Patterns of Care and Data Quality in a National Registry of Black and White Patients with Merkel Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Tumor Characteristics
3.3. Treatment Patterns
3.4. Data Quality
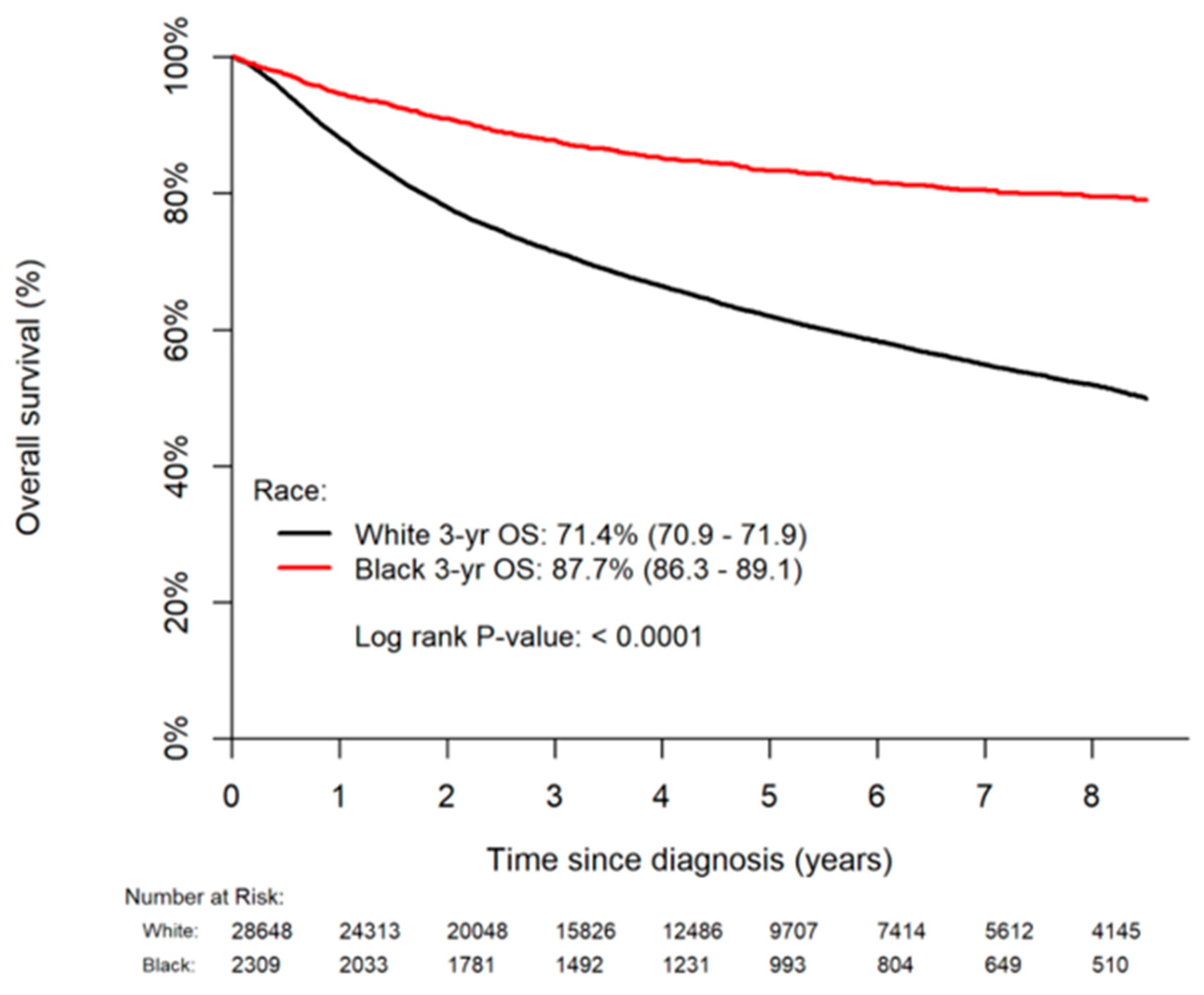
3.5. Overall Survival
3.6. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2017, 154, 457–463. [Google Scholar] [CrossRef]
- Agbai, O.N.; Buster, K.; Sanchez, M.; Hernandez, C.; Kundu, R.V.; Chiu, M.; Roberts, W.E.; Draelos, Z.D.; Bhushan, R.; Taylor, S.C.; et al. Skin cancer and photoprotection in people of color: A review and recommendations for physicians and the public. J. Am. Acad. Dermatol. 2014, 70, 748–762. [Google Scholar] [CrossRef]
- Madankumar, R.; Criscito, M.C.; Martires, K.J.; Stein, J.A. A population-based cohort study of the influence of socioeconomic factors and race on survival in Merkel cell carcinoma. J. Am. Acad. Dermatol. 2017, 76, 166–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sridharan, V.; Muralidhar, V.; Margalit, D.N.; Tishler, R.B.; DeCaprio, J.A.; Thakuria, M.; Rabinowits, G.; Schoenfeld, J.D. Merkel cell carcinoma: A population analysis on survival. J. Natl. Compr. Canc. Netw. 2016, 14, 1247–1257. [Google Scholar] [CrossRef]
- Tripathi, R.; Bordeaux, J.S.; Nijhawan, R.I. Factors associated with time to treatment for Merkel cell carcinoma. J. Am. Acad. Dermatol. 2021, 84, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Simon, M.; Krist, A.H. Addressing systemic racism through clinical preventive service recommendations from the US Preventive Services Task Force. JAMA 2021, 325, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.G.; Stahl, K.; Olecki, E.J.; Holguin, R.P.; Pameijer, C.; Shen, C. Survival benefit of guideline-concordant postoperative radiation for local Merkel cell carcinoma. J. Surg. Res. 2021, 266, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.X.; Khera, R.; Miccio, J.A.; Jairam, V.; Chang, E.; Yu, J.B.; Park, H.S.; Krumholz, H.M.; Aneja, S. Prevalence of missing data in the national cancer database and association with overall survival. JAMA Netw. Open. 2021, 4, e211793. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Vyas, D.A.; Eisenstein, L.G.; Jones, D.S. hidden in plain sight—Reconsidering the use of race correction in clinical algorithms. N. Engl. J. Med. 2020, 383, 874–882. [Google Scholar] [CrossRef]
- Flanagin, A.; Frey, T.; Christiansen, S.L.; AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021, 326, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Becker, J.C.; Nghiem, P.; Ferlay, J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. Eur. J. Cancer 2018, 94, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.B.; McKenzie, G.; Rattani, A.; Tennant, P.; Bumpous, J.; Miller, D.; Dunlap, N. Merkel cell carcinoma of the head and neck: Epidemiology, pathogenesis, current state of treatment and future directions. Cancers 2021, 13, 3506. [Google Scholar] [CrossRef]
- Yusuf, M.B.; Gaskins, J.; Rattani, A.; McKenzie, G.; Mandish, S.; Wall, W.; Farley, A.; Tennant, P.; Bumpous, J.; Dunlap, N. Immune status in Merkel cell carcinoma: Relationships with clinical factors and independent prognostic value. Ann. Surg. Oncol. 2021, 28, 6154–6165. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.; Luu, M.; Barker, C.A.; Gharavi, N.M.; Hamid, O.; Shiao, S.L.; Nguyen, A.T.; Lu, D.J.; Ho, A.S.; Zumsteg, Z.S. Improved survival in women versus men with Merkel cell carcinoma. J. Am. Acad. Dermatol. 2021, 84, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.D.; Carpenter, D.J.; Hong, J.C.; Havrilesky, L.J.; Sosa, J.A.; Chino, J.P. Radiation Records in the National Cancer Database: Variations in Coding and/or Practice Can Significantly Alter Survival Results. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, T.; Aulakh, S.; Baksh, M.; Nguyen, K.; Ailawadhi, M.; Samreen, A.; Parrondo, R.; Sher, T.; Roy, V.; Manochakian, R.; et al. Landmark Cancer Clinical Trials and Real-World Patient Populations: Examining Race and Age Reporting. Cancers 2021, 13, 5770. [Google Scholar] [CrossRef] [PubMed]
- Aldrighetti, C.M.; Niemierko, A.; Van Allen, E.; Willers, H.; Kamran, S.C. Racial and Ethnic Disparities Among Participants in Precision Oncology Clinical Studies. JAMA Netw. Open. 2021, 4, e2133205. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.M.; Anand, S.; Dasari, A.; Unger, J.M.; Gothwal, A.; Ellis, L.M.; Varadhachary, G.; Kopetz, S.; Overman, M.J.; Raghav, K. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncol. 2019, 5, e191870. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Bilimoria, K.Y. Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J. Surg. Oncol. 2014, 109, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; She, H.; Martin Kasyanju Carrero, L.; Ma, W.; Zhou, B. Nomogram prediction for the overall survival and cancer-specific survival of patients diagnosed with Merkel cell carcinoma. Ann. Transl. Med. 2021, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- van Veenendaal, L.M.; van Akkooi, A.C.J.; Verhoef, C.; Grünhagen, D.J.; Klop, W.M.C.; Valk, G.D.; Tesselaar, M.E.T. Merkel cell carcinoma: Clinical outcome and prognostic factors in 351 patients. J. Surg. Oncol. 2018, 117, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Allen, P.J.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann. Surg. 2011, 254, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cao, D.; Zhao, J.; Zhu, B.; Xie, J. Clinical features and prognosis of Merkel cell carcinoma in elderly patients. Med. Sci. Monit. 2020, 26, e924570. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Vats, P.; Verhaegen, M.E.; Robinson, D.R.; Wu, Y.M.; Dhanasekaran, S.M.; Palanisamy, N.; Siddiqui, J.; Cao, X.; Su, F.; et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015, 75, 3720–3727. [Google Scholar] [CrossRef] [PubMed]

| Total | White | Black | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Prop | N | Prop | N | Prop | p-Value | ||
| 34,503 | 31,947 | 93% | 2556 | 7% | ||||
| Age | <0.0001 | |||||||
| 49 or less | 5439 | 16% | 4239 | 13% | 1200 | 47% | ||
| 50–59 | 4267 | 12% | 3782 | 12% | 485 | 19% | ||
| 60–69 | 6729 | 20% | 6313 | 20% | 416 | 16% | ||
| 70–79 | 8751 | 25% | 8456 | 26% | 295 | 12% | ||
| 80+ | 9317 | 27% | 9157 | 29% | 160 | 6% | ||
| Sex | <0.0001 | |||||||
| Male | 20,528 | 59% | 19,350 | 61% | 1178 | 46% | ||
| Female | 13,975 | 41% | 12,597 | 39% | 1378 | 54% | ||
| Insurance Status | <0.0001 | |||||||
| Private Insurance | 11,769 | 35% | 10,772.8 | 34% | 1202.5 | 47% | ||
| Not Insured | 763 | 2% | 588.6 | 2% | 198.9 | 8% | ||
| Government | 21,427 | 63% | 20,585.6 | 64% | 1154.6 | 45% | ||
| Unknown | 544 | |||||||
| Analytic Stage | <0.0001 | |||||||
| Stage I | 9555 | 49% | 15,752.5 | 49% | 925.9 | 36% | ||
| Stage II | 3914 | 20% | 6622 | 21% | 792.2 | 31% | ||
| Stage III | 4602 | 24% | 7227.7 | 23% | 543.3 | 21% | ||
| Stage IV | 1432 | 7% | 2344.8 | 7% | 294.6 | 12% | ||
| Unknown | 15,000 | |||||||
| Definitive Resection | 0.3833 | |||||||
| No Definitive Resection | 17,450 | 51% | 16,179 | 51% | 1271 | 50% | ||
| Definitive Resection | 17,053 | 49% | 15,768 | 49% | 1285 | 50% | ||
| Definitive Resection (within first 42 days) | 0.4932 | |||||||
| No Definitive Resection | 23,657 | 69% | 21,810 | 68% | 1847 | 72% | ||
| Definitive Resection | 10,846 | 31% | 10,137 | 32% | 709 | 28% | ||
| Time to Definitive Resection Quartile | <0.0001 | |||||||
| Within 21 days | 4765 | 28% | 4431 | 28% | 334 | 26% | ||
| 22–35 days | 4330 | 25% | 4066 | 26% | 264 | 21% | ||
| 36–56 days | 4160 | 24% | 3890 | 25% | 270 | 21% | ||
| Greater than 57 days | 3798 | 22% | 3381 | 21% | 417 | 32% | ||
| No Definitive Resection | 17,450 | 16,179 | 1271 | |||||
| Radiation | <0.0001 | |||||||
| No Radiation | 23,775 | 69% | 21,583 | 68% | 2192 | 86% | ||
| Radiation | 10,728 | 31% | 10,364 | 32% | 364 | 14% | ||
| Time to Radiation Quartile | <0.0001 | |||||||
| Within 60 days | 2934 | 27% | 2855 | 28% | 79 | 22% | ||
| 61–80 days | 2402 | 22% | 2341 | 23% | 61 | 17% | ||
| 81–110 days | 2710 | 25% | 2636 | 25% | 74 | 20% | ||
| Greater than 110 days | 2682 | 25% | 2532 | 24% | 150 | 41% | ||
| No RT | 23,775 | 21,583 | 2192 | |||||
| Chemotherapy | <0.0001 | |||||||
| No Chemo | 32,049 | 93% | 29,606 | 93% | 2443 | 96% | ||
| Chemotherapy | 2454 | 7% | 2341 | 7% | 113 | 4% | ||
| Time to Chemo Quartile | 0.8801 | |||||||
| Within 30 days | 520 | 21% | 495 | 21% | 25 | 22% | ||
| 31–60 days | 701 | 29% | 671 | 29% | 30 | 27% | ||
| 61–100 days | 625 | 25% | 598 | 26% | 27 | 24% | ||
| Greater than 100 days | 608 | 25% | 577 | 25% | 31 | 27% | ||
| No Chemo | 32,049 | 29,606 | 2443 | |||||
| Charlson/Deyo Comorbidity Score | 0.0323 | |||||||
| 0 | 27,188 | 79% | 25,122 | 79% | 2066 | 81% | ||
| 1 | 5101 | 15% | 4757 | 15% | 344 | 13% | ||
| 2 or more | 2214 | 6% | 2068 | 6% | 146 | 6% | ||
| Distance to Treatment Center | <0.0001 | |||||||
| Less than 10 mi | 13,880 | 45% | 13,560.2 | 42% | 1572.7 | 62% | ||
| 10–25 mi | 8487 | 27% | 8860.3 | 28% | 574.1 | 22% | ||
| 25–50 mi | 4210 | 14% | 4575.7 | 14% | 199.9 | 8% | ||
| 50–100 mi | 2657 | 9% | 2916.7 | 9% | 126.4 | 5% | ||
| 100 mi or more | 1849 | 6% | 2034.1 | 6% | 82.9 | 3% | ||
| Unknown | 3420 | |||||||
| Zip Code Income Level | <0.0001 | |||||||
| $63,000+ | 11,407 | 37% | 12,120 | 38% | 492.3 | 19% | ||
| $48,000–63,000 | 8470 | 27% | 8901.3 | 28% | 527 | 21% | ||
| $38,000–48,000 | 6904 | 22% | 7096.2 | 22% | 598 | 23% | ||
| $0–38,000 | 4263 | 14% | 3829.5 | 12% | 938.7 | 37% | ||
| Unknown | 3459 | |||||||
| Diagnosis Year | 0.0705 | |||||||
| 2006–2008 | 6883 | 20% | 6324 | 20% | 559 | 22% | ||
| 2009–2011 | 7769 | 23% | 7201 | 23% | 568 | 22% | ||
| 2012–2014 | 9185 | 27% | 8510 | 27% | 675 | 26% | ||
| 2015–2017 | 10,666 | 31% | 9912 | 31% | 754 | 29% | ||
| Tumor Size | <0.0001 | |||||||
| No mass found/Microscopic | 554 | 3% | 1209.3 | 4% | 32.8 | 1% | ||
| Less than 2 cm | 11,323 | 56% | 18,606.2 | 58% | 916.7 | 36% | ||
| 2–5 cm | 6135 | 30% | 9132.6 | 29% | 1001.2 | 39% | ||
| Greater than 5 cm | 2221 | 11% | 2998.9 | 9% | 605.3 | 24% | ||
| Size unknown | 14,270 | |||||||
| Resection Margins | <0.0001 | |||||||
| No Residual Tumor | 26,339 | 88% | 25,401.2 | 88% | 2005.2 | 84% | ||
| Residual Tumor | 3630 | 12% | 3510.8 | 12% | 373.8 | 16% | ||
| No Definitive Resection | 3212 | 3035 | 177 | |||||
| Definitive Resection w/unknown margins | 1322 | |||||||
| Primary Site | <0.0001 | |||||||
| Head/Neck | 14,812 | 43% | 14,199 | 44% | 613 | 24% | ||
| Trunk | 7164 | 21% | 6132 | 19% | 1032 | 40% | ||
| Limbs | 10,879 | 32% | 10,034 | 31% | 845 | 33% | ||
| Other/NOS | 1648 | 5% | 1582 | 5% | 66 | 3% | ||
| Immune Suppression | 0.0062 | |||||||
| Immunocompetent | 5692 | 89% | 27,386.1 | 86% | 1847.8 | 72% | ||
| Immunosuppressed | 680 | 11% | 4560.9 | 14% | 708.2 | 28% | ||
| Unknown | 28,077 | |||||||
| Positive Lymph Nodes | 0.4507 | |||||||
| None positive | 7285 | 62% | 22,584.3 | 71% | 1806.1 | 71% | ||
| 1–3 positive | 3577 | 30% | 7468.6 | 23% | 626.6 | 25% | ||
| 4–8 positive | 603 | 5% | 1170 | 4% | 77.3 | 3% | ||
| 9 or more positive | 378 | 3% | 724.1 | 2% | 46 | 2% | ||
| Unknown | 22,660 | |||||||
| Facility Type | <0.0001 | |||||||
| Academic/Research Program | 15,206 | 48% | 15,451 | 48% | 1409.1 | 55% | ||
| Community Cancer Program | 12,759 | 41% | 12,978.6 | 41% | 788 | 31% | ||
| Integrated Network Program | 3525 | 11% | 3517.4 | 11% | 358.9 | 14% | ||
| Facility Type Suppressed (age < 40) a | 3013 | |||||||
| Facility Case Volume | <0.0001 | |||||||
| Lowest Third | 11,431 | 33% | 10,548 | 33% | 883 | 35% | ||
| Middle Third | 11,470 | 33% | 10,494 | 33% | 976 | 38% | ||
| Largest Third | 11,602 | 34% | 10,905 | 34% | 697 | 27% | ||
| Race Effect | Confidence Interval | p-Value | ||
|---|---|---|---|---|
| Disease Characteristics | ||||
| Cancer Stage A,1 | 1.41 | 1.25–1.59 | <0.0001 | |
| Tumor Size A,1 | 1.99 | 1.77–2.24 | <0.0001 | |
| Number of Positive Nodes A,1 | 1.14 | 0.96–1.38 | 0.1561 | |
| Subsite B,2 | <0.0001 | |||
| Head/Neck | ref | |||
| Trunk | 1.87 | 1.66–2.10 | <0.0001 | |
| Limbs | 1.24 | 1.10–1.39 | 0.0004 | |
| Other/NOS | 0.86 | 0.66–1.13 | 0.2950 | |
| Treatment Characteristics | ||||
| Facility Volume A,3 | 1.26 | 1.16–1.38 | <0.0001 | |
| Definitive Resection (within 42 days) C,4 | 0.85 | 0.77–0.94 | 0.0011 | |
| Time to Definitive Resection Quartile A,4,* | 1.22 | 1.09–1.37 | 0.0007 | |
| Positive Surgical Margins (DR pts only) C,4 | 1.20 | 0.93–1.53 | 0.1566 | |
| Radiation (DR pts only) C,4 | 0.51 | 0.42–0.61 | <0.0001 | |
| Time to Radiation Quartile (DR pts only) A,4,* | 1.80 | 1.30–2.50 | 0.0004 | |
| Radiation (no DR pts only) C,4 | 0.54 | 0.45–0.64 | <0.0001 | |
| Time to Radiation Quartile (no DR pts only) A,4,* | 1.30 | 0.98–1.73 | 0.0645 | |
| Chemo (DR only) C,5 | 0.50 | 0.31–0.79 | 0.0031 | |
| Time to Chemo Quartile (DR pts only) A,5,* | 2.31 | 0.97–6.14 | 0.0933 | |
| Chemo (no DR pts only) C,5 | 0.63 | 0.48–0.85 | 0.0020 | |
| Time to Chemo Quartile (no DR pts only) A,5,* | 1.16 | 0.77–1.76 | 0.4723 | |
| Data Quality | ||||
| Missing Cancer Stage C,6 | 1.69 | 1.52–1.88 | <0.0001 | |
| Missing Tumor Size C,6 | 0.87 | 0.79–0.96 | 0.0072 | |
| Missing Nodal Information C,6 | 2.10 | 1.84–2.40 | <0.0001 | |
| Adj HR | 95% CI | p-Values | |||||
|---|---|---|---|---|---|---|---|
| Race | <0.0001 | ||||||
| White | Reference | ||||||
| Black | 0.73 | 0.65 | 0.81 | <0.0001 | |||
| Age | <0.0001 | ||||||
| 49 or less | Reference | ||||||
| 50–59 | 2.93 | 2.54 | 3.37 | <0.0001 | |||
| 60–69 | 4.42 | 3.87 | 5.05 | <0.0001 | |||
| 70–79 | 7.16 | 6.26 | 8.19 | <0.0001 | |||
| 80+ | 14.92 | 13.05 | 17.07 | <0.0001 | |||
| Sex | <0.0001 | ||||||
| Male | Reference | ||||||
| Female | 0.78 | 0.75 | 0.81 | <0.0001 | |||
| Insurance Status | <0.0001 | ||||||
| Private Insurance | Reference | ||||||
| Not Insured | 1.38 | 1.15 | 1.66 | 0.0006 | |||
| Government | 1.35 | 1.27 | 1.43 | <0.0001 | |||
| Analytic Stage | <0.0001 | ||||||
| Stage I | Reference | ||||||
| Stage II | 1.19 | 1.10 | 1.30 | <0.0001 | |||
| Stage III | 1.29 | 1.10 | 1.51 | 0.0013 | |||
| Stage IV | 1.88 | 1.62 | 2.20 | <0.0001 | |||
| Procedure (time-varying covariate) | <0.0001 | ||||||
| No Procedure | Reference | ||||||
| Excision/Biopsy/Other | 0.64 | 0.59 | 0.68 | <0.0001 | |||
| Mohs | 0.56 | 0.51 | 0.62 | <0.0001 | |||
| Wide Local Excision | 0.61 | 0.57 | 0.65 | <0.0001 | |||
| Radiation (time-varying covariate) | 0.0065 | ||||||
| No Radiation | Reference | ||||||
| Radiation | 1.06 | 1.02 | 1.10 | 0.0065 | |||
| Chemotherapy (time-varying covariate) | <0.0001 | ||||||
| No Chemo | Reference | ||||||
| Chemotherapy | 1.66 | 1.55 | 1.78 | <0.0001 | |||
| Charlson/Deyo Comorbidity Score | <0.0001 | ||||||
| 0 | Reference | ||||||
| 1 | 1.25 | 1.19 | 1.32 | <0.0001 | |||
| 2 or more | 1.81 | 1.70 | 1.93 | <0.0001 | |||
| Distance to Treatment Center | 0.6678 | ||||||
| Less than 10 mi | Reference | ||||||
| 10–25 mi | 1.01 | 0.96 | 1.06 | 0.7172 | |||
| 25–50 mi | 0.96 | 0.90 | 1.02 | 0.2219 | |||
| 50–100 mi | 0.99 | 0.91 | 1.07 | 0.7285 | |||
| 100 mi or more | 0.97 | 0.89 | 1.07 | 0.5535 | |||
| Zip Code Income Level | <0.0001 | ||||||
| $63,000+ | Reference | ||||||
| $48,000–63,000 | 1.04 | 0.99 | 1.10 | 0.0955 | |||
| $38,000–48,000 | 1.11 | 1.05 | 1.16 | 0.0002 | |||
| $0–38,000 | 1.15 | 1.07 | 1.22 | <0.0001 | |||
| Diagnosis Year | <0.0001 | ||||||
| 2006–2008 | Reference | <0.0001 | |||||
| 2009–2011 | 1.03 | 0.97 | 1.08 | 0.3151 | |||
| 2012–2014 | 0.98 | 0.93 | 1.03 | 0.4569 | |||
| 2015–2016 | 0.86 | 0.81 | 0.92 | <0.0001 | |||
| Tumor Size | <0.0001 | ||||||
| No mass found/Microscopic | Reference | Reference | |||||
| Less than 2 cm | 1.30 | 1.10 | 1.53 | 0.0025 | |||
| 2–5 cm | 1.37 | 1.16 | 1.63 | 0.0003 | |||
| Greater than 5 cm | 1.60 | 1.33 | 1.92 | <0.0001 | |||
| Resection Margins | <0.0001 | ||||||
| No Residual Tumor | Reference | Reference | |||||
| Residual Tumor | 1.35 | 1.27 | 1.43 | <0.0001 | |||
| Primary Site | <0.0001 | ||||||
| Head/Neck | Reference | ||||||
| Trunk | 0.84 | 0.79 | 0.89 | <0.0001 | |||
| Limbs | 0.86 | 0.82 | 0.90 | <0.0001 | |||
| Other/NOS | 0.84 | 0.74 | 0.96 | 0.0103 | |||
| Immune Suppression | 0.0001 | ||||||
| Immunocompetent | Reference | ||||||
| Immunosuppressed | 1.15 | 1.07 | 1.23 | 0.0001 | |||
| Positive Lymph Nodes | 0.0080 | ||||||
| None positive | Reference | ||||||
| 1–3 positive | 1.11 | 0.95 | 1.31 | 0.1841 | |||
| 4–8 positive | 1.25 | 1.01 | 1.54 | 0.0382 | |||
| 9 or more positive | 1.53 | 1.20 | 1.96 | <0.0001 | |||
| Facility Type | 0.1433 | ||||||
| Academic/Research Program | Reference | ||||||
| Community Cancer Program | 1.06 | 1.00 | 1.12 | 0.0489 | |||
| Integrated Network Program | 1.04 | 0.97 | 1.11 | 0.3267 | |||
| Facility Case Volume | <0.0001 | ||||||
| Lowest Third | Reference | ||||||
| Middle Third | 0.96 | 0.91 | 1.01 | 0.0882 | |||
| Largest Third | 0.86 | 0.80 | 0.92 | <0.0001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattani, A.; Gaskins, J.; McKenzie, G.; Scharf, V.K.; Broman, K.; Pisu, M.; Holder, A.; Dunlap, N.; Schwartz, D.; Yusuf, M.B. Patterns of Care and Data Quality in a National Registry of Black and White Patients with Merkel Cell Carcinoma. Cancers 2022, 14, 5059. https://doi.org/10.3390/cancers14205059
Rattani A, Gaskins J, McKenzie G, Scharf VK, Broman K, Pisu M, Holder A, Dunlap N, Schwartz D, Yusuf MB. Patterns of Care and Data Quality in a National Registry of Black and White Patients with Merkel Cell Carcinoma. Cancers. 2022; 14(20):5059. https://doi.org/10.3390/cancers14205059
Chicago/Turabian StyleRattani, Abbas, Jeremy Gaskins, Grant McKenzie, Virginia Kate Scharf, Kristy Broman, Maria Pisu, Ashley Holder, Neal Dunlap, David Schwartz, and Mehran B. Yusuf. 2022. "Patterns of Care and Data Quality in a National Registry of Black and White Patients with Merkel Cell Carcinoma" Cancers 14, no. 20: 5059. https://doi.org/10.3390/cancers14205059
APA StyleRattani, A., Gaskins, J., McKenzie, G., Scharf, V. K., Broman, K., Pisu, M., Holder, A., Dunlap, N., Schwartz, D., & Yusuf, M. B. (2022). Patterns of Care and Data Quality in a National Registry of Black and White Patients with Merkel Cell Carcinoma. Cancers, 14(20), 5059. https://doi.org/10.3390/cancers14205059






