; Chen, B.; Lin, Y.; Zhou, Z.; Ge, R.; Zou, H.; Wang, J.; Wang, J. KIAA1199 as a Potential Diagnostic Biomarker of Rheumatoid Arthritis Related to Angiogenesis. Arthritis Res. Ther. 2015, 17, 140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Momoeda, M.; de Vega, S.; Kaneko, H.; Yoshinaga, C.; Shimoda, M.; Nakamura, T.; Endo, Y.; Yoshida, H.; Kaneko, K.; Ishijima, M.; et al. Deletion of Hybid (Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization) Results in Attenuation of Osteoarthritis in Mice. Am. J. Pathol. 2021, 191, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and Inflammation: Inseparable Actors of Cancer Progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- López-Nouoa, J.M.; Nieto, M.A. Inflammation and EMT: An Alliance towards Organ Fibrosis and Cancer Progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Rothley, M.; Schreiber, C.; Möller, S.; Roßwag, S.; Franz, S.; Garvalov, B.K.; Thiele, W.; Spataro, S.; Herskind, C.; et al. Sulfated Hyaluronic Acid Inhibits the Hyaluronidase CEMIP and Regulates the HA Metabolism, Proliferation and Differentiation of Fibroblasts. Matrix Biol. 2022, 109, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.; He, J.; Wang, L.; Lin, B.; Liang, Z.; Lu, B.; Chen, W.; Lu, G.; Li, F.; Lv, W.; et al. H3K27me3 Loss Plays a Vital Role in CEMIP Mediated Carcinogenesis and Progression of Breast Cancer with Poor Prognosis. Biomed. Pharmacother. 2020, 123, 109728. [Google Scholar] [CrossRef] [PubMed]
- Galamb, O.; Spisák, S.; Sipos, F.; Tóth, K.; Solymosi, N.; Wichmann, B.; Krenács, T.; Valcz, G.; Tulassay, Z.; Molnár, B. Reversal of Gene Expression Changes in the Colorectal Normal-Adenoma Pathway by NS398 Selective COX2 Inhibitor. Br. J. Cancer 2010, 102, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Qi, L.; Liang, J.; Zong, K.; Liu, W.; Li, R.; Feng, R.; Zhai, W. Lenvatinib Induces Anticancer Activity in Gallbladder Cancer by Targeting AKT. J. Cancer 2021, 12, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ding, B.; Zhao, Y.; Han, Y.; Sheng, Y.; Tao, L.; Shen, X.; Zhou, J.; Jiang, L.; Ding, Y. Tumor-Oriented Mathematical Models in Hydrogel Regulation for Precise Topical Administration Regimens. J. Control. Release 2022, 345, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of Pirfenidone Targeting the Tumor Microenvironment and Tumor-Stroma Interaction as a Novel Treatment for Non-Small Cell Lung Cancer. Sci. Rep. 2020, 10, 10900. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domanegg, K.; Sleeman, J.P.; Schmaus, A. CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer. Cancers 2022, 14, 5093. https://doi.org/10.3390/cancers14205093
Domanegg K, Sleeman JP, Schmaus A. CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer. Cancers. 2022; 14(20):5093. https://doi.org/10.3390/cancers14205093
Chicago/Turabian StyleDomanegg, Kevin, Jonathan P. Sleeman, and Anja Schmaus. 2022. "CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer" Cancers 14, no. 20: 5093. https://doi.org/10.3390/cancers14205093
APA StyleDomanegg, K., Sleeman, J. P., & Schmaus, A. (2022). CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer. Cancers, 14(20), 5093. https://doi.org/10.3390/cancers14205093






