Current Imaging Diagnosis of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
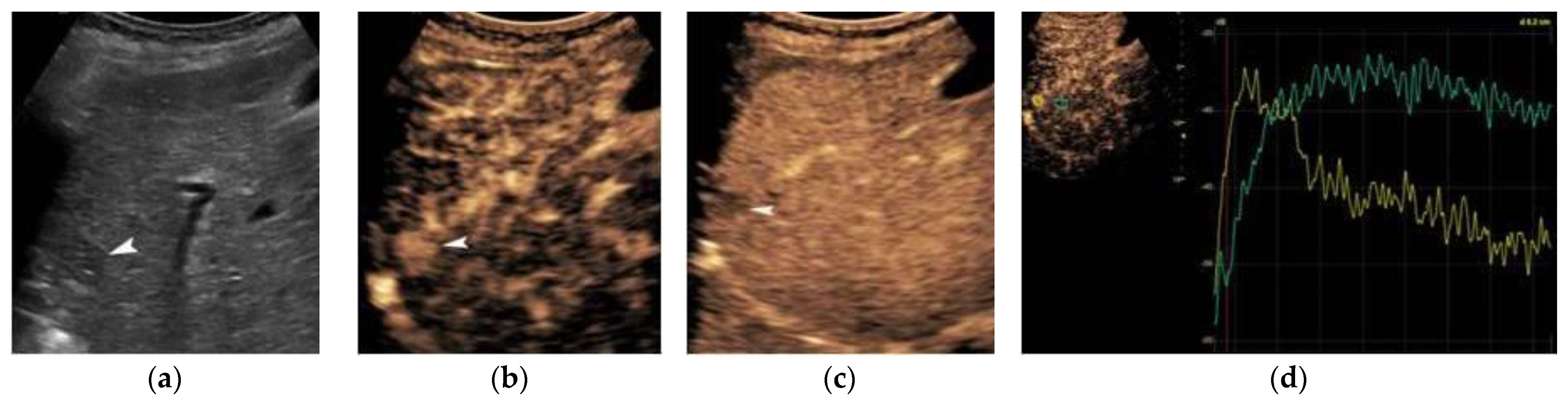
2. Ultrasound
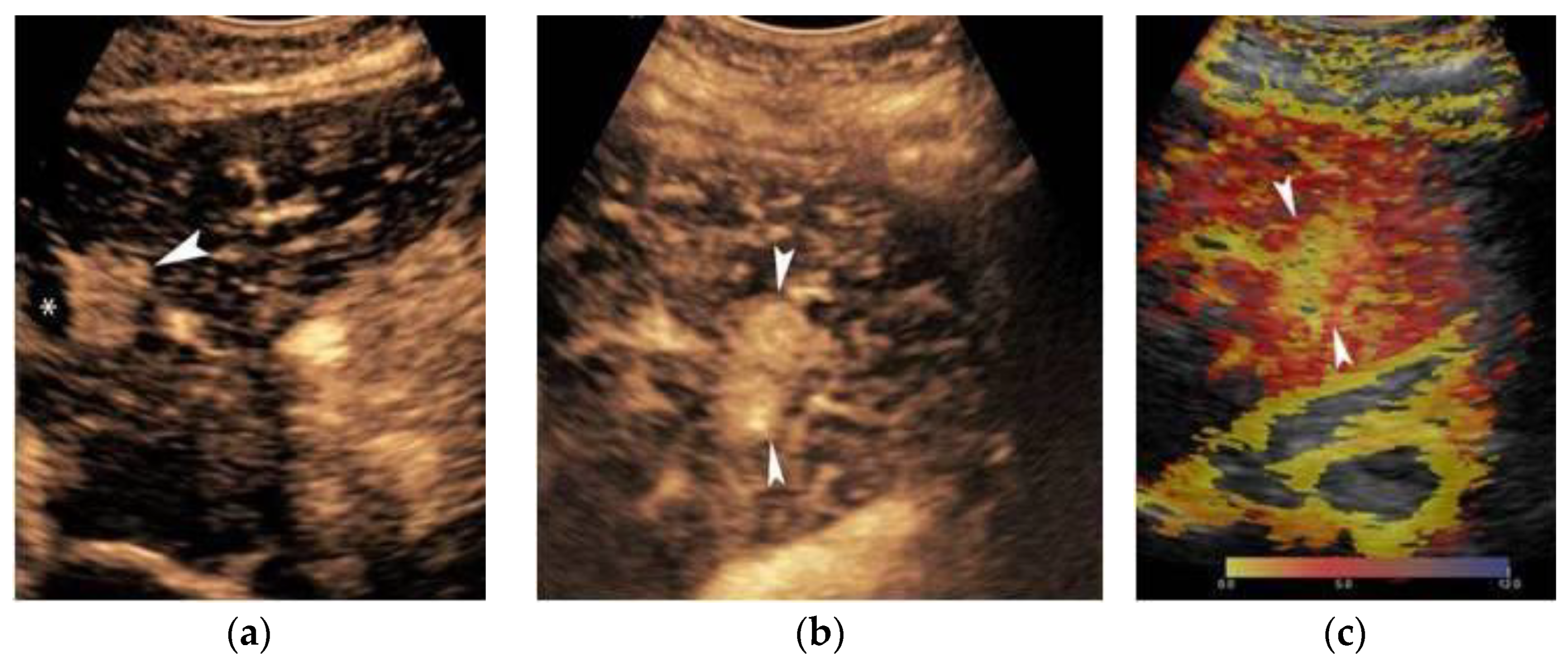
3. Contrast-Enhanced Ultrasound
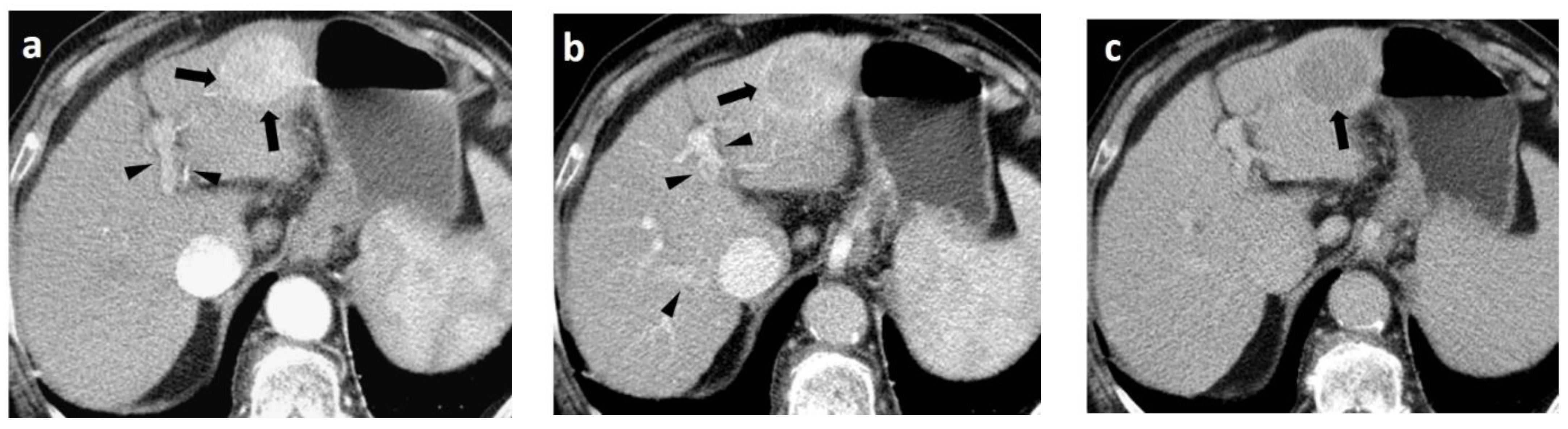
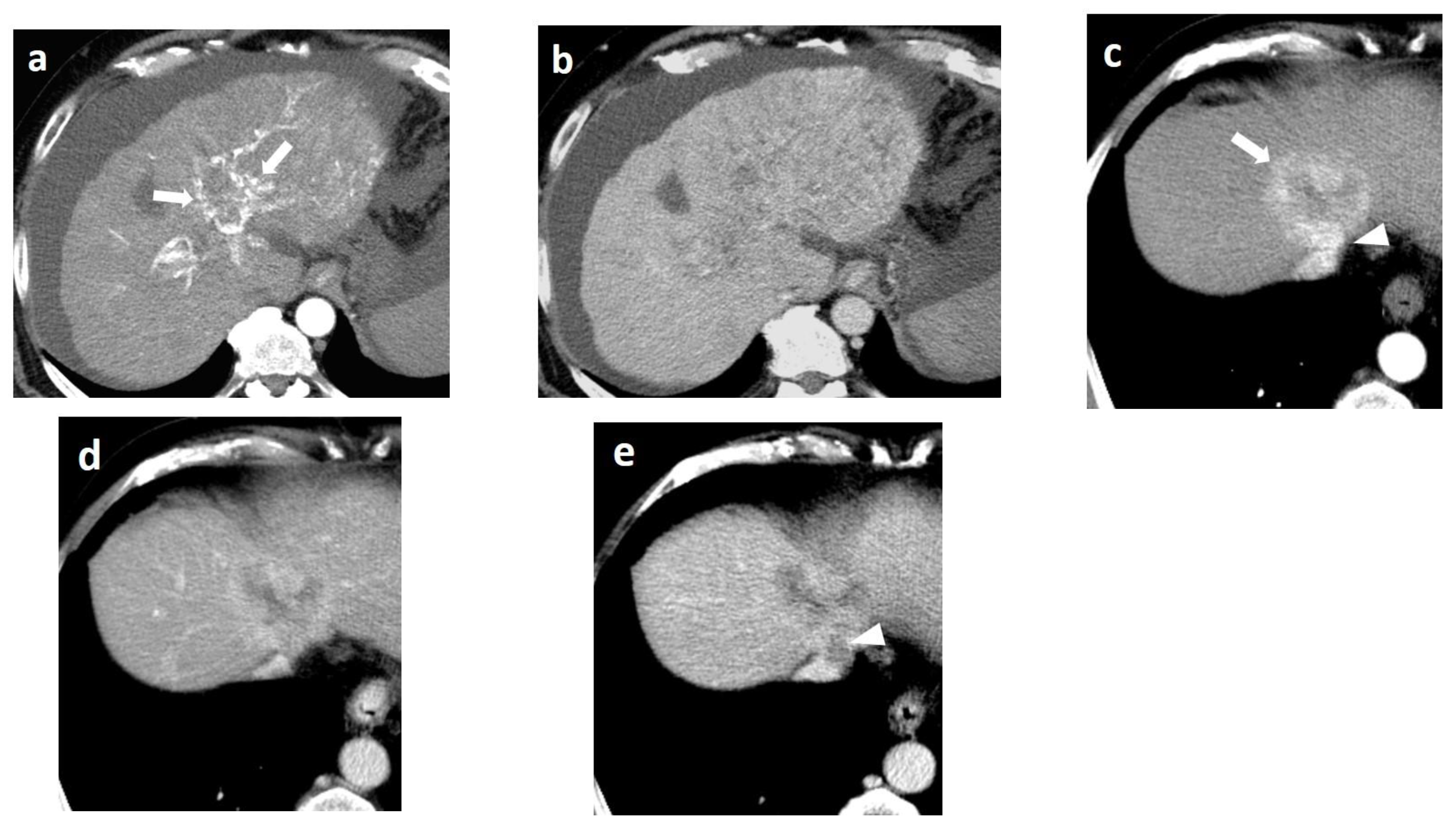
4. CT
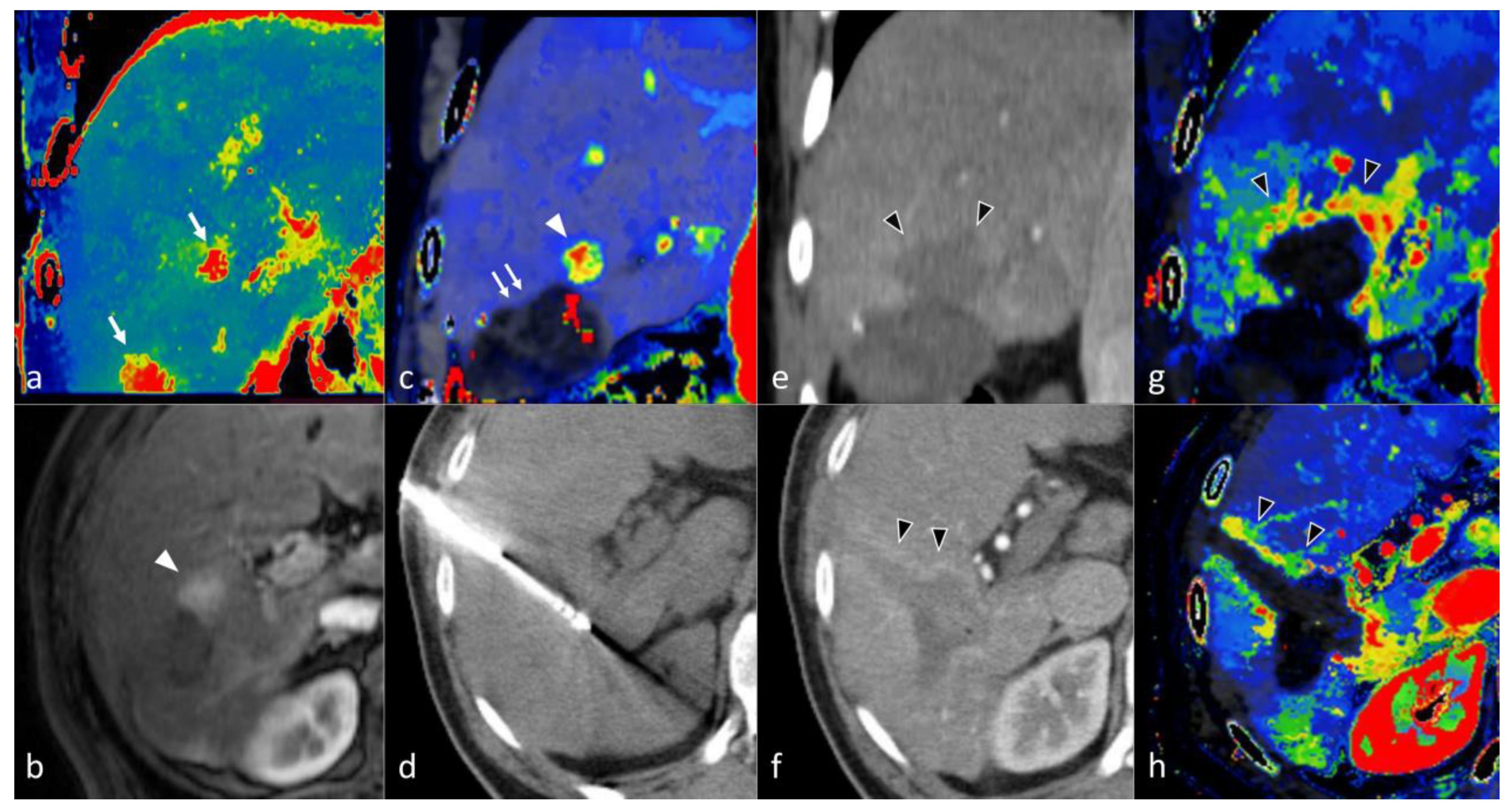
5. CT Perfusion
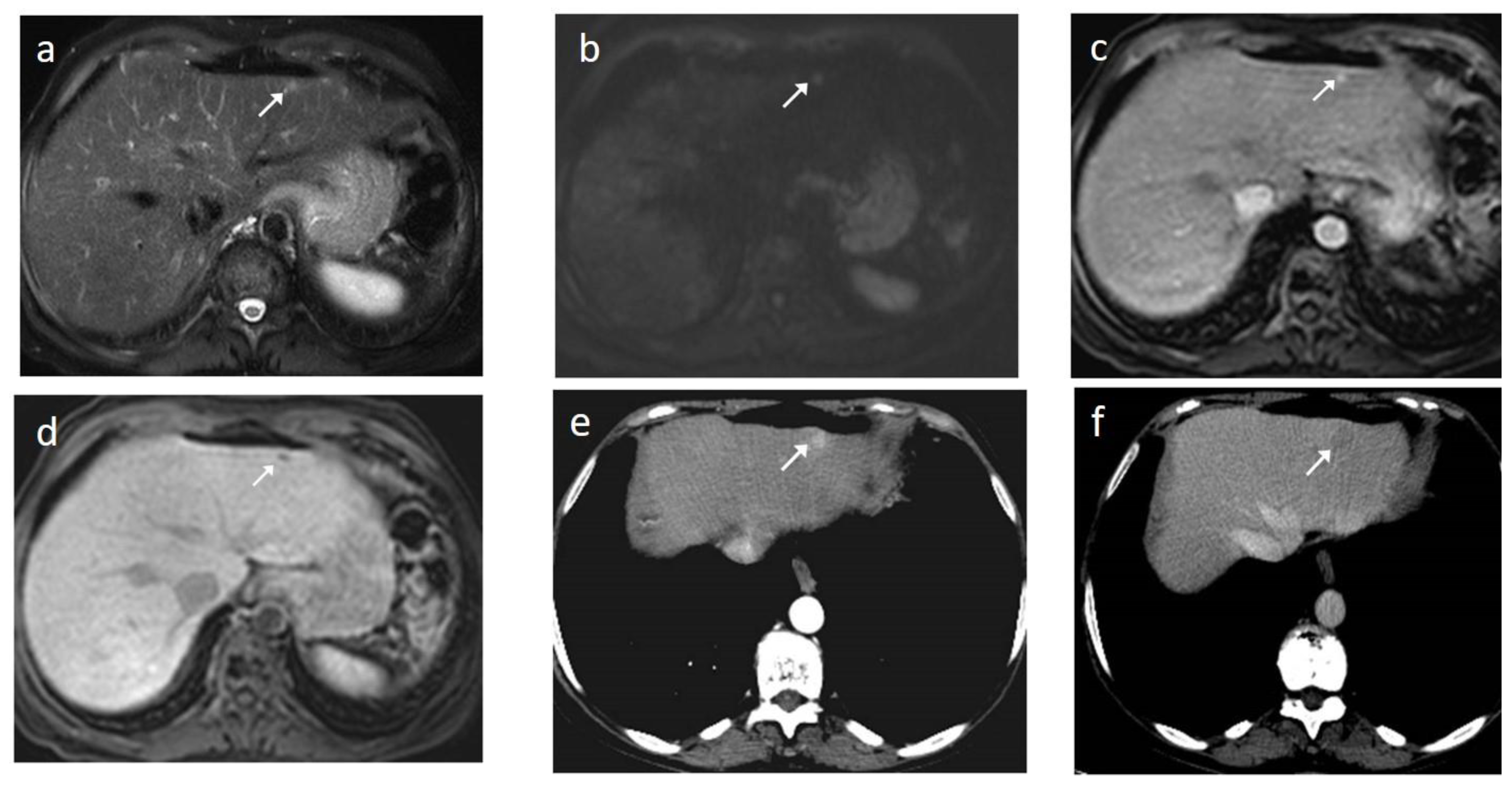
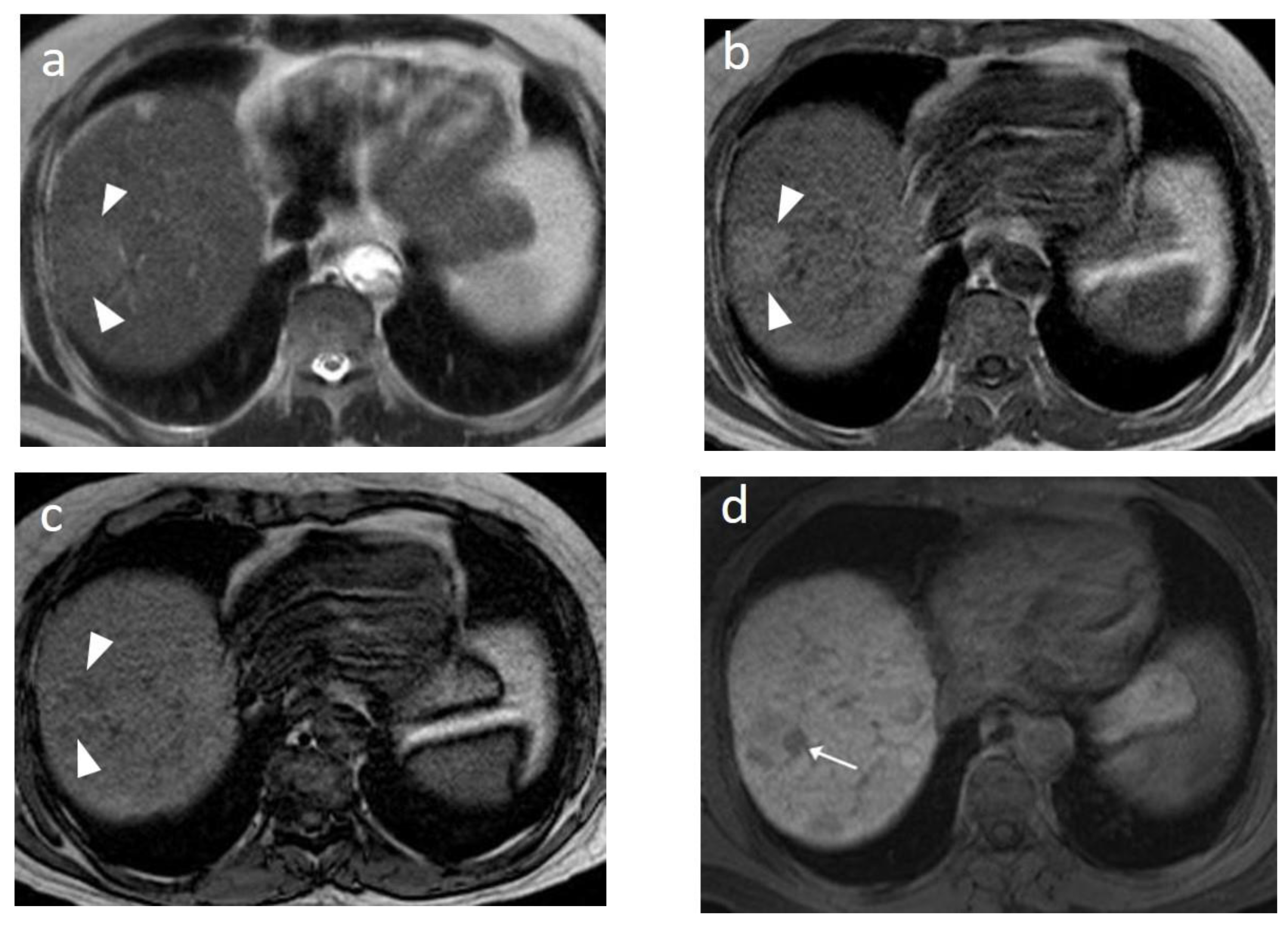
6. MRI
7. MR Perfusion
8. PET/CT
9. Artificial Intelligence
10. HCC Diagnostic Algorithms
11. HCC in Non-Cirrhotic Livers
12. Imaging Assessment of HCC following Percutaneous Locoregional Therapy
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Cancer Observatory. Available online: http://globocan.iarc.fr/Default.aspx (accessed on 5 August 2022).
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Masch, W.R.; Kampalath, R.; Parikh, N.; Shampain, K.A.; Aslam, A.; Chernyak, V. Imaging of treatment response during systemic therapy for hepatocellular carcinoma. Abdom. Radiol. 2021, 46, 3625–3633. [Google Scholar] [CrossRef]
- Sidhu, P. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Med. Eur. J. Ultrasound 2015, 36, 315–317. [Google Scholar] [CrossRef]
- Sparchez, Z.; Craciun, R.; Caraiani, C.; Horhat, A.; Nenu, I.; Procopet, B.; Sparchez, M.; Stefanescu, H.; Mocan, T. Ultrasound or Sectional Imaging Techniques as Screening Tools for Hepatocellular Carcinoma: Fall Forward or Move Forward? J. Clin. Med. 2021, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J. Med. Ultrason. 2020, 47, 239–255. [Google Scholar] [CrossRef]
- Makuuchi, M.; Hasegawa, H.; Yamazaki, S.; Bandai, Y.; Watanabe, G.; Ito, T. Ultrasonic characteristics of the small hepatocellular carcinoma. Ultrasound Med. Biol. 1983, (Suppl. S2), 489–491. [Google Scholar]
- Minami, Y. Hepatic malignancies: Correlation between sonographic findings and pathological features. World J. Radiol. 2010, 2, 249. [Google Scholar] [CrossRef]
- Hui, A.-M.; Takayama, T.; Sano, K.; Kubota, K.; Akahane, M.; Ohtomo, K.; Makuuchi, M. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J. Hepatol. 2000, 33, 975–979. [Google Scholar] [CrossRef]
- Shimada, M.; Rikimaru, T.; Hamatsu, T.; Yamashita, Y.-i.; Terashi, T.; Taguchi, K.-i.; Tanaka, S.; Shirabe, K.; Sugimachi, K. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am. J. Surg. 2001, 182, 177–182. [Google Scholar] [CrossRef]
- Tochio, H.; Kudo, M. Afferent and Efferent Vessels of Premalignant and Overt Hepatocellular Carcinoma: Observation by Color Doppler Imaging. Intervirology 2004, 47, 144–153. [Google Scholar] [CrossRef]
- Lencioni, R.; Pinto, F.; Armillotta, N.; Bartolozzi, C. Assessment of tumor vascularity in hepatocellular carcinoma: Comparison of power Doppler US and color Doppler US. Radiology 1996, 201, 353–358. [Google Scholar] [CrossRef]
- Chen, M.; Wang, D.; Zhao, Y.; Lu, D.M.; Li, H.X.; Liu, J.J.; Li, H. Preoperative color Doppler ultrasonography predicts early recurrence in AFP-positive hepatocellular carcinoma. Oncol. Lett. 2019, 18, 4703–4711. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Liu, C.; Mao, Y.; Mu, J.; Wei, X.; Jia, J.; Zhang, S.; Xin, X.; Tan, J. Superb microvascular imaging technique in depicting vascularity in focal liver lesions: More hypervascular supply patterns were depicted in hepatocellular carcinoma. Cancer Imaging 2019, 19, 92. [Google Scholar] [CrossRef]
- He, M.-N.; Lv, K.; Jiang, Y.-X.; Jiang, T.-A. Application of superb microvascular imaging in focal liver lesions. World J. Gastroenterol. 2017, 23, 7765–7775. [Google Scholar] [CrossRef]
- Rodgers, S.K.; Fetzer, D.T.; Gabriel, H.; Seow, J.H.; Choi, H.H.; Maturen, K.E.; Wasnik, A.P.; Morgan, T.A.; Dahiya, N.; O’Boyle, M.K.; et al. Role of US LI-RADS in the LI-RADS Algorithm. RadioGraphics 2019, 39, 690–708. [Google Scholar] [CrossRef]
- Delli Pizzi, A.; Mastrodicasa, D.; Cianci, R.; Serafini, F.L.; Mincuzzi, E.; Di Fabio, F.; Giammarino, A.; Mannetta, G.; Basilico, R.; Caulo, M. Multimodality Imaging of Hepatocellular Carcinoma: From Diagnosis to Treatment Response Assessment in Everyday Clinical Practice. Can. Assoc. Radiol. J. 2021, 72, 714–727. [Google Scholar] [CrossRef]
- Sangiovanni, A.; Del Ninno, E.; Fasani, P.; De Fazio, C.; Ronchi, G.; Romeo, R.; Morabito, A.; de Franchis, R.; Colombo, M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004, 126, 1005–1014. [Google Scholar] [CrossRef]
- Samoylova, M.L.; Mehta, N.; Roberts, J.P.; Yao, F.Y. Predictors of Ultrasound Failure to Detect Hepatocellular Carcinoma. Liver Transpl. 2018, 24, 1171–1177. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Salvatore, V.; Gianstefani, A.; Negrini, G.; Allegretti, G.; Galassi, M.; Piscaglia, F. Imaging Diagnosis of Hepatocellular Carcinoma: Recent Advances of Contrast-Enhanced Ultrasonography with SonoVue®. Liver Cancer 2015, 5, 55–66. [Google Scholar] [CrossRef]
- Dietrich, C.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. Eur. J. Ultrasound 2017, 38, e16–e47. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Li, J.-W.; Lu, Q.; Luo, Y.; Lin, L.; Shi, Y.-J.; Li, T.; Liu, J.-B.; Lyshchik, A. Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology 2020, 294, 329–339. [Google Scholar] [CrossRef]
- Lin, M.; Lu, D.S.; Duan, Y.; Liao, P.; Sayre, J.; Xie, X.; Kuang, M. Cirrhotic Nodule Transformation to Hepatocellular Carcinoma: Natural History and Predictive Biomarkers on Contrast-Enhanced Ultrasound. Am. J. Roentgenol. 2020, 214, 96–104. [Google Scholar] [CrossRef]
- Duan, Y.; Xie, X.; Li, Q.; Mercaldo, N.; Samir, A.E.; Kuang, M.; Lin, M. Differentiation of regenerative nodule, dysplastic nodule, and small hepatocellular carcinoma in cirrhotic patients: A contrast-enhanced ultrasound–based multivariable model analysis. Eur. Radiol. 2020, 30, 4741–4751. [Google Scholar] [CrossRef]
- Bartolotta, T.V.; Taibbi, A.; Midiri, M.; Lagalla, R. Contrast-enhanced ultrasound of hepatocellular carcinoma: Where do we stand? Ultrasonography 2019, 38, 200–214. [Google Scholar] [CrossRef]
- Fan, P.L.; Ding, H.; Mao, F.; Chen, L.L.; Dong, Y.; Wang, W.P. Enhancement patterns of small hepatocellular carcinoma (≤30 mm) on contrast-enhanced ultrasound: Correlation with clinicopathologic characteristics. Eur. J. Radiol. 2020, 132, 109341. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kim, T.K.; Burns, P.N.; Wilson, S.R. Enhancement Patterns of Hepatocellular Carcinoma at Contrast-enhanced US: Comparison with Histologic Differentiation. Radiology 2007, 244, 898–906. [Google Scholar] [CrossRef]
- Wilson, S.R.; Burns, P.N.; Kono, Y. Contrast-Enhanced Ultrasound of Focal Liver Masses: A Success Story. Ultrasound Med. Biol. 2020, 46, 1059–1070. [Google Scholar] [CrossRef]
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: A pictorial review. Insights Imaging 2020, 11, 9. [Google Scholar] [CrossRef]
- Hatanaka, K.; Kudo, M.; Minami, Y.; Maekawa, K. Sonazoid-Enhanced Ultrasonography for Diagnosis of Hepatic Malignancies: Comparison with Contrast-Enhanced CT. Oncology 2008, 75, 42–47. [Google Scholar] [CrossRef]
- Mandai, M.; Koda, M.; Matono, T.; Nagahara, T.; Sugihara, T.; Ueki, M.; Ohyama, K.; Murawaki, Y. Assessment of hepatocellular carcinoma by contrast-enhanced ultrasound with perfluorobutane microbubbles: Comparison with dynamic CT. Br. J. Radiol. 2011, 84, 499–507. [Google Scholar] [CrossRef]
- Numata, K.; Fukuda, H.; Miwa, H.; Ishii, T.; Moriya, S.; Kondo, M.; Nozaki, A.; Morimoto, M.; Okada, M.; Takebayashi, S.; et al. Contrast-enhanced ultrasonography findings using a perflubutane-based contrast agent in patients with early hepatocellular carcinoma. Eur. J. Radiol. 2014, 83, 95–102. [Google Scholar] [CrossRef]
- Maruyama, H.; Takahashi, M.; Ishibashi, H.; Yoshikawa, M.; Yokosuka, O. Contrast-enhanced ultrasound for characterisation of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br. J. Radiol. 2012, 85, 351–357. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Klopffleisch, T.; Nierhoff, J.; Herrmann, E.; Vermehren, J.; Schneider, M.D.; Zeuzem, S.; Bojunga, J. Contrast-Enhanced Ultrasound for the differentiation of benign and malignant focal liver lesions: A meta-analysis. Liver Int. 2013, 33, 739–755. [Google Scholar] [CrossRef]
- Terzi, E.; Iavarone, M.; Pompili, M.; Veronese, L.; Cabibbo, G.; Fraquelli, M.; Riccardi, L.; De Bonis, L.; Sangiovanni, A.; Leoni, S.; et al. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1006 nodules. J. Hepatol. 2018, 68, 485–492. [Google Scholar] [CrossRef]
- Sugimoto, K.; Moriyasu, F.; Kamiyama, N.; Metoki, R.; Yamada, M.; Imai, Y.; Iijima, H. Analysis of morphological vascular changes of hepatocellular carcinoma by microflow imaging using contrast-enhanced sonography. Hepatol. Res. 2008, 38, 790–799. [Google Scholar] [CrossRef]
- Schwarz, S.; Clevert, D.-A.; Ingrisch, M.; Geyer, T.; Schwarze, V.; Rübenthaler, J.; Armbruster, M. Quantitative Analysis of the Time–Intensity Curve of Contrast-Enhanced Ultrasound of the Liver: Differentiation of Benign and Malignant Liver Lesions. Diagnostics 2021, 11, 1244. [Google Scholar] [CrossRef]
- Dong, Y.; Qiu, Y.; Yang, D.; Yu, L.; Zuo, D.; Zhang, Q.; Tian, X.; Wang, W.-P.; Jung, E.M. Potential application of dynamic contrast enhanced ultrasound in predicting microvascular invasion of hepatocellular carcinoma. Clin. Hemorheol. Microcirc. 2021, 77, 461–469. [Google Scholar] [CrossRef]
- Wildner, D.; Pfeifer, L.; Goertz, R.; Bernatik, T.; Sturm, J.; Neurath, M.; Strobel, D. Dynamic Contrast-Enhanced Ultrasound (DCE-US) for the Characterization of Hepatocellular Carcinoma and Cholangiocellular Carcinoma. Ultraschall Med. Eur. J. Ultrasound 2014, 35, 522–527. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.-P.; Mao, F.; Zhang, Q.; Yang, D.; Tannapfel, A.; Meloni, M.F.; Neye, H.; Clevert, D.-A.; Dietrich, C.F. Imaging Features of Fibrolamellar Hepatocellular Carcinoma with Contrast-Enhanced Ultrasound. Ultraschall Med. Eur. J. Ultrasound 2021, 42, 306–313. [Google Scholar] [CrossRef]
- Tang, A.; Cruite, I.; Mitchell, D.G.; Sirlin, C.B. Hepatocellular carcinoma imaging systems: Why they exist, how they have evolved, and how they differ. Abdom. Radiol. 2018, 43, 3–12. [Google Scholar] [CrossRef]
- Kulkarni, N.M.; Fung, A.; Kambadakone, A.R.; Yeh, B.M. Computed Tomography Techniques, Protocols, Advancements, and Future Directions in Liver Diseases. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 305–320. [Google Scholar] [CrossRef]
- Kambadakone, A.R.; Fung, A.; Gupta, R.T.; Hope, T.A.; Fowler, K.J.; Lyshchik, A.; Ganesan, K.; Yaghmai, V.; Guimaraes, A.R.; Sahani, D.V.; et al. LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound. Abdom. Radiol. 2018, 43, 56–74. [Google Scholar] [CrossRef]
- Bae, K.T. Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lee, J.-M.; Sirlin, C.B. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part I. Development, Growth, and Spread: Key Pathologic and Imaging Aspects. Radiology 2014, 272, 635–654. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- American College of Radiology (ACR). Liver Imaging Reporting and Data System Version 2018. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018 (accessed on 8 July 2022).
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Cui, J.; Papadatos, D.; Sirlin, C.B.; Santillan, C.S. Hepatocarcinogenesis and LI-RADS. Abdom. Radiol. 2018, 43, 158–168. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lee, J.-M.; Sirlin, C.B. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part II. Extracellular Agents, Hepatobiliary Agents, and Ancillary Imaging Features. Radiology 2014, 273, 30–50. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.M.; Kim, S.J.; Baek, J.H.; Yun, S.H.; Kim, K.W.; Han, J.K.; Choi, B.I. Enhancement patterns of hepatocellular carcinomas on multiphasic multidetector row CT: Comparison with pathological differentiation. Br. J. Radiol. 2012, 85, e573–e583. [Google Scholar] [CrossRef]
- Zhu, F.; Yang, F.; Li, J.; Chen, W.; Yang, W. Incomplete tumor capsule on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: A systematic review and meta-analysis. Abdom. Radiol. 2019, 44, 3049–3057. [Google Scholar] [CrossRef]
- Galia, M.; Taibbi, A.; Marin, D.; Furlan, A.; Burgio, M.D.; Agnello, F.; Cabibbo, G.; Beers, B.E.V.; Bartolotta, T.V.; Midiri, M.; et al. Focal lesions in cirrhotic liver: What else beyond hepatocellular carcinoma? Diagn. Interv. Radiol. 2014, 20, 222–228. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Porrello, G.; Calandra, A.; Midiri, M.; Furlan, A.; Brancatelli, G. Uncommon imaging evolutions of focal liver lesions in cirrhosis. Abdom. Radiol. 2019, 44, 3069–3077. [Google Scholar] [CrossRef]
- Tang, M.; Li, Y.; Lin, Z.; Shen, B.; Huang, M.; Li, Z.-P.; Li, X.; Feng, S.-T. Hepatic nodules with arterial phase hyperenhancement and washout on enhanced computed tomography/magnetic resonance imaging: How to avoid pitfalls. Abdom. Radiol. 2020, 45, 3730–3742. [Google Scholar] [CrossRef]
- Huang, B.; Wu, L.; Lu, X.-Y.; Xu, F.; Liu, C.-F.; Shen, W.-F.; Jia, N.-Y.; Cheng, H.-Y.; Yang, Y.-F.; Shen, F. Small Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma in Cirrhotic Livers May Share Similar Enhancement Patterns at Multiphase Dynamic MR Imaging. Radiology 2016, 281, 150–157. [Google Scholar] [CrossRef]
- Xu, J.; Igarashi, S.; Sasaki, M.; Matsubara, T.; Yoneda, N.; Kozaka, K.; Ikeda, H.; Kim, J.; Yu, E.; Matsui, O.; et al. Intrahepatic cholangiocarcinomas in cirrhosis are hypervascular in comparison with those in normal livers. Liver Int. 2012, 32, 1156–1164. [Google Scholar] [CrossRef]
- Kim, I.; Kim, M.-J. Histologic Characteristics of Hepatocellular Carcinomas Showing Atypical Enhancement Patterns on 4-Phase MDCT Examination. Korean J. Radiol. 2012, 13, 586. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.K.; Lee, W.J.; Park, M.J.; Kim, S.H.; Rhim, H.; Choi, D. Hypovascular Hypointense Nodules on Hepatobiliary Phase Gadoxetic Acid–enhanced MR Images in Patients with Cirrhosis: Potential of DW Imaging in Predicting Progression to Hypervascular HCC. Radiology 2012, 265, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, Y.H.; Kim, C.S.; Han, Y.M. Added diagnostic value of T2-weighted MR imaging to gadolinium-enhanced three-dimensional dynamic MR imaging for the detection of small hepatocellular carcinomas. Eur. J. Radiol. 2008, 67, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, B.I.; Lee, E.S.; Park, S.B.; Lee, J.B. How to Differentiate Borderline Hepatic Nodules in Hepatocarcinogenesis: Emphasis on Imaging Diagnosis. Liver Cancer 2017, 6, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Yoon, J.H.; Lee, J.M. Emerging Role of Hepatobiliary Magnetic Resonance Contrast Media and Contrast-Enhanced Ultrasound for Noninvasive Diagnosis of Hepatocellular Carcinoma: Emphasis on Recent Updates in Major Guidelines. Korean J. Radiol. 2019, 20, 863. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Kim, S.Y.; Kang, T.W.; Kim, Y.K.; Park, B.J.; Lee, Y.J.; Choi, J.-I.; Lee, C.-H.; Park, H.S.; Lee, K.; et al. Radiologic-Pathologic Correlation of Hepatobiliary Phase Hypointense Nodules without Arterial Phase Hyperenhancement at Gadoxetic Acid–enhanced MRI: A Multicenter Study. Radiology 2020, 296, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Joo, I.; Lee, J.M. Atypical Appearance of Hepatocellular Carcinoma and Its Mimickers: How to Solve Challenging Cases Using Gadoxetic Acid-Enhanced Liver Magnetic Resonance Imaging. Korean J. Radiol. 2019, 20, 1019. [Google Scholar] [CrossRef]
- Basha, M.A.A.; AlAzzazy, M.Z.; Ahmed, A.F.; Yousef, H.Y.; Shehata, S.M.; El Sammak, D.A.E.A.; Fathy, T.; Obaya, A.A.; Abdelbary, E.H. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. Eur. Radiol. 2018, 28, 2592–2603. [Google Scholar] [CrossRef]
- Kojiro, M. ‘Nodule-in-Nodule’ Appearance in Hepatocellular Carcinoma: Its Significance as a Morphologic Marker of Dedifferentiation. Intervirology 2004, 47, 179–183. [Google Scholar] [CrossRef]
- Kitao, A.; Zen, Y.; Matsui, O.; Gabata, T.; Nakanuma, Y. Hepatocarcinogenesis: Multistep Changes of Drainage Vessels at CT during Arterial Portography and Hepatic Arteriography—Radiologic-Pathologic Correlation. Radiology 2009, 252, 605–614. [Google Scholar] [CrossRef]
- Chou, C.-T.; Chen, R.-C.; Lin, W.-C.; Ko, C.-J.; Chen, C.-B.; Chen, Y.-L. Prediction of Microvascular Invasion of Hepatocellular Carcinoma: Preoperative CT and Histopathologic Correlation. Am. J. Roentgenol. 2014, 203, W253–W259. [Google Scholar] [CrossRef]
- An, C.; Kim, M.-J. Imaging features related with prognosis of hepatocellular carcinoma. Abdom. Radiol. 2019, 44, 509–516. [Google Scholar] [CrossRef]
- Bello, H.R.; Mahdi, Z.K.; Lui, S.K.; Nandwana, S.B.; Harri, P.A.; Davarpanah, A.H. Hepatocellular Carcinoma with Atypical Imaging Features: Review of the Morphologic Hepatocellular Carcinoma Subtypes with Radiology-Pathology Correlation. J. Magn. Reson. Imaging 2021, 55, 681–697. [Google Scholar] [CrossRef]
- Fowler, K.J.; Burgoyne, A.; Fraum, T.J.; Hosseini, M.; Ichikawa, S.; Kim, S.; Kitao, A.; Lee, J.M.; Paradis, V.; Taouli, B.; et al. Pathologic, Molecular, and Prognostic Radiologic Features of Hepatocellular Carcinoma. RadioGraphics 2021, 41, 1611–1631. [Google Scholar] [CrossRef]
- Mulé, S.; Galletto Pregliasco, A.; Tenenhaus, A.; Kharrat, R.; Amaddeo, G.; Baranes, L.; Laurent, A.; Regnault, H.; Sommacale, D.; Djabbari, M.; et al. Multiphase Liver MRI for Identifying the Macrotrabecular-Massive Subtype of Hepatocellular Carcinoma. Radiology 2020, 295, 562–571. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, H.K.; Lee, W.J.; Choi, D.; Park, C.K. Scirrhous hepatocellular carcinoma: Comparison with usual hepatocellular carcinoma based on CT–pathologic features and long-term results after curative resection. Eur. J. Radiol. 2009, 69, 123–130. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Kim, S.H.; Park, C.K.; Min, J.H.; Lee, J.E.; Choi, Y.-H.; Lee, B.R. Imaging Features of Gadoxetic Acid–enhanced and Diffusion-weighted MR Imaging for Identifying Cytokeratin 19-positive Hepatocellular Carcinoma: A Retrospective Observational Study. Radiology 2018, 286, 897–908. [Google Scholar] [CrossRef]
- Reynolds, A.R.; Furlan, A.; Fetzer, D.T.; Sasatomi, E.; Borhani, A.A.; Heller, M.T.; Tublin, M.E. Infiltrative Hepatocellular Carcinoma: What Radiologists Need to Know. RadioGraphics 2015, 35, 371–386. [Google Scholar] [CrossRef]
- Raab, B.-W. The Thread and Streak Sign. Radiology 2005, 236, 284–285. [Google Scholar] [CrossRef]
- Lafitte, M.; Laurent, V.; Soyer, P.; Ayav, A.; Balaj, C.; Petit, I.; Hossu, G. MDCT features of hepatocellular carcinoma (HCC) in non-cirrhotic liver. Diagn. Interv. Imaging 2016, 97, 355–360. [Google Scholar] [CrossRef]
- Ippolito, D.; Pecorelli, A.; Querques, G.; Drago, S.G.; Maino, C.; Franzesi, C.T.; Hatzidakis, A.; Sironi, S. Dynamic Computed Tomography Perfusion Imaging: Complementary Diagnostic Tool in Hepatocellular Carcinoma Assessment from Diagnosis to Treatment Follow-up. Acad. Radiol. 2019, 26, 1675–1685. [Google Scholar] [CrossRef]
- Kim, S.H.; Kamaya, A.; Willmann, J.K. CT Perfusion of the Liver: Principles and Applications in Oncology. Radiology 2014, 272, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Ronot, M.; Clift, A.K.; Vilgrain, V.; Frilling, A. Functional imaging in liver tumours. J. Hepatol. 2016, 65, 1017–1030. [Google Scholar] [CrossRef]
- Ronot, M.; Leporq, B.; Van Beers, B.E.; Vilgrain, V. CT and MR perfusion techniques to assess diffuse liver disease. Abdom. Radiol. 2020, 45, 3496–3506. [Google Scholar] [CrossRef]
- Sahani, D.V.; Holalkere, N.-S.; Mueller, P.R.; Zhu, A.X. Advanced Hepatocellular Carcinoma: CT Perfusion of Liver and Tumor Tissue—Initial Experience. Radiology 2007, 243, 736–743. [Google Scholar] [CrossRef]
- Ippolito, D.; Sironi, S.; Pozzi, M.; Antolini, L.; Invernizzi, F.; Ratti, L.; Leone, E.B.; Fazio, F. Perfusion CT in cirrhotic patients with early stage hepatocellular carcinoma: Assessment of tumor-related vascularization. Eur. J. Radiol. 2010, 73, 148–152. [Google Scholar] [CrossRef]
- Fischer, M.A.; Kartalis, N.; Grigoriadis, A.; Loizou, L.; Stål, P.; Leidner, B.; Aspelin, P.; Brismar, T.B. Perfusion computed tomography for detection of hepatocellular carcinoma in patients with liver cirrhosis. Eur. Radiol. 2015, 25, 3123–3132. [Google Scholar] [CrossRef]
- Hatzidakis, A.; Perisinakis, K.; Kalarakis, G.; Papadakis, A.; Savva, E.; Ippolito, D.; Karantanas, A. Perfusion-CT analysis for assessment of hepatocellular carcinoma lesions: Diagnostic value of different perfusion maps. Acta Radiol. 2019, 60, 561–568. [Google Scholar] [CrossRef]
- Chen, B.-B.; Hsu, C.-Y.; Yu, C.-W.; Liang, P.-C.; Hsu, C.; Hsu, C.-H.; Cheng, A.-L.; Shih, T.T.-F. Dynamic Contrast-enhanced MR Imaging of Advanced Hepatocellular Carcinoma: Comparison with the Liver Parenchyma and Correlation with the Survival of Patients Receiving Systemic Therapy. Radiology 2016, 281, 454–464. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bhargava, P.; Kolokythas, O.; Mitsumori, L.M.; Maki, J.H. Computer-Assisted Evaluation of Contrast Kinetics for Detection of Hepatocellular Carcinoma on Magnetic Resonance Imaging. Curr. Probl. Diagn. Radiol. 2015, 44, 8–14. [Google Scholar] [CrossRef]
- Jajamovich, G.H.; Huang, W.; Besa, C.; Li, X.; Afzal, A.; Dyvorne, H.A.; Taouli, B. DCE-MRI of hepatocellular carcinoma: Perfusion quantification with Tofts model versus shutter-speed model—initial experience. Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Taouli, B.; Johnson, R.S.; Hajdu, C.H.; Oei, M.T.H.; Merad, M.; Yee, H.; Rusinek, H. Hepatocellular Carcinoma: Perfusion Quantification with Dynamic Contrast-Enhanced MRI. Am. J. Roentgenol. 2013, 201, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.; Bottino, A.; Nogueira, C.; Pannain, V. Analysis of morphological variables and arterialization in the differential diagnosis of hepatic nodules in explanted cirrhotic livers. Diagn. Pathol. 2007, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Matsui, O.; Kitao, A.; Kobayashi, S.; Nakayama, J.; Miyagawa, S.; Kadoya, M. Tumor Hemodynamics and Hepatocarcinogenesis: Radio-Pathological Correlations and Outcomes of Carcinogenic Hepatocyte Nodules. ISRN Hepatol. 2014, 2014, 607628. [Google Scholar] [CrossRef]
- Ma, G.-L.; Bai, R.-J.; Jiang, H.-J.; Hao, X.-J.; Dong, X.-P.; Li, D.-Q.; Liu, X.-D.; Wei, L. Early changes of hepatic hemodynamics measured by functional CT perfusion in a rabbit model of liver tumor. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 407–411. [Google Scholar] [CrossRef]
- Li, J.-P.; Feng, G.-L.; Li, D.-Q.; Wang, H.-B.; Zhao, D.-L.; Wan, Y.; Jiang, H.-J. Detection and differentiation of early hepatocellular carcinoma from cirrhosis using CT perfusion in a rat liver model. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 612–618. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Aggarwal, N.; Sood, R.G.; Sood, S.; Sidhu, R. Role of Perfusion CT Differentiating Hemangiomas from Malignant Hepatic Lesions. J. Clin. Imaging Sci. 2014, 4, 10. [Google Scholar] [CrossRef]
- Guo, M.; Yu, Y. Application of 128 Slice 4D CT Whole Liver Perfusion Imaging in Hepatic Tumor. Cell Biochem. Biophys. 2014, 70, 173–178. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Gao, Z.; Zhu, K.; Yin, X. Evaluation of the blood flow in common hepatic tumors by multi-slice spiral CT whole-liver perfusion imaging. Zhonghua Zhong Liu Za Zhi 2015, 37, 904–908. [Google Scholar]
- Hayano, K.; Desai, G.S.; Kambadakone, A.R.; Fuentes, J.M.; Tanabe, K.K.; Sahani, D.V. Quantitative characterization of hepatocellular carcinoma and metastatic liver tumor by CT perfusion. Cancer Imaging 2013, 13, 512–519. [Google Scholar] [CrossRef]
- Guyennon, A. Perfusion characterization of liver metastases from endocrine tumors: Computed tomography perfusion. World J. Radiol. 2010, 2, 449. [Google Scholar] [CrossRef]
- Reiner, C.S.; Goetti, R.; Burger, I.A.; Fischer, M.A.; Frauenfelder, T.; Knuth, A.; Pfammatter, T.; Schaefer, N.; Alkadhi, H. Liver Perfusion Imaging in Patients with Primary and Metastatic Liver Malignancy. Acad. Radiol. 2012, 19, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Marquez, H.P.; Gordic, S.; Leidner, B.; Klotz, E.; Aspelin, P.; Alkadhi, H.; Brismar, T.B. Arterio-portal shunts in the cirrhotic liver: Perfusion computed tomography for distinction of arterialized pseudolesions from hepatocellular carcinoma. Eur. Radiol. 2017, 27, 1074–1080. [Google Scholar] [CrossRef]
- Alıcıoglu, B.; Guler, O.; Bulakbası, N.; Akpınar, S.; Tosun, O.; Comunoglu, C. Utility of semiquantitative parameters to differentiate benign and malignant focal hepatic lesions. Clin. Imaging 2013, 37, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Si, Y.; Zhao, K.; Shi, X.; Bi, W.; Liu, S.-e.; Hua, H. Evaluation of quantitative parameters of dynamic contrast-enhanced magnetic resonance imaging in qualitative diagnosis of hepatic masses. BMC Med. Imaging 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Ghodasara, S.; Pahwa, S.; Dastmalchian, S.; Gulani, V.; Chen, Y. Free-Breathing 3D Liver Perfusion Quantification Using a Dual-Input Two-Compartment Model. Sci. Rep. 2017, 7, 17502. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.S.; Pialat, J.B.; Wiart, M.; Duboeuf, F.; Mabrut, J.-Y.; Bancel, B.; Rode, A.; Ducerf, C.; Baulieux, J.; Berthezene, Y. Characterization of hepatocellular carcinoma and colorectal liver metastasis by means of perfusion MRI. J. Magn. Reson. Imaging 2008, 28, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, W.M.; Kaufmann, S.; Kloth, C.; Nikolaou, K.; Bösmüller, H.; Horger, M. VEGFR-2 expression in HCC, dysplastic and regenerative liver nodules, and correlation with pre-biopsy Dynamic Contrast Enhanced CT. Eur. J. Radiol. 2016, 85, 2036–2041. [Google Scholar] [CrossRef]
- Bai, R.-J.; Li, J.-P.; Ren, S.-H.; Jiang, H.-J.; Liu, X.-D.; Ling, Z.-S.; Huang, Q.; Feng, G.-L. A correlation of computed tomography perfusion and histopathology in tumor edges of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 612–617. [Google Scholar] [CrossRef]
- Borgheresi, A.; Gonzalez-Aguirre, A.; Brown, K.T.; Getrajdman, G.I.; Erinjeri, J.P.; Covey, A.; Yarmohammadi, H.; Ziv, E.; Sofocleous, C.T.; Boas, F.E. Does Enhancement or Perfusion on Preprocedure CT Predict Outcomes After Embolization of Hepatocellular Carcinoma? Acad. Radiol. 2018, 25, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Marquez, H.P.; Puippe, G.; Mathew, R.P.; Alkadhi, H.; Pfammatter, T.; Fischer, M.A. CT Perfusion for Early Response Evaluation of Radiofrequency Ablation of Focal Liver Lesions: First Experience. Cardiovasc. Interv. Radiol. 2017, 40, 90–98. [Google Scholar] [CrossRef]
- Lv, Y.; Jin, Y.; Yan, Q.; Yuan, D.; Wang, Y.; Li, X.; Shen, Y. The value of 64-slice spiral CT perfusion imaging in the treatment of liver cancer with argon-helium cryoablation. Oncol. Lett. 2016, 12, 4584–4588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ippolito, D.; Querques, G.; Pecorelli, A.; Talei Franzesi, C.; Okolicsanyi, S.; Strazzabosco, M.; Sironi, S. Diagnostic Value of Quantitative Perfusion Computed Tomography Technique in the Assessment of Tumor Response to Sorafenib in Patients with Advanced Hepatocellular Carcinoma. J. Comput. Assist. Tomogr. 2019, 43, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Popovic, P.; Leban, A.; Kregar, K.; Garbajs, M.; Dezman, R.; Bunc, M. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial chemoembolization of hepatocellular carcinoma. Radiol. Oncol. 2017, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Reiner, C.S.; Morsbach, F.; Sah, B.-R.; Puippe, G.; Schaefer, N.; Pfammatter, T.; Alkadhi, H. Early Treatment Response Evaluation after Yttrium-90 Radioembolization of Liver Malignancy with CT Perfusion. J. Vasc. Interv. Radiol. 2014, 25, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Klotz, E.; Haberland, U.; Glatting, G.; Schoenberg, S.O.; Fink, C.; Attenberger, U.; Henzler, T. Technical prerequisites and imaging protocols for CT perfusion imaging in oncology. Eur. J. Radiol. 2015, 84, 2359–2367. [Google Scholar] [CrossRef]
- Kalarakis, G.; Perisinakis, K.; Akoumianakis, E.; Karageorgiou, I.; Hatzidakis, A. CT liver perfusion in patients with hepatocellular carcinoma: Can we modify acquisition protocol to reduce patient exposure? Eur. Radiol. 2021, 31, 1410–1419. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Malavasi, S.; Vilgrain, V. Liver CT perfusion: Which is the relevant delay that reduces radiation dose and maintains diagnostic accuracy? Eur. Radiol. 2019, 29, 6550–6558. [Google Scholar] [CrossRef]
- Topcuoglu, O.M.; Karcaaltincaba, M.; Akata, D.; Ozmen, M.N. Reproducibility and variability of very low dose hepatic perfusion CT in metastatic liver disease. Diagn. Interv. Radiol. 2016, 22, 495–500. [Google Scholar] [CrossRef]
- Chung, C.Y.; Hu, R.; Peterson, R.B.; Allen, J.W. Automated Processing of Head CT Perfusion Imaging for Ischemic Stroke Triage: A Practical Guide to Quality Assurance and Interpretation. Am. J. Roentgenol. 2021, 217, 1401–1416. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, H.; Shao, J.; He, Q.; Su, W.; Wang, P.; Tang, Q.; Zeng, J.; Xu, S.; Zhao, J.; et al. Accuracy of Various Forms of Contrast-Enhanced MRI for Diagnosing Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 680691. [Google Scholar] [CrossRef]
- Rimola, J.; Forner, A.; Tremosini, S.; Reig, M.; Vilana, R.; Bianchi, L.; Rodríguez-Lope, C.; Solé, M.; Ayuso, C.; Bruix, J. Non-invasive diagnosis of hepatocellular carcinoma ⩽2cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J. Hepatol. 2012, 56, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Semaan, S.; Vietti Violi, N.; Lewis, S.; Chatterji, M.; Song, C.; Besa, C.; Babb, J.S.; Fiel, M.I.; Schwartz, M.; Thung, S.; et al. Hepatocellular carcinoma detection in liver cirrhosis: Diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. Eur. Radiol. 2020, 30, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.F.; Miloushev, V.Z.; Tang, A.; Finklestone, L.A.; Brejt, S.Z.; Sandhu, R.S.; Santillan, C.S.; Wolfson, T.; Gamst, A.; Sirlin, C.B. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom. Radiol. 2016, 41, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.R.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Heimbach, J.K.; Murad, M.H.; Mohammed, K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018, 67, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Aubé, C.; Oberti, F.; Lonjon, J.; Pageaux, G.; Seror, O.; N’Kontchou, G.; Rode, A.; Radenne, S.; Cassinotto, C.; Vergniol, J.; et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017, 37, 1515–1525. [Google Scholar] [CrossRef]
- Golfieri, R.; Renzulli, M.; Lucidi, V.; Corcioni, B.; Trevisani, F.; Bolondi, L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤2 cm) HCC in cirrhosis. Eur. Radiol. 2011, 21, 1233–1242. [Google Scholar] [CrossRef]
- Marin, D.; Di Martino, M.; Guerrisi, A.; De Filippis, G.; Rossi, M.; Ginanni Corradini, S.; Masciangelo, R.; Catalano, C.; Passariello, R. Hepatocellular Carcinoma in Patients with Cirrhosis: Qualitative Comparison of Gadobenate Dimeglumine–enhanced MR Imaging and Multiphasic 64-Section CT. Radiology 2009, 251, 85–95. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.M.; Lee, J.S.; Lee, H.Y.; Park, B.H.; Kim, Y.H.; Han, J.K.; Choi, B.I. Hepatocellular Carcinoma: Diagnostic Performance of Multidetector CT and MR Imaging—A Systematic Review and Meta-Analysis. Radiology 2015, 275, 97–109. [Google Scholar] [CrossRef]
- Sano, K.; Ichikawa, T.; Motosugi, U.; Sou, H.; Muhi, A.M.; Matsuda, M.; Nakano, M.; Sakamoto, M.; Nakazawa, T.; Asakawa, M.; et al. Imaging Study of Early Hepatocellular Carcinoma: Usefulness of Gadoxetic Acid–enhanced MR Imaging. Radiology 2011, 261, 834–844. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.M.; So, Y.H.; Hong, S.H.; Kim, S.J.; Han, J.K.; Choi, B.I. Multiphasic MDCT Enhancement Pattern of Hepatocellular Carcinoma Smaller Than 3 cm in Diameter: Tumor Size and Cellular Differentiation. Am. J. Roentgenol. 2009, 193, W482–W489. [Google Scholar] [CrossRef]
- Luca, A.; Caruso, S.; Milazzo, M.; Mamone, G.; Marrone, G.; Miraglia, R.; Maruzzelli, L.; Carollo, V.; Minervini, M.I.; Vizzini, G.; et al. Multidetector-row computed tomography (MDCT) for the diagnosis of hepatocellular carcinoma in cirrhotic candidates for liver transplantation: Prevalence of radiological vascular patterns and histological correlation with liver explants. Eur. Radiol. 2010, 20, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Hussain, H.K.; Johnson, T.D.; Weadock, W.J.; Pelletier, S.J.; Marrero, J.A. Value of delayed hypointensity and delayed enhancing rim in magnetic resonance imaging diagnosis of small hepatocellular carcinoma in the cirrhotic liver. J. Magn. Reson. Imaging 2010, 32, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Choi, J.-I.; Lee, Y.J.; Park, M.Y.; Rha, S.E.; Lall, C. MRI of Small Hepatocellular Carcinoma: Typical Features Are Less Frequent Below a Size Cutoff of 1.5 cm. Am. J. Roentgenol. 2017, 208, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.J.; Karimova, E.J.; Arauz, A.R.; Saad, N.E.; Brunt, E.M.; Chapman, W.C.; Heiken, J.P. Validation of Organ Procurement and Transplant Network (OPTN)/United Network for Organ Sharing (UNOS) Criteria for Imaging Diagnosis of Hepatocellular Carcinoma. Transplantation 2013, 95, 1506–1511. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, D.; Park, C.K.; Lee, W.J.; Lim, H.K. Encapsulated hepatocellular carcinoma: CT-pathologic correlations. Eur. Radiol. 2006, 16, 2326–2333. [Google Scholar] [CrossRef]
- Iguchi, T.; Aishima, S.; Sanefuji, K.; Fujita, N.; Sugimachi, K.; Gion, T.; Taketomi, A.; Shirabe, K.; Maehara, Y.; Tsuneyoshi, M. Both Fibrous Capsule Formation and Extracapsular Penetration Are Powerful Predictors of Poor Survival in Human Hepatocellular Carcinoma: A Histological Assessment of 365 Patients in Japan. Ann. Surg. Oncol. 2009, 16, 2539–2546. [Google Scholar] [CrossRef]
- Honda, H.; Kaneko, K.; Maeda, T.; Kuroiwa, T.; Fukuya, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Takenaka, K.; Masuda, K. Small Hepatocellular Carcinoma on Magnetic Resonance Imaging: Relation of Signal Intensity to Angiographic and Clinicopathologic Findings. Investig. Radiol. 1997, 32, 161–168. [Google Scholar] [CrossRef]
- Shinmura, R.; Matsui, O.; Kobayashi, S.; Terayama, N.; Sanada, J.; Ueda, K.; Gabata, T.; Kadoya, M.; Miyayama, S. Cirrhotic Nodules: Association between MR Imaging Signal Intensity and Intranodular Blood Supply. Radiology 2005, 237, 512–519. [Google Scholar] [CrossRef]
- Honda, H.; Kaneko, K.; Kanazawa, Y.; Hayashi, T.; Fukuya, T.; Matsumata, T.; Maeda, T.; Masuda, K. MR imaging of hepatocellular carcinomas: Effect of Cu and Fe contents on signal intensity. Abdom. Imaging 1997, 22, 60–66. [Google Scholar] [CrossRef]
- Kew, M.C. Hepatic Iron Overload and Hepatocellular Carcinoma. Liver Cancer 2014, 3, 31–40. [Google Scholar] [CrossRef]
- International Consensus Group for Hepatocellular, Neoplasia Pathologic Diagnosis of Early Hepatocellular Carcinoma: A Report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology 2009, 49, 658–664. [CrossRef] [PubMed]
- Kutami, R.; Nakashima, Y.; Nakashima, O.; Shiota, K.; Kojiro, M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J. Hepatol. 2000, 33, 282–289. [Google Scholar] [CrossRef]
- Asayama, Y.; Nishie, A.; Ishigami, K.; Ushijima, Y.; Takayama, Y.; Okamoto, D.; Fujita, N.; Kubo, Y.; Aishima, S.; Yoshizumi, T.; et al. Fatty change in moderately and poorly differentiated hepatocellular carcinoma on MRI: A possible mechanism related to decreased arterial flow. Clin. Radiol. 2016, 71, 1277–1283. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, C.S.; Han, Y.M.; Lee, Y.H. Detection of liver malignancy with gadoxetic acid-enhanced MRI: Is addition of diffusion-weighted MRI beneficial? Clin. Radiol. 2011, 66, 489–496. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, A.M.; Catalano, M.; Pompili, M.; Marini, M.G.; Rodríguez Carnero, P.; Gullì, C.; Infante, A.; Iezzi, R.; Ponziani, F.R.; Cerrito, L.; et al. Critical analysis of major and ancillary features of LI-RADS v2018 in the differentiation of small (≤ 2 cm) hepatocellular carcinoma from dysplastic nodules with gadobenate dimeglumine-enhanced magnetic resonance imaging. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7786–7801. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Chuma, M.; Hige, S.; Omatsu, T.; Yokoo, H.; Nakanishi, K.; Kamiyama, T.; Kubota, K.; Haga, H.; Matsuno, Y.; et al. Relationship Between Diffusion-Weighted Magnetic Resonance Imaging and Histological Tumor Grading of Hepatocellular Carcinoma. Ann. Surg. Oncol. 2012, 19, 1302–1309. [Google Scholar] [CrossRef]
- Lim, K.S. Diffusion-weighted MRI of hepatocellular carcinoma in cirrhosis. Clin. Radiol. 2014, 69, 1–10. [Google Scholar] [CrossRef]
- Piana, G.; Trinquart, L.; Meskine, N.; Barrau, V.; Beers, B.V.; Vilgrain, V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J. Hepatol. 2011, 55, 126–132. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, Y.K.; Lee, M.W.; Lee, W.J.; Kim, Y.-S.; Kim, S.H.; Choi, D.; Rhim, H. Small Hepatocellular Carcinomas: Improved Sensitivity by Combining Gadoxetic Acid–enhanced and Diffusion-weighted MR Imaging Patterns. Radiology 2012, 264, 761–770. [Google Scholar] [CrossRef]
- Park, M.-S.; Kim, S.; Patel, J.; Hajdu, C.H.; Do, R.K.G.; Mannelli, L.; Babb, J.; Taouli, B. Hepatocellular carcinoma: Detection with diffusion-weighted versus contrast-enhanced magnetic resonance imaging in pretransplant patients. Hepatology 2012, 56, 140–148. [Google Scholar] [CrossRef]
- Kitao, A.; Matsui, O.; Yoneda, N.; Kozaka, K.; Shinmura, R.; Koda, W.; Kobayashi, S.; Gabata, T.; Zen, Y.; Yamashita, T.; et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: Correlation with gadoxetic acid enhanced MR imaging. Eur. Radiol. 2011, 21, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Izumi, N.; Kokudo, N.; Matsui, O.; Sakamoto, M.; Nakashima, O.; Kojiro, M.; Makuuchi, M. Management of Hepatocellular Carcinoma in Japan: Consensus-Based Clinical Practice Guidelines Proposed by the Japan Society of Hepatology (JSH) 2010 Updated Version. Dig. Dis. 2011, 29, 339–364. [Google Scholar] [CrossRef]
- Zech, C.J.; Ba-Ssalamah, A.; Berg, T.; Chandarana, H.; Chau, G.-Y.; Grazioli, L.; Kim, M.-J.; Lee, J.M.; Merkle, E.M.; Murakami, T.; et al. Consensus report from the 8th International Forum for Liver Magnetic Resonance Imaging. Eur. Radiol. 2020, 30, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yang, T.; Huang, Z.; Long, L.; Zhou, Z.; Li, W.; Gao, Y.; Wang, M.; Zhang, X. Hepatocellular carcinoma grading and recurrence prediction using T1 mapping on gadolinium-ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. Oncol. Lett. 2019, 18, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Mulé, S.; Chalaye, J.; Legou, F.; Tenenhaus, A.; Calderaro, J.; Galletto Pregliasco, A.; Laurent, A.; Kharrat, R.; Amaddeo, G.; Regnault, H.; et al. Hepatobiliary MR contrast agent uptake as a predictive biomarker of aggressive features on pathology and reduced recurrence-free survival in resectable hepatocellular carcinoma: Comparison with dual-tracer 18F-FDG and 18F-FCH PET/CT. Eur. Radiol. 2020, 30, 5348–5357. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Z.; Zhang, Y.; Lin, N.; Xiong, M.; Huang, X.; Chen, Q.; Cao, D. Hepatocellular Carcinoma: Retrospective Evaluation of the Correlation Between Gadobenate Dimeglumine–Enhanced Magnetic Resonance Imaging and Pathologic Grade. J. Comput. Assist. Tomogr. 2018, 42, 365–372. [Google Scholar] [CrossRef]
- Kitao, A.; Matsui, O.; Yoneda, N.; Kozaka, K.; Kobayashi, S.; Koda, W.; Inoue, D.; Ogi, T.; Yoshida, K.; Gabata, T. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: Molecular and genetic background. Eur. Radiol. 2020, 30, 3438–3447. [Google Scholar] [CrossRef]
- Yoneda, N.; Matsui, O.; Kitao, A.; Kita, R.; Kozaka, K.; Koda, W.; Kobayashi, S.; Gabata, T.; Ikeda, H.; Nakanuma, Y. Hypervascular hepatocellular carcinomas showing hyperintensity on hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging: A possible subtype with mature hepatocyte nature. Jpn. J. Radiol. 2013, 31, 480–490. [Google Scholar] [CrossRef]
- Aoki, T.; Nishida, N.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Yamada, A.; et al. Higher Enhancement Intrahepatic Nodules on the Hepatobiliary Phase of Gd-EOB-DTPA-Enhanced MRI as a Poor Responsive Marker of Anti-PD-1/PD-L1 Monotherapy for Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10, 615–628. [Google Scholar] [CrossRef]
- Kudo, M. Gd-EOB-DTPA-MRI Could Predict WNT/β-Catenin Mutation and Resistance to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma. Liver Cancer 2020, 9, 479–490. [Google Scholar] [CrossRef]
- Huang, M.; Liao, B.; Xu, P.; Cai, H.; Huang, K.; Dong, Z.; Xu, L.; Peng, Z.; Luo, Y.; Zheng, K.; et al. Prediction of Microvascular Invasion in Hepatocellular Carcinoma: Preoperative Gd-EOB-DTPA-Dynamic Enhanced MRI and Histopathological Correlation. Contrast Media Mol. Imaging 2018, 2018, 9674565. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.H.; Lee, J.E.; Sinn, D.H.; Park, C.K. Preoperative gadoxetic acid–enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J. Hepatol. 2017, 67, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Cannella, R.; Sartoris, R.; Grégory, J.; Garzelli, L.; Vilgrain, V.; Ronot, M.; Dioguardi Burgio, M. Quantitative magnetic resonance imaging for focal liver lesions: Bridging the gap between research and clinical practice. Br. J. Radiol. 2021, 94, 20210220. [Google Scholar] [CrossRef]
- Ippolito, D.; Inchingolo, R.; Grazioli, L.; Drago, S.G.; Nardella, M.; Gatti, M.; Faletti, R. Recent advances in non-invasive magnetic resonance imaging assessment of hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 2413–2426. [Google Scholar] [CrossRef]
- Khalifa, F.; Soliman, A.; El-Baz, A.; Abou El-Ghar, M.; El-Diasty, T.; Gimel’farb, G.; Ouseph, R.; Dwyer, A.C. Models and methods for analyzing DCE-MRI: A review: Models and methods for analyzing DCE-MRI. Med. Phys. 2014, 41, 124301. [Google Scholar] [CrossRef] [PubMed]
- Shukla-Dave, A.; Obuchowski, N.A.; Chenevert, T.L.; Jambawalikar, S.; Schwartz, L.H.; Malyarenko, D.; Huang, W.; Noworolski, S.M.; Young, R.J.; Shiroishi, M.S.; et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J. Magn. Reson. Imaging 2019, 49, e101–e121. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Xia, C.; Huang, Z.; Zuo, P.; Stemmer, A.; Song, B. Quantitative free-breathing dynamic contrast-enhanced MRI in hepatocellular carcinoma using gadoxetic acid: Correlations with Ki67 proliferation status, histological grades, and microvascular density. Abdom. Radiol. 2018, 43, 1393–1403. [Google Scholar] [CrossRef]
- Weiss, J.; Ruff, C.; Grosse, U.; Grözinger, G.; Horger, M.; Nikolaou, K.; Gatidis, S. Assessment of Hepatic Perfusion Using GRASP MRI: Bringing Liver MRI on a New Level. Investig. Radiol. 2019, 54, 737–743. [Google Scholar] [CrossRef]
- Izuishi, K.; Yamamoto, Y.; Mori, H.; Kameyama, R.; Fujihara, S.; Masaki, T.; Suzuki, Y. Molecular mechanisms of [18F]fluorodeoxyglucose accumulation in liver cancer. Oncol. Rep. 2014, 31, 701–706. [Google Scholar] [CrossRef]
- Signore, G.; Nicod-Lalonde, M.; Prior, J.O.; Bertagna, F.; Muoio, B.; Giovanella, L.; Furlan, C.; Treglia, G. Detection rate of radiolabelled choline PET or PET/CT in hepatocellular carcinoma: An updated systematic review and meta-analysis. Clin. Transl. Imaging 2019, 7, 237–253. [Google Scholar] [CrossRef]
- Chalaye, J.; Costentin, C.E.; Luciani, A.; Amaddeo, G.; Ganne-Carrié, N.; Baranes, L.; Allaire, M.; Calderaro, J.; Azoulay, D.; Nahon, P.; et al. Positron emission tomography/computed tomography with 18F-fluorocholine improve tumor staging and treatment allocation in patients with hepatocellular carcinoma. J. Hepatol. 2018, 69, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Lièvre, M.-A.; Franco, D.; Gervais, P.; Kuhnast, B.; Agostini, H.; Marthey, L.; Désarnaud, S.; Helal, B.-O. Diagnostic value of combining 11C-choline and 18F-FDG PET/CT in hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 852–859. [Google Scholar] [CrossRef]
- Ghidaglia, J.; Golse, N.; Pascale, A.; Sebagh, M.; Besson, F.L. 18F-FDG /18F-Choline Dual-Tracer PET Behavior and Tumor Differentiation in HepatoCellular Carcinoma. A Systematic Review. Front. Med. 2022, 9, 924824. [Google Scholar] [CrossRef]
- Gougelet, A.; Sartor, C.; Senni, N.; Calderaro, J.; Fartoux, L.; Lequoy, M.; Wendum, D.; Talbot, J.-N.; Prignon, A.; Chalaye, J.; et al. Hepatocellular Carcinomas with Mutational Activation of Beta-Catenin Require Choline and Can Be Detected by Positron Emission Tomography. Gastroenterology 2019, 157, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, J.Y.; Joung, J.-G.; Joh, J.-W.; Kim, J.M.; Hyun, S.H. Metabolism-Associated Gene Signatures for FDG Avidity on PET/CT and Prognostic Validation in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 845900. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, A.; De Lucia, D.R.; Pontillo, G.; Gatti, M.; Cocozza, S.; Ugga, L.; Cuocolo, R. State of the Art in Artificial Intelligence and Radiomics in Hepatocellular Carcinoma. Diagnostics 2021, 11, 1194. [Google Scholar] [CrossRef]
- Feng, B.; Ma, X.-H.; Wang, S.; Cai, W.; Liu, X.-B.; Zhao, X.-M. Application of artificial intelligence in preoperative imaging of hepatocellular carcinoma: Current status and future perspectives. World J. Gastroenterol. 2021, 27, 5341–5350. [Google Scholar] [CrossRef]
- Yao, S.; Ye, Z.; Wei, Y.; Jiang, H.-Y.; Song, B. Radiomics in hepatocellular carcinoma: A state-of-the-art review. World J. Gastrointest. Oncol. 2021, 13, 1599–1615. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, S.H.; Kim, S.Y.; Kim, M.-J.; Lee, S.S.; Byun, J.H. Gadoxetic Acid–enhanced MRI of Hepatocellular Carcinoma: Value of Washout in Transitional and Hepatobiliary Phases. Radiology 2019, 291, 651–657. [Google Scholar] [CrossRef]
- Joo, I.; Lee, J.M.; Lee, D.H.; Jeon, J.H.; Han, J.K. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: Can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur. Radiol. 2019, 29, 1724–1732. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Meyer, M.; Choudhoury, K.R.; Gonzáles, F.; Schwartz, F.R.; Gupta, R.T.; Bashir, M.R.; Furlan, A.; Marin, D. LI-RADS: Diagnostic Performance of Hepatobiliary Phase Hypointensity and Major Imaging Features of LR-3 and LR-4 Lesions Measuring 10–19 mm With Arterial Phase Hyperenhancement. Am. J. Roentgenol. 2019, 213, W57–W65. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Baglieri, J.; Brenner, D.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef]
- Bengtsson, B.; Widman, L.; Wahlin, S.; Stål, P.; Björkström, N.K.; Hagström, H. The risk of hepatocellular carcinoma in cirrhosis differs by etiology, age and sex: A Swedish nationwide population-based cohort study. UEG J. 2022, 10, 465–476. [Google Scholar] [CrossRef]
- Sharma, S.A.; Kowgier, M.; Hansen, B.E.; Brouwer, W.P.; Maan, R.; Wong, D.; Shah, H.; Khalili, K.; Yim, C.; Heathcote, E.J.; et al. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J. Hepatol. 2018, 68, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Madhoun, M.F.; Fazili, J.; Bader, T.; Roberts, D.N.; Bright, B.C.; Bronze, M.S. Hepatitis C Prevalence in Patients with Hepatocellular Carcinoma Without Cirrhosis. Am. J. Med. Sci. 2010, 339, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Saba, L.; Bosco, S.; Rossi, M.; Miles, K.A.; Di Miscio, R.; Lombardo, C.V.; Tamponi, E.; Piga, M.; Catalano, C. Hepatocellular carcinoma (HCC) in non-cirrhotic liver: Clinical, radiological and pathological findings. Eur. Radiol. 2014, 24, 1446–1454. [Google Scholar] [CrossRef]
- Jamwal, R.; Krishnan, V.; Kushwaha, D.S.; Khurana, R. Hepatocellular carcinoma in non-cirrhotic versus cirrhotic liver: A clinico-radiological comparative analysis. Abdom. Radiol. 2020, 45, 2378–2387. [Google Scholar] [CrossRef]
- Al-Sharhan, F.; Dohan, A.; Barat, M.; Feddal, A.; Terris, B.; Pol, S.; Mallet, V.; Soyer, P. MRI presentation of hepatocellular carcinoma in non-alcoholic steatohepatitis (NASH). Eur. J. Radiol. 2019, 119, 108648. [Google Scholar] [CrossRef]
- Thompson, S.M.; Garg, I.; Ehman, E.C.; Sheedy, S.P.; Bookwalter, C.A.; Carter, R.E.; Roberts, L.R.; Venkatesh, S.K. Non-alcoholic fatty liver disease-associated hepatocellular carcinoma: Effect of hepatic steatosis on major hepatocellular carcinoma features at MRI. Br. J. Radiol. 2018, 91, 20180345. [Google Scholar] [CrossRef]
- Gawrieh, S.; Dakhoul, L.; Miller, E.; Scanga, A.; deLemos, A.; Kettler, C.; Burney, H.; Liu, H.; Abu-Sbeih, H.; Chalasani, N.; et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: A United States multicentre study. Aliment Pharm. 2019, 50, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Bouda, D.; Barrau, V.; Raynaud, L.; Dioguardi Burgio, M.; Paulatto, L.; Roche, V.; Sibert, A.; Moussa, N.; Vilgrain, V.; Ronot, M. Factors Associated with Tumor Progression After Percutaneous Ablation of Hepatocellular Carcinoma: Comparison Between Monopolar Radiofrequency and Microwaves. Results of a Propensity Score Matching Analysis. Cardiovasc Interv. Radiol. 2020, 43, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, T.; He, J.-t.; Shao, H. TACE combined with microwave ablation therapy vs. TACE alone for treatment of early- and intermediate-stage hepatocellular carcinomas larger than 5 cm: A meta-analysis. Diagn. Interv. Radiol. 2020, 26, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.D.; Rizzo, A.; Bonucci, C.; Tavolari, S.; Palloni, A.; Frega, G.; Mollica, V.; Tober, N.; Mazzotta, E.; Felicani, C.; et al. The (Eternal) Debate on Microwave Ablation Versus Radiofrequency Ablation in BCLC-A Hepatocellular Carcinoma. In Vivo 2020, 34, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.-L.; Zheng, J.-P.; Guo, L.-W.; Chen, Y.-T.; Zeng, H.; Yao, Z. Evaluation of efficacy of transcatheter arterial chemoembolization combined with computed tomography-guided radiofrequency ablation for hepatocellular carcinoma using magnetic resonance diffusion weighted imaging and computed tomography perfusion imaging: A prospective study. Medicine 2017, 96, e5518. [Google Scholar] [CrossRef]
- Tan, W.; Deng, Q.; Lin, S.; Wang, Y.; Xu, G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2019, 36, 263–271. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Yang, W.; Hu, K.; Xie, H.; Hu, K.-Q.; Bai, W.; Dong, Z.; Lu, Y.; Zeng, Z.; et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma: HEPATOLOGY, Vol. 00, No. X, 2014. Hepatology 2015, 61, 1579–1590. [Google Scholar] [CrossRef]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Gerena, M.; Molvar, C.; Masciocchi, M.; Nandwana, S.; Sabottke, C.; Spieler, B.; Sharma, R.; Tsai, L.; Kielar, A. LI-RADS treatment response assessment of combination locoregional therapy for HCC. Abdom. Radiol. 2021, 46, 3634–3647. [Google Scholar] [CrossRef]
- Ahmed, O.; Pillai, A. Hepatocellular Carcinoma: A Contemporary Approach to Locoregional Therapy. Am. J. Gastroenterol. 2020, 115, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.S.; Tantawy, W.; Abbas, Y.A. MRI assessment of hepatocellular carcinoma after locoregional therapy. Insights Imaging 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Kielar, A.; Fowler, K.J.; Lewis, S.; Yaghmai, V.; Miller, F.H.; Yarmohammadi, H.; Kim, C.; Chernyak, V.; Yokoo, T.; Meyer, J.; et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom. Radiol. 2018, 43, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta-Lala, M.; Masch, W.R.; Shampain, K.; Zhang, A.; Jo, A.S.; Moorman, S.; Aslam, A.; Maturen, K.E.; Davenport, M.S. MRI Assessment of Hepatocellular Carcinoma after Local-Regional Therapy: A Comprehensive Review. Radiol. Imaging Cancer 2020, 2, e190024. [Google Scholar] [CrossRef]
- Kloeckner, R.; Galle, P.R.; Bruix, J. Local and Regional Therapies for Hepatocellular Carcinoma. Hepatology 2021, 73, 137–149. [Google Scholar] [CrossRef]
- Huber, T.C.; Bochnakova, T.; Koethe, Y.; Park, B.; Farsad, K. Percutaneous Therapies for Hepatocellular Carcinoma: Evolution of Liver Directed Therapies. J. Hepatocell. Carcinoma 2021, 8, 1181–1193. [Google Scholar] [CrossRef]
- Young, S.; Taylor, A.J.; Sanghvi, T. Post Locoregional Therapy Treatment Imaging in Hepatocellular Carcinoma Patients: A Literature-based Review. J. Clin. Transl. Hepatol. 2018, 6, 189–197. [Google Scholar] [CrossRef]
- Park, M.-h.; Rhim, H.; Kim, Y.-s.; Choi, D.; Lim, H.K.; Lee, W.J. Spectrum of CT Findings after Radiofrequency Ablation of Hepatic Tumors. RadioGraphics 2008, 28, 379–390. [Google Scholar] [CrossRef]
- Crocetti, L.; Della Pina, C.; Cioni, D.; Lencioni, R. Peri-intraprocedural imaging: US, CT, and MRI. Abdom. Imaging 2011, 36, 648–660. [Google Scholar] [CrossRef]
- Guan, Y.-S. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J. Gastroenterol. 2004, 10, 3543. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Katayama, K.; Hori, M.; Yakushijin, T.; Fujimoto, K.; Itoh, T.; Igura, T.; Sakakibara, M.; Takamura, M.; Tsurusaki, M.; et al. Prospective Comparison of Gd-EOB-DTPA-Enhanced MRI with Dynamic CT for Detecting Recurrence of HCC after Radiofrequency Ablation. Liver Cancer 2017, 6, 349–359. [Google Scholar] [CrossRef]
- Ippolito, D.; Bonaffini, P.A.; Capraro, C.; Leni, D.; Corso, R.; Sironi, S. Viable residual tumor tissue after radiofrequency ablation treatment in hepatocellular carcinoma: Evaluation with CT perfusion. Abdom. Imaging 2013, 38, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, D.E.; Kernagis, L.Y.; Soulen, M.C.; Geschwind, J.-F.H. Chemoembolization of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2002, 13, S211–S221. [Google Scholar] [CrossRef]
- Bolondi, L.; Burroughs, A.; Dufour, J.-F.; Galle, P.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.; Sangro, B. Heterogeneity of Patients with Intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a Subclassification to Facilitate Treatment Decisions. Semin. Liver Dis. 2013, 32, 348–359. [Google Scholar] [CrossRef]
- Willatt, J.; Ruma, J.A.; Azar, S.F.; Dasika, N.L.; Syed, F. Imaging of hepatocellular carcinoma and image guided therapies—How we do it. Cancer Imaging 2017, 17, 9. [Google Scholar] [CrossRef]
- Vossen, J.A.; Buijs, M.; Kamel, I.R. Assessment of Tumor Response on MR Imaging After Locoregional Therapy. Tech. Vasc. Interv. Radiol. 2006, 9, 125–132. [Google Scholar] [CrossRef]
- Lim, H.S.; Jeong, Y.Y.; Kang, H.K.; Kim, J.K.; Park, J.G. Imaging Features of Hepatocellular Carcinoma After Transcatheter Arterial Chemoembolization and Radiofrequency Ablation. Am. J. Roentgenol. 2006, 187, W341–W349. [Google Scholar] [CrossRef]
- Agnello, F. Imaging appearance of treated hepatocellular carcinoma. World J. Hepatol. 2013, 5, 417. [Google Scholar] [CrossRef]
- Chiu, R.Y.W.; Yap, W.W.; Patel, R.; Liu, D.; Klass, D.; Harris, A.C. Hepatocellular Carcinoma Post Embolotherapy: Imaging Appearances and Pitfalls on Computed Tomography and Magnetic Resonance Imaging. Can. Assoc. Radiol. J. 2016, 67, 158–172. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, J.-M.; He, C.; Li, Z.-F.; Xu, Y.-S.; Li, Z.; Liu, H.-F.; Lei, J.-Q. Utility of diffusion weighted imaging with the quantitative apparent diffusion coefficient in diagnosing residual or recurrent hepatocellular carcinoma after transarterial chemoembolization: A meta-analysis. Cancer Imaging 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, D.-Q.; He, W.; Zhang, B.-F.; Zhao, L.-Q. Computed tomography perfusion in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma. World J. Gastroenterol. 2008, 14, 5738. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, X.; Tan, B.; Liu, M.; Dong, G.; Zhai, Z. Computed Tomographic Perfusion Imaging for the Therapeutic Response of Chemoembolization for Hepatocellular Carcinoma. J. Comput. Assist. Tomogr. 2012, 36, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Fior, D.; Franzesi, C.T.; Capraro, C.; Casiraghi, A.; Leni, D.; Vacirca, F.; Corso, R.; Sironi, S. Tumour-related neoangiogenesis: Functional dynamic perfusion computed tomography for diagnosis and treatment efficacy assessment in hepatocellular carcinoma. Dig. Liver Dis. 2014, 46, 916–922. [Google Scholar] [CrossRef]
- Syha, R.; Gatidis, S.; Grözinger, G.; Grosse, U.; Maurer, M.; Zender, L.; Horger, M.; Nikolaou, K.; Ketelsen, D. C-arm computed tomography and volume perfusion computed tomography (VPCT)-based assessment of blood volume changes in hepatocellular carcinoma in prediction of midterm tumor response to transarterial chemoembolization: A single center retrospective trial. Cancer Imaging 2016, 16, 30. [Google Scholar] [CrossRef]
- Rathmann, N.; Kara, K.; Budjan, J.; Henzler, T.; Smakic, A.; Schoenberg, S.O.; Diehl, S.J. Parenchymal Liver Blood Volume and Dynamic Volume Perfusion CT Measurements of Hepatocellular Carcinoma in Patients Undergoing Transarterial Chemoembolization. Anticancer Res. 2017, 37, 5681–5685. [Google Scholar] [CrossRef]
- Su, T.-H.; He, W.; Jin, L.; Chen, G.; Xiao, G.-W. Early Response of Hepatocellular Carcinoma to Chemoembolization: Volume Computed Tomography Liver Perfusion Imaging as a Short-Term Response Predictor. J. Comput. Assist. Tomogr. 2017, 41, 315–320. [Google Scholar] [CrossRef]









| Advantages | Disadvantages |
|---|---|
|
|
| Feature | Recommendation |
|---|---|
| CT Scanner Configuration | ≥8-row multidetector CT |
| Slice Thickness | 2–5 mm |
| Multiplanar Reformations | Suggested coronal and sagittal planes |
| Non-contrast Imaging | Suggested for initial diagnosis Required for patients with prior locoregional therapy |
| Dynamic Contrast-Enhanced Phases | Late Arterial Phase Portal-Venous Delayed Phase |
| Contrast Administration | ≥300 mgI/mL for a dose of 1.5–2 mL/kg body weight (521–647 mgI/kg) Injection rate ≥ 3 mL/s Saline chaser bolus (30–50 mL) |
 |
 Observations in this cell are categorized based on one additional major feature:
Observations in this cell are categorized based on one additional major feature: - LR-4 if enhancing “capsule”
- LR-5 if nonperipheral “washout” OR threshold growth
| Method | Late Arterial Phase | Portal Venous Phase | Delayed Phase |
|---|---|---|---|
| Bolus-Tracking Individualized scan delay | Image acquisition: 10–30 s after aortic threshold density of 100–150 HU | 60–80 s after start of injection or 45–60 s after aortic threshold | 3–5 min after start of injection |
| Test-Bolus Individualized scan delay | Image acquisition: 10–20 s after peaking aortic enhancement | 60–80 s after start of injection | 3–5 min after start of injection |
| Fixed Scan Delay Alternatively for young patients with no comorbidities | 35–45 s after start of injection | 60–80 s after start of injection | 3–5 min after start of injection |
| Lesion. | Comments | Imaging Features |
|---|---|---|
| Vascular Pseudolesions | Attributable to arterioportal shunts Particular common in cirrhotic livers | Peripheral, round, or wedge shaped APHE nodules Isodense on PVP |
| Hemangiomas | Rarely encountered Most demonstrate fibrotic involution (“sclerosed” hemangiomas) | “Sclerosed” hemangiomas demonstrate rim APHE Mimic non-HCC malignancies (~6% of LR-M observations) |
| FNH-like nodules | Particularly common in alcoholic cirrhosis SAA-HN-variant is potentially malignant | Nodules with APHE Isodense or “washout” on PVP |
| HGDN | Rarely depicted on MDCT Mimic early HCC | May demonstrate non-rim APHE and become isodense on PVP or depicted only as hypodense nodule on PVP |
| ICC | Comprise 10–15% of cancers in cirrhotic liver | Small ICCs (<3 cm) frequently demonstrate atypical enhancement pattern with global APHE and “washout” or isodensity on PVP |
| cHCC-CCA | Account for <5% of liver cancers | No constant imaging features Commonly have targetoid appearance but may also mimic HCC |
| Hypervascular Metastases | Very rarely encountered due to unfavorable microenvironment & altered portal venous flow | Lesions with APHE Isodense or “washout” on PVP |
| Parameter (Unit) | Definition/Biological Significance | Expected Change in HCC |
|---|---|---|
| CT Liver Perfusion/DCE-MRI Perfusion parameters based on pharmacokinetic models (model-based approach) | [86,87,88,89]/[90,91,92,93] | |
| Blood Flow, or Total Perfusion (mL/100 g/min) | Total flow rate of blood in liver tissue. Reflects hypervascularity. | ↑↑ |
| Blood Volume (mL/100 g) | Intravascular blood volume. Reflects hypervascularity. | ↑↑ |
| Mean Transit Time (s) | Residence time of contrast agent in tissue. Shorter MTT might suggest hypervascularity and presence of intratumoral arteriovenous shunts. | ↓ |
| Hepatic Arterial Blood Flow, or Arterial Liver Perfusion (mL/100 g/min) | Blood flow derived from hepatic artery. High in lesions with a predominant hepatic arterial supply. | ↑↑ |
| Portal Liver Perfusion (mL/100 g/min) | Blood flow derived from portal vein. High in normal liver tissue. Low in lesions with a predominant hepatic arterial supply. | ↓↓ |
| Hepatic Arterial Fraction, or Hepatic Perfusion Index (%) | Percentage of blood input contributed by hepatic artery. Low in normal liver tissue. Increased in lesions with arterioportal imbalance. | ↑↑ |
| Permeability Surface area product (mL/100 g/min) | Reflects leakage rate of blood from vascular into interstitial space. Virtually zero in normal liver parenchyma where fenestrated sinusoids permit free communication between the intravascular and interstitial space. Countable in liver fibrosis and liver tumors. | ↑ |
| Ktrans (s−1) | Transfer constant from plasma to interstitial space. Reported in studies that employ single-input dual-compartment models. Related to vessel permeability. | ↓/↑ |
| Kep (s−1) | Reflux constant from interstitial space to plasma. Reported in studies that employ single-input dual-compartment models. Inverse relation to Ktrans. | ↑ |
| ve (%) | Extra-vascular extra-cellular volume fraction. Related to cell density. | ↓ |
| Descriptive perfusion parameters (model-free approach) | ||
| Area Under the Curve (unitless) | Area under pixel density curve. High in lesions with vivid enhancement. | ↑↑ |
| Slope of Increase, or wash-in (s−1) | Running average of the slope of the tissue density—time curve *. High in hypervascular tumors with rapid and vivid enhancement. | ↑↑ |
| Slope of Decrease, or Wash-out (s−1) | The slope of the line connecting the point of maximum enhancement and the last point of the tissue density-time curve *. Low in lesions that display washout. | ↓↓ |
| Time to peak (s) | Time interval between onset of afferent vessel enhancement and peak of the tissue density-time curve *. Short in hypervascular lesions with rapid enhancement. | ↓↓ |
| Positive Enhancement Integral (%) | The area under the tissue density curve in each tissue voxel, divided by the area under the curve corresponding to a reference vein ROI. | ↑ |
| CT Liver Perfusion/ DCE-MRI Parameter | Reported Behavior in Common Focal Liver Lesions | ||||
|---|---|---|---|---|---|
| HCC | Hemangioma | Hypovascular Metastasis | Hypervascular Metastasis | Arterioportal Shunt | |
| [98,99,100]/[105,106] | [101,102,103]/[107,108] | [101,102,103] | [104] | ||
| Blood Flow, or Total Perfusion | ↑↑ | ↑↑↑ | ↓ | ↑↑↑ | ↑↑ |
| Blood Volume | ↑↑ | ↑↑↑ | ↑↑ | ↑↑↑ | - |
| Mean Transit Time | ↓ | ↓↓ | ↑↑ | ↓↓↓ | - |
| Hepatic Arterial Blood Flow, or Arterial Liver Perfusion | ↑↑ | ↑↑↑ | ↑ | ↑↑ * | ↑↑ |
| Portal Liver Perfusion | ↓↓ | ↓ | ↓ | ↓↓ * | ↓ |
| Hepatic Arterial Fraction, or Hepatic Perfusion Index | ↑↑ | ↑↑↑ | ↑ | ↑↑ * | ↑↑ |
| Permeability Surface area product | ↑ | ↑↑ | ↓/↑ | ↑ * | - |
| Slope of Increase, or wash-in | ↑↑ | ↑↑↑ | - | - | - |
| Slope of Decrease, or wash-out | ↓↓ | ↓ | - | - | - |
| Positive Enhancement Integral | ↑ | ↑↑↑ | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chartampilas, E.; Rafailidis, V.; Georgopoulou, V.; Kalarakis, G.; Hatzidakis, A.; Prassopoulos, P. Current Imaging Diagnosis of Hepatocellular Carcinoma. Cancers 2022, 14, 3997. https://doi.org/10.3390/cancers14163997
Chartampilas E, Rafailidis V, Georgopoulou V, Kalarakis G, Hatzidakis A, Prassopoulos P. Current Imaging Diagnosis of Hepatocellular Carcinoma. Cancers. 2022; 14(16):3997. https://doi.org/10.3390/cancers14163997
Chicago/Turabian StyleChartampilas, Evangelos, Vasileios Rafailidis, Vivian Georgopoulou, Georgios Kalarakis, Adam Hatzidakis, and Panos Prassopoulos. 2022. "Current Imaging Diagnosis of Hepatocellular Carcinoma" Cancers 14, no. 16: 3997. https://doi.org/10.3390/cancers14163997
APA StyleChartampilas, E., Rafailidis, V., Georgopoulou, V., Kalarakis, G., Hatzidakis, A., & Prassopoulos, P. (2022). Current Imaging Diagnosis of Hepatocellular Carcinoma. Cancers, 14(16), 3997. https://doi.org/10.3390/cancers14163997






