Obesity-Related Cross-Talk between Prostate Cancer and Peripheral Fat: Potential Role of Exosomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Cross-Talk between PCa and PPAT
2.1. PPAT Promotes Survival and Progression of Prostate Cancer
2.2. PCa Affects the State and Production of PPAT
3. Exosomes as a Mediator of Positive Feedback between Adipose Tissue and Cancer
3.1. Tumor Exosomes Mediate the Alteration of Adipose Tissue and Release of Biomolecules
3.2. Adipose Exosomes Induce the Development of Multiple Tumors
3.3. Exosomes Mediate Cross-Talk between PPAT and PCa
4. Obesity Alters the Number and Structure of Exosomes Involved in the Cross-Talk between Adiposity and Cancer
4.1. Obesity Affects the Number and Status of Adipose Exosomes
4.2. Obesity Alters the Cargo and Function of Adipose Exosomes
4.3. Adipose Exosomes Mediate PCa Progression in Obese Patients
5. Conclusions
6. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, C.; Lee, H.J.; Ren, S.; Zi, X.; Zhang, Z.; Wang, H.; Yu, Y.; Yang, C.; Gao, X.; et al. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature 2020, 580, 93–99. [Google Scholar] [CrossRef]
- Zhong, S.; Yan, X.; Wu, Y.; Zhang, X.; Chen, L.; Tang, J.; Zhao, J. Body mass index and mortality in prostate cancer patients: A dose-response meta-analysis. Prostate Cancer Prostatic Dis. 2016, 19, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.B.; Liu, S.H.; Jiang, H.W.; Bai, P.D.; Ding, Q. Obesity affects the biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: A dose-response meta-analysis of 29,464 patients. PLoS ONE 2014, 9, e106677. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Howard, L.E.; Song, H.J.; Sourbeer, K.N.; Koontz, B.F.; Salama, J.K.; Freedland, S.J. Racial differences in adipose tissue distribution and risk of aggressive prostate cancer among men undergoing radiotherapy. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Bhindi, B.; Trottier, G.; Elharram, M.; Fernandes, K.A.; Lockwood, G.; Toi, A.; Hersey, K.M.; Finelli, A.; Evans, A.; van der Kwast, T.H.; et al. Measurement of peri-prostatic fat thickness using transrectal ultrasonography (TRUS): A new risk factor for prostate cancer. BJU Int. 2012, 110, 980–986. [Google Scholar] [CrossRef]
- Zadra, G.; Photopoulos, C.; Loda, M. The fat side of prostate cancer. Biochim. Et Biophys. Acta 2013, 1831, 1518–1532. [Google Scholar] [CrossRef]
- Grossmann, M.; Wittert, G. Androgens, diabetes and prostate cancer. Endocr.-Relat. Cancer 2012, 19, F47–F62. [Google Scholar] [CrossRef]
- Billis, A. Intraprostatic fat: Does it exist? Hum. Pathol. 2004, 35, 525. [Google Scholar] [CrossRef]
- van Roermund, J.G.; Bol, G.H.; Witjes, J.A.; Ruud Bosch, J.L.; Kiemeney, L.A.; van Vulpen, M. Periprostatic fat measured on computed tomography as a marker for prostate cancer aggressiveness. World J. Urol. 2010, 28, 699–704. [Google Scholar] [CrossRef]
- Salji, M.; Hendry, J.; Patel, A.; Ahmad, I.; Nixon, C.; Leung, H.Y. Peri-prostatic Fat Volume Measurement as a Predictive Tool for Castration Resistance in Advanced Prostate Cancer. Eur. Urol. Focus 2018, 4, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Monteiro, C.; Silvestre, R.; Castela, A.; Coutinho, H.; Fraga, A.; Príncipe, P.; Lobato, C.; Costa, C.; Cordeiro-da-Silva, A.; et al. Human periprostatic white adipose tissue is rich in stromal progenitor cells and a potential source of prostate tumor stroma. Exp. Biol. Med. Maywood 2012, 237, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Finley, D.S.; Calvert, V.S.; Inokuchi, J.; Lau, A.; Narula, N.; Petricoin, E.F.; Zaldivar, F.; Santos, R.; Tyson, D.R.; Ornstein, D.K. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J. Urol 2009, 182, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- van Roermund, J.G.; Hinnen, K.A.; Tolman, C.J.; Bol, G.H.; Witjes, J.A.; Bosch, J.L.; Kiemeney, L.A.; van Vulpen, M. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011, 107, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, L.J.; Qi, J.; Yang, Z.G.; Huang, T.; Huo, R.C. Periprostatic adiposity measured on magnetic resonance imaging correlates with prostate cancer aggressiveness. Urol. J. 2014, 11, 1793–1799. [Google Scholar]
- Woo, S.; Cho, J.Y.; Kim, S.Y.; Kim, S.H. Periprostatic fat thickness on MRI: Correlation with Gleason score in prostate cancer. AJR Am. J. Roentgenol. 2015, 204, W43–W47. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, M.; Chen, Y.; Yu, W.; Fan, Y.; Liu, Q.; Gao, G.; Zhao, Z.; Wang, X.; Jin, J. The combination of prostate imaging reporting and data system version 2 (PI-RADS v2) and periprostatic fat thickness on multi-parametric MRI to predict the presence of prostate cancer. Oncotarget 2017, 8, 44040–44049. [Google Scholar] [CrossRef]
- Dahran, N.; Szewczyk-Bieda, M.; Wei, C.; Vinnicombe, S.; Nabi, G. Normalized periprostatic fat MRI measurements can predict prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localised disease. Sci. Rep. 2017, 7, 4630. [Google Scholar]
- Gregg, J.R.; Surasi, D.S.; Childs, A.; Moll, N.; Ward, J.F.; Kim, J.; Daniel, C.R.; Logothetis, C.; Bathala, T.; Davis, J.W. The Association of Periprostatic Fat and Grade Group Progression in Men with Localized Prostate Cancer on Active Surveillance. J Urol. 2021, 205, 122–128. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugino, Y.; Kato, M.; Nishikawa, K.; Kanda, H. Pre-treatment ratio of periprostatic to subcutaneous fat thickness on MRI is an independent survival predictor in hormone-naïve men with advanced prostate cancer. Int. J. Clin. Oncol. 2020, 25, 370–376. [Google Scholar] [CrossRef]
- Ribeiro, R.; Monteiro, C.; Cunha, V.; Oliveira, M.J.; Freitas, M.; Fraga, A.; Príncipe, P.; Lobato, C.; Lobo, F.; Morais, A.; et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J. Exp. Clin. Cancer Res. 2012, 31, 32. [Google Scholar] [CrossRef] [PubMed]
- La Civita, E.; Liotti, A.; Cennamo, M.; Crocetto, F.; Ferro, M.; Liguoro, P.; Cimmino, A.; Imbimbo, C.; Beguinot, F.; Formisano, P.; et al. Peri-Prostatic Adipocyte-Released TGFβ Enhances Prostate Cancer Cell Motility by Upregulation of Connective Tissue Growth Factor. Biomedicines 2021, 9, 1692. [Google Scholar] [CrossRef] [PubMed]
- Figiel, S.; Pinault, M.; Domingo, I.; Guimaraes, C.; Guibon, R.; Besson, P.; Tavernier, E.; Blanchet, P.; Multigner, L.; Bruyère, F.; et al. Fatty acid profile in peri-prostatic adipose tissue and prostate cancer aggressiveness in African-Caribbean and Caucasian patients. Eur. J. Cancer Oxf. Engl. 1990 2018, 91, 107–115. [Google Scholar] [CrossRef]
- Liotti, A.; La Civita, E.; Cennamo, M.; Crocetto, F.; Ferro, M.; Guadagno, E.; Insabato, L.; Imbimbo, C.; Palmieri, A.; Mirone, V.; et al. Periprostatic adipose tissue promotes prostate cancer resistance to docetaxel by paracrine IGF-1 upregulation of TUBB2B beta-tubulin isoform. Prostate 2021, 81, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Toulet, A.; Attané, C.; Milhas, D.; Dauvillier, S.; Zaidi, F.; Clement, E.; Cinato, M.; Le Gonidec, S.; Guérard, A.; et al. Periprostatic Adipose Tissue Favors Prostate Cancer Cell Invasion in an Obesity-Dependent Manner: Role of Oxidative Stress. Mol. Cancer Res. MCR 2019, 17, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, M.; Chen, X.; Zhu, R.; Yang, D.R.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O. Adipocytes affect castration-resistant prostate cancer cells to develop the resistance to cytotoxic action of NK cells with alterations of PD-L1/NKG2D ligand levels in tumor cells. Prostate 2018, 78, 353–364. [Google Scholar] [CrossRef]
- Fontaine, A.; Bellanger, D.; Guibon, R.; Bruyère, F.; Brisson, L.; Fromont, G. Lipophagy and prostate cancer: Association with disease aggressiveness and proximity to periprostatic adipose tissue. J. Pathol. 2021, 255, 166–176. [Google Scholar] [CrossRef]
- Sacca, P.A.; Creydt, V.P.; Choi, H.; Mazza, O.N.; Fletcher, S.J.; Vallone, V.B.; Scorticati, C.; Chasseing, N.A.; Calvo, J.C. Human periprostatic adipose tissue: Its influence on prostate cancer cells. Cell Physiol Biochem. 2012, 30, 113–122. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metab. Clin. Exp. 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Kelly, S.P.; Graubard, B.I.; Andreotti, G.; Younes, N.; Cleary, S.D.; Cook, M.B. Prediagnostic Body Mass Index Trajectories in Relation to Prostate Cancer Incidence and Mortality in the PLCO Cancer Screening Trial. J. Natl. Cancer Inst. 2017, 109, djw225. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Freedland, S.J.; Miano, L.; Finazzi Agrò, E.; Bañez, L.; Tubaro, A. The uncertain relationship between obesity and prostate cancer: An Italian biopsy cohort analysis. Eur. J. Surg. Oncol. 2011, 37, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.; Roswall, N.; Van Hemelrijck, M.; Larsen, S.B.; Cuzick, J.; Holmberg, L.; Overvad, K.; Tjønneland, A. Prostate cancer incidence, clinical stage and survival in relation to obesity: A prospective cohort study in Denmark. Int. J. Cancer 2015, 136, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Burow, M.E.; Gimble, J.M.; Bunnell, B.A. Concise review: The obesity cancer paradigm: Exploration of the interactions and crosstalk with adipose stem cells. Stem Cells Dayt. Ohio 2015, 33, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Chen, M.; Xu, K.; Chen, B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell. Mol. Life Sci. CMLS 2021, 78, 7275–7288. [Google Scholar] [CrossRef]
- Fontana, F.; Anselmi, M.; Carollo, E.; Sartori, P.; Procacci, P.; Carter, D.; Limonta, P. Adipocyte-Derived Extracellular Vesicles Promote Prostate Cancer Cell Aggressiveness by Enabling Multiple Phenotypic and Metabolic Changes. Cells 2022, 11, 2388. [Google Scholar] [CrossRef]
- Kubo, H. Extracellular Vesicles in Lung Disease. Chest 2018, 153, 210–216. [Google Scholar] [CrossRef]
- Couzin, J. Cell biology: The ins and outs of exosomes. Science 2005, 308, 1862–1863. [Google Scholar] [CrossRef]
- Hong, H.; Koch, M.O.; Foster, R.S.; Bihrle, R.; Gardner, T.A.; Fyffe, J.; Ulbright, T.M.; Eble, J.N.; Cheng, L. Anatomic distribution of periprostatic adipose tissue: A mapping study of 100 radical prostatectomy specimens. Cancer 2003, 97, 1639–1643. [Google Scholar] [CrossRef]
- Byar, D.P.; Mostofi, F.K. Carcinoma of the prostate: Prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer 1972, 30, 5–13. [Google Scholar] [CrossRef]
- Cheng, L.; Darson, M.F.; Bergstralh, E.J.; Slezak, J.; Myers, R.P.; Bostwick, D.G. Correlation of margin status and extraprostatic extension with progression of prostate carcinoma. Cancer 1999, 86, 1775–1782. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Abd Elmageed, Z.Y.; Yang, Y.; Thomas, R.; Ranjan, M.; Mondal, D.; Moroz, K.; Fang, Z.; Rezk, B.M.; Moparty, K.; Sikka, S.C.; et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem. Cells 2014, 32, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Moreira, Â.; Pereira, S.S.; Costa, M.; Morais, T.; Pinto, A.; Fernandes, R.; Monteiro, M.P. Adipocyte secreted factors enhance aggressiveness of prostate carcinoma cells. PLoS ONE 2015, 10, e0123217. [Google Scholar] [CrossRef]
- Onuma, M.; Bub, J.D.; Rummel, T.L.; Iwamoto, Y. Prostate cancer cell-adipocyte interaction: Leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J. Biol. Chem. 2003, 278, 42660–42667. [Google Scholar] [CrossRef]
- Ito, Y.; Ishiguro, H.; Kobayashi, N.; Hasumi, H.; Watanabe, M.; Yao, M.; Uemura, H. Adipocyte-derived monocyte chemotactic protein-1 (MCP-1) promotes prostate cancer progression through the induction of MMP-2 activity. Prostate 2015, 75, 1009–1019. [Google Scholar] [CrossRef]
- Pettersson, A.; Lis, R.T.; Meisner, A.; Flavin, R.; Stack, E.C.; Fiorentino, M.; Finn, S.; Graff, R.E.; Penney, K.L.; Rider, J.R.; et al. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. J. Natl. Cancer Inst. 2013, 105, 1881–1890. [Google Scholar] [CrossRef]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Telci Caklili, O.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Guérard, A.; Mazerolles, C.; Le Gonidec, S.; Toulet, A.; Nieto, L.; Zaidi, F.; Majed, B.; Garandeau, D.; Socrier, Y.; et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016, 7, 10230. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Maguire, T.M.; Hill, A.; McDermott, E.; O’Higgins, N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. BCR 2000, 2, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Satoh, Y.; Fujiyama, C.; Toda, S.; Sugihara, H.; Masaki, Z. Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU Int. 2003, 91, 716–720. [Google Scholar] [CrossRef]
- Kaneko, A.; Satoh, Y.; Tokuda, Y.; Fujiyama, C.; Udo, K.; Uozumi, J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2010, 17, 369–376. [Google Scholar] [CrossRef]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Sivashanmugam, P.; Tang, L.; Daaka, Y. Interleukin 6 mediates the lysophosphatidic acid-regulated cross-talk between stromal and epithelial prostate cancer cells. J. Biol. Chem. 2004, 279, 21154–21159. [Google Scholar] [CrossRef]
- Xu, H.; Hu, M.B.; Bai, P.D.; Zhu, W.H.; Liu, S.H.; Hou, J.Y.; Xiong, Z.Q.; Ding, Q.; Jiang, H.W. Proinflammatory cytokines in prostate cancer development and progression promoted by high-fat diet. BioMed Res. Int. 2015, 2015, 249741. [Google Scholar] [CrossRef]
- Gucalp, A.; Iyengar, N.M.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Wang, H.; Williams, S.; Krasne, M.D.; Yaghnam, I.; Kunzel, B.; et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, K.; Fitchev, P.; Brendler, C.; Plunkett, B.; Dangle, P.; McGuire, M.; Shevrin, D.; Cornwell, M.; Quinn, M.; Kaul, K. 314 Active triacylglycerol metabolism and increased infiltrating adipocyte density in high grade prostate cancer. J. Urol. 2012, 187, e127. [Google Scholar] [CrossRef]
- Gazi, E.; Gardner, P.; Lockyer, N.P.; Hart, C.A.; Brown, M.D.; Clarke, N.W. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J. Lipid Res. 2007, 48, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Iordanescu, G.; Brendler, C.; Crawford, S.E.; Wyrwicz, A.M.; Venkatasubramanian, P.N.; Doll, J.A. MRS measured fatty acid composition of periprostatic adipose tissue correlates with pathological measures of prostate cancer aggressiveness. J. Magn. Reson. Imaging JMRI 2015, 42, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Et Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef]
- Susa, T.; Ikaga, R.; Kajitani, T.; Iizuka, M.; Okinaga, H.; Tamamori-Adachi, M.; Okazaki, T. Wild-type and specific mutant androgen receptor mediates transcription via 17β-estradiol in sex hormone-sensitive cancer cells. J. Cell. Physiol. 2015, 230, 1594–1606. [Google Scholar] [CrossRef]
- Capurso, C.; Capurso, A. From excess adiposity to insulin resistance: The role of free fatty acids. Vasc. Pharmacol. 2012, 57, 91–97. [Google Scholar] [CrossRef]
- de Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Zhang, Y.; Daquinag, A.; Traktuev, D.O.; Amaya-Manzanares, F.; Simmons, P.J.; March, K.L.; Pasqualini, R.; Arap, W.; Kolonin, M.G. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009, 69, 5259–5266. [Google Scholar] [CrossRef]
- Klopp, A.H.; Zhang, Y.; Solley, T.; Amaya-Manzanares, F.; Marini, F.; Andreeff, M.; Debeb, B.; Woodward, W.; Schmandt, R.; Broaddus, R.; et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin. Cancer Res. 2012, 18, 771–782. [Google Scholar] [CrossRef]
- Venkatasubramanian, P.N.; Crawford, S.E.; Fitchev, P.S.; Brendler, C.B.; Plunkett, B.A.; Abroe, B.; Mafi, M.; Morgan, G.; Cornwell, M.L.; O’Leary, J. Periprostatic fat from obese patients promotes prostate cancer growth. Cancer Res. 2012, 72, 1499. [Google Scholar] [CrossRef]
- Zhang, T.; Tseng, C.; Zhang, Y.; Sirin, O.; Corn, P.G.; Li-Ning-Tapia, E.M.; Troncoso, P.; Davis, J.; Pettaway, C.; Ward, J.; et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 2016, 7, 11674. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, T.O.; Richardson, R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 4449. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.J.; Monteiro, C.P.; Cunha, V.F.; Azevedo, A.S.; Oliveira, M.J.; Monteiro, R.; Fraga, A.M.; Príncipe, P.; Lobato, C.; Lobo, F.; et al. Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell. Physiol. Biochem. 2012, 29, 233–240. [Google Scholar] [CrossRef]
- Lin, G.; Yang, R.; Banie, L.; Wang, G.; Ning, H.; Li, L.C.; Lue, T.F.; Lin, C.S. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate 2010, 70, 1066–1073. [Google Scholar] [CrossRef]
- Zhau, H.E.; He, H.; Wang, C.Y.; Zayzafoon, M.; Morrissey, C.; Vessella, R.L.; Marshall, F.F.; Chung, L.W.; Wang, R. Human prostate cancer harbors the stem cell properties of bone marrow mesenchymal stem cells. Clin. Cancer Res. 2011, 17, 2159–2169. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuédé, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- Taussky, D.; Barkati, M.; Campeau, S.; Zerouali, K.; Nadiri, A.; Saad, F.; Delouya, G. Changes in periprostatic adipose tissue induced by 5α-reductase inhibitors. Andrology 2017, 5, 511–515. [Google Scholar]
- De Rosa, V.; Procaccini, C.; Calì, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, C.M.; Azrak, Z.; Hanache, S.; Abou-Kheir, W.; Zeidan, A. Differential Role of Leptin and Adiponectin in Cardiovascular System. Int. J. Endocrinol. 2015, 2015, 534320. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Careaga Quiroga, V.P.; Sacca, P.A.; Mazza, O.N.; Scorticati, C.; Vitagliano, G.; Fletcher, S.J.; Maier, M.S.; Calvo, J.C. Fatty acid composition of human periprostatic adipose tissue from argentine patients and its relationship to prostate cancer and benign prostatic hyperplasia. Res. Cancer Tumor 2015, 4, 4,1–6. [Google Scholar]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Attané, C.; Carrié, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; Le Gonidec, S.; et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Guo, J.; Fei, X.; Yu, L.; Ma, S. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget 2017, 8, 81880–81891. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Ou, S.; Chen, J.; Chen, L. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J. Physiol. Biochem. 2019, 75, 391–401. [Google Scholar] [CrossRef]
- Au Yeung, C.L.; Co, N.N.; Tsuruga, T.; Yeung, T.L.; Kwan, S.Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.K.; et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 2016, 7, 11150. [Google Scholar] [CrossRef]
- La Camera, G.; Gelsomino, L.; Malivindi, R.; Barone, I.; Panza, S.; De Rose, D.; Giordano, F.; D’Esposito, V.; Formisano, P.; Bonofiglio, D.; et al. Adipocyte-derived extracellular vesicles promote breast cancer cell malignancy through HIF-1α activity. Cancer Lett. 2021, 521, 155–168. [Google Scholar] [CrossRef]
- Guo, G.; Tan, Z.; Liu, Y.; Shi, F.; She, J. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res. Ther. 2022, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Ge, S.; Liu, Y.; Bai, M.; Zhu, K.; Fan, Q.; Li, J.; Ning, T.; Tian, F.; et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene 2019, 38, 2844–2859. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Hashemi, S.M.; Ghanbarian, H.; Sharifi, K.; Salehi, M.; Mohammadi-Yeganeh, S. Delivery of miR-381-3p Mimic by Mesenchymal Stem Cell-Derived Exosomes Inhibits Triple Negative Breast Cancer Aggressiveness; an In Vitro Study. Stem. Cell Rev. Rep. 2021, 17, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, H.; Sun, H.; Hua, Y.; Zhang, G.; Jiang, J.; Wang, X. Adipose Mesenchymal Stem Cell-Derived Exosomal microRNA-1236 Reduces Resistance of Breast Cancer Cells to Cisplatin by Suppressing SLC9A1 and the Wnt/β-Catenin Signaling. Cancer Manag. Res. 2020, 12, 8733–8744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, Y.; Li, K.; Zhang, G.; Guo, Z.; Wu, X.; Qiu, C.; Li, Y.; Wan, X.; Sui, J.; et al. Exosomes Secreted by Adipose-Derived Mesenchymal Stem Cells Foster Metastasis and Osteosarcoma Proliferation by Increasing COLGALT2 Expression. Front. Cell Dev. Biol. 2020, 8, 353. [Google Scholar] [CrossRef]
- Fan, X.; Wang, J.; Qin, T.; Zhang, Y.; Liu, W.; Jiang, K.; Huang, D. Exosome miR-27a-3p secreted from adipocytes targets ICOS to promote antitumor immunity in lung adenocarcinoma. Thorac. Cancer 2020, 11, 1453–1464. [Google Scholar] [CrossRef]
- Reza, A.; Choi, Y.J.; Yasuda, H.; Kim, J.H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Li, Z.; Sun, S.; Zhu, S.; Wang, L.; Wu, J.; Yuan, J.; Zhang, Y.; Sun, S.; et al. Exosomes from the tumour-adipocyte interplay stimulate beige/brown differentiation and reprogram metabolism in stromal adipocytes to promote tumour progression. J. Exp. Clin. Cancer Res. CR 2019, 38, 223. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, S.; Li, Z.; Yang, Q.; Li, B.; Zhu, S.; Wang, L.; Wu, J.; Yuan, J.; Yang, C.; et al. Tumour-originated exosomal miR-155 triggers cancer-associated cachexia to promote tumour progression. Mol. Cancer 2018, 17, 155. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.; Bai, M.; Liu, Y.; Zhan, Y.; Deng, T.; Yang, H.; Sun, W.; Wang, X.; Zhu, K.; et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int. J. Cancer 2019, 144, 2501–2515. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ru, Z.; Zhou, Y.; Xiao, W.; Sun, R.; Zhang, S.; Gao, Y.; Li, X.; Zhang, X.; Yang, H. Lung cancer-derived extracellular vesicles induced myotube atrophy and adipocyte lipolysis via the extracellular IL-6-mediated STAT3 pathway. Biochim. Et Biophys. Acta. Mol. Cell Biol. Lipids 2019, 1864, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xiong, H.; Ru, Z.; Zhao, Y.; Zhou, Y.; Xie, K.; Xiao, W.; Xiong, Z.; Wang, C.; Yuan, C.; et al. Extracellular vesicles-released parathyroid hormone-related protein from Lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis. 2021, 12, 134. [Google Scholar] [CrossRef]
- Liu, A.; Pan, W.; Zhuang, S.; Tang, Y.; Zhang, H. Cancer cell-derived exosomal miR-425-3p induces white adipocyte atrophy. Adipocyte 2022, 11, 487–500. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Deng, T.; Liu, R.; Ning, T.; Bai, M.; Ying, G.; Zhang, H.; Ba, Y. Exosomal miR-155 from gastric cancer induces cancer-associated cachexia by suppressing adipogenesis and promoting brown adipose differentiation via C/EPBβ. Cancer Biol. Med. 2022, 19, 9. [Google Scholar] [CrossRef]
- Ba, L.; Xue, C.; Li, X.; Zhang, M.; Yang, Y.; Han, Q.; Sun, Z.; Zhao, R.C. Gastric Cancer Cell-Derived Exosomes Can Regulate the Biological Functions of Mesenchymal Stem Cells by Inducing the Expression of Circular RNA circ_0004303. Stem. Cells Dev. 2021, 30, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Zhang, W.; Zhu, B.; Li, X.; Tang, Q.; Zhou, Y. Colorectal cancer prompted adipose tissue browning and cancer cachexia through transferring exosomal miR-146b-5p. J. Cell. Physiol. 2021, 236, 5399–5410. [Google Scholar] [CrossRef]
- Luo, F.; Sun, Z.; Han, Q.; Xue, C.; Bai, C. Effect of Human Hepatocellular Carcinoma HepG2 Cell-derived Exosome on the Differentiation of Mesenchymal Stem Cells and Their Interaction. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Acad. Med. Sinicae 2017, 39, 312–317. [Google Scholar]
- Sagar, G.; Sah, R.P.; Javeed, N.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Giorgadze, N.; Tchkonia, T.; Kirkland, J.L.; Chari, S.T.; et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 2016, 65, 1165–1174. [Google Scholar] [CrossRef]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, A.; Dumbali, S.P.; Wenzel, P.L. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front. Cell Dev. Biol. 2020, 8, 603292. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Xiao, X.; Wang, L.; Sun, Z.; Guan, M.; Zhao, R.C. Pancreatic cancer cell exosomes induce lipidomics changes in adipocytes. Adipocyte 2022, 11, 346–355. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Li, X.; Su, X.; Xiao, X.; Keating, A.; Zhao, R.C. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J. Hematol. Oncol. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Z.; Li, T.; Zhu, L.; Li, Z.; Tian, N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am. J. Transl. Res. 2017, 9, 5012–5021. [Google Scholar] [PubMed]
- Zheng, Z.; Liu, L.; Zhan, Y.; Yu, S.; Kang, T. Adipose-derived stem cell-derived microvesicle-released miR-210 promoted proliferation, migration and invasion of endothelial cells by regulating RUNX3. Cell Cycle Georget. Tex. 2018, 17, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- McNeal, J.E.; Villers, A.A.; Redwine, E.A.; Freiha, F.S.; Stamey, T.A. Capsular penetration in prostate cancer. Significance for natural history and treatment. Am. J. Surg. Pathol. 1990, 14, 240–247. [Google Scholar] [CrossRef]
- Takahara, K.; Ii, M.; Inamoto, T.; Nakagawa, T.; Ibuki, N.; Yoshikawa, Y.; Tsujino, T.; Uchimoto, T.; Saito, K.; Takai, T.; et al. microRNA-145 Mediates the Inhibitory Effect of Adipose Tissue-Derived Stromal Cells on Prostate Cancer. Stem. Cells Dev. 2016, 25, 1290–1298. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Pérez de Rojas, J.; Martínez-Ruiz, V.; Arrabal-Polo, M.; Pérez-Gómez, B.; Jiménez-Moleón, J.J. Obesity and biochemical recurrence in clinically localised prostate cancer: A systematic review and meta-analysis of 86,490 patients. Prostate Cancer Prostatic Dis. 2022, 25, 411–421. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, M.U.; Chae, H.K.; Nam, W.; Kim, S.W.; Yu, H.; Kim, H.G.; Kang, G.H.; Park, J.Y. Overweight and obesity as risk factors for biochemical recurrence of prostate cancer after radical prostatectomy. Int. J. Clin. Oncol. 2022, 27, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.; Clément, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Fat Cell and Fatty Acid Turnover in Obesity. Adv. Exp. Med. Biol. 2017, 960, 135–160. [Google Scholar] [PubMed]
- Durcin, M.; Fleury, A.; Taillebois, E.; Hilairet, G.; Krupova, Z.; Henry, C.; Truchet, S.; Trötzmüller, M.; Köfeler, H.; Mabilleau, G.; et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1305677. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Su, H.; Li, J.; Lyon, C.; Tang, W.; Wan, M.; Hu, T.Y. Clinical applications of exosome membrane proteins. Precis. Clin. Med. 2020, 3, 54–66. [Google Scholar] [CrossRef]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N.; et al. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci. Signal. 2021, 14, eabj2807. [Google Scholar]
- Wen, Z.; Li, J.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Hypertrophic Adipocyte-Derived Exosomal miR-802-5p Contributes to Insulin Resistance in Cardiac Myocytes Through Targeting HSP60. Obes. Silver Spring Md. 2020, 28, 1932–1940. [Google Scholar] [CrossRef]
- Figueira, I.; Godinho-Pereira, J.; Galego, S.; Maia, J.; Haskó, J.; Molnár, K.; Malhó, R.; Costa-Silva, B.; Wilhelm, I.; Krizbai, I.A.; et al. MicroRNAs and Extracellular Vesicles as Distinctive Biomarkers of Precocious and Advanced Stages of Breast Cancer Brain Metastases Development. Int. J. Mol. Sci. 2021, 22, 5214. [Google Scholar] [CrossRef]
- Sano, S.; Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nakamura, Y.; Wei, M.; Wanibuchi, H.; et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2014, 445, 327–333. [Google Scholar] [CrossRef]
- Müller, G.; Schneider, M.; Biemer-Daub, G.; Wied, S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell. Signal. 2011, 23, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Daquinag, A.C.; Amaya-Manzanares, F.; Sirin, O.; Tseng, C.; Kolonin, M.G. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012, 72, 5198–5208. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Monteiro, C.; Catalán, V.; Hu, P.; Cunha, V.; Rodríguez, A.; Gómez-Ambrosi, J.; Fraga, A.; Príncipe, P.; Lobato, C.; et al. Obesity and prostate cancer: Gene expression signature of human periprostatic adipose tissue. BMC Med. 2012, 10, 108. [Google Scholar] [CrossRef]
- Hoy, A.J.; Balaban, S.; Saunders, D.N. Adipocyte–tumor cell metabolic crosstalk in breast cancer. Trends Mol. Med. 2017, 23, 381–392. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef]
- Nead, K.T.; Sharp, S.J.; Thompson, D.J.; Painter, J.N.; Savage, D.B.; Semple, R.K.; Barker, A.; Perry, J.R.; Attia, J.; Dunning, A.M.; et al. Evidence of a Causal Association Between Insulinemia and Endometrial Cancer: A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2015, 107, djv178. [Google Scholar] [CrossRef]
- Pan, S.Y.; Johnson, K.C.; Ugnat, A.-M.; Wen, S.W.; Mao, Y. Association of obesity and cancer risk in Canada. Am. J. Epidemiol. 2004, 159, 259–268. [Google Scholar] [CrossRef]
- Bitting, R.L.; Armstrong, A.J. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr. Relat. Cancer 2013, 20, R83–R99. [Google Scholar] [CrossRef]
- Marhold, M.; Tomasich, E.; El-Gazzar, A.; Heller, G.; Spittler, A.; Horvat, R.; Krainer, M.; Horak, P. HIF1α Regulates mTOR Signaling and Viability of Prostate Cancer Stem CellsHIF1α in Prostate Cancer Stem Cells. Mol. Cancer Res. 2015, 13, 556–564. [Google Scholar] [CrossRef]
- Ranasinghe, W.K.; Baldwin, G.S.; Shulkes, A.; Bolton, D.; Patel, O. Normoxic regulation of HIF-1α in prostate cancer. Nat Rev Urol. 2014, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Anselmi, M.; Limonta, P. Molecular mechanisms and genetic alterations in prostate cancer: From diagnosis to targeted therapy. Cancer Lett. 2022, 534, 215619. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Rios-Colon, L.; Arthur, E.; Niture, S.; Qi, Q.; Moore, J.T.; Kumar, D. The Role of Exosomes in the Crosstalk between Adipocytes and Liver Cancer Cells. Cells 2020, 9, 1988. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Boo, L.; Ky, H.; Satharasinghe, D.A.; Tan, S.W.; Cheong, S.K.; Huang, H.D.; Lan, K.C.; et al. Adipose MSCs Suppress MCF7 and MDA-MB-231 Breast Cancer Metastasis and EMT Pathways Leading to Dormancy via Exosomal-miRNAs Following Co-Culture Interaction. Pharm. Basel Switz. 2020, 14, 8. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Lim, S.K. Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 2014, 5, 518. [Google Scholar] [CrossRef]
- Pelosi, M.A., 3rd; Pelosi, M.A., 2nd. Liposuction. Obstet. Gynecol. Clin. N. Am. 2010, 37, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem. Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. MSCs-derived exosomes: Cell-secreted nanovesicles with regenerative potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Lee, J.; Won, Y.; Duncan, D.I.; Jin, R.C.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Park, B.C. Skin brightening efficacy of exosomes derived from human adipose tissue-derived stem/stromal cells: A prospective, split-face, randomized placebo-controlled study. Cosmetics 2020, 7, 90. [Google Scholar] [CrossRef]


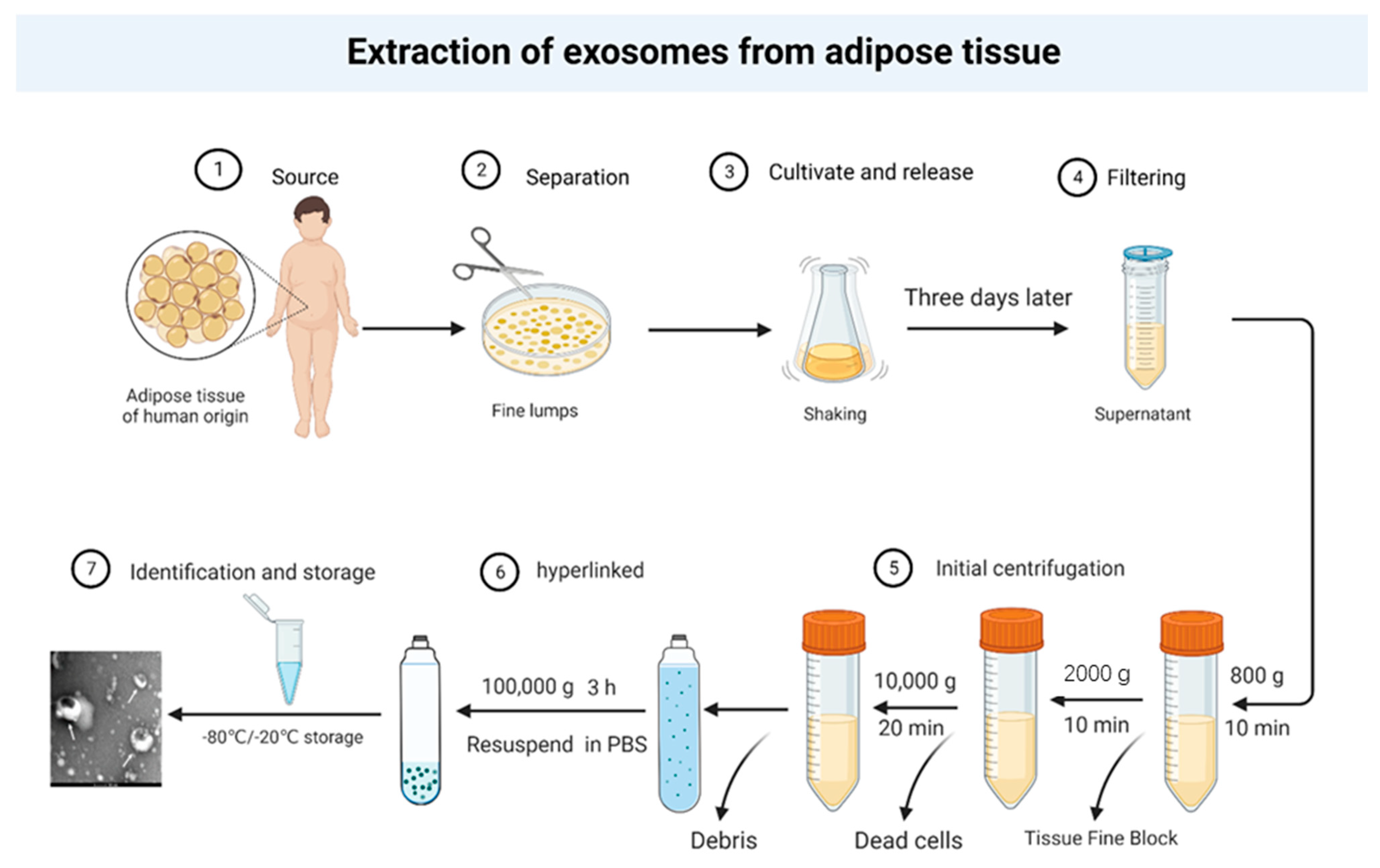
| Type of Research | Times | Research Subjects | Conclusion | References |
|---|---|---|---|---|
| Clinical Research | 2011 | 932 patients treated with brachytherapy or radiation therapy. | There was an association between thickness and size of PPAT and high risk of PCa. | [14] |
| Clinical Research | 2012 | 652 prostate cancer patients | The thickness of PPAT was associated with the detection rate of prostate cancer, especially high-grade PCa. | [6] |
| Clinical Research | 2014 | 184 patients who underwent radical retropubic prostatectomy. | PPAT area and ratio (PPAT volume/prostate volume) were associated with high-risk PCa. | [15] |
| Clinical Research | 2014 | 308 patients treated with radiotherapy. | PPAT regions were associated with PCade aggressiveness and were associated with skin color. | [5] |
| Clinical Research | 2015 | 190 PCa patients undergoing MRI. | PPAT thickness was an independent predictor of PCa and high-grade PCa. | [16] |
| Clinical Research | 2017 | 371 patients with PCa, 292 patients with high-grade Pca. | PPAT thickness was a potential detection metric for PCa and advanced PCa. | [17] |
| Clinical Research | 2017 | 162 patients who underwent MRI prior to prostatectomy. | PPAT fat ratio correlated with PCa aggressiveness. | [18] |
| Clinical Research | 2021 | 175 prostate cancer patients (mean age 62.5 years, mean prostate-specific antigen 5.4 ng/dL). | A higher periprostatic fat ratio was found to be significantly associated with a higher Gleason score by parametric magnetic resonance imaging (mpMRI). | [19] |
| Clinical Research | 2021 | 175 prostate cancer patients (mean age 62.5 years, mean prostate-specific antigen 5.4 ng/dL). | Increased periprostatic fat volume was associated with disease progression in prostate cancer patients. | [19] |
| Clinical Research | 2020 | 85 men with advanced PCa receiving ADT who had not received hormone therapy. | PPAT thickness was a predictor of survival in patients with advanced PCa not receiving hormonal therapy. | [20] |
| Basic Studies | 2012 | PPAT in prostate cancer patients, PC3, LNCaP. | PPAT-derived factors increased migration of PC3 and LNCaP cell lines, while PPAT had a strong proliferative effect on PC3 cell lines. | [21] |
| Basic Studies | 2021 | PPAT from 14 PCa patients (median age 62 years, median BMI 28.3) who underwent radical prostatectomy, DU145, PC3 | Conditioned medium (CM) culture of PPAT promoted migration of human androgen non-dependent PCa cell lines and upregulated CTGF expression. | [22] |
| Basic Studies | 2018 | DU145, PPAT in 36 Caucasians and 36 African-Caribbeans | Fatty acid (FA) content in PPAT is associated with PCa progression. | [23] |
| Basic Studies | 2021 | PPAT in vitro culture collection of conditioned medium, DU145, PC3. | PPAT secreted IGF-1 to upregulate TUBB2B β-microtubulin heterodimer to promote resistance to doxorubicin in prostate cancer. | [24] |
| Basic Studies | 2012 | PPAT, PC-3, and LNCaP cell lines from prostate cancer patients. | PPAT increased MMP (matrix metalloproteinase) activity to regulate the microenvironment of extraprostatic tumor cells and promoted prostate cancer cell survival and migration. | [21] |
| Basic Studies | 2019 | Prostate cancer cell lines C4-2B, Du-145, and PC-3. | Free fatty acids released by PPAT promoted tumor progression by affecting the HIF1/MMP14 pathway by stimulating NOX5/ROS. | [25] |
| Basic Studies | 2018 | Primary NK cells, C4-2, 3T3-L1. | Inhibition of the IL-6/leptin-JAK/Stat3 signaling axis in adipocytes enhanced immune killing of CRPC (castration-resistant prostate cancer) cells by NK cells. | [26] |
| Basic Studies | 2021 | Adipocytes isolated from PCa patients and PC3, 22RV1 | Decreased autophagic activity and increased intracellular lipid droplet content in PC3 cells after co-culture with adipocytes. | [27] |
| Basic Studies | 2012 | PPAT, LNCaP, PC3 in PCa patients. | PPAT-released pro-MMP-9 induced invasiveness of LNCaP (androgen-dependent) cells. | [28] |
| Basic Studies | 2009 | PPAT collected from patients undergoing radical prostatectomy. | PPAT regulated the aggressiveness of prostate cancer by providing IL-6. | [13] |
| Exosome Cargo | Source | Role in Tumor | References |
|---|---|---|---|
| MMP3 | 3T3-L1 adipocytes | Induction of lung cancer metastasis through activation of the MMP3/MMP9 process. | [87] |
| miRNA-21 | Cancer-associated adipocytes | Inhibition of the apoptotic process in ovarian cancer cells. | [89] |
| - | 3T3-L1 adipocytes | Reducing degradation of caspase 3/PARP molecules in PCa and improving resistance to doxorubicin in prostate cancer. | [37] |
| - | Adipocytes in the obese state | Enhanced estrogen receptor expression and growth, motility and invasion, stem-cell-like properties and epithelial–mesenchymal transition of triple-negative breast cancer cells through induction of HIF-1α activity. | [90] |
| miR-3940-5p, miR-22-3p, miR-16-5p | Adipose mesenchymal stem cells | Inhibiting the proliferation and migration of rectal cancer. | [91] |
| circ-DB | 3T3-L1 adipocytes | Inhibiting miR-34a and activating USP7/Cyclin A2 signaling pathway promote hepatocellular carcinoma growth and reduce DNA damage. | [92] |
| miR-381-3p | Adipose mesenchymal stem cells | Inhibition of apoptosis and progression of triple-negative breast cancer cells. | [93] |
| microRNA-1236 | Adipose mesenchymal stem cells | Inhibiting SLC9A1 and Wnt/β-linked protein signaling to reduce cisplatin resistance in breast cancer cells. | [94] |
| - | Adipose mesenchymal stem cells | Increasing COLGALT2 expression to promote osteosarcoma proliferation and metastasis. | [95] |
| miR-27a-3p | 3T3-L1 adipocytes | Inhibiting ICOS+ T cell proliferation and IFN-γ secretion to alter the immune microenvironment of lung adenocarcinoma. | [96] |
| miR-23a/b | 3T3-L1 adipocytes | Targeting the VHL/HIF axis to promote HCC cell growth and migration. | [88] |
| hsa-miR-124-3p | Adipose mesenchymal stem cells | Inhibiting the growth and proliferation of ovarian cancer cells. | [97] |
| microRNA-21 | Cancer-associated adipocytes | Targeting APAF1 promotes paclitaxel resistance in ovarian cancer cells. | [89] |
| - | Adipose mesenchymal stem cells | Mediated Wnt signaling pathway induces migration of breast cancer cells. | [98] |
| Exosome Cargo | Source | Role in Adipose | References |
|---|---|---|---|
| miRNA-126 | Breast cancer | Decreases the uptake of glucose by fat cells and increases their secretion of lactate and pyruvate. | [99] |
| miRNA-155 | Breast cancer | Promotes beige/brown differentiation and remodeling of adipocytes through downregulation of PPARγ expression. | [100] |
| ciRS-133 | Gastric cancer | Regulating preadipocytes and regulating preadipocyte differentiation. | [101] |
| IL-6 | Lung cancer | Inducing adipocyte lipolysis by mediating the STAT3 pathway. | [102] |
| Parathyroid hormone-related protein | Lewis lung carcinoma | Inducting lipolysis and adipose tissue browning through the PKA pathway. | [103] |
| miR-425-3p | A549, H1299 and AGS | Inducing white adipocyte atrophy. | [104] |
| miR-155 | Stomach cancer cells | Inhibiting adipogenesis and promoting brown adipose differentiation via C/EPBβ pathway in adipose mesenchymal stem cells. | [105] |
| ciRS-133 | Gastric cancer cells | Activation of PRDM16 and inhibition of miR-133 promote differentiation of preadipocytes into brown adipocytes. | [101] |
| circ_0004303 | Gastric cancer cells | Promoting migration and invasion of adipose mesenchymal stem cells. | [106] |
| miR-146b-5p | Human colorectal cancer tissue | Promoting adipose tissue browning and inhibiting HOXC10 to accelerate lipolysis. | [107] |
| - | HepG2 | Inducing adipose MSCs to differentiate into cancer-associated myofibroblasts. | [108] |
| Adrenomedullin | Human pancreatic cancer tissue exosomes | Activating p38 and ERK1/2 MAPK and promoting lipolysis by phosphorylating hormone-sensitive lipase. | [109] |
| H-ras, miR-125b, miR-155, and GTPases | C4-2B prostate cancer cells | Inducing prostate-tumor-like transformation of adipose stem cells. | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Lou, K.; Luo, C.; Zou, J.; Zou, X.; Zhang, G. Obesity-Related Cross-Talk between Prostate Cancer and Peripheral Fat: Potential Role of Exosomes. Cancers 2022, 14, 5077. https://doi.org/10.3390/cancers14205077
Feng S, Lou K, Luo C, Zou J, Zou X, Zhang G. Obesity-Related Cross-Talk between Prostate Cancer and Peripheral Fat: Potential Role of Exosomes. Cancers. 2022; 14(20):5077. https://doi.org/10.3390/cancers14205077
Chicago/Turabian StyleFeng, Shangzhi, Kecheng Lou, Cong Luo, Junrong Zou, Xiaofeng Zou, and Guoxi Zhang. 2022. "Obesity-Related Cross-Talk between Prostate Cancer and Peripheral Fat: Potential Role of Exosomes" Cancers 14, no. 20: 5077. https://doi.org/10.3390/cancers14205077
APA StyleFeng, S., Lou, K., Luo, C., Zou, J., Zou, X., & Zhang, G. (2022). Obesity-Related Cross-Talk between Prostate Cancer and Peripheral Fat: Potential Role of Exosomes. Cancers, 14(20), 5077. https://doi.org/10.3390/cancers14205077





