Simple Summary
Prostate cancer (PCa) relapse occurs in up to 50% of patients after radical treatment. Once PCa recurrence is detected, a precise identification of the number and sites of recurrence is necessary to tailor the treatment on the patient’s needs. Positron emission tomography (PET) plays a pivotal role in this clinical setting and new radiotracers have been developed to improve its performance. While 68Ga-PSMA is a well-established radiotracer for PCa recurrence detection, 68Ga-DOTA-RM2 is a recently proposed tracer that targets the gastrin-releasing peptide receptors that are overexpressed in prostate cancer. In this work, the performance of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in identifying recurrent disease were compared on the same cohort, using the same study protocol, as this is the only way to assess whether one outperforms the other and therefore should be preferred in clinical practice. Furthermore, the association between PET findings and clinical and histopathological characteristics was investigated to find potential biomarkers.
Abstract
The aim of the present study is to investigate and compare the performances of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in identifying recurrent prostate cancer (PCa) after primary treatment and to explore the association of dual-tracer PET findings with clinical and histopathological characteristics. Thirty-five patients with biochemical relapse (BCR) of PCa underwent 68Ga PSMA PET/MRI for restaging purpose, with 31/35 also undergoing 68Ga-DOTA-RM2 PET/MRI scan within 16 days (mean: 3 days, range: 2–16 days). Qualitative and quantitative image analysis has been performed by comparing 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI findings both on a patient and lesion basis. Clinical and instrumental follow-up was used to validate PET findings. Fisher’s exact test and Mann-Whitney U test were used to investigate the association between dual-tracer PET findings, clinical and histopathological data. p-value significance was defined below the 0.05 level. Patients’ mean age was 70 years (range: 49–84) and mean PSA at time of PET/MR scans was 1.88 ng/mL (range: 0.21–14.4). A higher detection rate was observed for 68Ga-PSMA PET/MRI, with more lesions being detected compared to 68Ga-DOTA-RM2 PET/MRI (26/35 patients, 95 lesions vs. 15/31 patients, 41 lesions; p = 0.016 and 0.002). 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI findings were discordant in 11/31 patients; among these, 10 were 68Ga-PSMA positive (9/10 confirmed as true positive and 1/10 as false positive by follow-up examination). Patients with higher levels of PSA and shorter PSA doubling time (DT) presented more lesions on 68Ga-PSMA PET/MRI (p = 0.006 and 0.044), while no association was found between PET findings and Gleason score. 68Ga-PSMA has a higher detection rate than 68Ga-DOTA-RM2 in detecting PCa recurrence. The number of 68Ga-PSMA PET positive lesions is associated with higher levels of PSA and shorter PSA DT, thus representing potential prognostic factors.
1. Introduction
Prostate cancer (PCa) relapse affects up to 50% of patients treated with radical prostatectomy (RP) or external-beam radiotherapy (EBRT) as primary treatment for clinically localized disease [1].
When biochemical recurrence (BCR) is detected, an accurate identification of location and extent of cancer recurrence is mandatory in order to address patients to directed therapies with prolonged intervals of cancer-free survival, thus avoiding systemic treatments, including androgen deprivation therapy [2].
Prostate Specific Antigen (PSA) is the commonly used circulating biomarkers identifying BCR; however, it is unable to provide information regarding the exact location of disease recurrence.
Of note, patients with low values of PSA might benefit the most by receiving early local therapies with curative intent; however, conventional imaging techniques have limited sensitivity in detecting tumour recurrences at PSA values lower than 1 ng/mL [1]. On the other hand, patients presenting metastatic disease should be candidate to systemic therapies. Therefore, the distinction between local or systemic disease is of utmost importance to plan the most appropriate treatment [3]. In this scenario, imaging plays a fundamental role in the identification of local and/or distant metastases.
In the last decade, Prostate Specific Membrane Antigen (PSMA), a transmembrane protein with a significantly increased expression in PCa cells, has gained particular attention as a radiotracer for positron emission tomography (PET) imaging in PCa, in view of its better sensitivity and specificity in detecting of metastatic disease, compared to conventional imaging, also at low PSA values [4,5,6].
Additionally, gastrin releasing peptide receptors (GRPR) are G-protein coupled receptors overexpressed in prostate tumours both at the mRNA and the protein level [7]. For this reason, GRPR analogues have been recently identified as alterative targets for molecular imaging in prostate cancer [8]. So far, among the available radiotracers targeting GRPR, 68Ga-DOTA-RM2 has been the most investigated [9,10,11,12].
Very few studies have been reported on the combined use of both 68Ga-PSMA and 68Ga-DOTA-RM2 in prostate cancer setting, both in the staging [12,13] and restaging [10,14] phase of the disease, with the majority of them using PET/computed tomography (CT) scanners for imaging acquisition [10,13,14].
The recent introduction of fully hybrid PET/Magnetic Resonance Imaging (MRI) paves the way to a completely innovative imaging approach for PCa recurrence. The simultaneous acquisition of PET and multiparametric MR (mp-MR) provides metabolic, structural, and functional imaging information regarding PCa status in a whole-body single session examination, with better soft tissue contrast and reduced radiation exposure in comparison to PET/CT [15,16]. However, very limited data are currently available on its role in this specific clinical setting. In particular, studies using both 68Ga-PSMA and 68Ga-DOTA-RM2 with PET/MRI are still anecdotal [10,12,14]
Therefore, the aim of our study was to compare the performances of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in the identification of recurrent PCa after primary treatment. Furthermore, we also investigated the association between 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI positive findings and clinical, as well as histopathological characteristics.
2. Materials and Methods
2.1. Patients
In this prospective clinical study, 35 consecutive patients with biochemically recurrent PCa were enrolled from June 2020 to March 2021 at IRCCS San Raffaele Scientific Institute.
Inclusion criteria were age greater than 18 years at the time of PET/MRI scan, previous radical treatment for PCa and biochemical failure, defined as two consecutive PSA measurements ≥ 0.2 ng/mL after RP or an increase by ≥2 ng/mL above the nadir PSA for patients that underwent RT [17].
Exclusion criteria were medical condition possibly interfering and significantly affecting study compliance and contraindications to undergo MRI scan (i.e., severe claustrophobia).
All recruited patients underwent 68Ga-PSMA PET/MRI and 31/35 also 68Ga-DOTA-RM2 PET/MRI in two different scan sessions, with at least 48 h interval.
PSA level at different time points and Gleason Score (GS) after RP, whenever available, were collected for all patients.
This study was approved by the Institutional Ethics Committee of IRCCS San Raffaele Scientific Institute (EudraCT 2018-001036-21), and all patients gave written informed consent to participate to the study.
The data presented in the present manuscript are part of a single-centre prospective study still ongoing at IRCCS San Raffaele Scientific Institute (Project GR-2016-02363991).
2.2. 68Ga-PSMA PET/MRI Acquisition Protocol
68Ga-PSMA was synthetized according to the procedure previously described. [12]
Fasting condition was requested on the day of 68Ga-PSMA PET/MRI scan. Images were acquired on a fully hybrid 3 Tesla PET/MRI system (SIGNA PET/MRI; General Electric Healthcare, Waukesha, WI, USA) from the skull base to mid-thigh in the following examined patients.
The 68Ga-PSMA PET/MRI scan started approximately 60 min (Mean ± SD, 63 ± 17 min) after injection of 129–288 MBq (mean ± SD, 170.56 ± 36.06 MBq) of 68Ga-PSMA [10,14].
The 68Ga-PSMA PET/MR examination protocol included a high statistic (HS) scan of the pelvis (20 min), covering a single bed position, that was simultaneously acquired with the following MRI sequences.
- an axial T2 weighted sequence with large field of view (FOV): FSE, TR = 10,235 ms; TE = 99.7 ms, FOV = 32 × 32 cm2; voxel size = 0.9 × 0.9 × 5 mm3,
- an axial T2 weighted sequence with small FOV: PROPELLER, TR = 9578 ms, TE = 151 ms, FOV = 18 × 18 cm2, voxel size = 0.6 × 0.6 × 3 mm3,
- a sagittal T2 weighted sequence with small FOV: PROPELLER, TR = 9578 ms, TE = 151 ms, FOV = 18 × 18 cm2, voxel size = 0.6 × 0.6 × 3 mm3,
- a diffusion weighted imaging (DWI) sequence with small FOV: TR = 6643 ms, TR = 79.5 ms, FOV = 18 × 18 cm2, voxel size = 1.8 × 1.8 × 3 mm3; b = 50, 800, 1400; 2000 s/mm2 and ADC maps,
- T1-Lava Flex sequence of the whole pelvic region pre-contrast and post-contrast: TR = 5 ms, TE = 1.7 ms, FOV: 44 × 35.2 cm2, voxel size = 1.3 × 1.2 × 2 mm3,
- a high temporal resolution T1 perfusion sequence after IV injection of 0.1 mmol/kg bolus of gadobutrol (Gadovist, Bayer Schering Pharma, Germany) at a flow rate of 3.5 mL/s: DISCO, TR = 5.1 ms, TE = 1.7 ms, FOV = 29 × 29 cm2, Voxel size = 1.9 × 2.2 × 3 mm 3, 88 dynamics.
Following the single bed HS acquisition, a total-body (TB) PET scan (5–6 FOVs, 4 min/FOV) was then simultaneously acquired to a MRI TB T1 Lava Flex sequence for anatomical localization, and a TB DWI with b = 50 and b = 1000 s/mm2.
PET images were reconstructed using a Bayesian penalized likelihood reconstruction algorithm with a reconstructed FOV of 60 cm and image matrix of 192 × 192. The algorithm includes a Point Spread Function and Time of Flight information.
Attenuation Correction (AC) of PET data was performed using MR-Based AC technique based on the processing of the LAVA-Flex sequences acquired simultaneously with the PET data.
2.3. 68Ga-DOTA-RM2 PET/MRI Acquisition Protocol
68Ga-DOTA-RM2 was synthetized according to the procedure previously described in [12].
68Ga-DOTA-RM2 PET/MRI scan was performed within sixteen days (mean: 3 days, range: 2–16 days) from 68Ga-PSMA PET/MRI; as for 68Ga-PSMA PET/MRI, fasting condition was requested on the day of the examination and the same PET/MRI scanner was used.
Image acquisition started approximatively 50 min (mean ± SD, 49 ± 13 min) after injection of 84–228 MBq (mean ± SD, 153.36 ± 36 MBq) of 68Ga-DOTA-RM2 [10,14]. The 68Ga-DOTA-RM2 PET/MR examination protocol included a HS scan of the pelvis (20 min), covering a single bed position, that was simultaneously acquired with an axial T2 weighted sequence with large FOV (32 × 32 cm2) for anatomical localisation.
Following the single bed acquisition, a TB PET scan (5–6 FOVs, 4 min/FOV) was acquired from the base of the skull to mid-thigh simultaneously with a MRI TB Lava Flex sequence and TB DWI with b = 50 and b = 1500 s/mm2.
Reconstruction and attenuation correction of PET images were performed by using the same algorithms and parameters used for 68Ga-PSMA PET images.
2.4. PET/MR Image Analysis
68Ga-PSMA and 68Ga-DOTA-RM2 image read-out was performed on the Advantage Workstation (AW, General Electric Healthcare, Waukesha, WI, USA), on which PET, MRI and fused PET/MR images could be visualized in axial, coronal, and sagittal planes. HS PET acquisition bed on the pelvic region and TB PET examination of both 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI images were qualitatively interpreted by two experienced (more than 10 years of experience) Nuclear Medicine physicians, with knowledge of all the available patients’ clinical and imaging information. MR examination being part of the patients’ clinical work-up, MR images were evaluated by a Radiologist Physician and a report was provided to each patient. As the present study is specifically focused on the use of 68Ga-PSMA and 68Ga-DOTA-RM2 PET in recurrent PCa, MRI data were not considered for the purpose of this work and therefore were not included in the analysis. MRI was just used for the localization of the lesions.
The whole-body distribution pattern of both 68Ga-PSMA and 68Ga-DOTA-RM2 was qualitatively assessed, and the presence of increased uptake was considered positive for malignancy, with the exception of areas of physiologically increased uptake [18,19]. The anatomical sites were defined based on MR images simultaneously acquired with PET examinations.
Regions of interest (ROI) were semi-automatically defined on transaxial PET images on the single site of recurrence in those patients presenting only one pathological finding and on the most representative site of pathological uptake in those patients presenting multiple lesions. Furthermore, SUVmax and SUVmean, calculated at the 40% threshold of SUVmax [20], were calculated from each ROI drawn on TB PET images, for both radiotracers.
A qualitative comparison between 68Ga-PSMA and 68Ga-DOTA-RM2 pathological uptakes in the different anatomical sites localized on MR images was performed in order to describe the possible concordances and discrepancies between 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MR imaging. Specifically, 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MR findings were defined concordant if the same pathological lesions were detected by both modalities or if both scans resulted negative for the presence of pathological findings. Moreover, 68Ga-PSMA and 68Ga-DOTA-RM2 findings were described as partially concordant if at least one lesion, but not all pathological findings, was detected by PET examination with both tracers. Finally, when pathological uptake was observed in one PET examination, but not in the other, the exams were considered discordant.
2.5. Lesion Validation
All PET findings were validated by using clinical and instrumental follow-up. Specifically, 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MR imaging findings were considered true positive when at least one of the following criteria was met: (a) histological confirmation on surgically resected specimen; (b) progression (increase in number of pathological 68Ga-PSMA uptake sites or increase in uptake intensity) on follow-up 68Ga-PSMA PET/CT or PET/MR studies associated with an increase in PSA level; (c) confirmation on conventional imaging either at baseline (including the diagnostic MR exam performed simultaneously to the 68Ga-PSMA scan) or during follow-up; (d) disappearance or considerable reduction (i.e., number and intensity) of 68Ga-PSMA uptake on follow-up PET/CT or PET/MR scans after local or systemic treatment associated with a decrease in PSA level greater than 50%; (e) a decrease in PSA level greater than 50% after selective irradiation of the site of pathological 68Ga-PSMA or 68Ga-DOTA-RM2 uptake.
Patients with negative 68Ga-PSMA and/or 68Ga-DOTA-RM2 PET/MR were considered true negative in the absence of evidence of disease on conventional imaging or 68Ga-PSMA PET/CT or PET/MR acquired during the follow-up period (mean follow-up duration for both positive and negative findings: 13.8 months, range: 8.2–17.9 months).
2.6. Statistical Analyses
Statistical analyses were performed with R statistical software [21], and Python version 3.7, Python Software Foundation, available at http://www.python.org. (accessed on 30 November 2021). McNemar’s test was used to test for the differences in detection rate of recurrent PCa according to 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI uptake on a patient basis in those patients examined with both radiotracers. Moreover, in the same patients, Wilcoxon-signed rank test was performed to compare the positive findings of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI on a lesion basis.
In order to study the association between 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI findings and clinical data, the sample was subdivided based on PSA concentration in three groups (PSA ≤ 0.5 ng/mL, 0.5–2 ng/mL, ≥2 ng/mL); based on PSA doubling time (PSA DT) in two groups (PSA DT <6 months and ≥ 6 months); and based on GS of the primary tumour (prior to radical therapy) in two groups (GS ≤ 3 + 4 and ≥4 + 3). Fisher’s exact test was used to correlate clinical and histopathological data with dual-tracer PET positive findings on a patient basis. Furthermore, Receiver Operating Characteristics (ROC) analysis was computed to investigate the predictive value of clinical and histopathological data (PSA, PSA DT, and GS) to predict 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI positivity. Corresponding area under the curve (AUC) with 95% confidence interval (CI) were derived.
The Mann-Whitney U test was used to investigate whether higher levels of PSA, GS, and lower PSA DT were related to a higher number of PET positive lesions. All p-values were corrected for multiple testing by false discovery rate and significance was defined below the 0.05 level.
3. Results
3.1. Patients
Thirty-five men (mean age: 70 years; range: 49–84) with BCR of PCa were enrolled in this prospective study. Mean PSA level at time of scanning was 1.88 ng/mL (range: 0.21–14.4). All patients underwent 68Ga PSMA PET/MRI, with 31/35 also undergoing 68Ga-DOTA-RM2 PET/MRI because of reduced compliance to the study protocol. Patients’ characteristics are reported in Table 1.

Table 1.
Patients’ characteristics.
3.2. PET/MRI Findings and Comparison between 68Ga-PSMA and 68Ga-DOTA-RM2
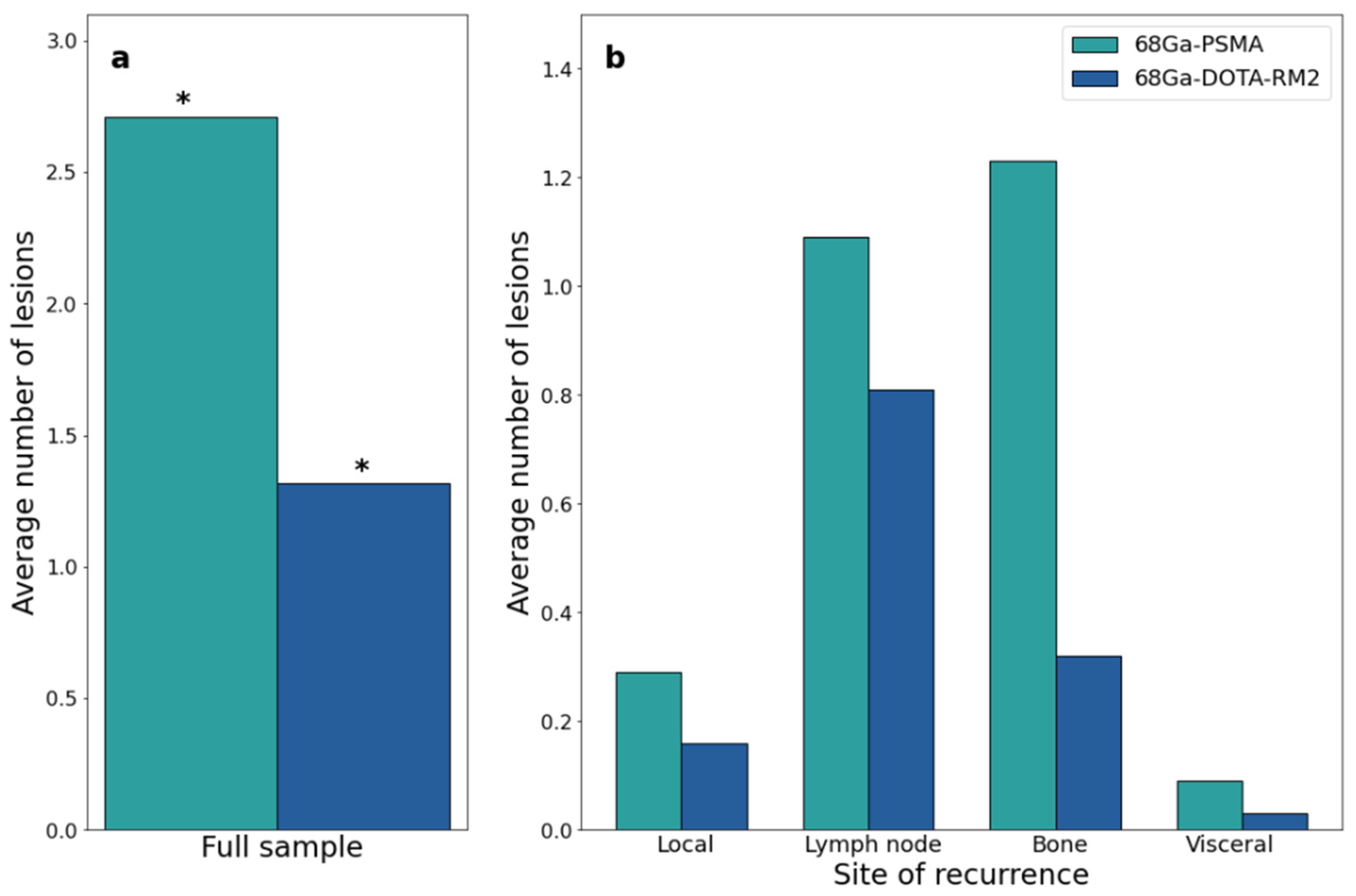
68Ga-PSMA PET/MRI was positive in 26/35 patients (74%), with a total number of 95 lesions being detected (mean SUVmax: 19.69, range: 1.91–112.37; mean SUVmean: 12.03, range: 1.04–65.83); 68Ga-DOTA-RM2 PET/MRI was positive in 15/31 patients (48%), with a total number of 41 detected lesions (mean SUVmax: 19.51, range: 3.41–146.62; mean SUVmean: 13.36, range: 2.04–101.41). 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI findings were concordant in 12/31 patients (6 patients with the same positive findings on both examinations, 6 patients with negative examinations; Figure 1 for an exemplar image), partially concordant in 8/31 patients and discordant in 11/31 patients. Specifically, regarding the discordant findings, 68Ga-PSMA detected 7 lesions in 5 patients having local recurrences, 5 lesions in 5 patients experiencing bone disease, 4 lesions in 3 patients with lymph nodal recurrence and 2 lesions in one patient with visceral metastases. Conversely, 68Ga-DOTA-RM2 PET/MRI detected only one lesion in one patient presenting bone involvement.
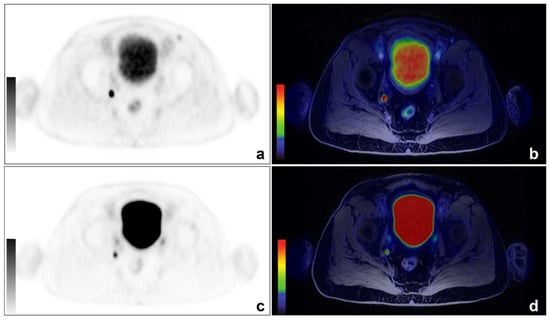
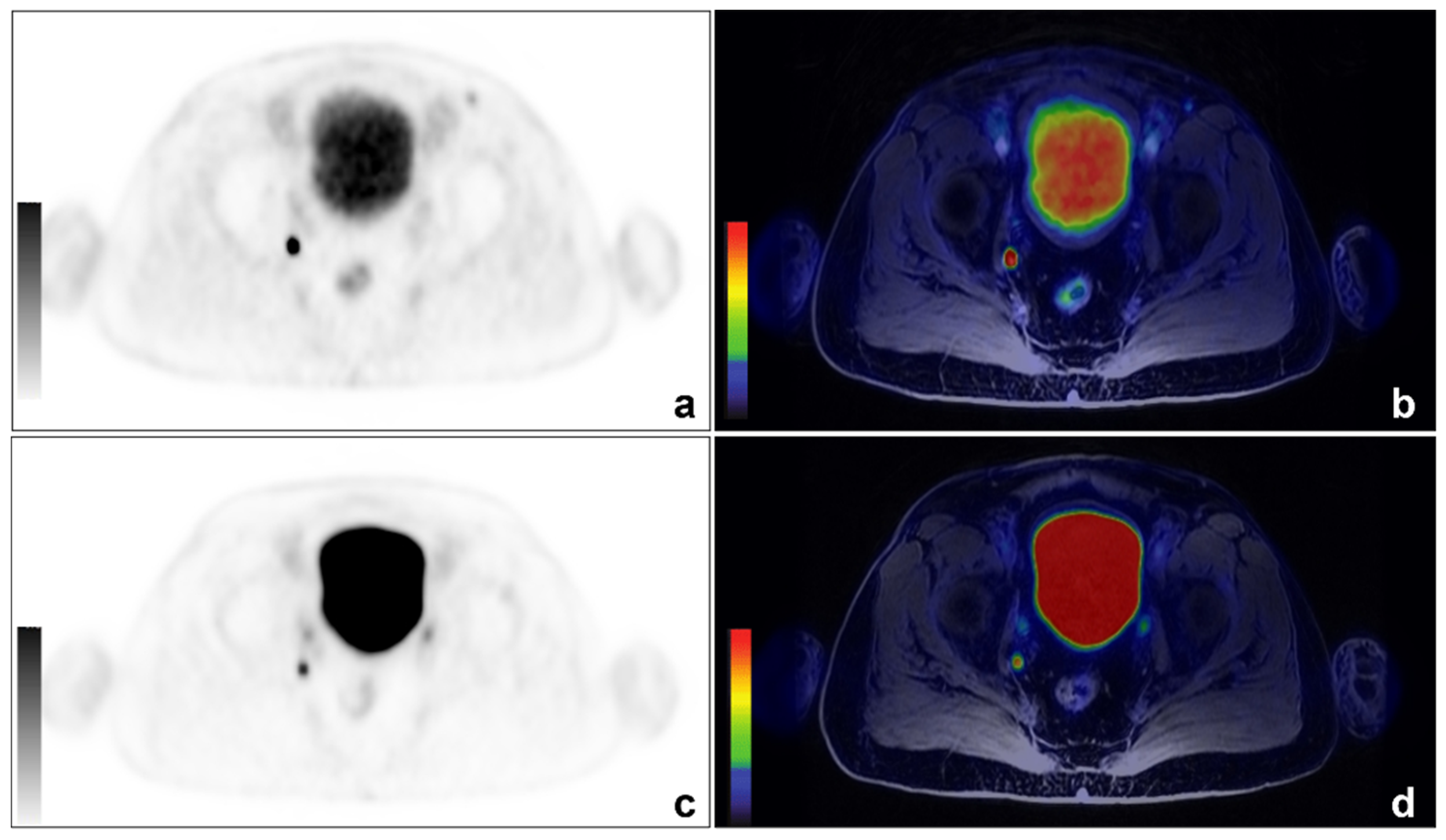
Figure 1.
An example of concordant findings between 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in detecting PCa lymph nodal recurrence. A 66-year-old patient previously treated with radical prostatectomy for a Gleason 7 (4 + 3) PCa (pT3aN0) experienced biochemical recurrence (PSA: 0.53 ng/mL). 68Ga-PSMA PET/MRI images showed a focal right obturator lymph nodal uptake ((a): axial 68Ga-PSMA PET; (b): axial 68Ga-PSMA PET/MRI). The same finding is visible on 68Ga-DOTA-RM2 PET/MRI images (c): axial 68Ga-DOTA-RM2 PET; (d): axial 68Ga-DOTA-RM2 PET/MRI).
Among the 12 concordant cases, 6/12 were confirmed as true positive and 4/12 true negative, whilst 2/12 patients were found to be false negative. Of note, regarding patient n. 9 who resulted to be true positive, further examination on conventional imaging confirmed only a minority of lesions detected by PET with both radiotracers.
Among patients presenting partially concordant uptake findings of the two radiotracers, in only one subject 68Ga-DOTA-RM2 detected more lesions compared to 68Ga-PSMA; notably, these additional findings were not confirmed by available evidence gathered in the follow-up period. Conversely, in 5/7 patients for whom 68Ga-PSMA identified more locations of PCa recurrence than 68Ga-DOTA-RM2, the results were confirmed by follow-up examination.
Among the 11/31 discordant findings, 10/11 were 68Ga-PSMA positive and 68Ga-DOTA-RM2 negative (Figure 2 and Figure 3 for exemplar images).
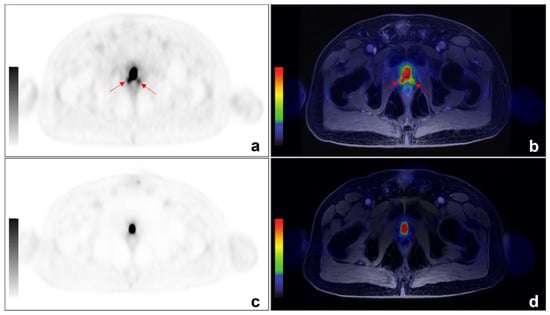
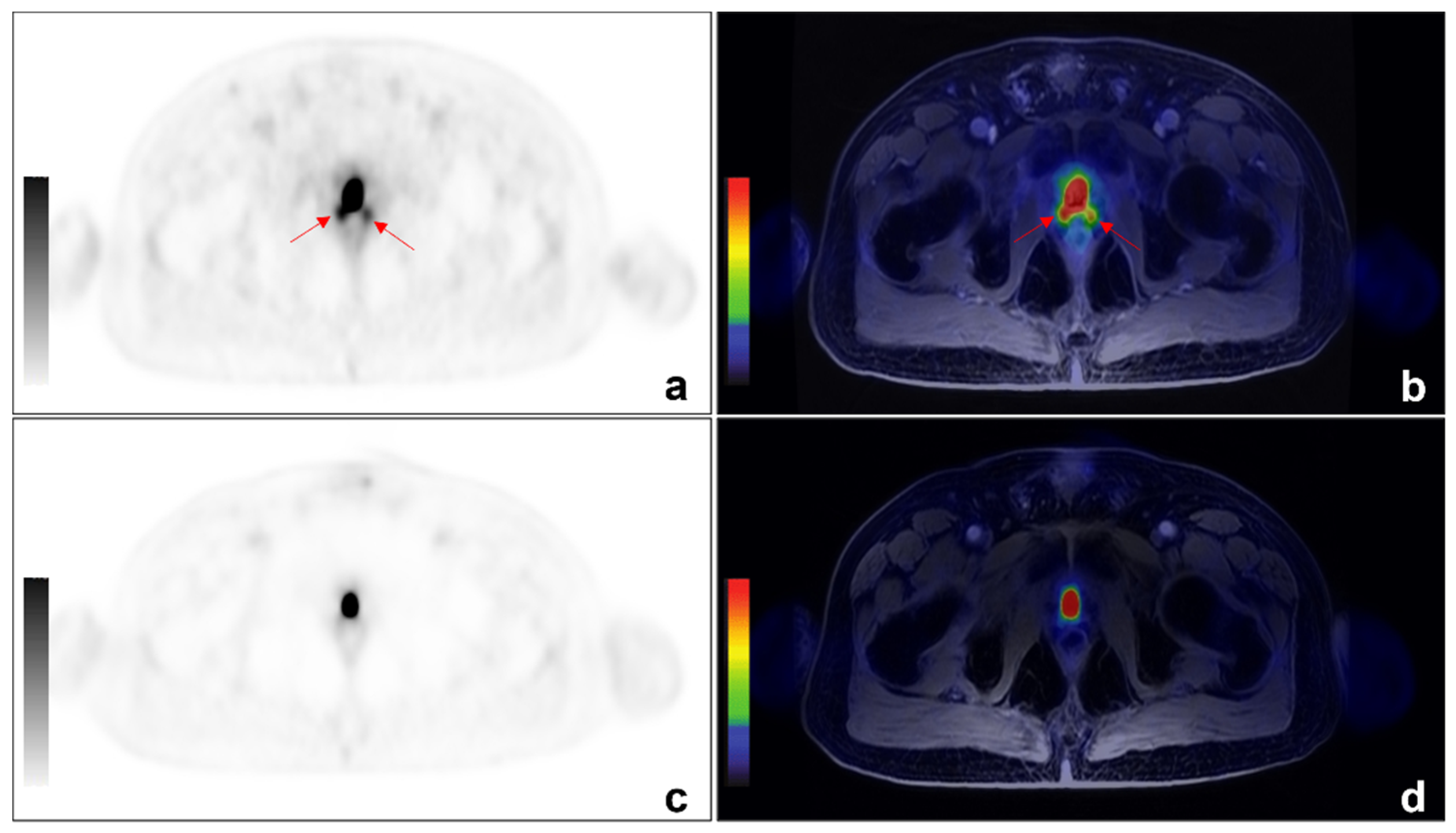
Figure 2.
An example of discordant findings between 68Ga-PSMA and 68-Ga-DOTA-RM2 PET/MRI in detecting PCa local recurrence. A 64 year-old patient previously treated with radical prostatectomy for PCa (Gleason 4 + 3) experienced biochemical recurrence of PCa (PSA = 2.5 ng/mL). 68Ga-PSMA PET/MRI showed radiotracer uptake (red arrows) in correspondence of the right and left posterior sides of the prostatic lodge. ((a): axial 68Ga-PSMA PET; (b): axial 68Ga-PSMA PET/MRI). No pathological uptake was detected on 68Ga-DOTA-RM2 PET/MR images (c): axial 68Ga-DOTA-RM2 PET; (d): axial 68Ga-DOTA-RM2 PET/MRI).
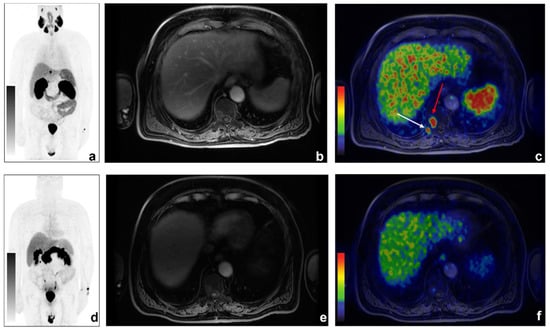
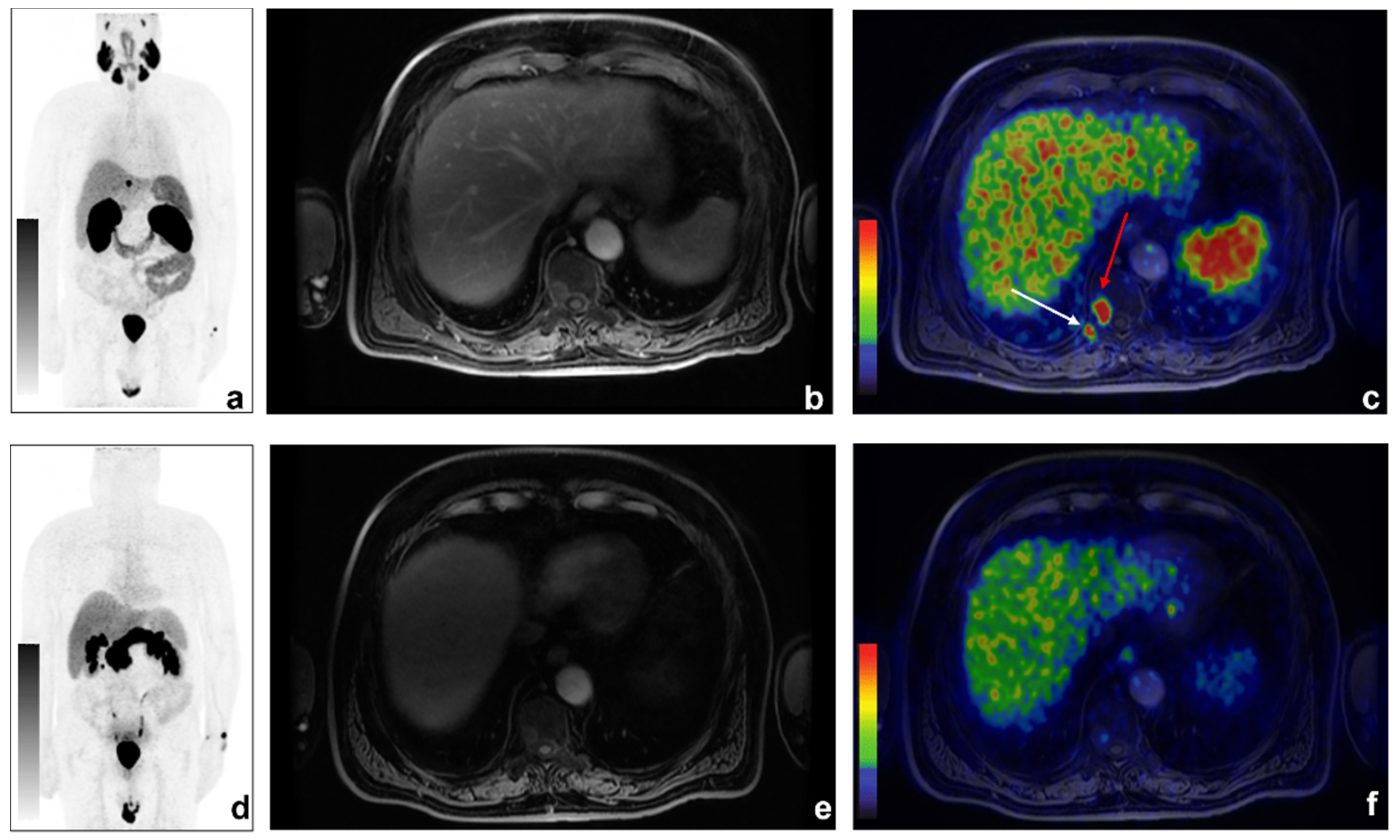
Figure 3.
An example of discordant findings between 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in bone metastases detection. A 70-year-old patient previously treated with radical prostatectomy for PCa (Gleason 5 + 4; pT3aN0) experienced biochemical recurrence of PCa (PSA = 2.16 ng/mL). 68Ga-PSMA PET/MRI showed radiotracer uptake in correspondence of right D10 hemisome ((a): 68Ga-PSMA MIP; (b): axial LAVA-FLEX MRI; (c): axial 68Ga-PSMA PET/MRI, red arrow) and the right posterior tract of the X rib (c): white arrow). Non-significant uptake was detected on 68Ga-DOTA-RM2 PET/MR images (d): 68Ga-DOTA-RM2 MIP; (e): axial LAVA-FLEX MRI; (f): 68Ga-DOTA-RM2 PET/MRI).
Nine/11 of these patients were confirmed to be true positive by follow-up examination, while 1/11 68Ga-PSMA positive finding was not supported by further evidence, thus being classified as false positive. The remaining patient presenting 68Ga-DOTA-RM2 positive finding with negative 68Ga-PSMA PET/MRI, finally resulted to be a true positive. See Table 2 for detailed information on the follow-up examination for each patient.

Table 2.
PET findings and PET findings validation.
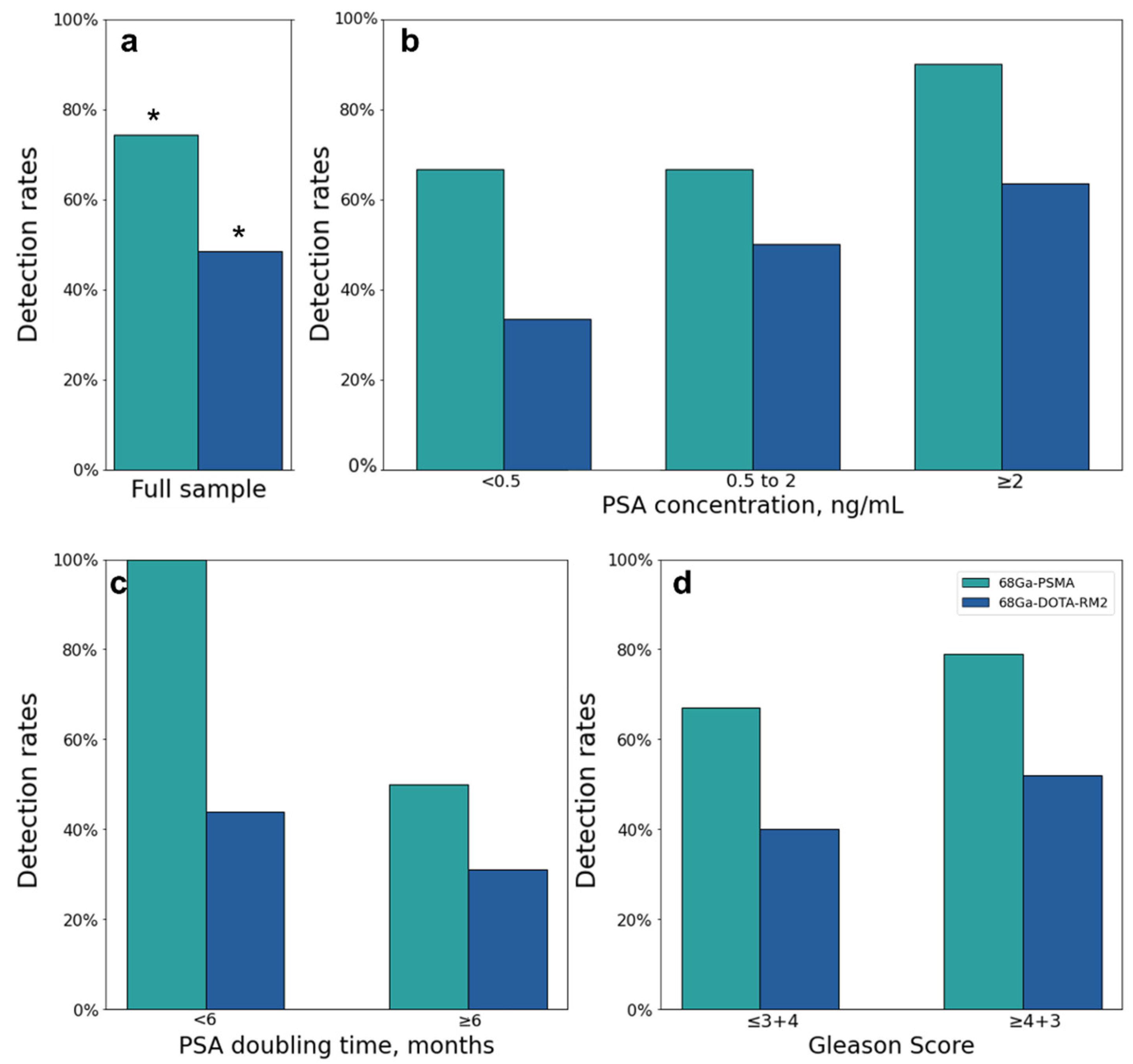
Both scans identified 26 lesions in 19/31 patients, while 68Ga-PSMA detected 66 additional lesions in 17/31 patients as compared to 68Ga-DOTA-RM2. Fifteen lesions in 3/31 patients were visible only on 68Ga-DOTA-RM2 PET/MRI. The patient-based detection rates (Figure 4a) and the average number of detected lesions (Figure 5a) were significantly different between the two imaging modalities (p = 0.016 and 0.002). The detection rates of these radiotracers stratified by PSA serum concentration, PSA DT and GS prior to radical treatment are depicted in Figure 4b, Figure 4c and Figure 4d, respectively. Moreover, in Figure 5b is shown the average number of lesions identified by dual-tracer PET stratified by site of recurrence.
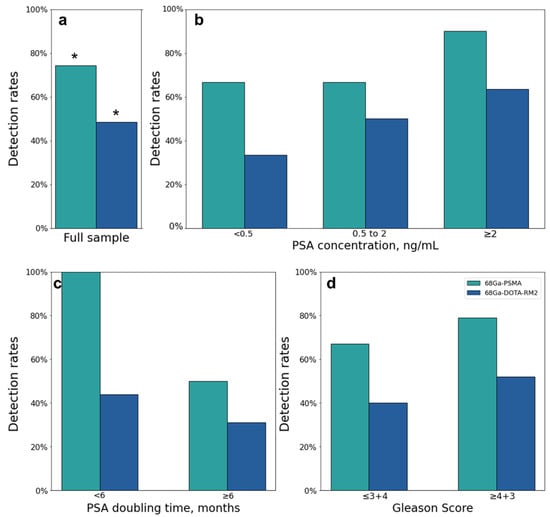
Figure 4.
68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI findings. (a): 68Ga-PSMA and 68Ga-DOTA-RM2 detection rates; (b): 68Ga-PSMA and 68Ga-DOTA-RM2 detection rates stratified by different PSA levels at time of scans; (c): 68Ga-PSMA and 68Ga-DOTA-RM2 detection rates stratified by PSA DT; (d): 68Ga-PSMA and 68Ga-DOTA-RM2 detection rates stratified by GS prior to radical treatment. * = p < 0.05.
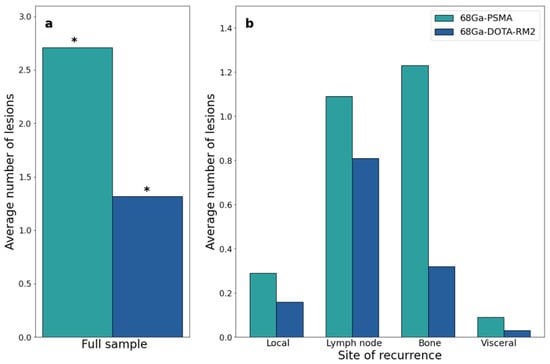
Figure 5.
68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI findings at the lesion level. (a): Average number of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI positive lesions; (b): Average number of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI positive lesions stratified by site. * = p < 0.05.
3.3. Associations between Semi-Quantitative Imaging Parameters and Clinical Data
Correlations between clinical data and 68Ga-PSMA and 68Ga-DOTA-RM2 detection rates on a patient basis are reported in Table 3. PSA level and GS were not significantly associated with 68Ga-PSMA PET/MRI detection rate, while patients with higher PSA DT, were less likely to be 68Ga-PSMA PET positive compared to those showing PSA DT < 6 months (OR < 0.01, p = 0.022). However, this association was lost after correction for multiple testing (adjusted p = 0.065). None of the investigated clinical variables was significantly associated with 68Ga-DOTA-RM2 PET/MRI detection rate on a patient basis.

Table 3.
Correlations between 68Ga-PSMA and 68Ga-DOTA-RM2 detection rates and clinical data on a patient basis.
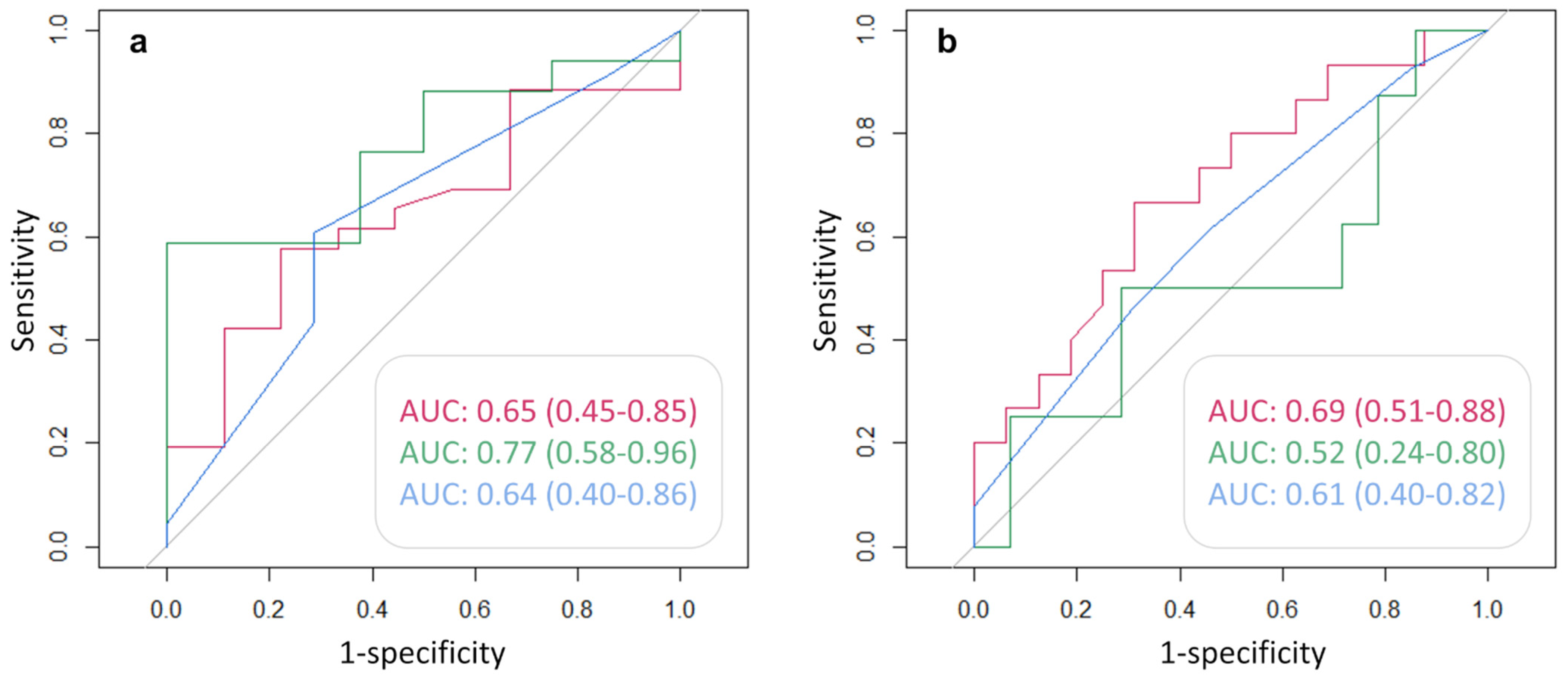
Figure 6 shows the AUC, and the corresponding 95% CI, of each clinical variable used to predict the positivity of either 68Ga-PSMA (Figure 6a) or 68Ga-DOTA-RM2 (Figure 6b) PET/MRI in the detection of recurrent PCa. PSA DT has an AUC = 0.77 (95% CI = 0.58, 0.96) for the prediction of positive 68Ga-PSMA PET, while PSA at time of scans presents an AUC = 0.69 for the prediction of 68Ga-DOTA-RM2 positive findings, although the 95% CI touches the diagonal (random classification). All the other investigated variables do not bear the potential to predict neither 68Ga-PSMA nor 68Ga-DOTA-RM2 PET positivity for the identification of recurrent PCa in our cohort.
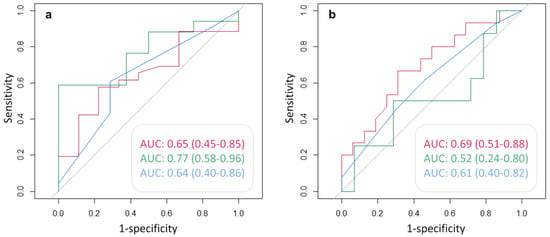
Figure 6.
ROC analysis for the prediction of (a): 68Ga-PSMA and (b): 68Ga-DOTA-RM2 PET positive findings. Red line: AUC of PSA at time of scans in the prediction of PET positivity; green line: AUC of PSA DT in the prediction of PET positivity; blue line: AUC of GS prior to radical treatment in the prediction of PET positivity. AUC and 95% CI are here reported.
Table 4 reports the number of lesions detected by PET/MRI stratified according to GS, PSA levels and kinetic.

Table 4.
Study of the differences in the number of lesions detected by 68Ga-PSMA and 68Ga-DOTA-RM2 and clinical data.
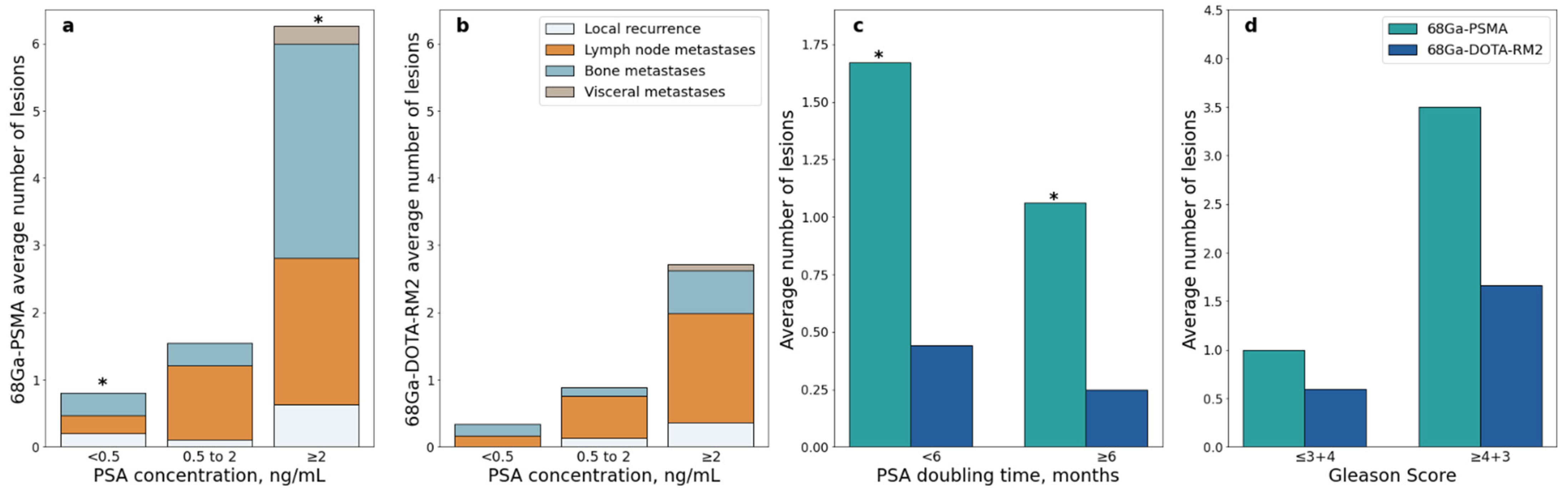
A significant difference in the number of lesions detected by 68Ga-PSMA PET/MRI was observed in patients with PSA value < 0.5 ng/mL vs. PSA value ≥ 2 ng/mL (Mann-Whitney U = 21.5, p = 0.001, Figure 7a); this difference remained significant after correction for multiple testing (adjusted p = 0.006). A significant difference was also observed in patients with PSA DT < 6 months vs. PSA DT ≥ 6 months (Mann-Whitney U = 119, adjusted p = 0.044; Figure 7c).
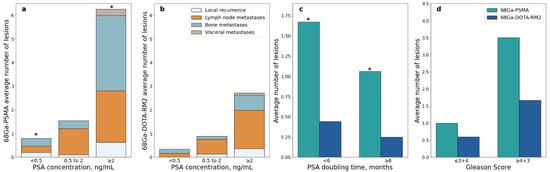
Figure 7.
68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI detection rates stratified according to clinical data; (a): Average number of 68Ga-PSMA PET/MRI positive lesions stratified by site and PSA level at time of scans; (b): Average number of 68Ga-DOTA-RM2 PET/MRI positive lesions stratified by site and PSA level at time of scans; (c): Average number of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI positive lesions stratified by PSA doubling time; (d): Average number of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI positive lesions stratified by Gleason Score, * = p < 0.05.
Similarly, higher PSA serum levels were associated with more PET positive lesions in 68Ga-DOTA-RM2 PET/MRI, but significance was lost after correction for multiple testing (Mann-Whitney U = 46, adjusted p = 0.17; Figure 7b). The number of PET positive lesions did not differ significantly across GS levels both on 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI (Mann-Whitney U = 56, adjusted p = 0.43 and U = 66.5, adjusted p = 0.65, respectively; Figure 7d).
4. Discussion
The present study investigated the diagnostic performance of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI for detection of recurrent PCa after primary treatment. Furthermore, we also studied the association between 68Ga-PSMA and 68Ga-DOTA-RM2 PET uptake and clinical as well as histopathological data.
So far, only few studies have investigated the combined use of both 68Ga-PSMA and 68Ga-DOTA-RM2 PET in PCa both in the staging and restaging setting, using PET/MRI and PET/CT alternatively [10,12,13,14].
Recently, Baratto et al. conducted the largest study to date investigating the use of 68Ga-PSMA and 68Ga-DOTA-RM2 in patients with recurrent PCa [10]. However, despite the lower number of patients included in the analysis, our study presents several methodological advantages. In fact, in the present clinical trial, all patients have been examined with both 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI, thus not alternatively using either 68Ga-PSMA or 18F-PSMA as previous groups have done; patients’ recruitment and data analysis have been prospectively performed and all findings were validated by clinical and/or instrumental follow-up.
In our cohort, 68Ga-PSMA identified PCa recurrent lesions in 74% of patients, while 68Ga-DOTA-RM2 PET/MRI was positive in only 48% of patients. Interestingly, 9/10 of the patients presenting 68Ga-PSMA, but not 68Ga-DOTA RM2 uptake were confirmed to be true positive by follow-up examination. This is in contrast with previous findings where PSMA and 68Ga-DOTA-RM2 PET had similar detection rates on a patient basis [10].
On a lesion-based analysis, our results are coherent with the ones reported by Baratto et al., which directly compared the performances of 68Ga-PSMA and 68Ga-DOTA-RM2 PET and found that PSMA detected 36 additional lesions in 13 patients, with 66 additional lesions in 17 patients observed in our study.
The ability of PET to detect BCR at low PSA levels allows a personalized treatment planning at an early stage of recurrent disease by permitting early local therapies with curative intent. Therefore, it is particularly relevant to investigate the performance of different PET tracers by stratifying their detection rate according to PSA concentration. For this purpose, most studies divided their sample according to PSA in 5 sub-groups (<0.5, 0.5–1, 1–2, 2–5, >5) [10,22,23]. Differently from previous published data, in the present work, in order to allow statistical analysis, the cohort was stratified according to PSA level in 3 groups (<0.5, 0.5–2, >2) because of the numerosity of the cohort. The missing association between 68Ga-PSMA PET/MRI positivity rate and PSA level found in our cohort is likely due to the limited numerosity of the investigated sample combined with the high detection rate of 68Ga-PSMA for low PSA values (66.7% for PSA < 0.5 ng/mL). These findings are in line with previous works assessing the performance of PSMA in detecting recurrent PCa in larger cohorts [24,25,26], but higher compared to the detection rates reported in similar settings [10,22,27,28].
However, similarly to previous studies [22,27], we observed that patients presenting PSA concentration ≥2 ng/mL at the time of scans showed a significantly higher number of 68Ga-PSMA PET/MRI positive lesions compared to patients with PSA level <0.5 ng/mL.
Although the association between 68Ga-DOTA-RM2 PET/MRI positivity rate and PSA concentration both on a patient and lesion basis was not significant, we observed a trend towards a higher rate of 68Ga-DOTA-RM2 PET/MRI positive findings at higher PSA values, confirmed also by ROC analysis, that will surely be an interesting matter of study in future investigations.
The link between PSA kinetics and 68Ga-PSMA PET findings is well established, with patients having slow PSA kinetics being less likely to be 68Ga-PSMA PET positive [29,30]. Accordingly, we reported that patients having a PSA DT ≥ 6 months present a significantly lower number of lesions compared to those having a faster rise in PSA levels after primary treatment; we observed the same trend on a patient-based analysis, although after adjustment for multiple testing, the p-value only approached the level of significance (p = 0.065). Furthermore, ROC analysis showed that PSA DT has a moderate predictive value in the identification of 68Ga-PSMA PET positivity, in line with the correlation previously investigated. Conversely, we did not detect any association between 68Ga-DOTA-RM2 PET/MRI positivity rate and PSA kinetics.
Currently, the possible association between GS of the primary tumour and 68Ga-PSMA PET positive findings in recurrent PCa has not been definitively demonstrated, with different studies reporting conflicting results [10,24]. In our study, we did not observe any association between the positivity rate of PET/MRI with either radiotracer and GS.
A constraint of the present study might be the relatively limited number of included patients. However, as mentioned above, our cohort is remarkably homogeneous as all patients have been examined on the same scanner and applying the same methodology (all PET/MRI studies and not both PET/MRI and PET/CT; exclusive use of 68Ga-PSMA and 68Ga-DOTA-RM2).
Another limitation might be the lack of post-hoc correlation of imaging findings with histopathological results for all patients; however, we consider this a relative limitation related to patients’ disease management, as a number of findings detected on PET/MRI are located in sites that are not usually considered for bioptic confirmation (i.e., bone) and/or are directly treated without any further confirmation (i.e., radiotherapy on positive lymph-nodes, ADT). Additionally, all PET findings were validated using clinical and instrumental follow-up. All images were acquired using the same Signa 3 Tesla PET/MRI scanner, but since this study is part of a single-centre prospective trial that is still ongoing at IRCCS San Raffaele Scientific Institute, and it was specifically focused on the use of 68Ga-PSMA and 68Ga-DOTA-RM2 PET in recurrent PCa, MRI data were not considered for the purpose of this work and therefore were not included in the analysis. The inclusion of MR data will surely constitute an improvement of the present work and will be a matter of investigation in future studies.
Finally, the two PET examinations were read separately, with the Nuclear Medicine physicians not being blind to the clinical history of the patients, thus resulting in a potential bias.
5. Conclusions
The possibility to identify different sites of recurrence of PCa after radical treatment by using a dual-tracer approach certainly improves the disease characterization and therefore it may ultimately have an impact on patients’ management and follow-up. Although in our cohort, 68Ga-PSMA had a higher detection rate than 68Ga-DOTA-RM2 in detecting recurrent PCa. Furthermore, the number of 68Ga-PSMA PET/MRI positive lesions was associated with higher levels of PSA and a shorter PSA DT, thus representing potential prognostic factors. GS, on the other hand, was not related to PET findings.
Author Contributions
Conceptualization, P.M. (Paola Mapelli), S.G. and M.P.; methodology, P.M. (Paola Mapelli), A.M.S.G., E.P., A.P., V.C., G.B., R.R., P.M. (Patrizia Magnani), E.T. and V.B.; software, S.G., C.B. and P.S.; formal analysis, S.G. and P.M. (Paola Mapelli); data curation, A.M.S.G., E.P., A.P., V.C., and G.B.; writing—original draft preparation, P.M. (Paola Mapelli) and S.G.; writing—review and editing, P.M. (Paola Mapelli), S.G., N.S., L.G., A.B., F.D.C., A.E., P.S. and M.P.; supervision, M.P., P.S., A.E., F.D.C. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health (Project GR-2016-02363991-EUDRACT number: 2018-001036-21). Fully hybrid 3 Tesla PET/MRI system (SIGNA PET/MRI; General Electric Healthcare, Waukesha, WI, USA) was purchased with funding from Italian Ministry of Health.
Institutional Review Board Statement
This study was approved by the Institutional Ethics Committee of IRCCS San Raffaele Scientific Institute (EudraCT 2018-001036-21) and all patients gave written informed consent to participate to the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All code needed to replicate our analyses is available upon request from the corresponding author.
Conflicts of Interest
All the authors have no conflicts of interest to disclose related to the present paper.
References
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Ploussard, G.; Almeras, C.; Briganti, A.; Giannarini, G.; Hennequin, C.; Ost, P.; Renard-Penna, R.; Salin, A.; Lebret, T.; Villers, A.; et al. Management of Node Only Recurrence after Primary Local Treatment for Prostate Cancer: A Systematic Review of the Literature. J. Urol. 2015, 194, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Strauss, D.S.; Sachpekidis, C.; Kopka, K.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Pharmacokinetic studies of [68Ga]Ga-PSMA-11 in patients with biochemical recurrence of prostate cancer: Detection, differences in temporal distribution and kinetic modelling by tissue type. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4472–4482. [Google Scholar] [CrossRef]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Ferdinandus, J.; Czernin, J.; Eiber, M.; Flavell, R.R.; Behr, S.C.; Wu, I.K.; Lawhn-Heath, C.; Pampaloni, M.H.; Reiter, R.E.; et al. Impact of 68Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J. Nucl. Med. 2020, 61, 1793–1799. [Google Scholar] [CrossRef]
- Mansi, R.; Fleischmann, A.; Macke, H.R.; Reubi, J.C. Targeting GRPR in urological cancers—From basic research to clinical application. Nat. Rev. Urol. 2013, 10, 235–244. [Google Scholar] [CrossRef]
- Mena, E.; Lindenberg, L.M.; Choyke, P.L. New Targets for PET Molecular Imaging of Prostate Cancer. Semin. Nucl. Med. 2019, 49, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Popp, I.; Christian Rischke, H.; Drendel, V.; Grosu, A.L.; Bartholoma, M.; Weber, W.A.; Mansi, R.; Wetterauer, U.; Schultze-Seemann, W.; et al. Diagnosis of recurrent prostate cancer with PET/CT imaging using the gastrin-releasing peptide receptor antagonist 68Ga-RM2: Preliminary results in patients with negative or inconclusive [(18)F]Fluoroethylcholine-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1463–1472. [Google Scholar] [CrossRef]
- Baratto, L.; Song, H.; Duan, H.; Hatami, N.; Bagshaw, H.; Buyyounouski, M.; Hancock, S.; Shah, S.A.; Srinivas, S.; Swift, P.; et al. PSMA- and GRPR-targeted PET: Results from 50 Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2021, 62, 1545–1549. [Google Scholar] [CrossRef]
- Iagaru, A. Will GRPR Compete with PSMA as a Target in Prostate Cancer? J. Nucl. Med. 2017, 58, 1883–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapelli, P.; Ghezzo, S.; Samanes Gajate, A.M.; Preza, E.; Brembilla, G.; Cucchiara, V.; Ahmed, N.; Bezzi, C.; Presotto, L.; Bettinardi, V.; et al. Preliminary Results of an Ongoing Prospective Clinical Trial on the Use of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in Staging of High-Risk Prostate Cancer Patients. Diagnostics 2021, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, T.F.; Schiller, F.; Zamboglou, C.; Drendel, V.; Kiefer, S.; Jilg, C.A.; Grosu, A.L.; Mix, M. Voxel-based comparison of [68Ga]Ga-RM2-PET/CT and [68Ga]Ga-PSMA-11-PET/CT with histopathology for diagnosis of primary prostate cancer. EJNMMI Res. 2020, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot Comparison of (6)(8)Ga-RM2 PET and (6)(8)Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Musio, D.; Forte, V.; Gentile, V.; Tombolini, V.; Catalano, C. Prostate cancer recurrence after radical prostatectomy: The role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur. Radiol. 2013, 23, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kim, C.K.; Park, S.Y.; Park, B.K.; Lee, H.M.; Cho, S.W. Prostate cancer: Role of pretreatment multiparametric 3-T MRI in predicting biochemical recurrence after radical prostatectomy. AJR Am. J. Roentgenol. 2014, 202, W459–W465. [Google Scholar] [CrossRef] [PubMed]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Baratto, L.; Duan, H.; Laudicella, R.; Toriihara, A.; Hatami, N.; Ferri, V.; Iagaru, A. Physiological 68Ga-RM2 uptake in patients with biochemically recurrent prostate cancer: An atlas of semi-quantitative measurements. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 115–122. [Google Scholar] [CrossRef]
- Demirci, E.; Sahin, O.E.; Ocak, M.; Akovali, B.; Nematyazar, J.; Kabasakal, L. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl. Med. Commun. 2016, 37, 1169–1179. [Google Scholar] [CrossRef]
- Draulans, C.; De Roover, R.; van der Heide, U.A.; Kerkmeijer, L.; Smeenk, R.J.; Pos, F.; Vogel, W.V.; Nagarajah, J.; Janssen, M.; Isebaert, S.; et al. Optimal 68Ga-PSMA and (18)F-PSMA PET window levelling for gross tumour volume delineation in primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 30 November 2021).
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.A.; Buchholz, H.G.; Wieler, H.J.; Miederer, M.; Rosar, F.; Fischer, N.; Muller-Hubenthal, J.; Trampert, L.; Pektor, S.; Schreckenberger, M. PSA and PSA Kinetics Thresholds for the Presence of 68Ga-PSMA-11 PET/CT-Detectable Lesions in Patients With Biochemical Recurrent Prostate Cancer. Cancers 2020, 12, 389. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; da Cunha, M.L.; Wagner, J.; Haberkorn, U.; Debus, N.; Weber, W.; Eiber, M.; Holland-Letz, T.; Rauscher, I. Performance of [68Ga]Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer after prostatectomy-a multi-centre evaluation of 2533 patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2925–2934. [Google Scholar] [CrossRef]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of (18)F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranzbuhler, B.; Nagel, H.; Becker, A.S.; Muller, J.; Huellner, M.; Stolzmann, P.; Muehlematter, U.; Guckenberger, M.; Kaufmann, P.A.; Eberli, D.; et al. Clinical performance of 68Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Buchholz, H.G.; Wieler, H.J.; Hofner, T.; Muller-Hubenthal, J.; Trampert, L.; Schreckenberger, M. The positivity rate of 68Gallium-PSMA-11 ligand PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Oncotarget 2019, 10, 6124–6137. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Picchio, M.; von Eyben, R.; Rhee, H.; Bauman, G. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Ceci, F.; Uprimny, C.; Nilica, B.; Geraldo, L.; Kendler, D.; Kroiss, A.; Bektic, J.; Horninger, W.; Lukas, P.; Decristoforo, C.; et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: Which factors are associated with PET/CT detection rate? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1284–1294. [Google Scholar] [CrossRef] [Green Version]
- Pereira Mestre, R.; Treglia, G.; Ferrari, M.; Pascale, M.; Mazzara, C.; Azinwi, N.C.; Llado, A.; Stathis, A.; Giovanella, L.; Roggero, E. Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: A meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13063. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).