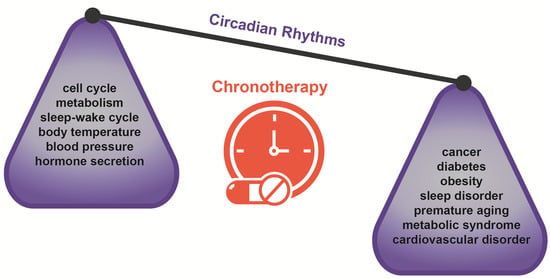
Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy
Abstract
:Simple Summary
Abstract
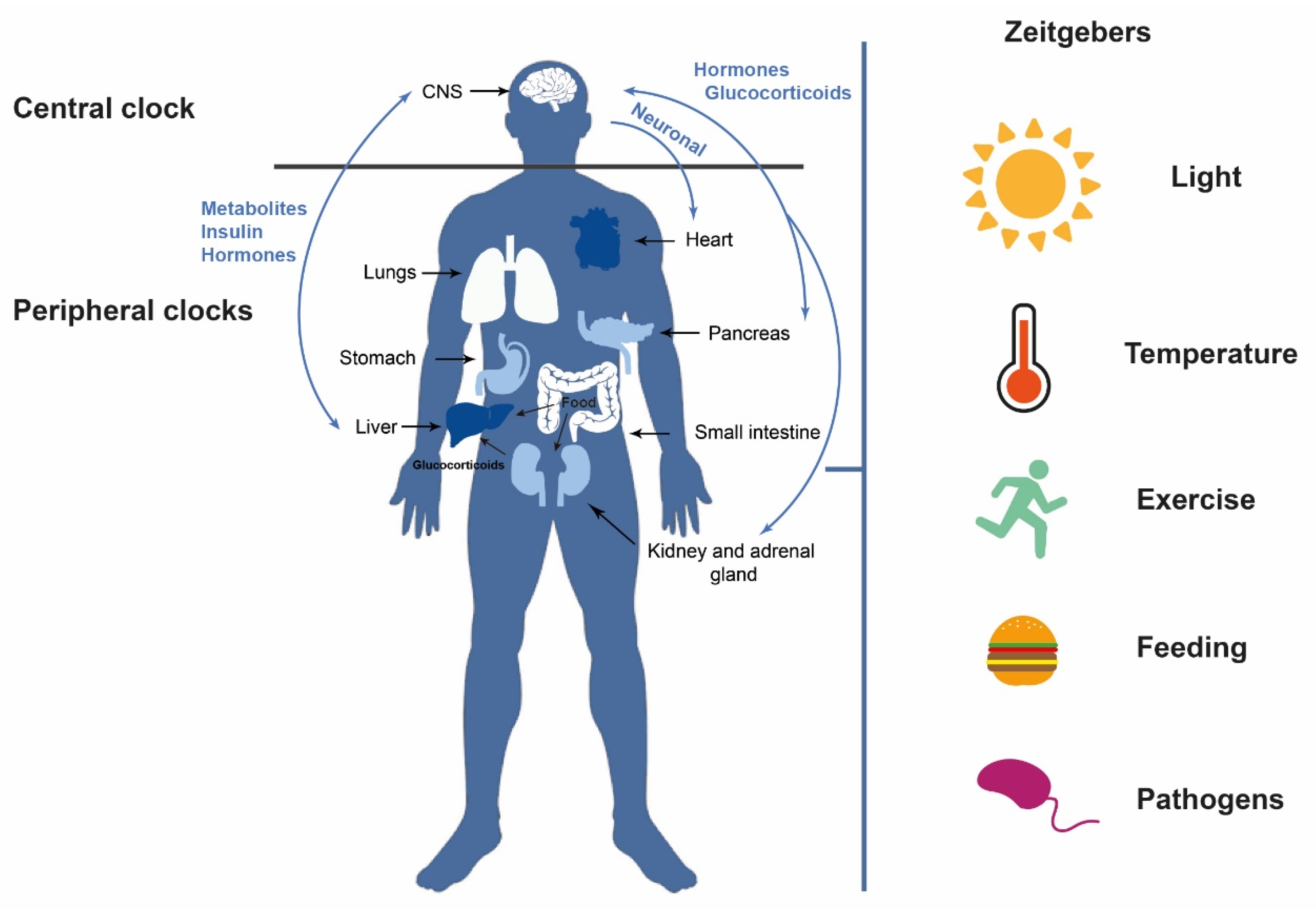
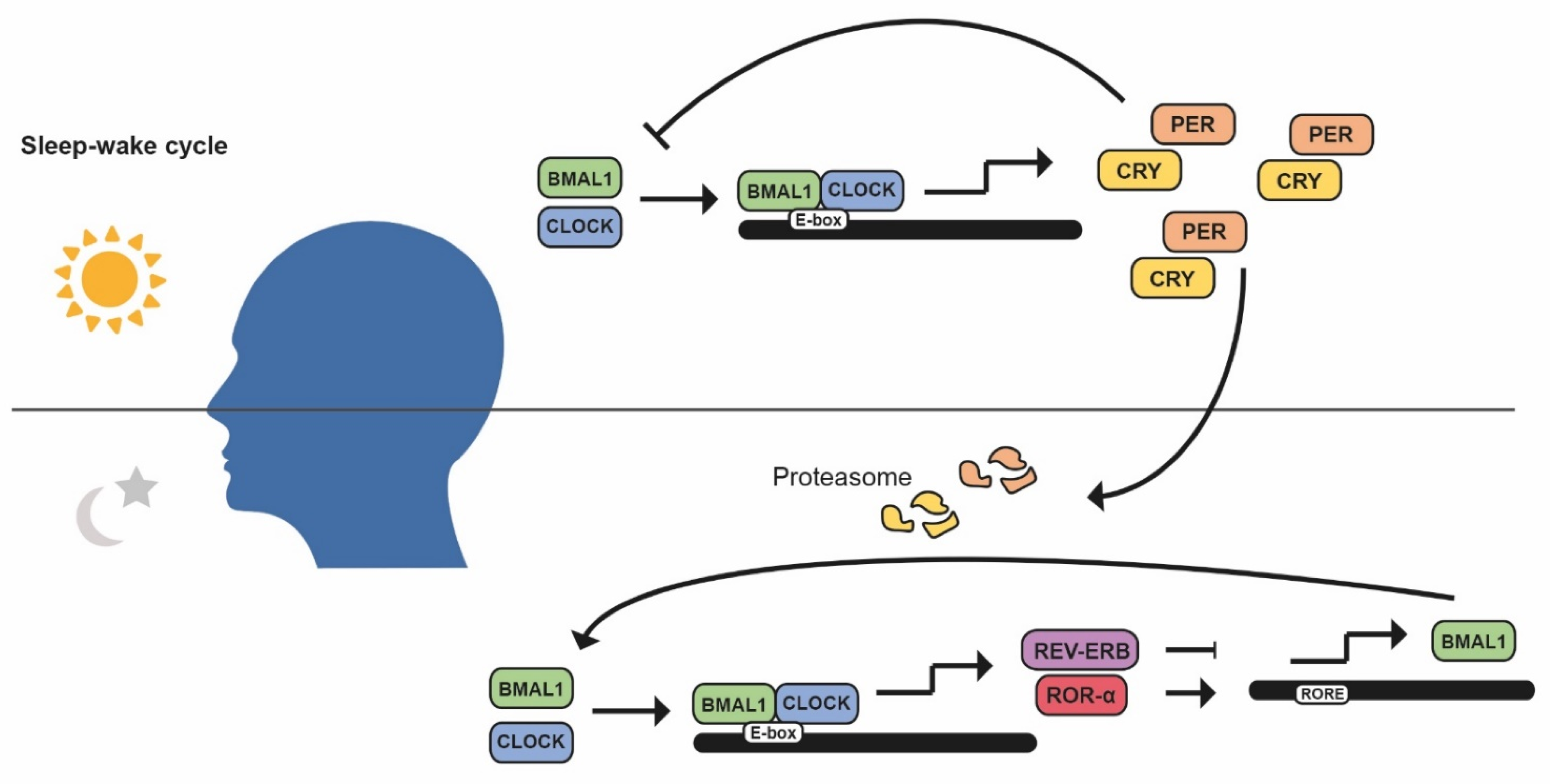
1. Introduction: Circadian Rhythms
2. Circadian Rhythms and Cancer
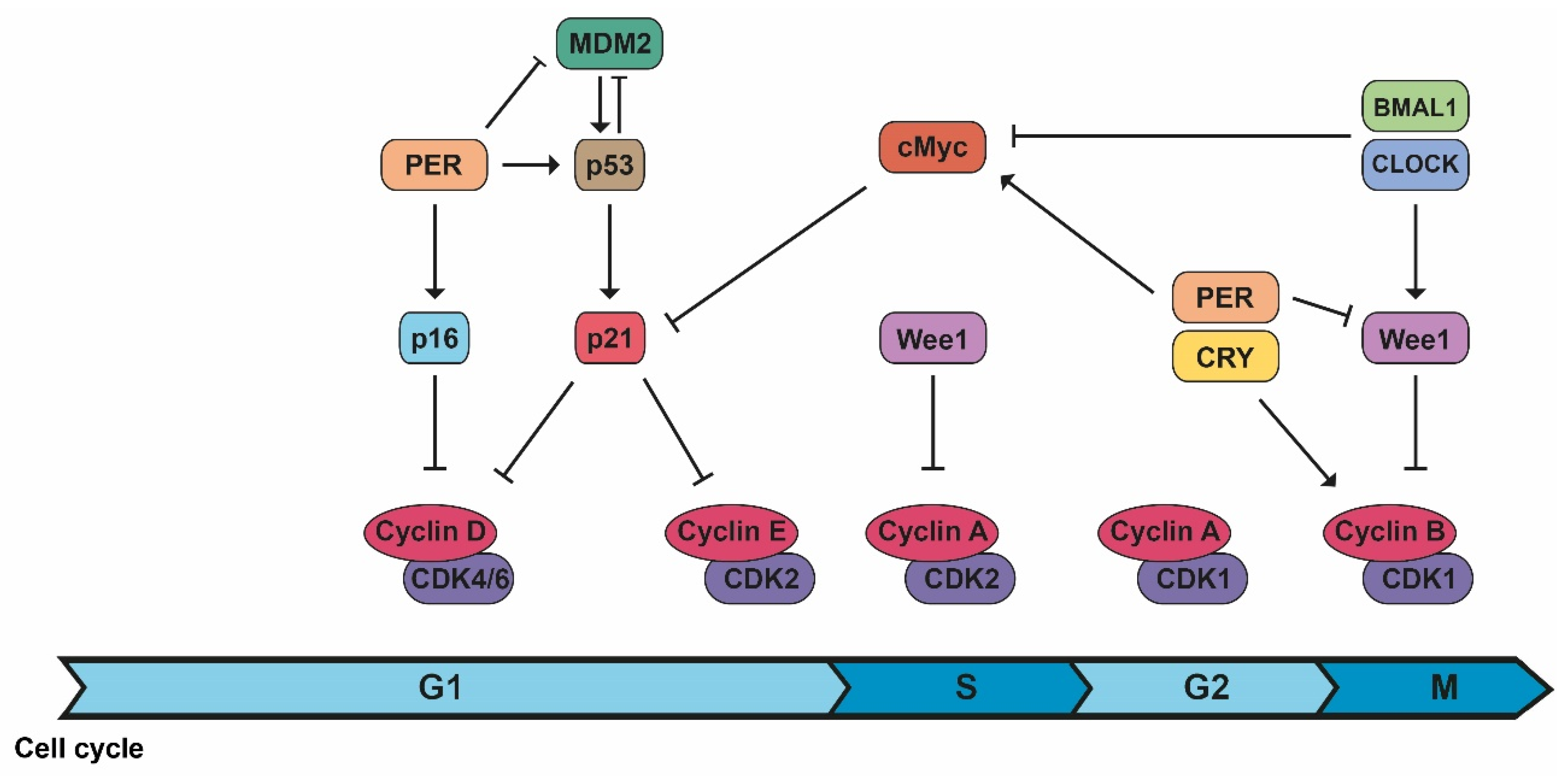
2.1. Cell Cycle Progression
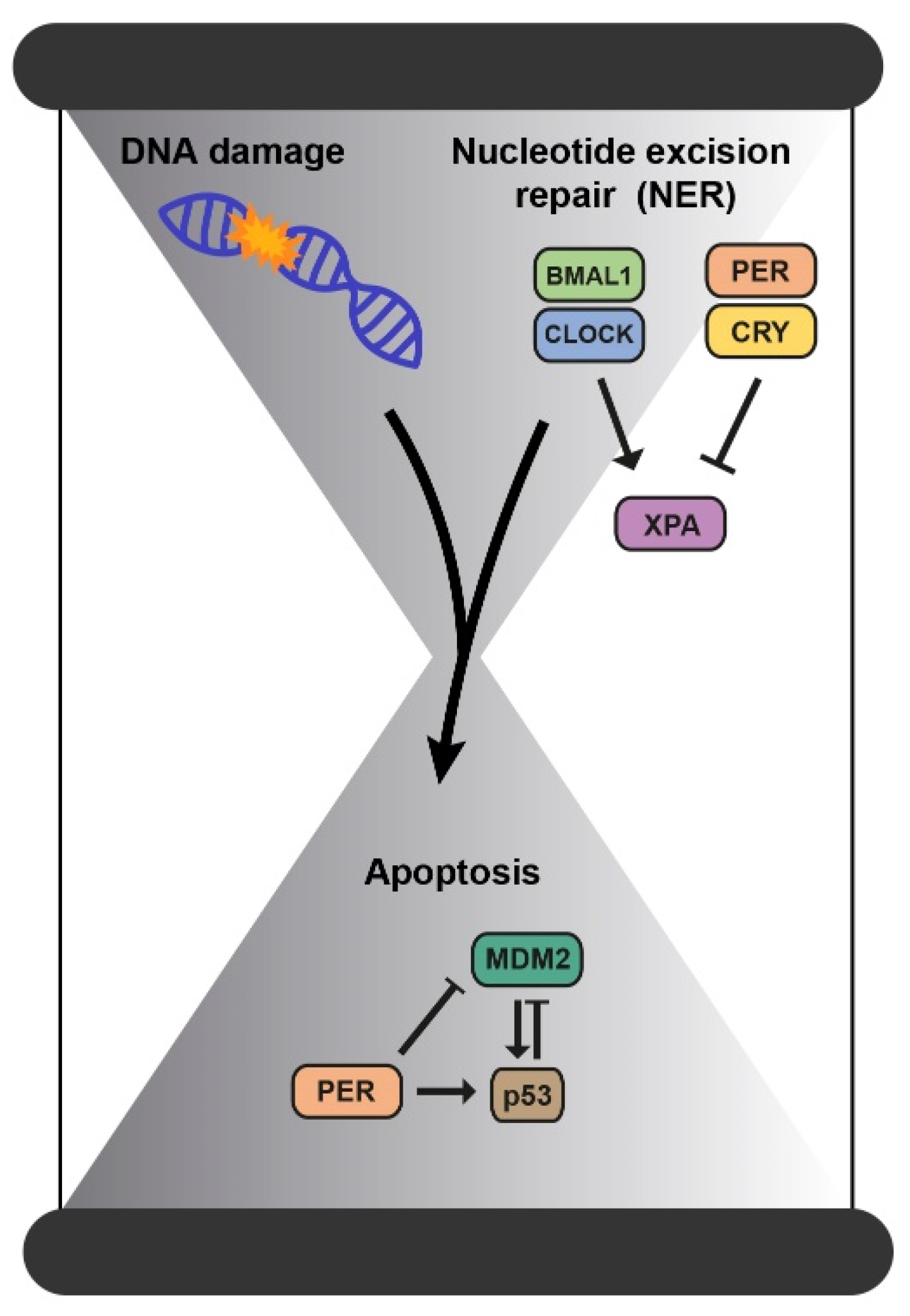
2.2. Mechanisms of DNA Repair
2.3. Mitochondrial Dysfunction
2.4. Reprogramming of Metabolism
2.5. The Immune System
| Cell Cycle Progression | ||
| Transitions from cell cycle phases are controlled by time windows established by the biological clock. | [26,27] | |
| CDK/cyclin B1 | Circadian controlled by Wee1, whose expression varies during the day because of CLOCK/BMAL1 activation and PER/CRY inhibition. | [2,3] |
| CDK/cyclin complexes | PER1 interacts with the checkpoint kinase Chk1 and controls the p16-INK4A gene, an inhibitor of CDK/cyclin complexes. c-Myc expression (controlled and inhibited by CLOCK/BMAL1 and stabilized by PER1), inhibits the expression of p21, another inhibitor of CDK/cyclin complexes. | [2,3] |
| Mechanisms of DNA repair | ||
| Mismatch repair (MMR) Double-strand breaks (DSBs) | Indirectly influenced by the clock as both occur during replication. | [33,36] |
| Nucleotide excision repair (NER) | Directly regulated by the clock through the repair factor XPA. | [36,39,40] |
| Mitochondrial dysfunction | ||
| BMAL1 knockout mice | Low levels of some mitochondrial fusion proteins. | [43] |
| PER1/2 knockout mice | Altered mitochondrial respiration. | [44] |
| Reprogramming of metabolism | ||
| Pancreas | Pancreatic differentiation is regulated by the biological clock through Wnt and Notch pathways and the cell cycle. | [48] |
| Misaligned meals uncouple insulin and corticosterone rhythms contributing to pancreas-associated conditions. | [49] | |
| Alterations in sleep habits are associated with high levels of Haemoglobin A1c (HbA1c) in young people with type 1 diabetes and with increased insulin requirements. | [49] | |
| The immune system | ||
| Sleep period | Highest quantity of undifferentiated T lymphocytes and NK cells. | [52] |
| Highest levels of proinflammatory cytokines (such as IL-1β and TNF-α). | [52,53] | |
| Active period | Highest levels of anti-inflammatory cytokines (such as IL-4 and IL-10). | [52,53] |
| Glucocorticoids, potent immunosuppressants, peak secretion. For example, cortisol levels are higher in the morning. | [51] | |
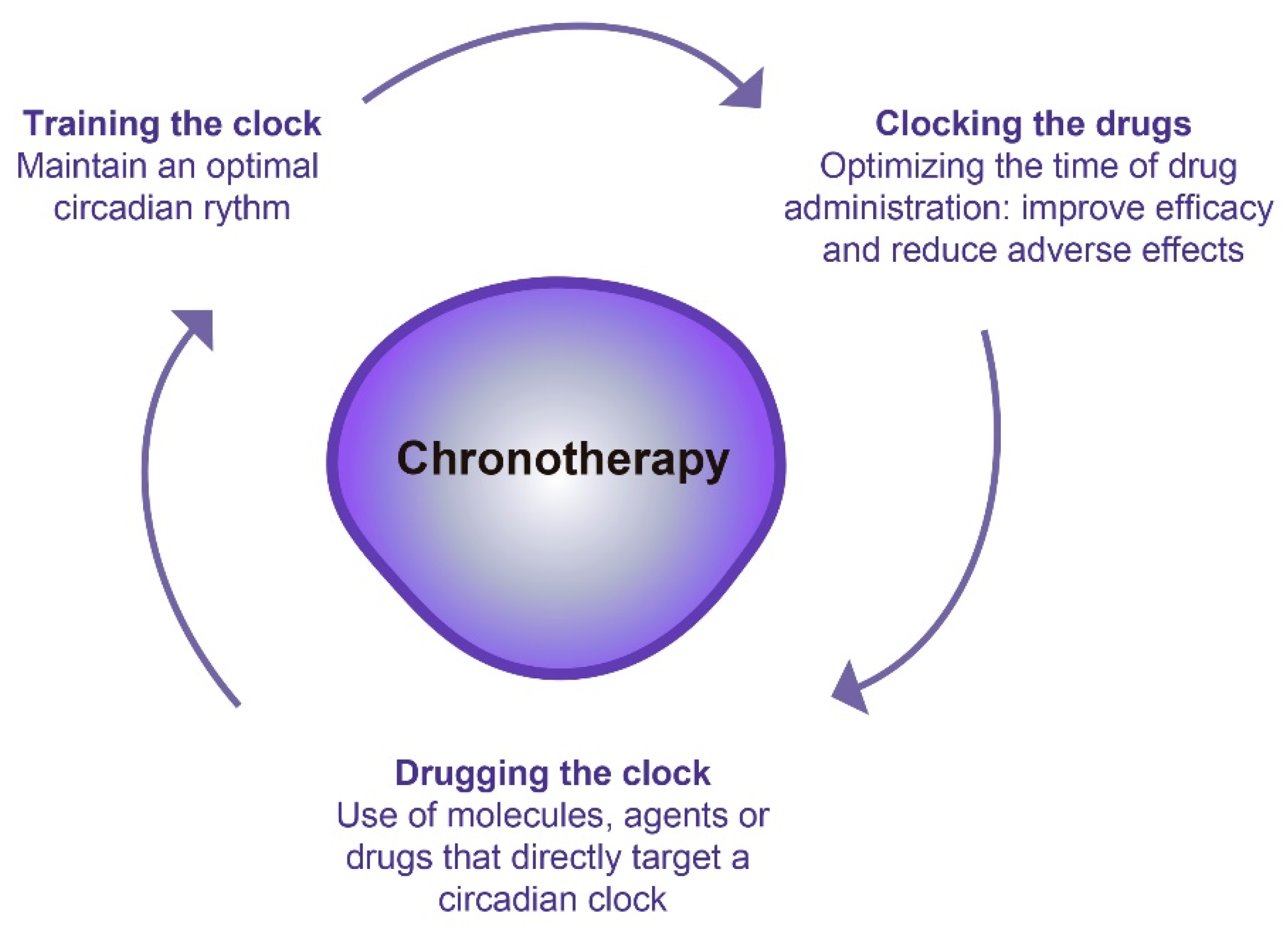
3. Chronotherapy: A Promising Therapeutic Option
3.1. Effect of Chronotherapy in Chemotherapy
3.2. Effect of Chronotherapy in Radiotherapy
3.3. Effect of Chronotherapy on the Blood–Brain Barrier
3.4. Effect of Chronotherapy on the Immune System
3.5. Other Uses of Chronotherapy in Cancer
| Effect of Chronotherapy in Chemotherapy | ||
| Oxaliplatin | Chronomodulated delivery: peak at 16:00 h. | [83,84,85,86,87,88,89] |
| Cisplatin | Non-small cell lung cancer: | |
| Low hematological and gastrointestinal adverse effects in the group following chronotherapy. | [90] | |
| Cisplatin + doxorubicin or pirarubicin | Ovarian cancer: | |
| Cisplatin in the evening 16:00–20:00) combined with doxorubicin or pirarubicin in the morning (06:00) cause less toxicity/side effects and high tumor response. | [60,81,91] | |
| Cisplatin + doxorubicin had also tumor response in endometrial carcinoma and bladder cancer. | [91] | |
| Fluorodeoxyuridine | Renal cell carcinoma: Circadian-modulated (68% of the daily dose administered in the evening) administration induces a durable tumor response with little toxicity. | [91] |
| 5-FU | Fewer adverse side effects in digestive cancers. Chronoadministration of oxaliplatin-5FU-leucovorin (ChronoFLO4) produced a survival advantage in males with colorectal cancer. | [88] |
| Irinotecan | Better tolerability after morning delivery in men and in the afternoon in women with metastatic colorectal cancer. | [93] |
| Effect of chronotherapy in radiotherapy | ||
| Brain metastasis in patients with non-small cell lung cancer: Better survival in patients treated in the morning (before 12:30 h). | [101] | |
| High-grade glioma: No differences in survival. | [102] | |
| Breast cancer: Radiotherapy in the afternoon induced less skin toxicity. | [104] | |
| Bone metastases: Females treated with radiotherapy in the morning exhibited a higher complete or partial response. | [105] | |
| Effect of chronotherapy on the blood–brain barrier | ||
| Temozolomide (TMZ) | Morning administration increases overall survival in patients with methylated MGMT, coinciding with the peak of BMAL1 expression. | [114] |
| Bortezomib | Night administration induces 70% tumor growth inhibition. | [115] |
| Effect of chronotherapy on the immune system | ||
| LYC-53772 and LYC-54143 | RORγ synthetic agonists: Activate BMAL1 transcription, induce T cells differentiation, block regulatory T cell-induced immunosuppression, elevate the secretion of cytokines, induced resistance to PD-L1 inhibition in T cells, and increase the cytotoxic activity of T cells. | [116,117] |
| SR1078 | RORα synthetic agonist: Increases CD8+ T cell response. | [119] |
| Interferon-β | Better antitumor effect during the day in mice. | [120] |
| Ipilimumab, Nivolumab, or Pembrolizumab | Melanoma: Morning or early afternoon administration extended overall survival. | [122] |
4. Challenges and Prospects for the Future of Chronotherapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Halberg, F. Susceptibility to Ouabain and Physiologic Circadian Periodicity. J. Minn. Acad. Sci. 1959, 27, 139–143. Available online: http://ci.nii.ac.jp/naid/10016410844/en/ (accessed on 8 July 2022).
- Ruby, C.L.; Major, R.J.; Hinrichsen, R.D. Regulation of Tissue Regeneration by the Circadian Clock. Eur. J. Neurosci. 2021, 53, 3576–3597. [Google Scholar] [CrossRef]
- García-Costela, M.; Escudero-Feliú, J.; Puentes-Pardo, J.D.; San Juán, S.M.; Morales-Santana, S.; Ríos-Arrabal, S.; Carazo, Á.; León, J. Circadian Genes as Therapeutic Targets in Pancreatic Cancer. Front. Endocrinol. 2020, 11, 638. [Google Scholar] [CrossRef]
- Foster, R.G. Sleep, Circadian Rhythms and Health. Interface Focus 2020, 10, 20190098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Chronotherapy. Handb. Clin. Neurol. 2021, 179, 357–370. [Google Scholar]
- Bass, J.; Takahashi, J. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhr, E.D.; Yoo, S.H.; Takahashi, J.S. Temperature as A Universal Resetting Cue for Mammalian Circadian Oscillators. Science 2010, 330, 379–385. Available online: http://science.sciencemag.org/content/330/6002/379.abstract (accessed on 8 July 2022). [CrossRef] [PubMed] [Green Version]
- Jacob, H.; Curtis, A.M.; Kearney, C.J. Therapeutics on the Clock: Circadian Medicine in the Treatment of Chronic Inflammatory Diseases. Biochem. Pharmacol. 2020, 182, 114254. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Maemura, K. Circadian Clock and Cardiovascular Disease. J. Cardiol. 2011, 57, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comas, M.; Gordon, C.J.; Oliver, B.G.; Stow, N.W.; King, G.; Sharma, P.; Ammit, A.J.; Grunstein, R.R.; Phillips, C.L. A Circadian Based Inflammatory Response—Implications for Respiratory Disease and Treatment. Sleep Sci. Pract. 2017, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Aroca-Siendones, M.I.; Moreno-Sanjuan, S.; Puentes-Pardo, J.D.; Verbeni, M.; Arnedo, J.; Escudero-Feliu, J.; García-Costela, M.; García-Robles, A.; Carazo, Á.; León, J. Core Circadian Clock Proteins as Biomarkers of Progression in Colorectal Cancer. Biomedicines 2021, 9, 967. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2009, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, D.W. Neuroscience in the 21st Century: From Basic to Clinical; Springer: Berlin, Germany, 2013; pp. 1–3111. [Google Scholar]
- Nagoshi, E. The Mammalian Circadian Timing System: From Gene Expression to Physiology. Chromosoma 2004, 113, 103–112. [Google Scholar]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Lévi, F.A. Systems Chronotherapeutics. Pharmacol. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to Immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular Architecture of the Mammalian Circadian Clock. Trends Cell Biol. 2014, 24, 90–99. Available online: https://www.sciencedirect.com/science/article/pii/S096289241300113x (accessed on 8 July 2022). [CrossRef] [PubMed] [Green Version]
- O-Neill, J.S.; Reddy, A.B. Circadian Clocks in Human Red Blood Cells. Nature 2011, 469, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Xiong, Y.; Tao, R.; Panayi, A.C.; Mi, B.; Liu, G. Emerging Insight Into the Role of Circadian Clock Gene Bmal1 in Cellular Senescence. Front. Endocrinol. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Mcclean, C.; Davison, G.W. Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots? Antioxidants 2022, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Reza, H.M.; Shinohara, K.; Nakahata, Y. Cellular Senescence and Its Impact on the Circadian Clock. J. Biochem. 2021, 171, 493–500. [Google Scholar] [CrossRef]
- Blacher, E.; Tsai, C.; Litichevskiy, L.; Shipony, Z.; Iweka, C.A.; Schneider, K.M.; Chuluun, B.; Heller, H.C.; Menon, V.; Thaiss, C.A.; et al. Aging Disrupts Circadian Gene Regulation and Function in Macrophages. Nat. Immunol. 2022, 23, 229–236. Available online: https://pubmed.ncbi.nlm.nih.gov/34949832/ (accessed on 12 July 2022). [CrossRef] [PubMed]
- Liang, C.; Ke, Q.; Liu, Z.; Ren, J.; Zhang, W.; Hu, J.; Wang, Z.; Chen, H.; Xia, K.; Lai, X.; et al. Bmal1 Moonlighting as A Gatekeeper for Line1 Repression and Cellular Senescence in Primates. Nucleic Acids Res. 2022, 50, 3323–3347. [Google Scholar] [PubMed]
- Randler, C.; Engelke, J. Gender Differences in Chronotype Diminish With Age: A Meta-Analysis Based On Morningness/Chronotype Questionnaires. Chronobiol. Int. 2019, 36, 888–905. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Destici, E.; Oklejewicz, M.; Saito, S.; Van Der Horst, G.T.J. Mammalian Cryptochromes Impinge On Cell Cycle Progression in A Circadian Clock-Independent Manner. Cell Cycle 2011, 10, 3788–3797. Available online: https://pubmed.ncbi.nlm.nih.gov/22033214/ (accessed on 8 July 2022). [CrossRef] [PubMed] [Green Version]
- Lévi, F.; Filipski, E.; Iurisci, I.; Li, X.M.; Innominato, P. Cross-Talks Between Circadian Timing System and Cell Division Cycle Determine Cancer Biology and Therapeutics. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 465–475. Available online: https://pubmed.ncbi.nlm.nih.gov/18419306/ (accessed on 8 July 2022). [CrossRef] [PubMed]
- Droin, C.; Paquet, E.R.; Naef, F. Low-Dimensional Dynamics of Two Coupled Biological Oscillators. Nat. Phys. 2019, 15, 1086–1094. [Google Scholar] [CrossRef]
- Altinok, A.; Lévi, F.; Goldbeter, A. A Cell Cycle Automaton Model for Probing Circadian Patterns of Anticancer Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 1036–1053. [Google Scholar] [CrossRef]
- Altinok, A.; Lévi, F.; Goldbeter, A. Identifying Mechanisms of Chronotolerance and Chronoefficacy for the Anticancer Drugs 5-Fluorouracil and Oxaliplatin by Computational Modeling. Eur. J. Pharm. Sci. 2009, 36, 20–38. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The Metastatic Spread of Breast Cancer Accelerates During Sleep. Nature 2022, 607, 156–162. Available online: https://pubmed.ncbi.nlm.nih.gov/35732738/ (accessed on 13 July 2022). [CrossRef]
- Antoch, M.P.; Gorbacheva, V.Y.; Vykhovanets, O.; Toshkov, I.A.; Kondratov, R.V.; Kondratova, A.A.; Lee, C.; Nikitin, A.Y. Disruption of the Circadian Clock Due to the Clock Mutation Has Discrete Effects On Aging and Carcinogenesis. Cell Cycle 2008, 7, 1197. Available online: /pmc/articles/pmc2744375/ (accessed on 5 July 2022). [CrossRef] [Green Version]
- Sancar, A.; Lindsey-Boltz, L.A.; Gaddameedhi, S.; Selby, C.P.; Ye, R.; Chiou, Y.Y.; Kemp, M.G.; Hu, J.; Lee, J.H.; Ozturk, N. Circadian Clock, Cancer, and Chemotherapy. Biochemistry 2015, 54, 110–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, H.L.; Linden, R.; Wang, J.Y. Dna Damage-Induced Cell Death: Lessons from the Central Nervous System. Cell Res. 2008, 18, 17. Available online: /Pmc/Articles/Pmc2626635/ (accessed on 7 July 2022). [CrossRef] [PubMed]
- Gotoh, T.; Vila-Caballer, M.; Santos, C.S.; Liu, J.; Yang, J.; Finkielstein, C.V. The Circadian Factor Period 2 Modulates P53 Stability and Transcriptional Activity in Unstressed Cells. Mol. Biol. Cell 2014, 25, 3081–3093. Available online: https://pubmed.ncbi.nlm.nih.gov/25103245/ (accessed on 6 July 2022). [CrossRef] [Green Version]
- Kang, T.H.; Reardon, J.T.; Kemp, M.; Sancar, A. Circadian Oscillation of Nucleotide Excision Repair in Mammalian Brain. Proc. Natl. Acad. Sci. USA 2009, 106, 2864–2867. [Google Scholar] [CrossRef] [Green Version]
- Brown, W.R. A Review and Mathematical Analysis of Circadian Rhythms in Cell Proliferation in Mouse, Rat, and Human Epidermis. J. Invest. Dermatol. 1991, 97, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbacheva, V.Y.; Kondratov, R.V.; Zhang, R.; Cherukuri, S.; Gudkov, A.V.; Takahashi, J.S.; Antoch, M.P. Circadian Sensitivity to the Chemotherapeutic Agent Cyclophosphamide Depends on the Functional Status of the Clock/Bmal1 Transactivation Complex. Proc. Natl. Acad. Sci. USA 2005, 102, 3407–3412. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.H. Circadian Rhythm of Ner and Atr Pathways. Biomolecules 2021, 11, 715. [Google Scholar] [CrossRef]
- Gaddameedhi, S.; Reardon, J.T.; Ye, R.; Ozturk, N.; Sancar, A. Cell Cycle Effect of Circadian Clock Mutations On Dna Damage Response in Mammalian Cells View Supplementary Material. Cell Cycle 2012, 3481, 3481–3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagoshi, E.; Brown, S.A.; Dibner, C.; Kornmann, B.; Schibler, U. Circadian Gene Expression in Cultured Cells. Methods Enzymol. 2005, 393, 543–557. [Google Scholar]
- Kozakiewicz, P.; Grzybowska-Szatkowska, L.; Ciesielka, M.; Rzymowska, J. The Role of Mitochondria in Carcinogenesis. Int. J. Mol. Sci. 2021, 22, 5100. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.S.; Valera-Alberni, M.; Cantó, C.; Pillon, N.J. Circadian Rhythms and Mitochondria: Connecting the Dots. Front. Genet. 2018, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Das, P.; Hashimoto, I.; Nakao, T.; Deguchi, Y.; Gouraud, S.S.; Waki, H.; Muragaki, Y.; Maeda, M. The Circadian Clock Maintains Cardiac Function by Regulating Mitochondrial Metabolism in Mice. PLoS ONE 2014, 9, E112811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld-Cohen, A.; Robles, M.S.; Aviram, R.; Manella, G.; Adamovich, Y.; Ladeuix, B.; Nir, D.; Rousso-Noori, L.; Kuperman, Y.; Golik, M.; et al. Circadian Control of Oscillations in Mitochondrial Rate-Limiting Enzymes and Nutrient Utilization by Period Proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E1673–E1682. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21376230 (accessed on 17 February 2019). [CrossRef] [Green Version]
- Walton, Z.E.; Patel, C.H.; Brooks, R.C.; Yu, Y.; Ibrahim-Hashim, A.; Riddle, M.; Porcu, A.; Jiang, T.; Ecker, B.L.; Tameire, F.; et al. Acid Suspends the Circadian Clock in Hypoxia Through Inhibition of Mtor. Cell 2018, 174, 72–87.E32. Available online: https://pubmed.ncbi.nlm.nih.gov/29861175/ (accessed on 13 July 2022). [CrossRef] [Green Version]
- Li, Z.; Ruan, L.; Lin, S.; Gittes, G.K. Clock Controls Timing of Mouse Pancreatic Differentiation Through Regulation of Wnt- and Notch-Based and Cell Division Components. Biochem. Biophys. Res. Commun. 2007, 359, 491–496. Available online: https://pubmed.ncbi.nlm.nih.gov/17559809/ (accessed on 13 July 2022). [CrossRef] [PubMed]
- Honzlová, P.; Novosadová, Z.; Houdek, P.; Sládek, M.; Sumová, A. Misaligned Feeding Schedule Elicits Divergent Circadian Reorganizations in Endo- and Exocrine Pancreas Clocks. Cell. Mol. Life Sci. 2022, 79, 1–16. Available online: https://pubmed.ncbi.nlm.nih.gov/35622158/ (accessed on 13 July 2022). [CrossRef]
- Von Schnurbein, J.; Boettcher, C.; Brandt, S.; Karges, B.; Dunstheimer, D.; Galler, A.; Denzer, C.; Denzer, F.; Vollbach, H.; Wabitsch, M.; et al. Sleep and Glycemic Control in Adolescents With Type 1 Diabetes. Pediatr. Diabetes 2018, 19, 143–149. Available online: https://pubmed.ncbi.nlm.nih.gov/28880049/ (accessed on 13 July 2022). [CrossRef]
- Cash, E.; Sephton, S.; Woolley, C.; Elbehi, A.M.; RI, A.; Ekine-Afolabi, B.; Kok, V.C. The Role of the Circadian Clock in Cancer Hallmark Acquisition and Immune-Based Cancer Therapeutics. J. Exp. Clin. Cancer Res. 2021, 40, 119. [Google Scholar] [CrossRef]
- Dimitrov, S.; Benedict, C.; Heutling, D.; Westermann, J.; Born, J.; Lange, T. Cortisol and Epinephrine Control Opposing Circadian Rhythms in T Cell Subsets. Blood 2009, 113, 5134–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zeng, P.; Gao, W.; Zhou, Q.; Feng, T.; Tian, X. Circadian Clock: A Regulator of the Immunity in Cancer. Cell Commun. Signal. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Schrepf, A.; Thaker, P.H.; Goodheart, M.J.; Bender, D.; Slavich, G.M.; Dahmoush, L.; Penedo, F.; Degeest, K.; Mendez, L.; Lubaroff, D.M.; et al. Diurnal Cortisol and Survival in Epithelial Ovarian Cancer. Psychoneuroendocrinology 2015, 53, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haus, E.; Smolensky, M.H. Biologic Rhythms in the Immune System. Chronobiol. Int. 1999, 16, 581–622. [Google Scholar] [CrossRef]
- Lemmer, B. Chronobiology, drug-delivery, and chronotherapeutics. Adv. Drug Deliv. Rev. 2007, 59, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Field, J.M.; Sehgal, A. Circadian Rhythms, Disease and Chronotherapy. J. Biol. Rhythms. 2021, 36, 503–531. [Google Scholar] [CrossRef]
- Dong, D.; Yang, D.; Lin, L.; Wang, S.; Wu, B. Circadian Rhythm in Pharmacokinetics and Its Relevance to Chronotherapy. Biochem. Pharmacol. 2020, 178, 114045. [Google Scholar] [CrossRef]
- Lu, D.; Wang, Z.; Wu, B. Pharmacokinetics-Based Chronotherapy. Curr. Drug Metab. 2022, 23, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Roles of Circadian Clocks in Cancer Pathogenesis and Treatment. Exp. Mol. Med. 2021, 53, 1529–1538. [Google Scholar] [CrossRef]
- Mahapatra, U.; Maiti, R.N.; Purkait, B.; Saha, D.; Mandal, S. Comparative Study On Efficacy and Safety of Morning Dose Versus Evening Dose of Levothyroxine in Treatment of Hypothyroidism: An Outpatient Department Based Prospective Interventional Study. IJBCP 2020, 9, 1554–1559. [Google Scholar] [CrossRef]
- Dallmann, R.; Okyar, A.; Lévi, F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol. Med. 2016, 22, 430–445. Available online: https://www.sciencedirect.com/science/article/pii/S1471491416000563 (accessed on 8 July 2022). [CrossRef] [PubMed] [Green Version]
- Hesse, J.; Martinelli, J.; Aboumanify, O.; Ballesta, A.; Relógio, A. A Mathematical Model of the Circadian Clock and Drug Pharmacology to Optimize Irinotecan Administration Timing in Colorectal Cancer. Comput. Struct. Biotechnol. J. 2021, 19, 5170–5183. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.K.; Seo, H.S.; Hyun, M.S.; Han, K.R.; Cho, S.W. Efficacy and Safety of Morning Versus Evening Dose of Controlled-Release Simvastatin Tablets in Patients With Hyperlipidemia: A Randomized, Double-Blind, Multicenter Phase Iii Trial. Clin. Ther. 2013, 35, 1350–1360.E1. [Google Scholar] [CrossRef]
- Hermida, R.C.; Calvo, C.; Ayala, D.E.; Domínguez, M.J.; Covelo, M.; Fernández, J.R.; Mojón, A.; López, J.E. Administration Time—Dependent Effects of Valsartan On Ambulatory Blood Pressure in Hypertensive Subjects. Hypertension 2003, 42, 283–290. [Google Scholar] [CrossRef]
- Pagani, L.; Schmitt, K.; Meier, F.; Izakovic, J.; Roemer, K.; Viola, A.; Cajochen, C. Serum Factors in Older Individuals Change Cellular Clock Properties. Proc. Natl. Acad. Sci. USA 2011, 108, 7218–7223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, J.; Vlasac, I.; Anderson, S.; Kyle, S.; Dixon, W.; Bechtold, D.; Gill, S.; Little, M.; Luik, A.; Loudon, A.; et al. Genome-Wide Association Analysis Identifies Novel Loci for Chronotype in 100,420 Individuals from the Uk Biobank. Nat. Commun. 2016, 7, 10889. [Google Scholar] [CrossRef] [Green Version]
- Ohdo, S. Chronotherapeutic Strategy: Rhythm Monitoring, Manipulation and Disruption. Adv. Drug Deliv. Rev. 2010, 62, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, F.; Guo, L.; Chen, M.; Yuan, X.; Wu, B. Small Heterodimer Partner Regulates Circadian Cytochromes P450 and Drug-Induced Hepatotoxicity. Theranostics 2018, 8, 5246–5258. [Google Scholar] [CrossRef]
- Lu, D.; Zhao, M.; Chen, M.; Wu, B. Circadian Clock-Controlled Drug Metabolism: Implications for Chronotherapeutics. Drug Metab. Dispos. Drug Metab. Dispos. 2020, 48, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lin, Y.; Gao, L.; Yang, Z.; Wang, S.; Wu, B. Cyp3a11 Metabolism-Based Chronotoxicity of Brucine in Mice. Toxicol. Lett. 2019, 313, 188–195. Available online: https://www.Sciencedirect.com/science/article/pii/S0378427419301882 (accessed on 8 July 2022). [CrossRef]
- Lin, Y.; Wang, S.; Zhou, Z.; Guo, L.; Yu, F.; Wu, B. Bmal1 Regulates Circadian Expression of Cytochrome P450 3a11 and Drug Metabolism in Mice. Commun. Biol. 2019, 2, 378. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, J.; Dulong, S.; Li, X.M.; Teboul, M.; Soliman, S.; Lévi, F.; Fages, F.; Ballesta, A. Model Learning to Identify Systemic Regulators of the Peripheral Circadian Clock. Bioinformatics 2021, 37 (Suppl. S1), I401–I409. [Google Scholar] [CrossRef] [PubMed]
- Innominato, P.F.; Lévi, F.A.; Bjarnason, G.A. Chronotherapy and the Molecular Clock: Clinical Implications in Oncology. Adv. Drug Deliv. Rev. 2010, 62, 979–1001. Available online: https://www.sciencedirect.com/science/article/pii/S0169409x10001389 (accessed on 8 July 2022). [CrossRef] [PubMed]
- Ruben, M.D.; Smith, D.F.; Fitzgerald, G.A.; Hogenesch, J.B. Dosing Time Matters. Science 2019, 365, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ren, L.; Jiang, M.; Chu, Y. Anti-Hypertensive Efficacy of Amlodipine Dosing During Morning Versus Evening: A Meta-Analysis. Rev. Cardiovasc. Med. 2019, 20, 91–98. [Google Scholar]
- Inhibitor, T. Safety and Efficacy of Elobixibat, An Ileal Bile Acid Transporter Inhibitor, in Elderly Patients With Chronic Idiopathic Constipation According to Administration Time: Interim Analysis of Post-Marketing Surveillance. J. Neurogastroenterol. Motil. 2022, 28, 3–9. [Google Scholar]
- Li, J.; Chen, R.; Yao, Q.Y.; Liu, S.J.; Tian, X.Y.; Hao, C.Y.; Lu, W.; Zhou, T.Y. Time-Dependent Pharmacokinetics of Dexamethasone and Its Efficacy in Human Breast Cancer Xenograft Mice: A Semi-Mechanism-Based Pharmacokinetic/Pharmacodynamic Model. Acta Pharmacol. Sin. 2018, 39, 472–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A Circadian Gene Expression Atlas in Mammals: Implications for Biology and Medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunil, S.A.; Srikanth, M.V.; Rao, N.S.; Uhumwangho, M.U.; Latha, K.; Murthy, K.V.R. Chronotherapeutic Drug Delivery Systems: An Approach to Circadian Rhythms Diseases. Curr. Drug Deliv. 2011, 8, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F. Circadian Chronotherapy for Human Cancers. Lancet Oncol. 2001, 2, 307–315. [Google Scholar] [CrossRef]
- Extra, J.M.; Espie, M.; Calvo, F.; Ferme, C.; Mignot, L.; Marty, M. Phase I Study of Oxaliplatin in Patients With Advanced Cancer. Cancer Chemother. Pharmacol. 1990, 25, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Filipski, E.; King Vm Li, X.; Granda, T.G.; Mormont, M.C.; Liu, X.; Claustrat, B.; Hastings, M.H.; Lévi, F. Host Circadian Clock as A Control Point in Tumor Progression. J. Natl. Cancer Inst. 2002, 94, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Caussanel, J.P.; Lévi, F.; Brienza, S.; Misset, J.L.; Itzhaki, M.; Adam, R.; Milano, G.; Hecquet, B.; Mathé, G. Phase I Trial of 5-Day Continuous Venous Infusion of Oxaliplatin At Circadian Rhythm-Modulated Rate Compared With Constant Rate. J. Natl. Cancer Inst. 1990, 82, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Misset, J.L.; Brienza, S.; Adam, R.; Metzger, G.; Itzakhi, M.; Caussanel, J.P.; Kunstlinger, F.; Lecouturier, S.; Descorps-Declère, A. A Chronopharmacologic Phase Ii Clinical Trial With 5-Fluorouracil, Folinic Acid, and Oxaliplatin Using An Ambulatory Multichannel Programmable Pump. High Antitumor Effectiveness Against Metastatic Colorectal Cancer. Cancer 1992, 69, 893–900. [Google Scholar] [CrossRef]
- Mormont, M.C.; Lévi, F. Circadian-System Alterations During Cancer Processes: A Review. Int. J. Cancer 1997, 70, 241–247. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; Hogenesch, J.B.; et al. Medicine in the Fourth Dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef]
- Giacchetti, S.; Bjarnason, G.; Garufi, C.; Genet, D.; Iacobelli, S.; Tampellini, M.; Smaaland, R.; Focan, C.; Coudert, B.; Humblet, Y.; et al. Phase Iii Trial Comparing 4-Day Chronomodulated Therapy Versus 2-Day Conventional Delivery of Fluorouracil, Leucovorin, and Oxaliplatin as First-Line Chemotherapy of Metastatic Colorectal Cancer: The European Organisation for Research and Treatment of Ca. J. Clin. Oncol. 2006, 24, 3562–3569. [Google Scholar] [CrossRef]
- Lévi, F.; Zidani, R.; Misset, J.L. Randomised Multicentre Trial of Chronotherapy With Oxaliplatin, Fluorouracil, and Folinic Acid in Metastatic Colorectal Cancer. International Organization for Cancer Chronotherapy. Lancet 1997, 350, 681–686. Available online: https://pubmed.ncbi.nlm.nih.gov/9291901/ (accessed on 13 July 2022). [CrossRef]
- Li, J.; Chen, R.; Ji, M.; Zou, S.L.; Zhu, L.N. Cisplatin-Based Chronotherapy for Advanced Non-Small Cell Lung Cancer Patients: A Randomized Controlled Study and Its Pharmacokinetics Analysis. Cancer Chemother. Pharmacol. 2015, 76, 651–655. [Google Scholar] [CrossRef]
- Kobayashi, M.; Wood, P.A.; Hrushesky, W.J.M. Circadian Chemotherapy for Gynecological and Genitourinary Cancers. Chronobiol. Int. 2002, 19, 237–251. [Google Scholar] [CrossRef]
- Giacchetti, S.; Dugué, P.A.; Innominato, P.F.; Bjarnason, G.A.; Focan, C.; Garufi, C.; Tumolo, S.; Coudert, B.; Iacobelli, S.; Smaaland, R.; et al. Sex Moderates Circadian Chemotherapy Effects On Survival of Patients With Metastatic Colorectal Cancer: A Meta-Analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Innominato, P.F.; Ballesta, A.; Huang, Q.; Focan, C.; Chollet, P.; Karaboué, A.; Giacchetti, S.; Bouchahda, M.; Adam, R.; Garufi, C.; et al. Sex-Dependent Least Toxic Timing of Irinotecan Combined With Chronomodulated Chemotherapy for Metastatic Colorectal Cancer: Randomized Multicenter Eortc 05011 Trial. Cancer Med. 2020, 9, 4148–4159. Available online: https://pubmed.ncbi.nlm.nih.gov/32319740/ (accessed on 13 July 2022). [CrossRef] [PubMed] [Green Version]
- Slat, E.A.; Sponagel, J.; Marpegan, L.; Simon, T.; Kfoury, N.; Kim, A.; Binz, A.; Herzog, E.D.; Rubin, J.B. Cell-Intrinsic, Bmal1-Dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma. J. Biol. Rhythm. 2017, 32, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulong, S.; Ballesta, A.; Okyar, A.; Levi, F. Identification of Circadian Determinants of Cancer Chronotherapy Through in Vitro Chronopharmacology and Mathematical Modeling. Mol. Cancer Ther. 2015, 14, 2154–2164. Available online: https://pubmed.ncbi.nlm.nih.gov/26141947/ (accessed on 4 July 2022). [CrossRef] [Green Version]
- Zeng, Z.L.; Luo Hy Yang, J.; Wu, W.J.; Chen, D.L.; Huang, P.; Xu, R.H. Overexpression of the Circadian Clock Gene Bmal1 Increases Sensitivity to Oxaliplatin in Colorectal Cancer. Clin. Cancer Res. 2014, 20, 1042–1052. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/24277452/ (accessed on 4 July 2022). [CrossRef] [Green Version]
- Tang, Q.; Cheng, B.; Xie, M.; Chen, Y.; Zhao, J.; Zhou, X.; Chen, L. Circadian Clock Gene Bmal1 Inhibits Tumorigenesis and Increases Paclitaxel Sensitivity in Tongue Squamous Cell Carcinoma. Cancer Res. 2017, 77, 532–544. Available online: https://pubmed.ncbi.nlm.nih.gov/27821487/ (accessed on 4 July 2022). [CrossRef]
- Harper, E.; Talbot, C.J. Is It Time to Change Radiotherapy: The Dawning of Chronoradiotherapy? Clin. Oncol. R Coll. Radiol. 2019, 31, 326–335. [Google Scholar] [CrossRef]
- Shuboni-Mulligan, D.D.; Breton, G.; Smart, D.; Gilbert, M.; Armstrong, T.S. Radiation Chronotherapy-Clinical Impact of Treatment Time-Of-Day: A Systematic Review. J. Neurooncol. 2019, 145, 415–427. [Google Scholar] [CrossRef]
- Squire, T.; Buchanan, G.; Rangiah, D.; Davis, I.; Yip, D.; Chua, Y.J.; Rich, T.; Elsaleh, H. Does Chronomodulated Radiotherapy Improve Pathological Response in Locally Advanced Rectal Cancer? Chronobiol. Int. 2017, 34, 492–503. [Google Scholar] [CrossRef]
- Rahn, D.A., 3rd; Ray, D.K.; Schlesinger, D.J.; Steiner, L.; Sheehan, J.P.; O’quigley, J.M.; Rich, T. Gamma Knife Radiosurgery for Brain Metastasis of Nonsmall Cell Lung Cancer: Is there a Difference in Outcome Between Morning and Afternoon Treatment? Cancer 2011, 117, 414–420. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Nasra, K.; Berry, R.; Danesh, L.; Little, T.; Abu-Isa, E. Clinical Effects of Morning and Afternoon Radiotherapy On High-Grade Gliomas. Chronobiol. Int. 2021, 38, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Karaboué, A.; Collon, T.; Pavese, I.; Bodiguel, V.; Cucherousset, J.; Zakine, E.; Innominato, P.F.; Bouchahda, M.; Adam, R.; Lévi, F. Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results From A Pilot Study in Patients With Metastatic Non-Small-Cell Lung Cancer. Cancers 2022, 14, 896. Available online: https://pubmed.ncbi.nlm.nih.gov/35205644/ (accessed on 13 July 2022). [CrossRef] [PubMed]
- Fuzissaki, M.A.; Paiva, C.E.; Oliveira, M.A.; Maia, M.A.; Canto, P.P.L.; Maia, Y.C.P. A Protective Effect of Morning Radiotherapy On Acute Skin Toxicity in Patients With Breast Cancer: A Prospective Cohort Study. Medicine 2021, 100, E27155. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Zhang, L.; Rowbottom, L.; Mcdonald, R.; Bjarnason, G.A.; Tsao, M.; Barnes, E.; Danjoux, C.; Popovic, M.; Lam, H.; et al. Effects of Circadian Rhythms and Treatment Times on the Response of Radiotherapy for Painful Bone Metastases. Ann. Palliat. Med. 2017, 6, 14–25. [Google Scholar] [CrossRef]
- Chan, S.; Rowbottom, L.; Mcdonald, R.; Zhang, L.; Bjarnason, G.A.; Tsao, M.; Danjoux, C.; Barnes, E.; Lam, H.; Popovic, M.; et al. Could Time of Whole Brain Radiotherapy Delivery Impact Overall Survival in Patients With Multiple Brain Metastases? Ann. Palliat. Med. 2016, 5, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.M.; Hou, W.H.; Huang, C.Y.; Wang, C.C.; Tsai, C.L.; Tsai, Y.C.; Yu, H.J.; Pu, Y.S.; Cheng, J.C.H. Differences in Toxicity and Outcome Associated With Circadian Variations Between Patients Undergoing Daytime and Evening Radiotherapy for Prostate Adenocarcinoma. Chronobiol. Int. 2016, 33, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. Cbtrus Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-oncology 2019, 21 (Suppl. S5), V1–V100. Available online: https://pubmed.ncbi.nlm.nih.gov/31675094/ (accessed on 4 July 2022). [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. Mgmt Gene Silencing and Benefit From Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/15758010/ (accessed on 4 July 2022). [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/15758009/ (accessed on 4 July 2022). [CrossRef] [Green Version]
- Sulli, G.; Rommel, A.; Wang, X.; Kolar, M.J.; Puca, F.; Saghatelian, A.; Plikus, M.V.; Verma, I.M.; Panda, S. Pharmacological Activation of Rev-Erbs Is Lethal in Cancer and Oncogene-Induced Senescence. Nature 2018, 553, 351–355. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/29320480/ (accessed on 4 July 2022). [CrossRef]
- Dong, Z.; Zhang, G.; Qu, M.; Gimple, R.C.; Wu, Q.; Qiu, Z.; Prager, B.C.; Wang, X.; Kim, L.J.Y.; Morton, A.R.; et al. Targeting Glioblastoma Stem Cells Through Disruption of the Circadian Clock. Cancer Discov. 2019, 9, 1556–1573. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/31455674/ (accessed on 4 July 2022). [CrossRef] [PubMed] [Green Version]
- Ballesta, A.; Zhou, Q.; Zhang, X.; Lv, H.; Gallo, J.M. Multiscale Design of Cell-Type-Specific Pharmacokinetic/Pharmacodynamic Models for Personalized Medicine: Application to Temozolomide in Brain Tumors. CPT Pharmacomet. Syst. Pharmacol. 2014, 3, e112. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/24785551/ (accessed on 4 July 2022). [CrossRef]
- Damato, A.R.; Luo, J.; Katumba, R.G.N.; Talcott, G.R.; Rubin, J.B.; Herzog, E.D.; Campian, J.L. Temozolomide Chronotherapy in Patients With Glioblastoma: A Retrospective Single-Institute Study. Neuro-Oncol. Adv. 2021, 3, Vdab041. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.M.; Prucca, C.G.; Velazquez, F.N.; Sosa Alderete, L.G.; Caputto, B.L.; Guido, M.E. Temporal Regulation of Tumor Growth in Nocturnal Mammals: In Vivo Studies and Chemotherapeutical Potential. FASEB J. 2021, 35, E21231. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Hao, L.Y.; Liu, X.; Lesch, C.A.; Sanchez, B.M.; Wendling, J.M.; Morgan, R.W.; Aicher, T.D.; Carter, L.L.; et al. Sterol Metabolism Controls T(H)17 Differentiation by Generating Endogenous Rorγ Agonists. Nat. Chem. Biol. 2015, 11, 141–147. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/25558972/ (accessed on 12 July 2022). [CrossRef] [PubMed]
- Hu, X.; Liu, X.; Moisan, J.; Wang, Y.; Lesch, C.A.; Spooner, C.; Morgan, R.W.; Zawidzka, E.M.; Mertz, D.; Bousley, D.; et al. Synthetic Rorγ Agonists Regulate Multiple Pathways to Enhance Antitumor Immunity. Oncoimmunology 2016, 5, e1254854. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/28123897/ (accessed on 12 July 2022). [CrossRef]
- Mahalingam, D.; Wang, J.S.; Hamilton, E.P.; Sarantopoulos, J.; Nemunaitis, J.; Weems, G.; Carter, L.; Hu, X.; Schreeder, M.; Wilkins, H.J. Phase 1 Open-Label, Multicenter Study of First-In-Class Rorγ Agonist Lyc-55716 (Cintirorgon): Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin. Cancer Res. 2019, 25, 3508–3516. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/30819679/ (accessed on 12 July 2022). [CrossRef] [Green Version]
- Lee, I.K.; Song, H.; Kim, H.; Kim, I.S.; Tran, N.L.; Kim, S.H.; Oh, S.J.; Lee, J.M. Rorα Regulates Cholesterol Metabolism of Cd8 + T Cells for Anticancer Immunity. Cancers 2020, 12, 1733. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/32610705/ (accessed on 12 July 2022). [CrossRef] [PubMed]
- Takane, H.; Ohdo, S.; Yamada, T.; Yukawa, E.; Higuchi, S. Chronopharmacology of Antitumor Effect Induced by Interferon-Beta in Tumor-Bearing Mice—Pubmed. J. Pharmacol. Exp. Ther. 2000, 294, 746–752. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/10900256/ (accessed on 12 July 2022). [PubMed]
- Re, G.L.; Santeufemia, D.A.; Re, F.L.; Bortolus, R.; Doretto, P.; Marus, W.; Buttazzi, L.; Lenardon, O.; Falda, A.; Piazza, R.; et al. Interleukin-2 Chronotherapy for Metastatic Renal Cell Carcinoma: Results of A Phase I-Ii Study. Cytokine 2020, 128, 154984. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/31972343/ (accessed on 12 July 2022). [CrossRef] [PubMed]
- Qian, D.C.; Kleber, T.; Brammer, B.; Xu, K.M.; Switchenko, J.M.; Janopaul-Naylor, J.R.; Zhong, J.; Yushak, M.L.; Harvey, R.D.; Paulos, C.M.; et al. Effect of Immunotherapy Time-Of-Day Infusion On Overall Survival Among Patients With Advanced Melanoma in the Usa (Memoir): A Propensity Score-Matched Analysis of A Single-Centre, Longitudinal Study. Lancet Oncol. 2021, 22, 1777–1786. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/34780711/ (accessed on 13 July 2022). [CrossRef]
- Yang, H.; Xia, L.; Chen, J.; Zhang, S.; Martin, V.; Li, Q.; Lin, S.; Chen, J.; Calmette, J.; Lu, M.; et al. Stress-Glucocorticoid-Tsc22d3 Axis Compromises Therapy-Induced Antitumor Immunity. Nat. Med. 2019, 25, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Victoria Lai, W.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids On Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/30125216/ (accessed on 12 July 2022). [CrossRef]
- Parakh, S.; Park, J.J.; Mendis, S.; Rai, R.; Xu, W.; Lo, S.; Drummond, M.; Rowe, C.; Wong, A.; Mcarthur, G.; et al. Efficacy of Anti-Pd-1 Therapy in Patients With Melanoma Brain Metastases. Br. J. Cancer 2017, 116, 1558–1563. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/28524161/ (accessed on 12 July 2022). [CrossRef] [Green Version]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.B. Melatonin for the Prevention and Treatment of Cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [Green Version]
- Targhazeh, N.; Reiter, R.J.; Rahimi, M.; Qujeq, D.; Yousefi, T.; Shahavi, M.H.; Mir, S.M. Oncostatic Activities of Melatonin: Roles in Cell Cycle, Apoptosis, and Autophagy. Biochimie, 2022, in press. [CrossRef] [PubMed]
- Önder, G.Ö. Melatonin Has An Inhibitory Effect On Mcf-7 and Mda-Mb-231 Human Breast Cancer Cell Lines by Inducing Autophagy and Apoptosis. Fundam. Clin. Pharmacol. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.; Nunes, M.; Trombley, C.; Flôres Defl Wu, G.; Taleb, Z.; Alkhateeb, A.; Banskota, S.; Harris, C.; Love, O.P.; Khan, W.I.; et al. The Circadian Clock Gene, Bmal1, Regulates Intestinal Stem Cell Signaling and Represses Tumor Initiation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1847–1872.E0. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Jin, B.Z.; Ai, F.; Duan, C.H.; Lu, Y.Z.; Dong, T.F.; Fu, Q.L. The Efficacy and Safety of Melatonin in Concurrent Chemotherapy Or Radiotherapy for Solid Tumors: A Meta-Analysis of Randomized Controlled Trials. Cancer Chemother. Pharmacol. 2012, 69, 1213–1220. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/22271210/ (accessed on 4 July 2022). [CrossRef]
- Liang, Y.; Liu, C.; Lu, M.; Dong, Q.; Wang, Z.; Wang, Z.; Xiong, W.; Zhang, N.; Zhou, J.; Liu, Q.; et al. Calorie Restriction Is the Most Reasonable Anti-Ageing Intervention: A Meta-Analysis of Survival Curves. Sci. Rep. 2018, 8, 5779. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/29636552/ (accessed on 4 July 2022). [CrossRef] [PubMed] [Green Version]
- Alidadi, M.; Banach, M.; Guest, P.C.; Bo, S.; Jamialahmadi, T.; Sahebkar, A. The Effect of Caloric Restriction and Fasting On Cancer. Semin. Cancer Biol. 2021, 73, 30–44. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/32977005/ (accessed on 4 July 2022). [CrossRef]
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Recent Results Cancer Res. 2016, 207, 241–266. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/27557543/ (accessed on 4 July 2022).
- Zhao, X.; Yang, J.; Huang, R.; Guo, M.; Zhou, Y.; Xu, L. The Role and Its Mechanism of Intermittent Fasting in Tumors: Friend Or Foe? Cancer Biol. Med. 2021, 18, 63–73. Available online: Https://Pubmed.Ncbi.Nlm.Nih.Gov/33628585/ (accessed on 4 July 2022). [CrossRef] [PubMed]
- Alonso-González, C.; González, A.; Martínez-Campa, C.; Gómez-Arozamena, J.; Cos, S. Melatonin Sensitizes Human Breast Cancer Cells to Ionizing Radiation by Downregulating Proteins Involved in Double-Strand Dna Break Repair. J. Pineal Res. 2015, 58, 189–197. [Google Scholar] [CrossRef]
- Wu, G.; Ruben, M.D.; Schmidt, R.E.; Francey, L.J.; Smith, D.F.; Anafi, R.C.; Hughey, J.J.; Tasseff, R.; Sherrill, J.D.; Oblong, J.E.; et al. Population-Level Rhythms in Human Skin With Implications for Circadian Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 12313–12318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, Y.; Kasukawa, T.; Kakazu, Y.; Iigo, M.; Sugimoto, M.; Ikeda, S.; Yasui, A.; Van Der Horst, G.T.J.; Soga, T.; Ueda, H.R. Measurement of Internal Body Time by Blood Metabolomics. Proc. Natl. Acad. Sci. USA 2009, 106, 9890–9895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.T.; Ladurner, A.G. Exploiting the Circadian Clock for Improved Cancer Therapy: Perspective From A Cell Biologist. Front. Genet. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Lee, Y.; Fong, S.Y.; Shon, J.; Zhang, S.L.; Brooks, R.; Lahens, N.F.; Chen, D.; Van Dang, C.; Field, J.M.; Sehgal, A. Time-Of-Day Specificity of Anticancer Drugs May Be Mediated by Circadian Regulation of the Cell Cycle. Sci. Adv. 2021, 7, eabd2645. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the Human Circadian Clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Facer-Childs, E.R.; Middleton, B.; Bagshaw, A.P.; Skene, D.J. Human Circadian Phenotyping and Diurnal Performance Testing in the Real World. J. Vis. Exp. 2020, 158, e60448. [Google Scholar] [CrossRef] [Green Version]
- Molina, T.A.; Burgess, H.J. Calculating the Dim Light Melatonin Onset: The Impact of Threshold and Sampling Rate. Chronobiol. Int. 2011, 28, 714–718. [Google Scholar] [CrossRef]
- Wittenbrink, N.; Ananthasubramaniam, B.; Münch, M.; Koller, B.; Maier, B.; Weschke, C.; Bes, F.; De Zeeuw, J.; Nowozin, C.; Wahnschaffe, A.; et al. High-Accuracy Determination of Internal Circadian Time From A Single Blood Sample. J. Clin. Invest. 2018, 128, 3826–3839. [Google Scholar] [CrossRef]
- Li, D.; Xu, W.; Guo, Y.; Xu, Y. Fluctuations Induced Extinction and Stochastic Resonance Effect in A Model of Tumor Growth With Periodic Treatment. Phys. Lett. A 2011, 375, 886–890. [Google Scholar] [CrossRef]
- Ledzewicz, U.; Schättler, H.; Gahrooi, M.R.; Dehkordi, S.M. On the Mtd Paradigm and Optimal Control for Multi-Drug Cancer Chemotherapy. Math. Biosci Eng. 2013, 10, 803–819. [Google Scholar] [PubMed]
- Lecca, P. Control Theory and Cancer Chemotherapy: How They Interact. Front. Bioeng. Biotechnol. 2021, 8, 621269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiama-Roig, A.; Verdugo-Sivianes, E.M.; Carnero, A.; Blanco, J.-R. Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy. Cancers 2022, 14, 5071. https://doi.org/10.3390/cancers14205071
Amiama-Roig A, Verdugo-Sivianes EM, Carnero A, Blanco J-R. Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy. Cancers. 2022; 14(20):5071. https://doi.org/10.3390/cancers14205071
Chicago/Turabian StyleAmiama-Roig, Ana, Eva M. Verdugo-Sivianes, Amancio Carnero, and José-Ramón Blanco. 2022. "Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy" Cancers 14, no. 20: 5071. https://doi.org/10.3390/cancers14205071
APA StyleAmiama-Roig, A., Verdugo-Sivianes, E. M., Carnero, A., & Blanco, J.-R. (2022). Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy. Cancers, 14(20), 5071. https://doi.org/10.3390/cancers14205071