Clinical Strategies for Enhancing the Efficacy of CAR T-Cell Therapy for Hematological Malignancies
Abstract
Simple Summary
Abstract
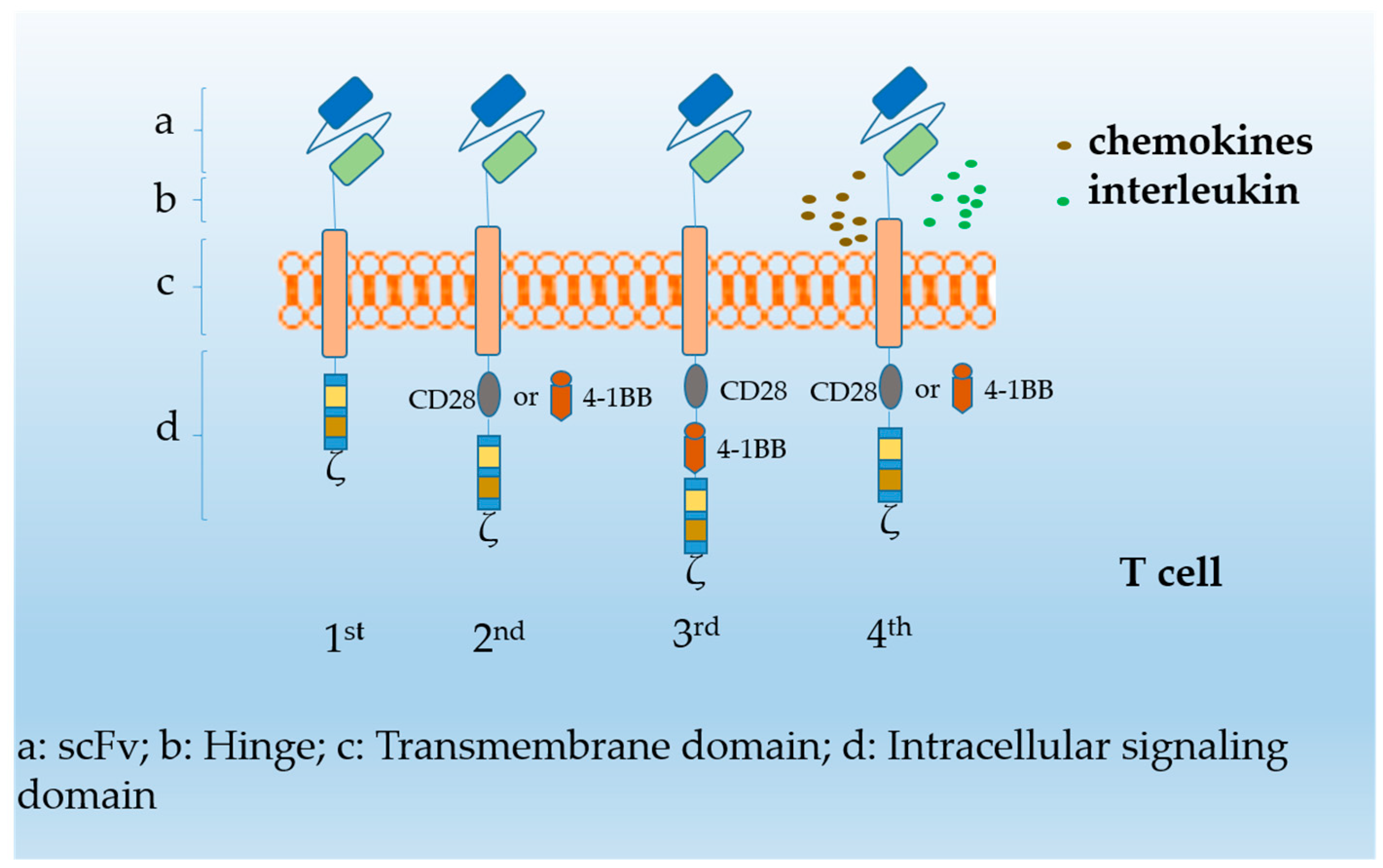
1. Introduction
| CAR T-Cells | Clinical Trials | Malignancy | Enrolled Patients | Lymphodepletion Regimens | Dosage Regimens (CAR T Cells) | Efficacy | Side Effect (CRS, NE) |
|---|---|---|---|---|---|---|---|
| Axicabtagene ciloleucel | NCT02348216 [20] | DLBCL PMBCL TFL 2017 | Adults 101/111 | 30 mg/m2 Flu and 500 mg/m2 Cy; On days -5, -4, and -3 | 2 × 106 cells/kg on day 0; a single infusion | ORR 82% CR 54% | CRS 13%, NE 28% |
| NCT02348216 [6] | DLBCL PMBCL TFL 2019 | Adults 108/119 | 30 mg/m2 Flu and 500 mg/m2 Cy; On days -5, -4, and -3 | 2 × 106 cells/kg on day 0; a single infusion | ORR 83% ORR 58% c | CRS 11%, NE 32% | |
| NCT03105336 [21] | I-NHL 2022 | Adults 148/153 | 30 mg/m2 Flu and 500 mg/m2 Cy; On days -5, -4, and -3 | 2 × 106 cells/kg on day 0; a single infusion | OR 92% CR 74% | CRS 7%, NE 19% | |
| Lisocabtagene maraleucel | NCT02631044 [3] | r/r LBCL 2020 | Adults 269/344 | 30 mg/m2 Flu and 500 mg/m2 Cy for 3 days;2– 7 days before CAR T cell infusion | 50 × 106 cells (51 patients), 100 × 106 cells (177 patients), 150 × 106 cells (41 patients); Including CD8+ and CD4+ CAR T cells | ORR 73% CR 53% | CRS 2%, NE 10% |
| Tisagenlecleucel | NCT02445248 [22] | r/r LBCL 2021 | Adults 115/167 | 25 mg/m2 Flu and 250 mg/m2 Cy for 3 days; or bendamustine with 90 mg/m² for 2 days | 0.1–6 × 108 CAR T cells; a single infusion (target dose: 5 × 108 CAR T cells) | OR 53% CR 39% | CRS 23% |
| NCT03568461 [23] | r/r FL 2022 | Adults 97/98 | 25 mg/m2 Flu and 250 mg/m2 Cy or bendamustine with 90 mg/m² for 2 days | 0.6–6 × 108 CAR T cells; a single infusion | CR 69.1% OR 86.2% | CRS 48.5%, NE 37.1% | |
| Brexucabtagene autoleucel | NCT02614066 [24] | r/r ALL 2021 | Adults 65/71 | 25 mg/m2 Flu and 900 mg/m2 Cy days -4, -3, and -2 | 1 × 106 cells/kg on day 0; a single infusion | CR 56% | CRS 24%, NE 25% |
| NCT02601313 [25] | r/r ML 2020 | Adults 71/74 | 30 mg/m2 Flu and 500 mg/m2 Cy on days -5, -4, and -3 | 2 × 106 cells/kg on day 0; a single infusion | ORR 93% CR 67% | CRS 15%, NE 31% |
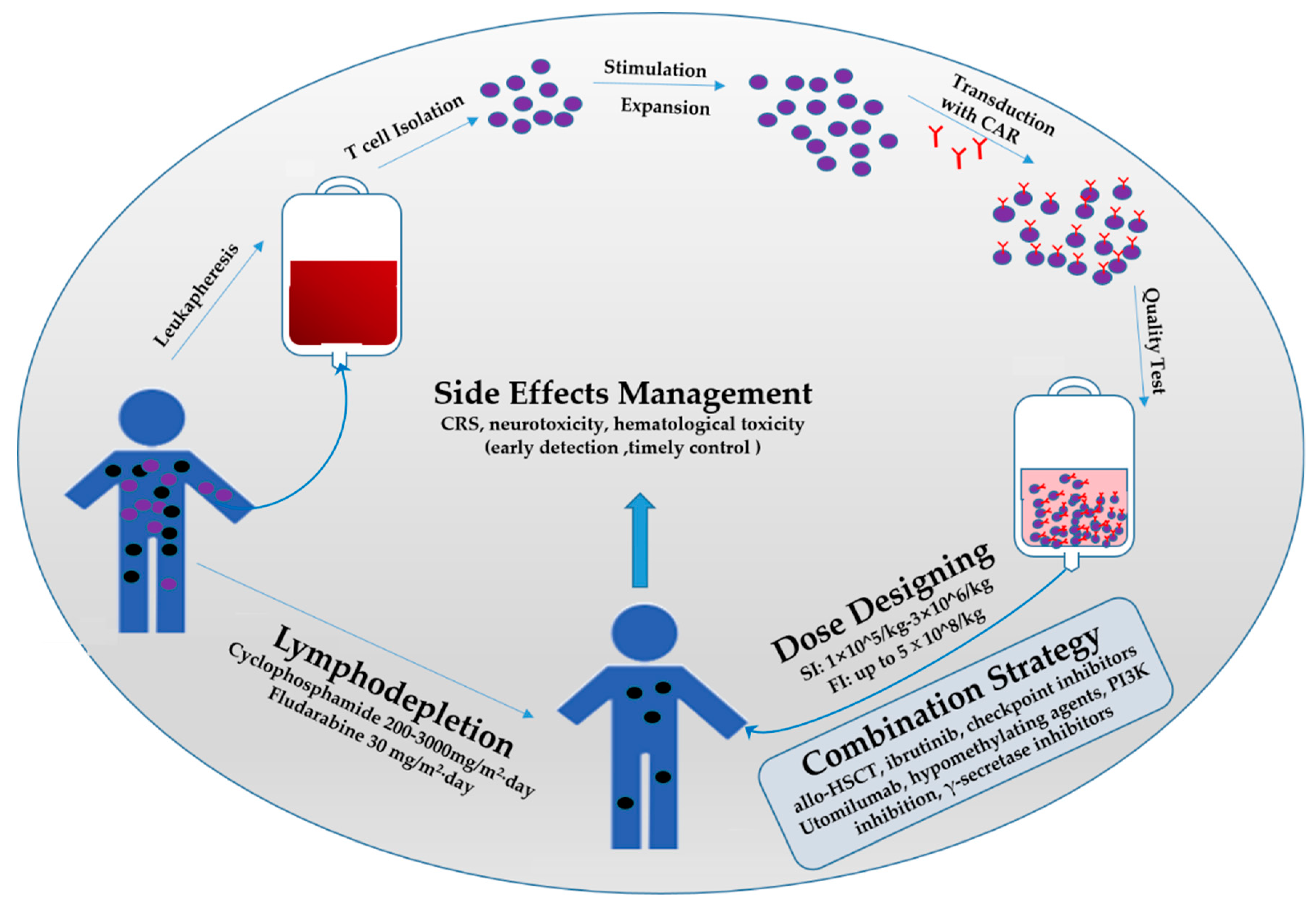
2. Lymphodepletion Regimen
3. Dosing Regimen of CAR T-Cells
4. Combination Strategies
5. Occurrence and Management of Side Effects
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Enblad, G.; Karlsson, H.; Gammelgård, G.; Wenthe, J.; Lövgren, T.; Amini, R.M.; Wikstrom, K.I.; Essand, M.; Savoldo, B.; Hallböök, H.; et al. A Phase I/IIa Trial Using CD19-Targeted Third-Generation CAR T Cells for Lymphoma and Leukemia. Clin. Cancer Res. 2018, 24, 6185–6194. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Wang, K.; Wei, C.; Feng, D.; Liu, Y.; He, Q.; Xu, X.; Wang, C.; Zhao, S.; Lv, L.; et al. The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Front. Immunol. 2021, 12, 609421. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Cordeiro, A.; Bezerra, E.D.; Hirayama, A.V.; Hill, J.A.; Wu, Q.V.; Voutsinas, J.; Sorror, M.L.; Turtle, C.J.; Maloney, D.G.; Bar, M. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol. Blood Marrow Transplant. 2020, 26, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Wan, W.; He, T.; Qi, F.; Liu, G.; Hu, K.; Lu, X.-A.; Yang, P.; Dong, F.; Wang, J.; et al. Autologous CD19-directed chimeric antigen receptor-T cell is an effective and safe treatment to refractory or relapsed diffuse large B-cell lymphoma. Cancer Gene Ther. 2019, 26, 248–255. [Google Scholar] [CrossRef]
- Heng, G.; Jia, J.; Li, S.; Fu, G.; Wang, M.; Qin, D.; Li, Y.; Pei, L.; Tian, X.; Zhang, J.; et al. Sustained Therapeutic Efficacy of Humanized Anti-CD19 Chimeric Antigen Receptor T Cells in Relapsed/Refractory Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2020, 26, 1606–1615. [Google Scholar] [CrossRef]
- Curran, K.J.; Margossian, S.P.; Kernan, N.A.; Silverman, L.B.; Williams, D.A.; Shukla, N.; Kobos, R.; Forlenza, C.J.; Steinherz, P.; Prockop, S.; et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 2019, 134, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef]
- Pan, J.; Zuo, S.; Wang, Z. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood 2020, 135, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nat. Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef]
- Rafiq, S.; Yeku, O.O.; Jackson, H.J.; Purdon, T.J.; Van Leeuwen, D.G.; Drakes, D.J.; Song, M.; Miele, M.M.; Li, Z.; Wang, P.; et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018, 36, 847–856. [Google Scholar] [CrossRef]
- Weng, J.; Lai, P.; Qin, L.; Lai, Y.; Jiang, Z.; Luo, C.; Huang, X.; Wu, S.; Shao, D.; Deng, C.; et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 11, 25. [Google Scholar] [CrossRef]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti–PD-1 Agent Pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, M.; Mao, Z.; Na Wang, N.; Cao, Y.; Xiao, Y.; Meng, F.; Sun, W.; Wang, Y.; Yang, X.; et al. Outcome of aggressive B-cell lymphoma with TP53 alterations administered with CAR T-cell cocktail alone or in combination with ASCT. Signal Transduct. Target. Ther. 2022, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef]
- Schuster, S.J.; Tam, C.S.; Borchmann, P.; Worel, N.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Damon, L.E.; et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: The phase 2 ELARA trial. Nat. Med. 2022, 28, 325–332. [Google Scholar] [CrossRef]
- Shah, B.D.; Bishop, M.R.; Oluwole, O.O.; Logan, A.C.; Baer, M.R.; Donnellan, W.B.; O’Dwyer, K.M.; Holmes, H.; Arellano, M.L.; Ghobadi, A.; et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood 2021, 138, 11–22. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Mackall, C.L.; Hakim, F.T.; Gress, R.E. Restoration of T-cell homeostasis after T-cell depletion. Semin. Immunol. 1997, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.; Khong, H.T.; Antony, P.A.; Palmer, D.C.; Restifo, N.P. Sinks, suppressors and antigen presenters: How lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005, 26, 111–117. [Google Scholar] [CrossRef]
- Dummer, W.; Niethammer, A.G.; Baccala, R.; Lawson, B.R.; Wagner, N.; Reisfeld, R.A.; Theofilopoulos, A.N. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Investig. 2002, 110, 185–192. [Google Scholar] [CrossRef]
- Muranski, P.; Boni, A.; Wrzesinski, C.; Citrin, D.; Rosenberg, S.A.; Childs, R.W.; Restifo, N.P. Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat. Clin. Pract. Oncol. 2006, 3, 668–681. [Google Scholar] [CrossRef]
- Wrzesinski, C.; Restifo, N.P. Less is more: Lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr. Opin. Immunol. 2005, 17, 195–201. [Google Scholar] [CrossRef]
- Gattinoni, L.; Finkelstein, S.E.; Klebanoff, C.; Antony, P.A.; Palmer, D.; Spiess, P.J.; Hwang, L.N.; Yu, Z.; Wrzesinski, C.; Heimann, D.M.; et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005, 202, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.A.; Gauthier, J.; Hirayama, A.V.; Voutsinas, J.M.; Wu, Q.; Li, D.; Gooley, T.A.; Cherian, S.; Chen, X.; Pender, B.S.; et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 2019, 133, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Strati, P. CAR T-Cell Therapy for B-Cell non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Clinical Trials and Real-World Experiences. Front. Oncol. 2020, 10, 849. [Google Scholar] [CrossRef]
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Siddiqi, T.; Chavez, J.C.; Hosing, C.M.; Ghobadi, A.; Budde, L.E.; Bot, A.; Rossi, J.M.; et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol. Ther. 2017, 25, 285–295. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Sun, Y.; Zhang, A.; Hu, G.-L.; Cao, W.; Wang, D.-H.; Zhang, B.; Chen, H. Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: A non-randomised, open-label phase I trial protocol. BMJ Open 2016, 6, e013904. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Wang, M.; Fu, G.; Li, Y.; Pei, L.; Xiong, Z.; Qin, D.; Zhang, R.; Tian, X.; et al. Treatment of acute lymphoblastic leukaemia with the second generation of CD19 CAR-T containing either CD28 or 4-1BB. Br. J. Haematol. 2018, 181, 360–371. [Google Scholar] [CrossRef]
- Ying, Z.; Huang, X.F.; Xiang, X.; Liu, Y.; Kang, X.; Song, Y.; Guo, X.; Liu, H.; Ding, N.; Zhang, T.; et al. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 2019, 25, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.B.; Rivière, I.; Sénéchal, B.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Halton, E.; Lamanna, N.; et al. Autologous CD19-Targeted CAR T Cells in Patients with Residual CLL following Initial Purine Analog-Based Therapy. Mol. Ther. 2018, 26, 1896–1905. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Hudecek, M.; Pender, B.; Robinson, E.; Hawkins, R.; Chaney, C.; Cherian, S.; Chen, X.; et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8 + and CD4 + CD19-specific chimeric antigen receptor–modified T cells. Sci. Transl. Med. 2016, 8, 355ra116. [Google Scholar] [CrossRef]
- Geyer, M.B.; Rivière, I.; Sénéchal, B.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Palomba, M.L.; Halton, E.; et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight 2019, 4, e122627. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.V.; Gauthier, J.; Hay, K.A.; Voutsinas, J.M.; Wu, Q.; Gooley, T.; Li, D.; Cherian, S.; Chen, X.; Pender, B.S.; et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 2019, 133, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, J.; Wei, G.; Luo, Y.; Shi, J.; Cui, Q.; Zhao, M.; Liang, A.; Zhang, Q.; Yang, J.; et al. Pre-transplant MRD negativity predicts favorable outcomes of CAR-T therapy followed by haploidentical HSCT for relapsed/refractory acute lymphoblastic leukemia: A multi-center retrospective study. J. Hematol. Oncol. 2020, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Somerville, R.P.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated with High Serum Interleukin-15 Levels. J. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, V.A.; Boelens, J.J.; Mauguen, A.; Baggott, C.; Prabhu, S.; Egeler, E.; Mavroukakis, S.; Pacenta, H.L.; Phillips, C.L.; Rossoff, J.; et al. Optimal fludarabine lymphodepletion is associated with improved outcomes after CAR T-cell therapy. Blood Adv. 2022, 6, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Dekker, L.; Calkoen, F.G.; Jiang, Y.; Blok, H.; Veldkamp, S.R.; De Koning, C.C.H.; Spoon, M.; Admiraal, R.; Hoogerbrugge, P.; Vormoor, B.J.; et al. Fludarabine exposure predicts outcome after CD19 CAR T-cell therapy in children and young adults with acute leukemia. Blood Adv. 2022, 6, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Dourthe, M.-E.; Rabian, F.; Yakouben, K.; Chevillon, F.; Cabannes-Hamy, A.; Méchinaud, F.; Grain, A.; Chaillou, D.; Rahal, I.; Caillat-Zucman, S.; et al. Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia 2021, 35, 3383–3393. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, H.; Shao, M.; Cui, Q.; Wu, Z.; Xiao, L.; Huang, H.; Hu, Y. Lymphodepletion chemotherapy revitalizes chimeric antigen receptor T cells contributing to regression of relapsed B-cell lymphoma: A case report. Medicine 2020, 99, e22510. [Google Scholar] [CrossRef]
- Sadelain, M.; Rivière, I.; Riddell, S. Therapeutic T cell engineering. Nature 2017, 545, 423–431. [Google Scholar] [CrossRef]
- Tu, S.; Huang, R.; Guo, Z.; Deng, L.; Song, C.; Zhou, X.; Yue, C.; Zhang, L.; He, Y.; Yang, J.; et al. Shortening the ex vivo culture of CD19-specific CAR T-cells retains potent efficacy against acute lymphoblastic leukemia without CAR T-cell-related encephalopathy syndrome or severe cytokine release syndrome. Am. J. Hematol. 2019, 94, E322–E325. [Google Scholar] [CrossRef]
- Tumaini, B.; Lee, D.W.; Lin, T.; Castiello, L.; Stroncek, D.F.; Mackall, C.; Wayne, A.; Sabatino, M. Simplified process for the production of anti–CD19-CAR–engineered T cells. Cytotherapy 2013, 15, 1406–1415. [Google Scholar] [CrossRef]
- Zhang, C.; He, J.; Liu, L.; Wang, J.; Wang, S.; Liu, L.; Ge, J.; Gao, L.; Kong, P.; Liu, Y.; et al. Novel CD19 chimeric antigen receptor T cells manufactured next-day for acute lymphoblastic leukemia. Blood Cancer J. 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef]
- Li, D.H.; Whitmore, J.B.; Guo, W.; Ji, Y. Toxicity and Efficacy Probability Interval Design for Phase I Adoptive Cell Therapy Dose-Finding Clinical Trials. Clin. Cancer Res. 2017, 23, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Pan, J.; Yang, J.F.; Deng, B.P.; Zhao, X.J.; Zhang, X.; Lin, Y.H.; Wu, Y.N.; Deng, Z.L.; Zhang, Y.L.; Liu, S.H.; et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017, 31, 2587–2593. [Google Scholar] [CrossRef]
- Yan, Z.-X.; Li, L.; Wang, W.; OuYang, B.-S.; Cheng, S.; Wang, L.; Wu, W.; Xu, P.-P.; Muftuoglu, M.; Hao, M.; et al. Clinical Efficacy and Tumor Microenvironment Influence in a Dose-Escalation Study of Anti-CD19 Chimeric Antigen Receptor T Cells in Refractory B-Cell Non-Hodgkin’s Lymphoma. Clin. Cancer Res. 2019, 25, 6995–7003. [Google Scholar] [CrossRef]
- Frey, N.V.; Shaw, P.A.; Hexner, E.O.; Pequignot, E.; Gill, S.; Luger, S.M.; Mangan, J.K.; Loren, A.W.; Perl, A.E.; Maude, S.L.; et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults with Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2020, 38, 415–422. [Google Scholar] [CrossRef]
- Anagnostou, T.; Riaz, I.B.; Hashmi, S.K.; Murad, M.H.; Kenderian, S.S. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: A systematic review and meta-analysis. Lancet Haematol. 2020, 7, e816–e826. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, S.; Fang, H.; Yu, K.; Jiang, S. A phase I study of CAR-T bridging HSCT in patients with acute CD19+ relapse/refractory B-cell leukemia. Oncol. Lett. 2020, 20, 20. [Google Scholar] [CrossRef]
- Li, Q.; Mu, J.; Yuan, J.; Yang, Z.; Wang, J.; Deng, Q. Low Level Donor Chimerism of CD19 CAR-T Cells Returned to Complete Donor Chimerism in Patients with Relapse After Allo-Hematopoietic Stem Cell Transplant. OncoTargets Ther. 2020, 13, 11471–11484. [Google Scholar] [CrossRef]
- Wen, S.; Niu, Z.; Xing, L.; Wang, Y.; Li, H.; Kuang, N.; Luo, J.; Zhang, X.; Wang, F. CAR-T bridging to allo-HSCT as a treatment strategy for relapsed adult acute B-lymphoblastic leukemia: A case report. BMC Cancer 2018, 18, 1143. [Google Scholar] [CrossRef]
- Hua, J.; Zhang, J.; Wu, X.; Zhou, L.; Bao, X.; Han, Y.; Miao, M.; Li, C.; Fu, Z.; Wu, D.; et al. Allogeneic Donor-Derived Anti-CD19 CAR T Cell Is a Promising Therapy for Relapsed/Refractory B-ALL After Allogeneic Hematopoietic Stem-Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2020, 20, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Zhang, J.; Zhang, X.; Wu, X.; Zhou, L.; Bao, X.; Han, Y.; Miao, M.; Li, C.; Fu, C.; et al. Donor-derived anti-CD19 CAR T cells compared with donor lymphocyte infusion for recurrent B-ALL after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2021, 56, 1056–1064. [Google Scholar] [CrossRef]
- González-Barca, E.; Boumendil, A.; Blaise, D.; Trněný, M.; Masszi, T.; Finel, H.; Michieli, M.; Bittenbring, J.T.; Gritti, G.; Snowden, J.A.; et al. Outcome in patients with diffuse large B-cell lymphoma who relapse after autologous stem cell transplantation and receive active therapy. A retrospective analysis of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2020, 55, 393–399. [Google Scholar] [CrossRef]
- Styczynski, J.; Tallamy, B.; Waxman, I.; Van De Ven, C.; Milone, M.C.; Shaw, L.M.; Harrison, L.; Morris, E.; Satwani, P.; Bhatia, M.; et al. A pilot study of reduced toxicity conditioning with BU, fludarabine and alemtuzumab before the allogeneic hematopoietic SCT in children and adolescents. Bone Marrow Transplant. 2011, 46, 790–799. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Chen, H.; Song, Y.; Tan, X.; Zhao, Y.; Liu, X.; Li, Z.; Yang, F.; Jiang, M.; Gao, Z.; et al. Chimeric antigen receptor T-cell therapy as a bridge to haematopoietic stem cell transplantation for refractory/relapsed B-cell acute lymphoblastic leukemia. Br. J. Haematol. 2020, 189, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, C.; Yin, P.; Guo, T.; Liu, L.; Xia, L.; Wu, Y.; Zhou, F.; Ai, L.; Shi, W.; et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am. J. Hematol. 2019, 94, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yan, L.; Shang, J.; Kang, L.; Yan, Z.; Jin, S.; Zhu, M.; Chang, H.; Gong, F.; Zhou, J.; et al. Anti-CD19 and anti-BCMA CAR T cell therapy followed by lenalidomide maintenance after autologous stem-cell transplantation for high-risk newly diagnosed multiple myeloma. Am. J. Hematol. 2022, 97, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Otáhal, P.; Prukova, D.; Král, V.; Fabry, M.; Vočková, P.; Latečková, L.; Trneny, M.; Klener, P. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. OncoImmunology 2016, 5, e1115940. [Google Scholar] [CrossRef] [PubMed]
- Works, M.; Soni, N.; Hauskins, C.; Sierra, C.; Baturevych, A.; Jones, J.C.; Curtis, W.; Carlson, P.; Johnstone, T.G.; Kugler, D.; et al. Anti–B-cell Maturation Antigen Chimeric Antigen Receptor T cell Function against Multiple Myeloma Is Enhanced in the Presence of Lenalidomide. Mol. Cancer Ther. 2019, 18, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Fraietta, J.A.; Beckwith, K.A.; Patel, P.R.; Ruella, M.; Zheng, Z.; Barrett, D.M.; Lacey, S.F.; Melenhorst, J.J.; McGettigan, S.E.; Cook, D.R.; et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016, 127, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib–Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hay, K.; Hanafi, L.-A.; Li, D.; Cherian, S.; Chen, X.; Wood, B.; Lozanski, A.; Byrd, J.C.; Heimfeld, S.; et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated with CD19-Specific Chimeric Antigen Receptor–Modified T Cells After Failure of Ibrutinib. J. Clin. Oncol. 2017, 35, 3010–3020. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Kenderian, S.S.; Shestova, O.; Fraietta, J.A.; Qayyum, S.; Zhang, Q.; Maus, M.V.; Liu, X.; Nunez-Cruz, S.; Klichinsky, M.; et al. The Addition of the BTK Inhibitor Ibrutinib to Anti-CD19 Chimeric Antigen Receptor T Cells (CART19) Improves Responses against Mantle Cell Lymphoma. Clin. Cancer Res. 2016, 22, 2684–2696. [Google Scholar] [CrossRef]
- Gauthier, J.; Hirayama, A.V.; Purushe, J.; Hay, K.A.; Lymp, J.; Li, D.H.; Yeung, C.C.S.; Sheih, A.; Pender, B.S.; Hawkins, R.M.; et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 2020, 135, 1650–1660. [Google Scholar] [CrossRef]
- Gulati, P.; Rühl, J.; Kannan, A.; Pircher, M.; Schuberth, P.; Nytko, K.J.; Pruschy, M.N.; Sulser, S.; Haefner, M.D.; Jensen, S.M.; et al. Aberrant Lck Signal via CD28 Costimulation Augments Antigen-Specific Functionality and Tumor Control by Redirected T Cells with PD-1 Blockade in Humanized Mice. Clin. Cancer Res. 2018, 24, 3981–3993. [Google Scholar] [CrossRef]
- Gargett, T.; Yu, W.; Dotti, G.; Yvon, E.S.; Christo, S.N.; Hayball, J.D.; Lewis, I.D.; Brenner, M.K.; Brown, M.P. GD2-specific CAR T Cells Undergo Potent Activation and Deletion Following Antigen Encounter but can be Protected from Activation-induced Cell Death by PD-1 Blockade. Mol. Ther. 2016, 24, 1135–1149. [Google Scholar] [CrossRef]
- Bansal, R.; Reshef, R. Revving the CAR—Combination strategies to enhance CAR T cell effectiveness. Blood Rev. 2021, 45, 100695. [Google Scholar] [CrossRef]
- Chou, C.K.; Turtle, C.J. Insight into mechanisms associated with cytokine release syndrome and neurotoxicity after CD19 CAR-T cell immunotherapy. Bone Marrow Transplant. 2019, 54, 780–784. [Google Scholar] [CrossRef]
- Sesques, P.; Ferrant, E.; Safar, V.; Wallet, F.; Tordo, J.; Dhomps, A.; Karlin, L.; Brisou, G.; Vercasson, M.; Msc, C.H.; et al. Commercial anti-CD19 CAR T cell therapy for patients with relapsed/refractory aggressive B cell lymphoma in a European center. Am. J. Hematol. 2020, 95, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Makita, S.; Kato, K.; Tokushige, K.; Fujita, T.; Akashi, K.; Izutsu, K.; Teshima, T. Efficacy and safety of tisagenlecleucel in Japanese adult patients with relapsed/refractory diffuse large B-cell lymphoma. Int. J. Clin. Oncol. 2020, 25, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, L.; Guo, T.; Wu, Y.; Ai, L.; Deng, J.; Dong, J.; Mei, H.; Hu, Y. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann. Hematol. 2019, 98, 1721–1732. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Z.; Luo, Y.; Shi, J.; Yu, J.; Pu, C.; Liang, Z.; Wei, G.; Cui, Q.; Sun, J.; et al. Potent Anti-leukemia Activities of Chimeric Antigen Receptor–Modified T Cells against CD19 in Chinese Patients with Relapsed/Refractory Acute Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 3297–3306. [Google Scholar] [CrossRef]
- Ma, F.; Ho, J.; Du, H.; Xuan, F.; Wu, X.; Wang, Q.; Wang, L.; Liu, Y.; Ba, M.; Wang, Y.; et al. Evidence of long-lasting anti-CD19 activity of engrafted CD19 chimeric antigen receptor–modified T cells in a phase I study targeting pediatrics with acute lymphoblastic leukemia. Hematol. Oncol. 2019, 37, 601–608. [Google Scholar] [CrossRef]
- Park, J.H.; Romero, F.A.; Taur, Y.; Sadelain, M.; Brentjens, R.J.; Hohl, T.M.; Seo, S.K. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated with Chimeric Antigen Receptor T Cells. Clin. Infect. Dis. 2018, 67, 533–540. [Google Scholar] [CrossRef]
- Magnani, C.F.; Gaipa, G.; Lussana, F.; Belotti, D.; Gritti, G.; Napolitano, S.; Matera, G.; Cabiati, B.; Buracchi, C.; Borleri, G.; et al. Sleeping Beauty–engineered CAR T cells achieve antileukemic activity without severe toxicities. J. Clin. Investig. 2020, 130, 6021–6033. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, J.; Wu, Z.; Yu, J.; Cui, Q.; Pu, C.; Liang, B.; Luo, Y.; Shi, J.; Jin, A.; et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J. Hematol. Oncol. 2016, 9, 70. [Google Scholar] [CrossRef]
- Wrzesinski, C.; Paulos, C.M.; Kaiser, A.; Muranski, P.; Palmer, D.; Gattinoni, L.; Yu, Z.; Rosenberg, S.A.; Restifo, N.P. Increased Intensity Lymphodepletion Enhances Tumor Treatment Efficacy of Adoptively Transferred Tumor-specific T Cells. J. Immunother. 2010, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Narala, N.; Huye, L.; Yagyu, S.; Savoldo, B.; Dotti, G.; Heslop, H.E.; Brenner, M.K.; Rooney, C.M.; Ramos, C.A. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 2015, 125, 3905–3916. [Google Scholar] [CrossRef]
- Ananthakrishnan, R.; Green, S.; Li, D.; LaValley, M. Extensions of the mTPI and TEQR designs to include non-monotone efficacy in addition to toxicity for optimal dose determination for early phase immunotherapy oncology trials. Contemp. Clin. Trials Commun. 2018, 10, 62–76. [Google Scholar] [CrossRef]
- Chiuzan, C.; Garrett-Mayer, E.; Nishimura, M.I. An Adaptive Dose-Finding Design Based on Both Safety and Immunologic Responses in Cancer Clinical Trials. Stat. Biopharm. Res. 2018, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Rovatti, P.E.; Gambacorta, V.; Lorentino, F.; Ciceri, F.; Vago, L. Mechanisms of Leukemia Immune Evasion and Their Role in Relapse After Haploidentical Hematopoietic Cell Transplantation. Front. Immunol. 2020, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Hombach, A.A.; Abken, H. Antigen-Specific T-Cell Activation Independently of the MHC: Chimeric Antigen Receptor-Redirected T Cells. Front. Immunol. 2013, 4, 371. [Google Scholar] [CrossRef]
- Leblanc, R.; Hideshima, T.; Catley, L.P.; Shringarpure, R.; Burger, R.; Mitsiades, N.; Mitsiades, C.; Cheema, P.; Chauhan, D.; Richardson, P.G.; et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood 2004, 103, 1787–1790. [Google Scholar] [CrossRef]
- Davenport, A.J.; Cross, R.S.; Watson, K.A.; Liao, Y.; Shi, W.; Prince, H.M.; Beavis, P.A.; Trapani, J.A.; Kershaw, M.H.; Ritchie, D.S.; et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA 2018, 115, E2068–E2076. [Google Scholar] [CrossRef]
- Loschinski, R.; Böttcher, M.; Stoll, A.; Bruns, H.; Mackensen, A.; Mougiakakos, D. IL-21 modulates memory and exhaustion phenotype of T-cells in a fatty acid oxidation-dependent manner. Oncotarget 2018, 9, 13125–13138. [Google Scholar] [CrossRef]
- Browning, R.L.; Byrd, W.H.; Gupta, N.; Jones, J.; Mo, X.; Hertlein, E.; Yu, L.; Muthusamy, N.; Byrd, J.C. Lenalidomide Induces Interleukin-21 Production by T Cells and Enhances IL21-Mediated Cytotoxicity in Chronic Lymphocytic Leukemia B Cells. Cancer Immunol. Res. 2016, 4, 698–707. [Google Scholar] [CrossRef]
- Chen, S.-S.; Chang, B.Y.; Chang, S.; Tong, T.; Ham, S.; Sherry, B.; Burger, J.A.; Rai, K.R.; Chiorazzi, N. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia 2016, 30, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Li, S.; Xue, L.; Wang, M.; Qiang, P.; Xu, H.; Zhang, X.; Kang, W.; You, F.; Xu, H.; Wang, Y.; et al. Decitabine enhances cytotoxic effect of T cells with an anti-CD19 chimeric antigen receptor in treatment of lymphoma. OncoTargets Ther. 2019, 12, 5627–5638. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.; Petukhov, A.; Staliarova, A.; Motorin, D.; Bulatov, E.; Shuvalov, O.; Soond, S.M.; Piacentini, M.; Melino, G.; Zaritskey, A.; et al. The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis. 2018, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Obstfeld, A.E.; Frey, N.V.; Mansfield, K.; Lacey, S.F.; June, C.H.; Porter, D.L.; Melenhorst, J.J.; Wasik, M.A. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: Clinicopathological insights. Blood 2017, 130, 2569–2572. [Google Scholar] [CrossRef]
- Mueller, K.T.; Maude, S.L.; Porter, D.L.; Frey, N.; Wood, P.; Han, X.; Waldron, E.; Chakraborty, A.; Awasthi, R.; Levine, B.L.; et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2017, 130, 2317–2325. [Google Scholar] [CrossRef]
- Taraseviciute, A.; Tkachev, V.; Ponce, R.; Turtle, C.J.; Snyder, J.M.; Liggitt, H.D.; Myerson, D.; Gonzalez-Cuyar, L.; Baldessari, A.; English, C.; et al. Chimeric Antigen Receptor T Cell–Mediated Neurotoxicity in Nonhuman Primates. Cancer Discov. 2018, 8, 750–763. [Google Scholar] [CrossRef]
- Wudhikarn, K.; Perales, M.-A. Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2022, 1–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, Z.; Wan, R.; Huang, W. Clinical Strategies for Enhancing the Efficacy of CAR T-Cell Therapy for Hematological Malignancies. Cancers 2022, 14, 4452. https://doi.org/10.3390/cancers14184452
Liu Q, Liu Z, Wan R, Huang W. Clinical Strategies for Enhancing the Efficacy of CAR T-Cell Therapy for Hematological Malignancies. Cancers. 2022; 14(18):4452. https://doi.org/10.3390/cancers14184452
Chicago/Turabian StyleLiu, Qianzhen, Zengping Liu, Rongxue Wan, and Wenhua Huang. 2022. "Clinical Strategies for Enhancing the Efficacy of CAR T-Cell Therapy for Hematological Malignancies" Cancers 14, no. 18: 4452. https://doi.org/10.3390/cancers14184452
APA StyleLiu, Q., Liu, Z., Wan, R., & Huang, W. (2022). Clinical Strategies for Enhancing the Efficacy of CAR T-Cell Therapy for Hematological Malignancies. Cancers, 14(18), 4452. https://doi.org/10.3390/cancers14184452






