Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value—Need for Biomarker Testing in High-Risk Patients?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Patients
2.2. Follow-Up
2.3. Tissue Microarray Construction
2.4. Immunohistochemistry
2.5. Semiquantitative Analysis of Nectin-4 Expression
2.6. Statistical Analysis
3. Results
3.1. Patients
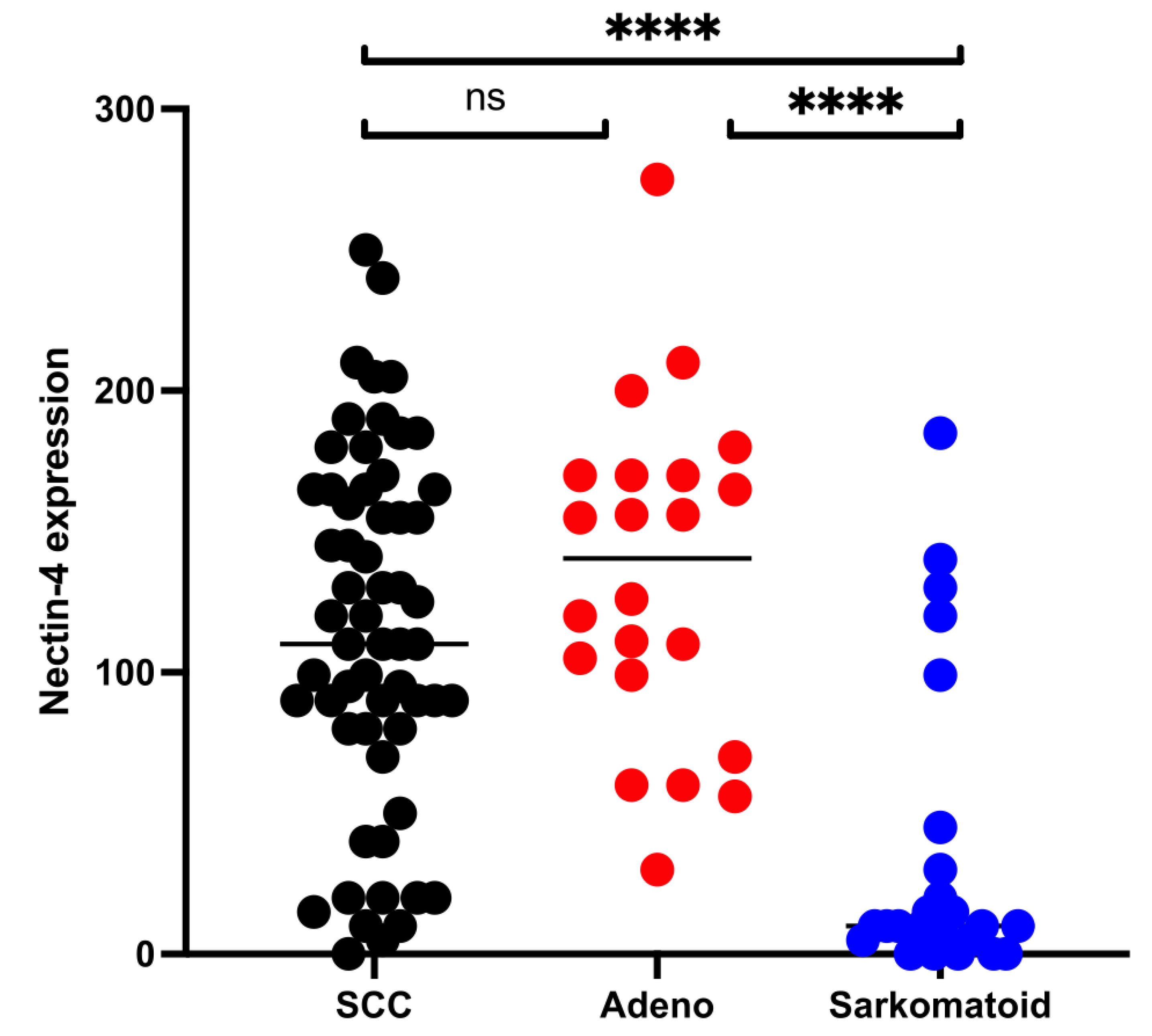
3.2. Expression of Nectin-4 in SCC, ADENO, and SARCO
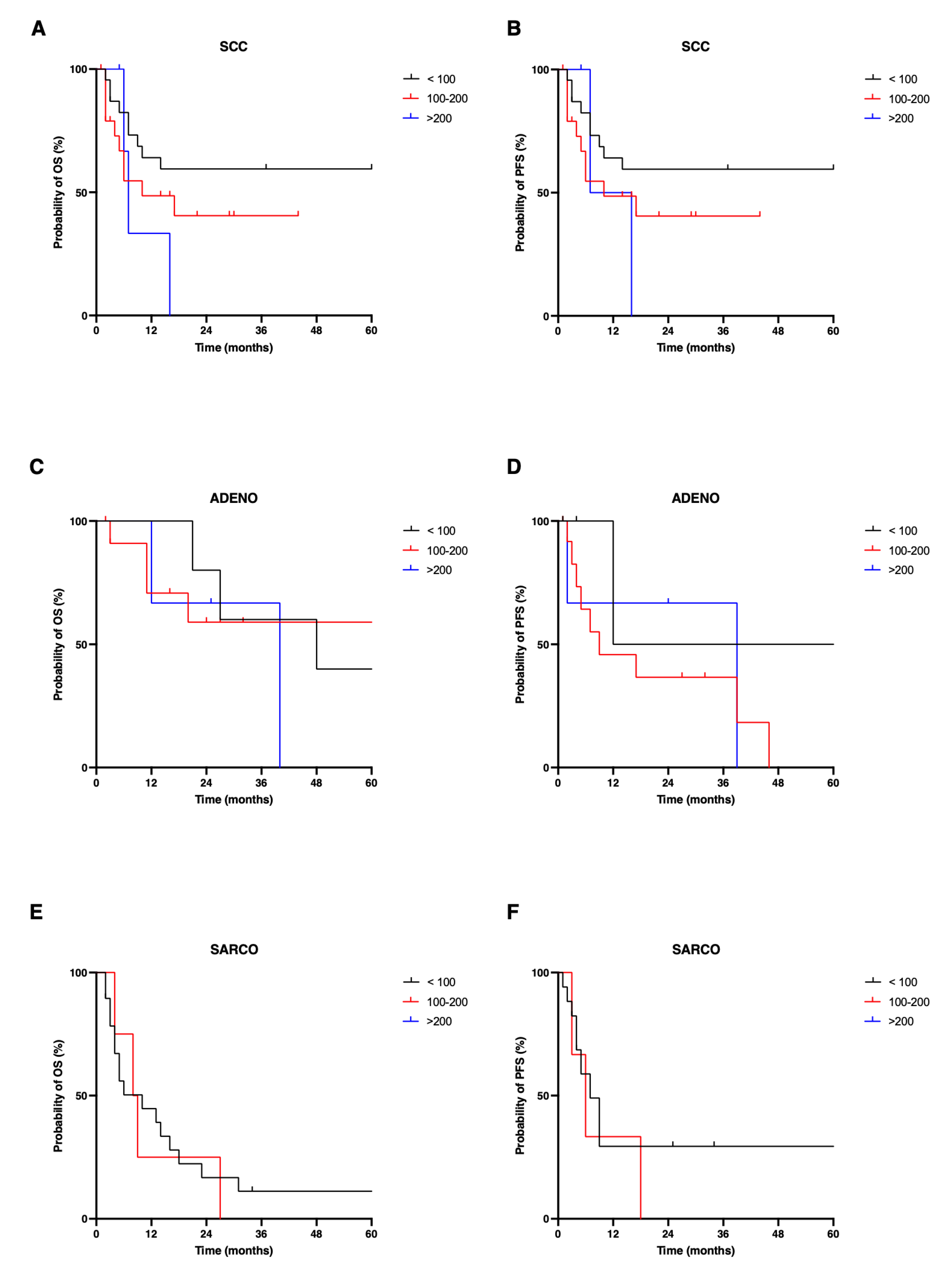
3.3. Nectin-4-Dependent Survival
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heath, E.I.; Rosenberg, J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat. Rev. Urol. 2021, 18, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Takai, Y. Nectin and nectin-like molecules: Biology and pathology. Am. J. Nephrol. 2007, 27, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Romero, D. Benefit of nectin-4 targeting with enfortumab vedotin confirmed. Nat. Rev. Urol. 2021, 18, 190. [Google Scholar] [CrossRef]
- Chu, C.E.; Sjöström, M.; Egusa, E.A.; Gibb, E.A.; Badura, M.L.; Zhu, J.; Koshkin, V.S.; Stohr, B.A.; Meng, M.V.; Pruthi, R.S.; et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin. Clin. Cancer Res. 2021, 27, 5123–5130. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.H.; Lombardo, K.A.; Parimi, V.; Kamanda, S.; Choi, W.; Hahn, N.M.; McConkey, D.J.; McGuire, B.M.; Bivalacqua, T.J.; Kates, M.; et al. Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 619–625. [Google Scholar] [CrossRef]
- Black, A.J.; Black, P.C. Variant histology in bladder cancer: Diagnostic and clinical implications. Transl. Cancer Res. 2020, 9, 6565–6575. [Google Scholar] [CrossRef]
- Moschini, M.; D’Andrea, D.; Korn, S.; Irmak, Y.; Soria, F.; Compérat, E.; Shariat, S.F. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 2017, 14, 651–668. [Google Scholar] [CrossRef]
- Lobo, N.; Shariat, S.F.; Guo, C.C.; Fernandez, M.I.; Kassouf, W.; Choudhury, A.; Gao, J.; Williams, S.B.; Galsky, M.D.; Taylor, J.A.; et al. What Is the Significance of Variant Histology in Urothelial Carcinoma? Eur. Urol. Focus 2020, 6, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.Y.; Petrylak, D.P.; O’Donnell, P.H.; Lee, J.-L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Case, K.; Martini, D.J.; Dababneh, M.; Nazha, B.; Brown, J.T.; Joshi, S.S.; Narayan, V.M.; Ogan, K.; Master, V.A.; Carthon, B.C.; et al. Expression of Nectin-4 and PD-L1 in bladder cancer with variant histology. J. Clin. Oncol. 2022, 40, 529. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Rodler, S.; Buchner, A.; Ledderose, S.T.; Eismann, L.; Volz, Y.; Pfitzinger, P.; Kretschmer, A.; Schulz, G.B.; Karl, A.; Schlenker, B.; et al. Prognostic value of pretreatment inflammatory markers in variant histologies of the bladder: Is inflammation linked to survival after radical cystectomy? World J. Urol. 2021, 39, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, S.; Rose, M.; Maurer, A.; Cassataro, M.A.; Seillier, L.; Morsch, R.; Hammad, E.; Baldia, P.H.; Ecke, T.H.; Vögeli, T.A.; et al. Evaluation of Therapeutic Targets in Histological Subtypes of Bladder Cancer. Int. J. Mol. Sci. 2021, 22, 11547. [Google Scholar] [CrossRef]
- Wong, J.L.; Rosenberg, J.E. Targeting nectin-4 by antibody-drug conjugates for the treatment of urothelial carcinoma. Expert Opin. Biol. 2021, 21, 863–873. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Rodriguez Pena, M.D.C.; Taheri, D.; Banno, E.; Kato, T.; Hatano, K.; Kawashima, A.; Ujike, T.; Uemura, M.; et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2020, 21, 5390. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2015, 34, 30. [Google Scholar]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- Cathomas, R.; Lorch, A.; Bruins, H.M.; Compérat, E.M.; Cowan, N.C.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Hernández, V.; Espinós, E.L.; et al. The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur. Urol. 2022, 81, 95–103. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, S.; Alexander, R.; Maclennan, G.T.; Hodges, K.B.; Harrison, B.T.; Lopez-Beltran, A.; Montironi, R. Sarcomatoid carcinoma of the urinary bladder: The final common pathway of urothelial carcinoma dedifferentiation. Am. J. Surg. Pathol. 2011, 35, e34–e46. [Google Scholar] [CrossRef] [PubMed]
- Sanfrancesco, J.; McKenney, J.K.; Leivo, M.Z.; Gupta, S.; Elson, P.; Hansel, D.E. Sarcomatoid Urothelial Carcinoma of the Bladder: Analysis of 28 Cases with Emphasis on Clinicopathologic Features and Markers of Epithelial-to-Mesenchymal Transition. Arch. Pathol. Lab. Med. 2016, 140, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Processali, T.; Diminutto, A.; Cerruto, M.A.; Antonelli, A. The impact of histological variants on bladder cancer outcomes. AME Med. J. 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Hayashi, T.; Hinata, N. Current status and future prospects of antibody-drug conjugates in urological malignancies. Int. J. Urol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Choi, W.; Lombardo, K.; Patel, S.; Epstein, G.; Feng, M.; Gabrielson, A.; Hahn, N.M.; Hoffman-Censits, J.; McConkey, D.; Bivalacqua, T.J.; et al. A Molecular Inquiry into the Role of Antibody-Drug Conjugates in Bacillus Calmette-Guérin-exposed Non–muscle-invasive Bladder Cancer. Eur. Urol. 2022, 81, 138–142. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.; Lombardo, K.; McConky, D.; Hahn, N.M.; Bashir, B.; Kelly, W.K.; Johnson, B.; Matoso, A. New and topics: Enfortumab vedotin mechanisms of response and resistance in urothelial cancer-What do we understand so far? Urol. Oncol. 2021, 39, 619–622. [Google Scholar] [CrossRef]


| Variable | SCC (n = 59) | ADENO (n = 22) | SARCO (n = 24) | p Value |
|---|---|---|---|---|
| Median follow-up (months) | 15 | 22.3 | 7 | 0.089 |
| Range | 0–175 | 0–93 | 0–121 | |
| Median age (y) | 66 | 69 | 61.5 | 0.497 |
| Range | 37–85 | 41–85 | 51–89 | |
| Gender | ||||
| Male | 30 | 15 | 11 | 0.267 |
| Female | 29 | 7 | 13 | |
| T-Stage | 0.635 | |||
| ≤pT1 | 2 | 1 | 0 | |
| pT2 | 8 | 1 | 1 | |
| pT3 | 34 | 14 | 18 | |
| pT4 | 15 | 6 | 5 | |
| Nodal stage | 0.005 | |||
| N0 | 35 | 8 | 13 | |
| N1 | 15 | 9 | 3 | |
| N2 | 1 | 0 | 5 | |
| Nx | 8 | 5 | 3 | |
| M-stage | 0.466 | |||
| M0 | 54 | 18 | 21 | |
| M1 | 5 | 4 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodler, S.; Eismann, L.; Schlenker, B.; Casuscelli, J.; Brinkmann, I.; Sendelhofert, A.; Waidelich, R.; Buchner, A.; Stief, C.; Schulz, G.B.; et al. Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value—Need for Biomarker Testing in High-Risk Patients? Cancers 2022, 14, 4411. https://doi.org/10.3390/cancers14184411
Rodler S, Eismann L, Schlenker B, Casuscelli J, Brinkmann I, Sendelhofert A, Waidelich R, Buchner A, Stief C, Schulz GB, et al. Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value—Need for Biomarker Testing in High-Risk Patients? Cancers. 2022; 14(18):4411. https://doi.org/10.3390/cancers14184411
Chicago/Turabian StyleRodler, Severin, Lennert Eismann, Boris Schlenker, Jozefina Casuscelli, Isabel Brinkmann, Andrea Sendelhofert, Raphaela Waidelich, Alexander Buchner, Christian Stief, Gerald Bastian Schulz, and et al. 2022. "Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value—Need for Biomarker Testing in High-Risk Patients?" Cancers 14, no. 18: 4411. https://doi.org/10.3390/cancers14184411
APA StyleRodler, S., Eismann, L., Schlenker, B., Casuscelli, J., Brinkmann, I., Sendelhofert, A., Waidelich, R., Buchner, A., Stief, C., Schulz, G. B., & Ledderose, S. (2022). Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value—Need for Biomarker Testing in High-Risk Patients? Cancers, 14(18), 4411. https://doi.org/10.3390/cancers14184411





