Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection of the Lifestyle Score
2.3. HRQoL, Fatigue and CIPN
2.4. Other Factors
2.5. Statistical Analysis
3. Results
3.1. Changes in Level of Adherence and HRQoL, Fatigue, and CIPN Outcomes up to 24 Months Post-Treatment
3.2. Longitudinal Associations of the Lifestyle Score with HRQoL, Fatigue, and CIPN
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ren, J.-S.; Masuyer, E.; Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 2012, 132, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef]
- Parry, C.; Kent, E.E.; Mariotto, A.B.; Alfano, C.M.; Rowland, J.H. Cancer survivors: A booming population. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1996–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, S.K.; Meng, X.; Youl, P.; Aitken, J.; Dunn, J.; Baade, P. A five-year prospective study of quality of life after colorectal cancer. Qual. Life Res. 2011, 21, 1551–1564. [Google Scholar] [CrossRef]
- Jansen, L.; Koch, L.; Brenner, H.; Arndt, V. Quality of life among long-term (>/=5 years) colorectal cancer survivors-systematic review. Eur. J. Cancer 2010, 46, 2879–2888. [Google Scholar] [CrossRef]
- Marventano, S.; Forjaz, M.J.; Grosso, G.; Mistretta, A.; Giorgianni, G.; Platania, A.; Gangi, S.; Basile, F.; Biondi, A. Health related quality of life in colorectal cancer patients: State of the art. BMC Surg. 2013, 13, S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thong, M.S.; Mols, F.; Wang, X.S.; Lemmens, V.E.; Smilde, T.J.; van de Poll-Franse, L.V. Quantifying fatigue in (long-term) colorectal cancer survivors: A study from the population-based Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry. Eur. J. Cancer 2013, 49, 1957–1966. [Google Scholar] [CrossRef] [Green Version]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; Macleod, M.R.; Colvin, L.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofthagen, C. Surviving chemotherapy for colon cancer and living with the consequences. J. Palliat. Med. 2010, 13, 1389–1391. [Google Scholar] [CrossRef]
- Bakitas, M.A. Background noise: The experience of chemotherapy-induced peripheral neuropathy. Nurs. Res. 2007, 56, 323–331. [Google Scholar] [CrossRef]
- Mols, F.; Beijers, T.; Lemmens, V.; Hurk, C.J.V.D.; Vreugdenhil, G.; van de Poll-Franse, L.V. Chemotherapy-Induced neuropathy and its association with quality of life among 2- to 11-Year colorectal cancer survivors: Results from the population-based PROFILES registry. J. Clin. Oncol. 2013, 31, 2699–2707. [Google Scholar] [CrossRef]
- World Cander ResearchFund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective-Continuous Update Project Expert Report 2018. Available online: https://www.wcrf.org/dietandcancer (accessed on 13 November 2021).
- Romaguera, D.; Ward, H.; Wark, P.A.; Vergnaud, A.C.; Peeters, P.H.; van Gils, C.H.; Ferrari, P.; Fedirko, V.; Jenab, M.; Boutron-Ruault, M.C.; et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: A cohort study. BMC Med. 2015, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 792–802. [Google Scholar] [CrossRef] [Green Version]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Blarigan, E.L.; Meyerhardt, J.A. Role of physical activity and diet after colorectal cancer diagnosis. J. Clin. Oncol. 2015, 33, 1825–1834. [Google Scholar] [CrossRef] [Green Version]
- Breedveld-Peters, J.J.L.; Koole, J.L.; Müller-Schulte, E.; van der Linden, B.W.A.; Windhausen, C.; Bours, M.J.L.; van Roekel, E.H.; Weijenberg, M.P. Colorectal cancers survivors’ adherence to lifestyle recommendations and cross-sectional associations with health-related quality of life. Br. J. Nutr. 2018, 120, 188–197. [Google Scholar] [CrossRef]
- van Veen, M.R.; Mols, F.; Bours, M.J.L.; Weijenberg, M.P.; Kampman, E.; Beijer, S. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among long-term colorectal cancer survivors: Results of the PROFILES registry. Supportive Care Cancer 2019, 27, 4565–4574. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, S.; Walter, J.; Hampe, J.; von Schönfels, W.; Hinz, S.; Küchler, T.; Jacobs, G.; Schafmayer, C.; Nöthlings, U. Lifestyle factors and health-related quality of life in colorectal cancer survivors. Cancer Causes Control 2013, 25, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Grimmett, C.; Bridgewater, J.; Steptoe, A.; Wardle, J. Lifestyle and quality of life in colorectal cancer survivors. Qual. Life Res. 2011, 20, 1237–1245. [Google Scholar] [CrossRef]
- Greenlee, H.; Hershman, D.L.; Shi, Z.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Kushi, L.H. BMI, lifestyle factors and taxane-induced neuropathy in breast cancer patients: The pathways study. JNCI J. Natl. Cancer Inst. 2016, 109. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, C.M.; Courneya, K.S.; Stein, K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- van Roekel, E.H.; Winkler, E.A.; Bours, M.J.; Lynch, B.M.; Willems, P.J.; Meijer, K.; Kant, I.; Beets, G.J.; Sanduleanu, S.; Healy, G.N.; et al. Associations of sedentary time and patterns of sedentary time accumulation with health-related quality of life in colorectal cancer survivors. Prev. Med. Rep. 2016, 4, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenkhuis, M.-F.; van Roekel, E.H.; Koole, J.L.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; van Duijnhoven, F.J.B.; Mols, F.; Weijenberg, M.P.; et al. Increases in adipose tissue and muscle function are longitudinally associated with better quality of life in colorectal cancer survivors. Sci. Rep. 2021, 11, 12440. [Google Scholar] [CrossRef]
- Kenkhuis, M.F.; Van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Van Duijnhoven, F.J.B.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Sedentary Behavior and Physical Activity with Quality of Life in Colorectal Cancer Survivors. Med. Sci. Sports Exerc. 2021, 53, 2298–2308. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; Van Duijnhoven, F.J.B.; Van Roekel, E.H.; Breedveld-Peters, J.J.; Breukink, S.O.; Janssen-Heijnen, M.L.; Keulen, E.T.P.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal associations of fibre, vegetable, and fruit intake with quality of life and fatigue in colorectal cancer survivors up to 24 months post-treatment. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; Mols, F.; Van Roekel, E.H.; Breedveld-Peters, J.J.; Breukink, S.O.; Janssen-Heijnen, M.L.; Keulen, E.T.P.; Van Duijnhoven, F.J.B.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal associations of fast foods, red and processed meat, alcohol, and sugar-sweetened drinks with quality of life and symptoms in colorectal cancer survivors up to 24 months post-treatment. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P.; Romaguera, D.; Brown, S.; Bender, A.; Kahle, L.L.; Reedy, J. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Roekel, E.H.; Bours, M.J.; de Brouwer, C.P.; Ten Napel, H.; Sanduleanu, S.; Beets, G.L.; Kant, I.; Weijenberg, M.P. The applicability of the international classification of functioning, disability, and health to study lifestyle and quality of life of colorectal cancer survivors. Cancer Epidemiol Biomark. Prev. 2014, 23, 1394–1405. [Google Scholar] [CrossRef] [Green Version]
- Wendel-Vos, G.C.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, B.A.J.; Hendriks, M.R.C.; Meijer, K.; Plasqui, G.; Schaper, N.C.; Savelberg, H.H.C.M. Which activity monitor to use? Validity, reproducibility and user friendliness of three activity monitors. BMC Public Health 2014, 14, 749. [Google Scholar] [CrossRef] [Green Version]
- Annegarn, J.; Spruit, M.A.; Uszko-Lencer, N.H.; Vanbelle, S.; Savelberg, H.H.; Schols, A.M.; Wouters, E.F.; Meijer, K. Objective physical activity assessment in patients with chronic organ failure: A validation study of a new single-unit activity monitor. Arch. Phys. Med. Rehabil. 2011, 92, 1852–1857.e1. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.; Granat, M. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture 2010, 31, 82–86. [Google Scholar] [CrossRef] [PubMed]
- RIVM/Netherlands Nutrition Center. Dutch Food Composition (NEVO) Table 2011; Netherlands Nutrition Center: The Hague, The Netherlands, 2011; Volume 339. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Kenkhuis, M.-F.; van der Linden, B.W.A.; Breedveld-Peters, J.J.L.; Koole, J.L.; van Roekel, E.H.; Breukink, S.O.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Associations of the dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations with patient-reported outcomes in colorectal cancer survivors 2–10 years post-diagnosis: A cross-sectional analysis. Br. J. Nutr. 2020, 125, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Pompili, C.; Koller, M.; Velikova, G.; Franks, K.; Absolom, K.; Callister, M.; Robson, J.; Imperatori, A.; Brunelli, A. EORTC QLQ-C30 summary score reliably detects changes in QoL three months after anatomic lung resection for Non-Small Cell Lung Cancer (NSCLC). Lung Cancer 2018, 123, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Giesinger, J.; Kieffer, J.; Fayers, P.; Grønvold, M.; Petersen, M.A.; Scott, N.; Sprangers, M.A.; Velikova, G.; Aaronson, N.K. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2015, 69, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Vercoulen, J.H.; Hommes, O.R.; Swanink, C.M.; Jongen, P.J.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. The measurement of fatigue in patients with multiple sclerosis. A multidimensional comparison with patients with chronic fatigue syndrome and healthy subjects. Arch. Neurol. 1996, 53, 642–649. [Google Scholar] [CrossRef]
- Servaes, P.; Van Der Werf, S.; Prins, J.; Verhagen, S.; Bleijenberg, G. Fatigue in disease-free cancer patients compared with fatigue in patients with Chronic Fatigue Syndrome. Supportive Care Cancer 2001, 9, 11–17. [Google Scholar] [CrossRef]
- Postma, T.; Aaronson, N.; Heimans, J.; Muller, M.; Hildebrand, J.; Delattre, J.; Hoang-Xuan, K.; Lanteri-Minet, M.; Grant, R.; Huddart, R.; et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef]
- Lavoie Smith, E.M.; Barton, D.L.; Qin, R.; Steen, P.D.; Aaronson, N.K.; Loprinzi, C.L. Assessing patient-reported peripheral neuropathy: The reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual. Life Res. 2013, 22, 2787–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangha, O.; Stucki, G.; Liang, M.H.; Fossel, A.H.; Katz, J.N. The self-administered comorbidity questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Care Res. 2003, 49, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Twisk, J.W.; de Vente, W. Hybrid models were found to be very elegant to disentangle longitudinal within-and between-subject relationships. J. Clin. Epidemiol. 2019, 107, 66–70. [Google Scholar] [CrossRef] [PubMed]
- van Zutphen, M.; Boshuizen, H.C.; Kok, D.E.; van Baar, H.; Geijsen, A.J.; Wesselink, E.; Winkels, R.M.; van Halteren, H.K.; de Wilt, J.H.W.; Kampman, E.; et al. Colorectal cancer survivors only marginally change their overall lifestyle in the first 2 years following diagnosis. J. Cancer Surviv. 2019, 13, 956–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesselink, E.; Van Baar, H.; Van Zutphen, M.; Tibosch, M.; Kouwenhoven, E.A.; Keulen, E.T.P.; Kok, D.E.; Van Halteren, H.K.; Breukink, S.O.; De Wilt, J.H.W.; et al. Inflammation Is a Mediating Factor in the Association between Lifestyle and Fatigue in Colorectal Cancer Patients. Cancers 2020, 12, 3701. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.; Velikova, G.; de Castro, G., Jr.; St-James, M.M.; Fayers, P.; Brown, J.M. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur. J. Cancer 2012, 48, 1713–1721. [Google Scholar] [CrossRef]
- Revicki, D.; Hays, R.D.; Cella, D.; Sloan, J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J. Clin. Epidemiol. 2008, 61, 102–109. [Google Scholar] [CrossRef]
- Yeo, F.; Ng, C.C.; Loh, K.W.J.; Molassiotis, A.; Cheng, H.L.; Au, J.S.K.; Leung, K.T.; Li, Y.C.; Wong, K.-H.; Suen, L.; et al. Minimal clinically important difference of the EORTC QLQ-CIPN20 for worsening peripheral neuropathy in patients receiving neurotoxic chemotherapy. Supportive Care Cancer 2019, 27, 4753–4762. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef]
| 2018 WCRF/AICR Recommendations | Operationalization of Recommendations | Adherence to the Individual Recommendations | |||||
|---|---|---|---|---|---|---|---|
| Points | 6 Weeks n (%) a | 6 Months n (%) a | 12 Months n (%) a | 24 Months n (%) a | |||
| 1. | Be a healthy weight | BMI (kg/m2): | |||||
| 18.5–24.9 25–29.9 <18.5 or ≥30 | 0.5 0.25 0 | 116 (29.5) 172 (43.8) 105 (26.7) | 89 (25.7) 151 (43.6) 106 (30.6) | 61 (21.6) 130 (46.1) 91 (32.3) | 49 (24.0) 85 (41.7) 70 (34.3) | ||
| Waist circumference (cm): | |||||||
| Men: <94 or women: <80 Men: 94- < 102 or women: 80- < 88 Men: ≥102 or women: ≥88 | 0.5 0.25 0 | 87 (22.0) 88 (22.3) 220 (55.7) | 61 (17.6) 85 (24.5) 201 (57.9) | 49 (17.2) 62 (21.8) 174 (61.1) | 41 (20.0) 39 (19.0) 125 (61.0) | ||
| 2. | Be physically active | Total self-reported moderate-vigorous physical activity (min/wk): | |||||
| ≥150 75- < 150 <75 | 0.5 0.25 0 | 320 (82.1) 28 (7.2) 42 (10.8) | 302 (87.5) 13 (3.8) 30 (8.7) | 255 (90.1) 11 (3.9) 17 (6.0) | 181 (90.5) 8 (4.0) 11 (5.5) | ||
| Accelerometer-assessed prolonged sedentary behavior (h/day): | |||||||
| ≤3 >3–6 >6 | 0.5 0.25 0 | 70 (21.5) 148 (45.5) 107 (32.9) | 85 (29.3) 154 (53.1) 51 (17.6) | 58 (24.8) 126 (53.9) 50 (21.4) | 45 (25.7) 93 (53.1) 37 (21.1) | ||
| 3. | Eat a diet rich in wholegrains, vegetables, fruit and beans | Fruits and vegetables (g/day): | |||||
| ≥400 200- < 400 <200 | 0.5 0.25 0 | 44 (11.5) 198 (51.7) 141 (36.8) | 45 (13.5) 146 (43.8) 142 (42.6) | 38 (13.9) 123 (44.9) 113 (41.2) | 28 (14.4) 88 (45.4) 78 (40.2) | ||
| Total dietary fiber (g/day): | |||||||
| ≥30 15- < 30 <15 | 0.5 0.25 0 | 28 (7.3) 298 (77.8) 57 (14.9) | 21 (6.3) 263 (79.0) 49 (14.7) | 19 (6.9) 208 (75.9) 47 (17.2) | 13 (6.7) 143 (73.7) 38 (19.6) | ||
| 4. | Limit consumption of “fast foods” and other processed foods high in fat, starches or sugars | Percent of total kcal from ultra-processed foods (UPFs): | |||||
| Tertile 1 Tertile 2 Tertile 3 | 1 0.5 0 | 128 (33.5) 127 (33.3) 127 (33.3) | 120 (36.0) 121 (36.3) 92 (27.6) | 102 (37.2) 101 (36.9) 71 (25.9) | 78 (40.2) 66 (34.0) 50 (25.8) | ||
| 5. | Limit consumption of red and processed meat | Total red meat (g/wk) and processed meat (g/wk): | |||||
| Red meat < 500 and processed meat < 21 Red meat < 500 and processed meat 21-<100 Red meat > 500 or processed meat ≥ 100 | 1 0.5 0 | 11 (2.9) 23 (6.0) 349 (91.1) | 9 (2.7) 25 (7.5) 299 (89.8) | 11 (4.0) 22 (8.0)241 (88.0) | 9 (4.6) 14 (7.2) 171 (88.1) | ||
| 6. | Limit consumption of sugar-sweetened drinks | Total sugar-sweetened drinks (g/day): | |||||
| 0 >0- ≤ 250 >250 | 1 0.5 0 | 67 (17.5) 248 (64.8) 68 (17.8) | 71 (21.3) 218 (65.5) 44 (13.2) | 71 (25.9) 173 (63.1) 30 (11.0) | 44 (22.7) 126 (65.0) 24 (12.4) | ||
| 7. | Limit alcohol consumption | Total ethanol (g/day): | |||||
| 0 >0- ≤ 10 >10 | 1 0.5 0 | 122 (31.9) 98 (25.6) 163 (42.6) | 104 (31.2) 84 (25.2) 145 (43.5) | 75 (27.4) 72 (26.3) 127 (46.4) | 58 (29.9) 49 (25.3) 87 (44.9) | ||
| The lifestyle scoreb—Mean (SD) Range | 0–7 | 3.0 (0.8) 0.75–5.5 | 3.1 (0.8) 0.5–5.75 | 3.1 (0.8) 1–5.75 | 3.1 (0.8) 1.25–5.25 | ||
| All Participants at 6 Weeks Post-Treatment (n = 396) a | 1 Tertile Lifestyle Score < 2.75 (n = 102) a | 2nd Tertile Lifestyle Score 2.75–3.5 (n = 107) a | 3rd Tertile Lifestyle Score > 3.5 (n = 106) a | p-Value c | Missing Lifestyle Score (n = 81) a | |
|---|---|---|---|---|---|---|
| Socio-demographic | ||||||
| Sex (male) [n (%)] | 270 (68.2) | 75 (73.5) | 66 (61.7) | 71 (67.0) | 0.19 | 58 (71.6) |
| Age (years) [mean (SD)] | 67.0 (9.1) | 66.7 (9.3) | 65.9 (8.5) | 68.4 (8.7) | 0.62 | 67.2 (10.0) |
| Comorbidities | 0.15 | |||||
| 0 | 91 (23.0) | 19 (18.6) | 26 (24.3) | 23 (21.7) | 23 (28.8) | |
| 1 | 102 (25.8) | 24 (23.5) | 36 (33.6) | 24 (22.6) | 18 (22.5) | |
| ≥2 | 202 (51.1) | 59 (57.8) | 45 (42.1) | 59 (55.7) | 39 (48.8) | |
| Education [n (%)] | 0.36 | |||||
| Low | 107 (27.1) | 29 (28.4) | 25 (23.4) | 30 (28.3) | 23 (28.8) | |
| Medium | 149 (37.7) | 36 (35.3) | 51 (47.7) | 38 (35.9) | 24 (30.0) | |
| High | 139 (35.2) | 37 (36.3) | 31 (29.0) | 38 (35.9) | 33 (41.3) | |
| Clinical | ||||||
| Cancer type [n (%)] | 0.80 | |||||
| Colon | 250 (63.1) | 66 (64.7) | 70 (65.4) | 65 (61.3) | 49 (60.5) | |
| Rectosigmoid and rectum | 146 (36.9) | 36 (35.3) | 37 (34.6) | 41 (38.7) | 32 (39.5) | |
| Tumour stage [n (%)] | 0.17 | |||||
| Stage I | 124 (31.3) | 41 (40.2) | 32 (29.9) | 31 (29.3) | 20 (24.7) | |
| Stage II | 100 (25.3) | 18 (17.7) | 28 (26.2) | 33 (31.1) | 21 (25.9) | |
| Stage III | 172 (43.4) | 43 (42.2) | 47 (43.9) | 42 (39.6) | 40 (49.4) | |
| Treatment [n (%)] | ||||||
| Surgery (yes) | 354 (89.4) | 85 (83.3) | 97 (90.7) | 95 (89.6) | 0.22 | 77 (95.1) |
| Chemotherapy (yes) | 155 (39.1) | 35 (34.3) | 43 (40.2) | 36 (34.0) | 0.57 | 41 (50.6) |
| Radiotherapy (yes) | 101 (25.5) | 20 (19.6) | 28 (26.2) | 26 (24.5) | 0.51 | 27 (33.3) |
| Stoma (yes) | 110 (28.4) | 29 (28.4) | 30 (28.0) | 26 (24.8) | 0.81 | 25 (33.8) |
| Lifestyle | ||||||
| Smoking [n (%)] | 0.59 | |||||
| Never | 118 (30.5) | 27 (26.5) | 30 (28.0) | 35 (33.3) | 26 (35.6) | |
| Former | 235 (60.7) | 65 (63.7) | 70 (65.4) | 59 (56.2) | 41 (56.2) | |
| Current | 34 (8.8) | 10 (9.8) | 7 (6.5) | 11 (10.5) | 6 (8.2) | |
| Body mass index, kg/m2 [mean (SD)] | 27.8 (4.6) | 29.1 (4.2) | 27.4 (4.1) | 26.1 (4.7) | 0.39 | 28.8 (4.7) |
| Underweight &Healthy weight: <25 | 119 (31.1) | 16 (15.7) | 30 (28.0) | 52 (49.1) | 0.00 * | 21 (26.3) |
| Overweight: 25–29.9 | 173 (43.8) | 47 (46.1) | 52 (48.6) | 41 (38.7) | 33 (41.3) | |
| Obese: ≥30 | 103 (26.1) | 39 (38.2) | 25 (23.4) | 13 (12.3) | 26 (32.5) | |
| Waist circumference (cm) [mean (SD)] | 100.1 (12.9) | 104.9 (12.5) | 98.8 (11.7) | 95.4 (12.3) | 0.76 | 101.9 (13.6) |
| MVPA (min/w) [median (IQR) | 7 (10.8) | 6 (8.2) | 7.5 (12.5) | 8.8 (11.0) | 0.00 * | 6.3 (9.6) |
| Prolonged sedentary behaviour (h/d) [mean (SD)] b | 5.3 (2.7) * (n = 325) | 5.9 (2.8) | 5.1 (2.4) | 5.0 (2.9) | 0.14 | 5.9 (1.9) (n = 10) |
| Fruit & vegetables (g/d) [mean (SD)] | 251.0 (124.7) | 182.5 (96.3) | 258.9 (115.6) | 320.3 (133.6) | 0.01 * | 233.2 (101.9) |
| Dietary fibre (g/d) [mean (SD)] | 20.9 (5.8) | 18.2 (4.6) | 21.4 (5.3) | 23.7 (6.6) | 0.00 * | 19.9 (4.9) |
| Sugary drinks (g/d) [mean (SD)] | 133.7 (157.9) | 188.6 (201.4) | 133.5 (157.2) | 95.3 (114.6) | 0.00 * | 111.4 (117.8) |
| Alcohol (g/d) [mean (SD)] | 13.1 (19.1) | 15.0 (14.3) | 10.8 (16.0) | 10.6 (19.5) | 0.01 * | 17.8 (26.9) |
| Red meat (g/w) [mean (SD)] | 593.4 (294.8) | 634.3 (303.8) | 629.6 (294.2) | 529.3 (292.6) | 0.92 | 574.9 (271.4) |
| Processed meat (g/w) [mean (SD)] | 325.1 (209.4) | 399.4 (225.9) | 324.7 (194.4) | 272.2 (199.4) | 0.26 | 296.7 (193.2) |
| Ultra-processed food (EN%) [mean (SD)] | 35.4 (10.8) | 40.4 (8.8) | 36.6 (11.2) | 28.8 (9.6) | 0.04 * | 36.2 (9.6) |
| EORTC QLQ-C30 | Checklist Individual Strength | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Global QoL (0–100) | Physical Functioning (0–100) | Role Functioning (0–100) | Social Functioning (0–100) | Summary Score (0–100) | Fatigue (EORTC) (0–100) | Fatigue (CIS) (20–140) | Subjective Fatigue (CIS) (8–56) | Activity Fatigue (CIS) (3–21) | |
| β (95% CI) b | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Lifestyle score (per 1 point increase) | |||||||||
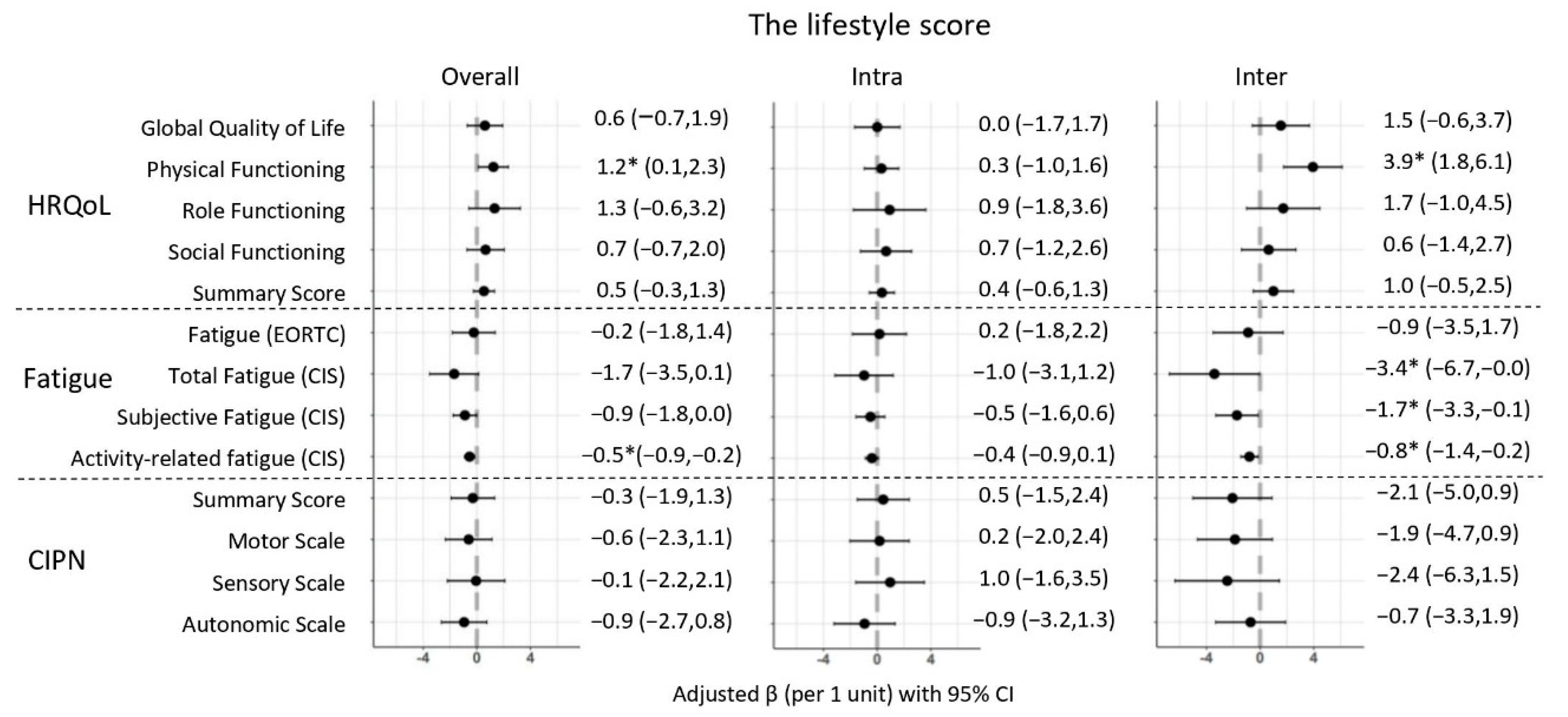
| Adjusted a | 0.6 (−0.7,1.9) | 1.2 * (0.1,2.3) | 1.3 (−0.6,3.2) | 0.7 (−0.7,2.0) | 0.5 (−0.3,1.3) | −0.2 (−1.8,1.4) | −1.7 (−3.5,0.1) | −0.9 (−1.8,0.0) | −0.5 * (−0.9,−0.2) |
| Additional adjustment for: | |||||||||
| Physical activity c | −0.5 (−1.8,0.9) | 0.3 (−0.9,1.4) | −0.5 (−2.5,1.4) | −0.3 (−1.7,1.2) | −0.1 (−0.9,0.7) | 0.9 (−0.8,2.5) | −0.3 (−2.1,1.6) | −0.2 (−1.1,0.7) | −0.2 (−0.6,0.2) |
| MVPA | −0.0 (−1.3,1.3) | 0.7 (−0.4,1.8) | 0.1 (−1.8,2.0) | 0.1 (−1.3,1.5) | 0.2 (−0.6,1.0) | 0.3 (−1.3,1.9) | −1.0 (−2.9,0.8) | −0.5 (−1.4,0.4) | −0.4 * (−0.8,−0.0) |
| Sedentary behaviour | 0.1 (−1.3,1.4) | 0.7 (−0.4,1.8) | 0.5 (−1.5,2.5) | 0.2 (−1.2,1.6) | 0.2 (−0.6,1.0) | 0.5 (−1.2,2.1) | −0.8 (−2.6,1.1) | −0.6 (−1.5,0.3) | −0.1 (−0.5,0.4) |
| Body composition d | 0.6 (−0.8,2.0) | 1.5 * (0.3,2.6) | 1.4 (−0.6,3.5) | 0.8 (−0.7,2.3) | 0.6 (−0.2,1.4) | 0.0 (−1.7,1.6) | −1.6 (−3.5,0.4) | −0.8 (−1.7,0.2) | −0.5 * (−0.9,−0.1) |
| Plant-based foods e | 0.6 (−1.0,2.1) | 1.0 (−0.3,2.3) | 1.4 (−0.9,3.6) | 0.6 (−1.1,2.2) | 0.3 (−0.6,1.2) | −0.3 (−2.1,1.6) | −1.6 (−3.7,0.5) | −0.8 (−1.8,0.3) | −0.5 * (−1.0,−0.1) |
| Ultra-processed foods | 0.4 (−1.2,2.0) | 1.0 (−0.3,2.4) | 0.8 (−1.5,3.1) | −0.1 (−1.8,1.5) | 0.1 (−0.8,1.1) | 0.5 (−1.4,2.4) | −1.4 (−3.6,0.8) | −0.8 (−1.8,0.3) | −0.5 * (−1.0,0.1) |
| Red and processed meat | 0.6 (−0.8,2.0) | 1.2 * (0.0,2.4) | 1.5 (−0.5,3.5) | 0.8 (−0.7,2.2) | 0.7 (−0.2,1.5) | −0.6 (−2.3,1.1) | −2.2 (−4.1,−0.3) | −1.1 (−2.0,−0.2) | −0.6 * (−1.0,−0.1) |
| Sugar-sweetened drinks | 0.4 (−1.1,1.8) | 0.9 (−0.3,2.1) | 0.7 (−1.4,2.8) | 0.2 (−1.3,1.8) | 0.2 (−0.7,1.1) | 0.2 (−1.5,2.0) | −0.9 (−2.9,1.1) | −0.7 (−1.6,0.3) | −0.5 * (−0.9,−0.0) |
| Alcohol | 1.9 * (0.5,3.3) | 2.6 * (1.4,3.8) | 3.3 * (1.3,5.3) | 2.0 * (0.5,3.4) | 1.7 * (0.8,2.5) | −1.9 * (−3.6,−0.2) | −3.7* (−5.7,−1.8) | −1.8 * (−2.8,−0.9) | −0.9 * (−1.3,−0.5) |
| Body composition and alcohol | 2.1 * (0.6,3.5) | 3.0 * (1.7,4.2) | 3.7 * (1.5,5.8) | 2.3 * (0.8,3.9) | 1.8 * (0.9,2.7) | −1.9 * (−3.6,−0.1) | −3.7 * (−5.8,−1.7) | −1.7 * (−2.7,−0.8) | −0.9 * (−1.3,−0.5) |
| EORTC QLQ-C30 | Checklist Individual Strength | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Global QOL (0–100) | Physical Functioning (0–100) | Role Functioning (0–100) | Social Functioning (0–100) | Summary Score (0–100) | Fatigue (EORTC) (0–100) | Fatigue (CIS) (20–140) | Subjective Fatigue (CIS) (8–56) | Activity Fatigue (CIS) (3–21) | |
| β (95% CI) b | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Model with the three subscores including alcohol in the dietary subscore | |||||||||
| Physical activity subscore a,c | 3.3 * (2.2,4.4) | 3.3 * (2.5,4.2) | 5.5 * (3.9,7.1) | 2.7 * (1.6, 3.9) | 2.0 * (1.4,2.7) | −3.2 * (−4.5,−1.9) | −4.5 * (−6.0,−3.0) | −2.1 * (−2.9,−1.4) | −1.2 * (−1.5,−0.9) |
| Body composition subscore a,d | −0.0 (−1.0,1.0) | −0.6 (−1.5,0.3) | −0.2 (−1.5,1.1) | −0.2 (−1.2,0.7) | −0.1 (−0.7,0.6) | −0.3 (−1.5,1.0) | −0.5 (−2.0,0.9) | −0.4 (−1.1,0.3) | −0.2 (−0.5,0.1) |
| Dietary subscore a,e | −0.1 (−0.5,0.2) | 0.1 (−0.2,0.4) | −0.1 (−0.7,0.4) | −0.0 (−0.4,0.3) | −0.0 (−0.2,0.2) | 0.3 (−0.2,0.7) | −0.0 (−0.5,0.5) | −0.0 (−0.3,0.2) | −0.0 (−0.1,0.1) |
| Model with the three subscores excluding alcohol in the dietary subscore | |||||||||
| Physical activity subscore a,c | 3.3 * (2.2,4.4) | 3.3 * (2.4,4.2) | 5.4 * (3.8,7.0) | 2.7 * (1.6, 3.9) | 2.0 * (1.4,2.7) | −3.2 * (−4.5,−1.9) | −4.5 * (−6.0,−3.0) | −2.1 * (−2.8,−1.4) | −1.2 * (−1.5,−0.8) |
| Body composition subscore a,d | −0.0 (−1.0,1.0) | −0.5 (−1.4,0.4) | −0.2 (−1.5,1.1) | −0.2 (−1.2,0.8) | −0.1 (−0.7,0.6) | −0.3 (−1.5,0.9) | −0.6 (−2.1,0.9) | −0.5 (−1.2,0.2) | −0.2 (−0.5,0.1) |
| Dietary subscore without alcohol a,f | 0.2 (−0.2,0.6) | 0.5 * (0.1,0.8) | 0.5 (−0.1,1.0) | 0.4 (−0.0,0.8) | 0.3 * (0.1,0.5) | −0.2 (−0.7,0.2) | −0.6 * (−1.1,−0.0) | −0.3 * (−0.5,−0.0) | −0.1 * (−0.2,−0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenkhuis, M.-F.; Mols, F.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; van Duijnhoven, F.J.B.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers 2022, 14, 417. https://doi.org/10.3390/cancers14020417
Kenkhuis M-F, Mols F, van Roekel EH, Breedveld-Peters JJL, Breukink SO, Janssen-Heijnen MLG, Keulen ETP, van Duijnhoven FJB, Weijenberg MP, Bours MJL. Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers. 2022; 14(2):417. https://doi.org/10.3390/cancers14020417
Chicago/Turabian StyleKenkhuis, Marlou-Floor, Floortje Mols, Eline H. van Roekel, José J. L. Breedveld-Peters, Stéphanie O. Breukink, Maryska L. G. Janssen-Heijnen, Eric T. P. Keulen, Fränzel J. B. van Duijnhoven, Matty P. Weijenberg, and Martijn J. L. Bours. 2022. "Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment" Cancers 14, no. 2: 417. https://doi.org/10.3390/cancers14020417
APA StyleKenkhuis, M.-F., Mols, F., van Roekel, E. H., Breedveld-Peters, J. J. L., Breukink, S. O., Janssen-Heijnen, M. L. G., Keulen, E. T. P., van Duijnhoven, F. J. B., Weijenberg, M. P., & Bours, M. J. L. (2022). Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers, 14(2), 417. https://doi.org/10.3390/cancers14020417






