Temporal Heterogeneity of HER2 Expression and Spatial Heterogeneity of 18F-FDG Uptake Predicts Treatment Outcome of Pyrotinib in Patients with HER2-Positive Metastatic Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. IHC Evaluation
2.3. PET/CT Imaging
2.4. Image Interpretation
2.5. Statistical Analyses
3. Results
3.1. Patient Baseline Characteristics and Their Association with PFS
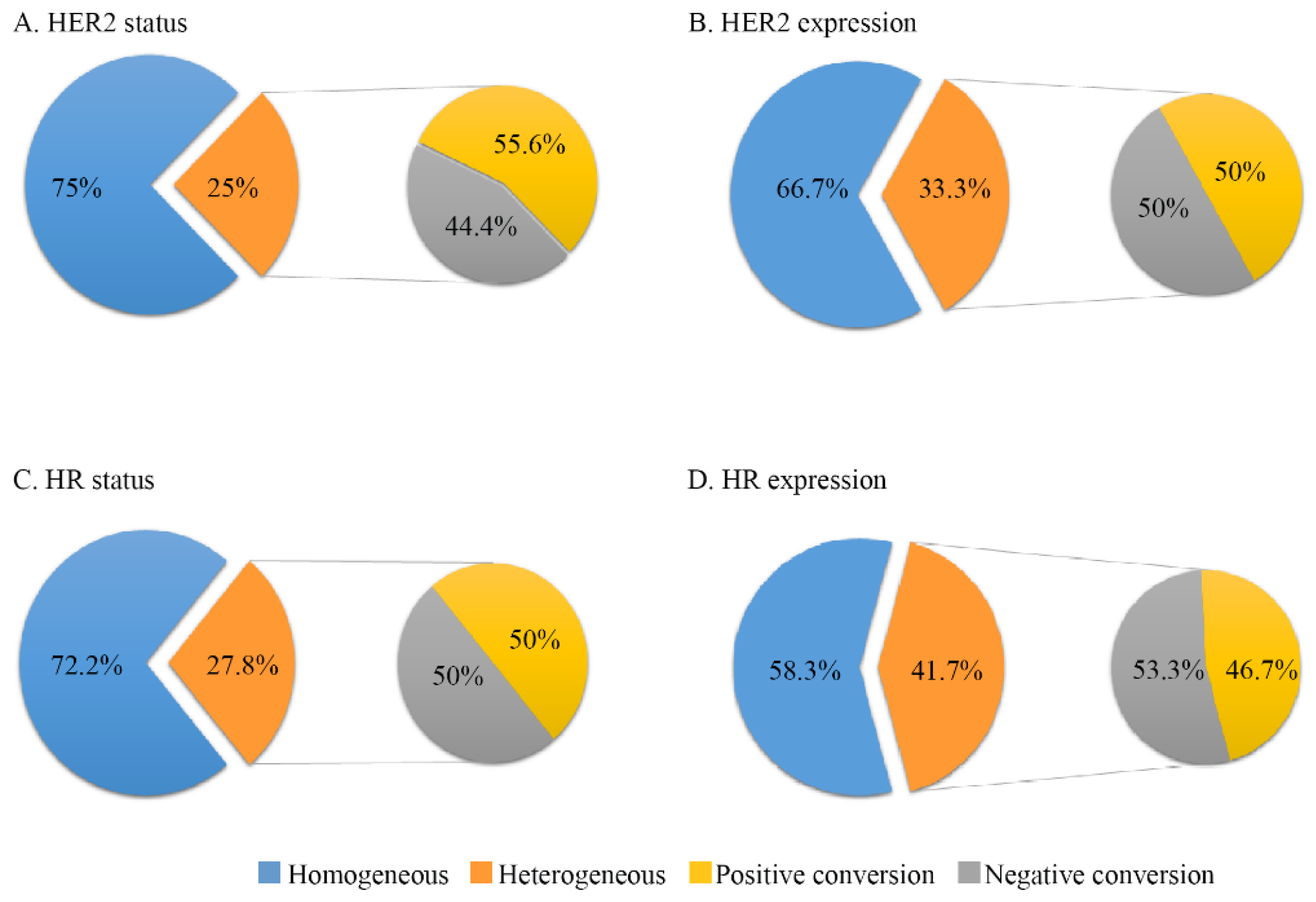
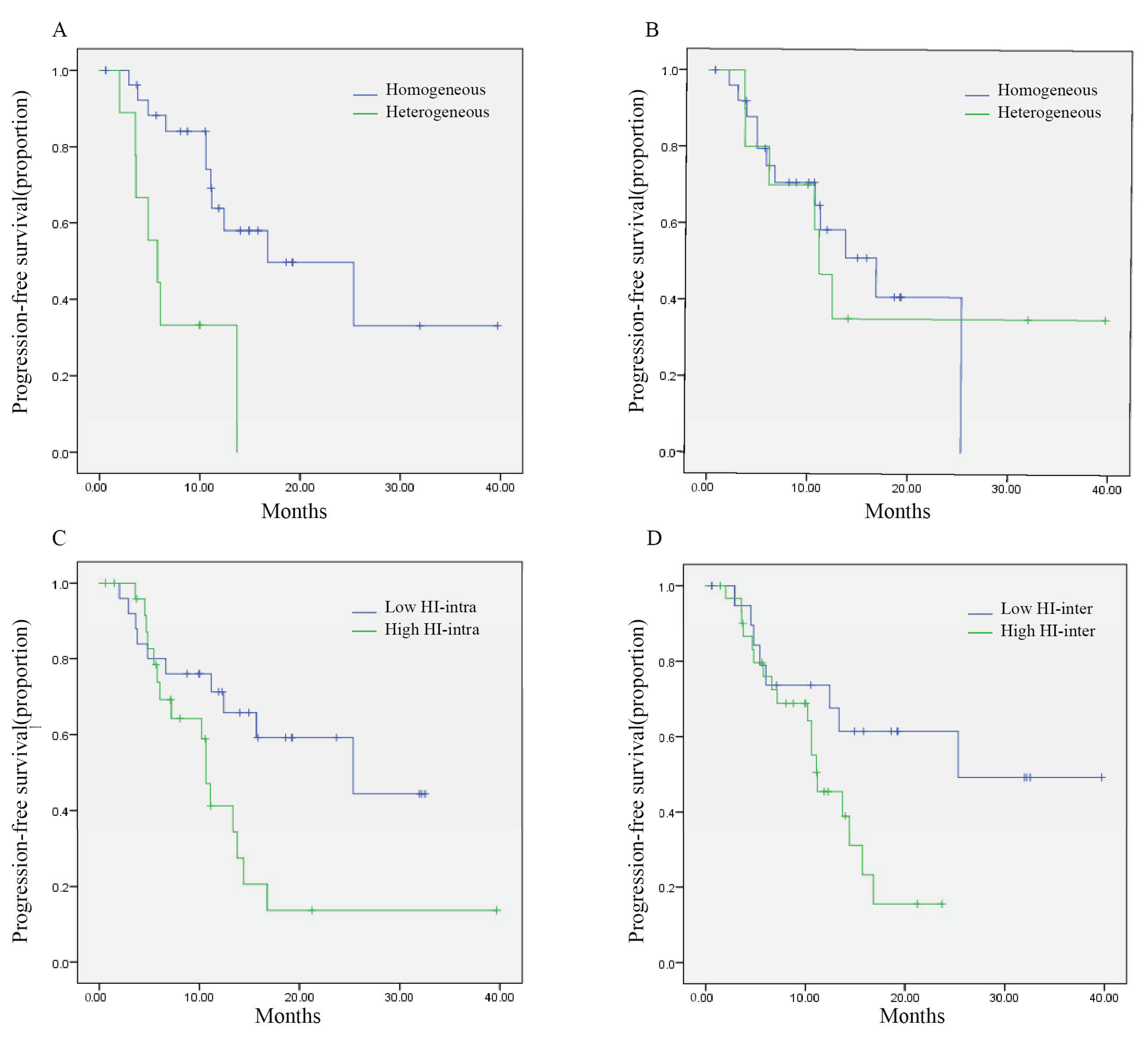
3.2. Temporal Tumor Heterogeneity and Its Association with PFS
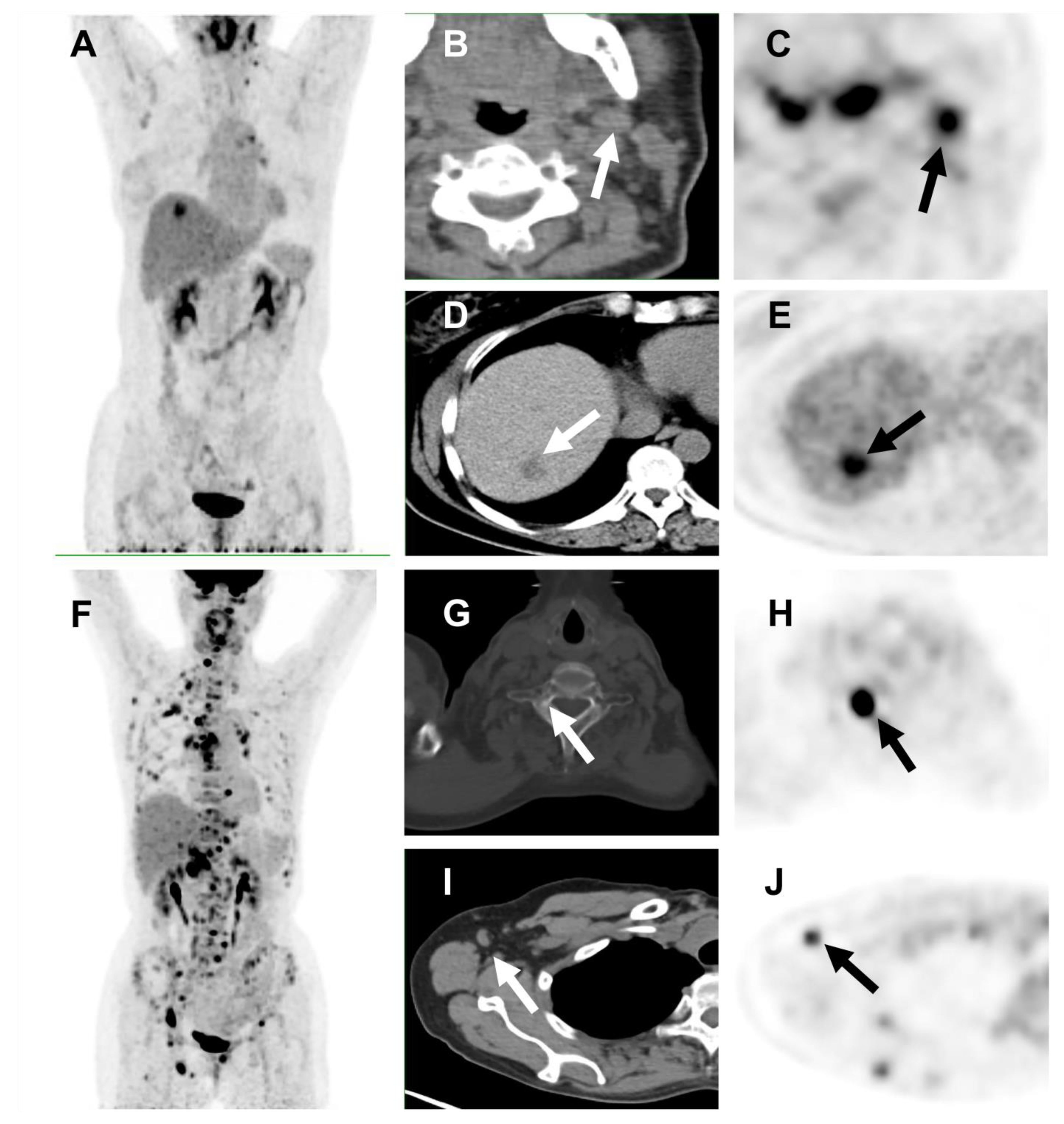
3.3. Spatial Tumor Heterogeneity and Its Association with PFS
3.4. Association between Temporal and Spatial Heterogeneity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, Z.; Tagawa, T.; Somlo, G. Human epidermal growth factor receptor family-targeted therapies in the treatment of her2-overexpressing breast cancer. Oncologist 2014, 19, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses her2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in her2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for her2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Ma, F.; Ouyang, Q.; Li, W.; Jiang, Z.; Tong, Z.; Liu, Y.; Li, H.; Yu, S.; Feng, J.; Wang, S.; et al. Pyrotinib or lapatinib combined with capecitabine in her2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: A randomized, phase ii study. J. Clin. Oncol. 2019, 37, 2610–2619. [Google Scholar] [CrossRef]
- Yan, M.; Bian, L.; Hu, X.; Zhang, Q.; Ouyang, Q.; Feng, J.; Yin, Y.; Sun, T.; Tong, Z.; Wang, X.; et al. Pyrotinib plus capecitabine for human epidermal factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (phenix): A randomized, double-blind, placebo-controlled phase 3 study. Transl. Breast Cancer Res. 2020, 1, 13. [Google Scholar] [CrossRef]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of her2-positive metastatic breast cancer (phoebe): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef]
- Li, Y.; Tong, Z.; Ouyang, Q.; Wu, X.; Li, W.; Cai, L.; Yu, Z.; Han, Z.; Wang, X.; Li, M.; et al. Abstract p2-13-40: Treatment patterns and adverse events of pyrotinib-based therapy in her2-positive breast cancer patients in china: Results from a multicenter, real-world study. Cancer Res. 2022, 82, P2-13-40. [Google Scholar]
- Chen, Q.; Ouyang, D.; Anwar, M.; Xie, N.; Wang, S.; Fan, P.; Qian, L.; Chen, G.; Zhou, E.; Guo, L.; et al. Effectiveness and safety of pyrotinib, and association of biomarker with progression-free survival in patients with her2-positive metastatic breast cancer: A real-world, multicentre analysis. Front. Oncol. 2020, 10, 811. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, M.; Zhang, J.; Wang, B.; Tao, Z.; Du, Y.; Zhang, S.; Cao, J.; Wang, L.; Hu, X. Real-world data of pyrotinib-based therapy in metastatic her2-positive breast cancer: Promising efficacy in lapatinib-treated patients and in brain metastasis. Cancer Res. Treat. 2020, 52, 1059–1066. [Google Scholar] [CrossRef]
- Shipitsin, M.; Campbell, L.L.; Argani, P.; Weremowicz, S.; Bloushtain-Qimron, N.; Yao, J.; Nikolskaya, T.; Serebryiskaya, T.; Beroukhim, R.; Hu, M.; et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 2007, 11, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Vollebergh, M.A.; de Hoon, B.; de Ronde, J.; Schouten, P.C.; Kersbergen, A.; Zander, S.A.; Pajic, M.; Jaspers, J.E.; Jonkers, M.; et al. Impact of intertumoral heterogeneity on predicting chemotherapy response of brca1-deficient mammary tumors. Cancer Res. 2012, 72, 2350–2361. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Hilton, J.; Clemons, M.; Mazzarello, S.; Hutton, B.; Haggar, F.; Addison, C.L.; Kuchuk, I.; Zhu, X.; Gelmon, K.; et al. Estrogen, progesterone, and her2/neu receptor discordance between primary and metastatic breast tumours-a review. Cancer Metastasis Rev. 2016, 35, 427–437. [Google Scholar] [CrossRef]
- Grinda, T.; Joyon, N.; Lusque, A.; Lefevre, S.; Arnould, L.; Penault-Llorca, F.; Macgrogan, G.; Treilleux, I.; Vincent-Salomon, A.; Haudebourg, J.; et al. Phenotypic discordance between primary and metastatic breast cancer in the large-scale real-life multicenter french esme cohort. NPJ Breast Cancer 2021, 7, 41. [Google Scholar] [CrossRef]
- Schrijver, W.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor conversion in distant breast cancer metastases: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef]
- Tixier, F.; Le Rest, C.C.; Hatt, M.; Albarghach, N.; Pradier, O.; Metges, J.P.; Corcos, L.; Visvikis, D. Intratumor heterogeneity characterized by textural features on baseline 18f-fdg pet images predicts response to concomitant radiochemotherapy in esophageal cancer. J. Nucl. Med. 2011, 52, 369–378. [Google Scholar] [CrossRef]
- Kang, S.R.; Song, H.C.; Byun, B.H.; Oh, J.R.; Kim, H.S.; Hong, S.P.; Kwon, S.Y.; Chong, A.; Kim, J.; Cho, S.G.; et al. Intratumoral metabolic heterogeneity for prediction of disease progression after concurrent chemoradiotherapy in patients with inoperable stage iii non-small-cell lung cancer. Nucl. Med. Mol. Imaging 2014, 48, 16–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kidd, E.A.; Grigsby, P.W. Intratumoral metabolic heterogeneity of cervical cancer. Clin. Cancer Res. 2008, 14, 5236–5241. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Park, S.; Bang, J.I.; Kim, E.K.; Lee, H.Y. Metabolic radiomics for pretreatment 18f-fdg pet/ct to characterize locally advanced breast cancer: Histopathologic characteristics, response to neoadjuvant chemotherapy, and prognosis. Sci. Rep. 2017, 7, 1556. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Kim, D.H.; Hong, C.M.; Kim, C.Y.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Prognostic implication of intratumoral metabolic heterogeneity in invasive ductal carcinoma of the breast. BMC Cancer 2014, 14, 585. [Google Scholar] [CrossRef]
- Cook, G.J.; Yip, C.; Siddique, M.; Goh, V.; Chicklore, S.; Roy, A.; Marsden, P.; Ahmad, S.; Landau, D. Are pretreatment 18f-fdg pet tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J. Nucl. Med. 2013, 54, 19–26. [Google Scholar] [CrossRef]
- Vaidya, M.; Creach, K.M.; Frye, J.; Dehdashti, F.; Bradley, J.D.; El Naqa, I. Combined pet/ct image characteristics for radiotherapy tumor response in lung cancer. Radiother. Oncol. 2012, 102, 239–245. [Google Scholar] [CrossRef]
- Watabe, T.; Tatsumi, M.; Watabe, H.; Isohashi, K.; Kato, H.; Yanagawa, M.; Shimosegawa, E.; Hatazawa, J. Intratumoral heterogeneity of f-18 fdg uptake differentiates between gastrointestinal stromal tumors and abdominal malignant lymphomas on pet/ct. Ann. Nucl. Med. 2012, 26, 222–227. [Google Scholar] [CrossRef]
- El Naqa, I.; Grigsby, P.; Apte, A.; Kidd, E.; Donnelly, E.; Khullar, D.; Chaudhari, S.; Yang, D.; Schmitt, M.; Laforest, R.; et al. Exploring feature-based approaches in pet images for predicting cancer treatment outcomes. Pattern Recognit. 2009, 42, 1162–1171. [Google Scholar] [CrossRef]
- Van Velden, F.H.; Cheebsumon, P.; Yaqub, M.; Smit, E.F.; Hoekstra, O.S.; Lammertsma, A.A.; Boellaard, R. Evaluation of a cumulative suv-volume histogram method for parameterizing heterogeneous intratumoural fdg uptake in non-small cell lung cancer pet studies. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1636–1647. [Google Scholar] [CrossRef]
- Miwa, K.; Inubushi, M.; Wagatsuma, K.; Nagao, M.; Murata, T.; Koyama, M.; Koizumi, M.; Sasaki, M. Fdg uptake heterogeneity evaluated by fractal analysis improves the differential diagnosis of pulmonary nodules. Eur. J. Radiol. 2014, 83, 715–719. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Xu, X.; Zhang, Y.; Zhang, J.; Xue, J.; Wang, M.; Yuan, H.; Hu, S.; Shi, W.; et al. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18f-fluoroestradiol pet/ct. Clin. Nucl. Med. 2017, 42, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Ma, G.; Hu, X.; Zhang, Y.; Wang, Z.; Zhang, J.; Zhao, Y.; Li, Y.; Xie, Y.; Yang, Z.; et al. Pretreatment (18)f-fdg uptake heterogeneity predicts treatment outcome of first-line chemotherapy in patients with metastatic triple-negative breast cancer. Oncologist 2018, 23, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gu, B.; Hu, X.; Zhang, Y.; Zhang, J.; Wang, Z.; Zhao, Y.; Gong, C.; Li, Y.; Yang, Z.; et al. Heterogeneity of targeted lung lesion predicts platinum-based first-line therapy outcomes and overall survival for metastatic triple-negative breast cancer patients with lung metastasis: A “pet biopsy” method. Cancer Manag. Res. 2019, 11, 6019–6027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, C.; Zhang, Y.; Gong, C.; Li, Y.; Xie, Y.; Wu, B.; Yang, Z.; Wang, B. Prognostic value of tumor heterogeneity on 18f-fdg pet/ct in hr+her2- metastatic breast cancer patients receiving 500 mg fulvestrant: A retrospective study. Sci. Rep. 2018, 8, 14458. [Google Scholar] [CrossRef]
- Shaoxian, T.; Baohua, Y.; Xiaoli, X.; Yufan, C.; Xiaoyu, T.; Hongfen, L.; Rui, B.; Xiangjie, S.; Ruohong, S.; Wentao, Y. Characterisation of gata3 expression in invasive breast cancer: Differences in histological subtypes and immunohistochemically defined molecular subtypes. J. Clin. Pathol. 2017, 70, 926–934. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Salamon, J.; Derlin, T.; Bannas, P.; Busch, J.D.; Herrmann, J.; Bockhorn, M.; Hagel, C.; Friedrich, R.E.; Adam, G.; Mautner, V.F. Evaluation of intratumoural heterogeneity on (1)(8)f-fdg pet/ct for characterization of peripheral nerve sheath tumours in neurofibromatosis type 1. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 685–692. [Google Scholar] [CrossRef]
- Tahari, A.K.; Alluri, K.C.; Quon, H.; Koch, W.; Wahl, R.L.; Subramaniam, R.M. Fdg pet/ct imaging of oropharyngeal squamous cell carcinoma: Characteristics of human papillomavirus-positive and -negative tumors. Clin. Nucl. Med. 2014, 39, 225–231. [Google Scholar] [CrossRef]
- Bundschuh, R.A.; Dinges, J.; Neumann, L.; Seyfried, M.; Zsoter, N.; Papp, L.; Rosenberg, R.; Becker, K.; Astner, S.T.; Henninger, M.; et al. Textural parameters of tumor heterogeneity in (1)(8)f-fdg pet/ct for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J. Nucl. Med. 2014, 55, 891–897. [Google Scholar] [CrossRef]
- Adams, H.; Tzankov, A.; Lugli, A.; Zlobec, I. New time-dependent approach to analyse the prognostic significance of immunohistochemical biomarkers in colon cancer and diffuse large b-cell lymphoma. J. Clin. Pathol. 2009, 62, 986–997. [Google Scholar] [CrossRef]
- Aparicio, S.; Caldas, C. The implications of clonal genome evolution for cancer medicine. N. Engl. J. Med. 2013, 368, 842–851. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Arnedos, M.; Vicier, C.; Loi, S.; Lefebvre, C.; Michiels, S.; Bonnefoi, H.; Andre, F. Precision medicine for metastatic breast cancer--limitations and solutions. Nat. Rev. Clin. Oncol. 2015, 12, 693–704. [Google Scholar] [CrossRef]
- Beca, F.; Polyak, K. Intratumor heterogeneity in breast cancer. Adv. Exp Med. Biol. 2016, 882, 169–189. [Google Scholar]
- Asselin, M.C.; O’Connor, J.P.; Boellaard, R.; Thacker, N.A.; Jackson, A. Quantifying heterogeneity in human tumours using mri and pet. Eur. J. Cancer 2012, 48, 447–455. [Google Scholar] [CrossRef]
- Liu, Z.H.; Wang, K.; Lin, D.Y.; Xu, J.; Chen, J.; Long, X.Y.; Ge, Y.; Luo, X.L.; Zhang, K.P.; Liu, Y.H.; et al. Impact of the updated 2018 asco/cap guidelines on her2 fish testing in invasive breast cancer: A retrospective study of her2 fish results of 2233 cases. Breast Cancer Res. Treat. 2019, 175, 51–57. [Google Scholar] [CrossRef]
- Bartlett, A.I.; Starcyznski, J.; Robson, T.; Maclellan, A.; Campbell, F.M.; van de Velde, C.J.; Hasenburg, A.; Markopoulos, C.; Seynaeve, C.; Rea, D.; et al. Heterogeneous her2 gene amplification: Impact on patient outcome and a clinically relevant definition. Am. J. Clin. Pathol. 2011, 136, 266–274. [Google Scholar] [CrossRef]
- Seol, H.; Lee, H.J.; Choi, Y.; Lee, H.E.; Kim, Y.J.; Kim, J.H.; Kang, E.; Kim, S.W.; Park, S.Y. Intratumoral heterogeneity of her2 gene amplification in breast cancer: Its clinicopathological significance. Mod. Pathol. 2012, 25, 938–948. [Google Scholar] [CrossRef]
- Rye, I.H.; Trinh, A.; Saetersdal, A.B.; Nebdal, D.; Lingjaerde, O.C.; Almendro, V.; Polyak, K.; Borresen-Dale, A.L.; Helland, A.; Markowetz, F.; et al. Intratumor heterogeneity defines treatment-resistant her2+ breast tumors. Mol. Oncol. 2018, 12, 1838–1855. [Google Scholar] [CrossRef]
- Filho, O.M.; Viale, G.; Stein, S.; Trippa, L.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.M.; Waks, A.G.; et al. Impact of her2 heterogeneity on treatment response of early-stage her2-positive breast cancer: Phase ii neoadjuvant clinical trial of t-dm1 combined with pertuzumab. Cancer Discov. 2021, 11, 2474–2487. [Google Scholar] [CrossRef]
- De Haas, S.L.; Hurvitz, S.A.; Martin, M.; Kiermaier, A.; Lewis Phillips, G.; Xu, J.; Helms, H.J.; Slamon, D.; Press, M.F. Abstract nr P6-07-09: Biomarker analysis from the neoadjuvant kristine study in her2-positive early breast cancer (ebc). Cancer Res. 2017, 77, P6-07. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, Y.; Li, H.; Luo, T.; Li, W.; Wang, H.; Shao, B.; Wang, B.; Ge, R. Pyrotinib combined with vinorelbine in her2-positive metastatic breast cancer: A multicenter retrospective study. Front. Oncol. 2021, 11, 664429. [Google Scholar] [CrossRef]
- Perez, E.A.; de Haas, S.L.; Eiermann, W.; Barrios, C.H.; Toi, M.; Im, Y.H.; Conte, P.F.; Martin, M.; Pienkowski, T.; Pivot, X.B.; et al. Relationship between tumor biomarkers and efficacy in marianne, a phase iii study of trastuzumab emtansine +/- pertuzumab versus trastuzumab plus taxane in her2-positive advanced breast cancer. BMC Cancer 2019, 19, 517. [Google Scholar]
- Van Raemdonck, E.; Floris, G.; Berteloot, P.; Laenen, A.; Vergote, I.; Wildiers, H.; Punie, K.; Neven, P. Efficacy of anti-her2 therapy in metastatic breast cancer by discordance of her2 expression between primary and metastatic breast cancer. Breast Cancer Res. Treat. 2021, 185, 183–194. [Google Scholar] [CrossRef]
- Bural, G.; Torigian, D.A.; Houseni, M.; Basu, S.; Srinivas, S.; Alavi, A. Tumor metabolism measured by partial volume corrected standardized uptake value varies considerably in primary and metastatic sites in patients with lung cancer. A new observation. Hell. J. Nucl. Med. 2009, 12, 218–222. [Google Scholar]
- Zhang, J.; Hu, X.-C.; Jia, Z.; Ragaz, J.; Zhang, Y.-J.; Zhou, M.; Zhang, Y.-P.; Li, G.; Wang, B.-Y.; Wang, Z.-H. The maximum standardized uptake value of {sup 18} f-fdg pet scan to determine prognosis of hormone-receptor positive metastatic breast cancer. BMC Cancer 2013, 13. [Google Scholar] [CrossRef]
- Ma, F.; Guan, Y.; Yi, Z.; Chang, L.; Li, Q.; Chen, S.; Zhu, W.; Guan, X.; Li, C.; Qian, H.; et al. Assessing tumor heterogeneity using ctdna to predict and monitor therapeutic response in metastatic breast cancer. Int. J. Cancer 2020, 146, 1359–1368. [Google Scholar] [CrossRef]
- Cacicedo, J.; Fernandez, I.; Del Hoyo, O.; Navarro, A.; Gomez-Iturriaga, A.; Pijoan, J.I.; Martinez-Indart, L.; Escudero, J.; Gomez-Suarez, J.; de Zarate, R.O.; et al. Prognostic value of maximum standardized uptake value measured by pretreatment 18f-fdg pet/ct in locally advanced head and neck squamous cell carcinoma. Clin. Transl. Oncol. 2017, 19, 1337–1349. [Google Scholar] [CrossRef]
- Lee, S.W.; Nam, S.Y.; Im, K.C.; Kim, J.S.; Choi, E.K.; Ahn, S.D.; Park, S.H.; Kim, S.Y.; Lee, B.J.; Kim, J.H. Prediction of prognosis using standardized uptake value of 2-[(18)f] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother. Oncol. 2008, 87, 211–216. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Pei, Y.; Huang, M.; Lu, F.; Zheng, Q.; Li, N.; Yang, Y. Impact of maximum standardized uptake value of non-small cell lung cancer on detecting lymph node involvement in potential stereotactic body radiotherapy candidates. J. Thorac. Dis. 2017, 9, 1023–1031. [Google Scholar] [CrossRef]
- Aogi, K.; Kadoya, T.; Sugawara, Y.; Kiyoto, S.; Shigematsu, H.; Masumoto, N.; Okada, M. Utility of (18)f fdg-pet/ct for predicting prognosis of luminal-type breast cancer. Breast Cancer Res. Treat. 2015, 150, 209–217. [Google Scholar] [CrossRef]
- Cokmert, S.; Tanriverdi, O.; Karapolat, I.; Demir, L.; Bayoglu, V.; Can, A.; Akyol, M.; Yilmaz, Y.; Oktay Tarhan, M. The maximum standardized uptake value of metastatic site in 18 f-fdg pet/ct predicts molecular subtypes and survival in metastatic breast cancer: An izmir oncology group study. J. BUON 2016, 21, 1410–1418. [Google Scholar]
- Ohara, M.; Shigematsu, H.; Tsutani, Y.; Emi, A.; Masumoto, N.; Ozaki, S.; Kadoya, T.; Okada, M. Role of fdg-pet/ct in evaluating surgical outcomes of operable breast cancer--usefulness for malignant grade of triple-negative breast cancer. Breast 2013, 22, 958–963. [Google Scholar] [CrossRef]
| Characteristics | Patients (n = 51) | |
|---|---|---|
| No. | % | |
| Age (years) | ||
| Median | 54 | |
| Range | 23–74 | |
| Menopausal status | ||
| Postmenopausal | 36 | 70.6 |
| Premenopausal | 15 | 29.4 |
| HR status a | ||
| Positive | 20 | 39.2 |
| Negative | 31 | 60.8 |
| De novo breast cancer | ||
| Yes | 6 | 11.8 |
| No | 45 | 88.2 |
| Histological Grade b | ||
| Grade 2 | 13 | 25.5 |
| Grade 3 | 35 | 68.6 |
| Disease-free interval | ||
| <24 months | 31 | 68.9 |
| >24 months | 14 | 31.1 |
| Number of metastatic sites | ||
| 1 | 16 | 31.4 |
| 2 | 14 | 27.5 |
| ≥3 | 21 | 41.2 |
| Metastatic sites | ||
| Lung | 10 | 19.6 |
| Liver | 12 | 23.5 |
| Bone | 23 | 45.1 |
| Brain | 10 | 19.6 |
| Visceral disease | 25 | 49.0 |
| Treatment line c | ||
| 1 | 21 | 41.2 |
| 2 | 23 | 45.1 |
| ≥3 | 7 | 13.7 |
| Previous anti-HER2 treatment | ||
| Trastuzumab | 47 | 92.2 |
| Pertuzumab | 13 | 25.5 |
| Lapatinib | 4 | 7.8 |
| Trastuzumab emtansine | 1 | 2.0 |
| Factors | No. of Patients | PFS (Months) | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Clinical risk factors | |||||
| Age | ≥54 years | 26 | 13.4 | 7.2–19.5 | 0.456 |
| <54 years | 25 | 14.4 | 9.1–19.7 | ||
| HR status a | Positive | 20 | 13.7 | 8.9–18.5 | 0.930 |
| Negative | 31 | 13.4 | 8.8–17.9 | ||
| Histological Grade | Grade 2 | 13 | 10.6 | 9.7–11.5 | 0.365 |
| Grade 3 | 35 | 13.7 | 10.8–16.6 | ||
| Disease-free interval | >24 months | 14 | 13.7 | 1.5–26.0 | 0.872 |
| <24 months | 31 | 12.4 | 9.3–15.6 | ||
| Treatment line b | First- or second-line | 44 | 15.7 | 4.1–27.3 | 0.017 * |
| Third- or later-line | 7 | 10.6 | 10.2–11.1 | ||
| Resistance to previous trastuzumab c | Yes | 26 | 12.4 | 3.6–21.2 | 0.432 |
| No | 25 | 15.7 | 9.4–22.06 | ||
| No. of metastatic sites | 1 | 16 | 25.3 | NR | 0.015 * |
| ≥2 | 35 | 11.2 | 8.9–13.5 | ||
| Visceral disease | Yes | 25 | 11.2 | 9.6–12.9 | 0.280 |
| No | 26 | 16.8 | 7.4–26.2 | ||
| Combinational agent | Capecitabine | 40 | 13.4 | 7.1–19.6 | 0.911 |
| Others | 11 | 14.4 | 8.7–20.1 | ||
| Tumor heterogeneity | |||||
| Temporal tumor heterogeneity between primary and metastatic disease | |||||
| HR status | heterogeneous | 10 | 11.1 | 8.6–13.6 | 0.887 |
| homogeneous | 26 | 16.8 | 8.5–25.1 | ||
| HR expression | heterogeneous | 15 | 13.7 | 9.5–18.0 | 0.541 |
| homogeneous | 21 | 16.8 | 8.5–25.1 | ||
| HER2 status | heterogeneous | 9 | 5.8 | 3.0–8.6 | 0.001 * |
| homogeneous | 27 | 16.8 | 4.5–29.1 | ||
| HER2 expression | heterogeneous | 12 | 5.8 | 3.7–8.0 | 0.001 * |
| homogeneous | 24 | NR | NR | ||
| Spatial tumor heterogeneity in terms of 18F-FDG uptake | |||||
| HI-intra | >1.69 | 26 | 10.6 | 9.5–11.7 | 0.023 * |
| <1.69 | 25 | 25.3 | 5.9–44.8 | ||
| HI-inter | >1.15 | 31 | 11.2 | 7.3–15.1 | 0.040 * |
| <1.15 | 20 | 25.3 | NR | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.; Liu, C.; Tao, Z.; Zhang, J.; Wang, L.; Cao, J.; Zhao, Y.; Xie, Y.; Hu, X.; Yang, Z.; et al. Temporal Heterogeneity of HER2 Expression and Spatial Heterogeneity of 18F-FDG Uptake Predicts Treatment Outcome of Pyrotinib in Patients with HER2-Positive Metastatic Breast Cancer. Cancers 2022, 14, 3973. https://doi.org/10.3390/cancers14163973
Gong C, Liu C, Tao Z, Zhang J, Wang L, Cao J, Zhao Y, Xie Y, Hu X, Yang Z, et al. Temporal Heterogeneity of HER2 Expression and Spatial Heterogeneity of 18F-FDG Uptake Predicts Treatment Outcome of Pyrotinib in Patients with HER2-Positive Metastatic Breast Cancer. Cancers. 2022; 14(16):3973. https://doi.org/10.3390/cancers14163973
Chicago/Turabian StyleGong, Chengcheng, Cheng Liu, Zhonghua Tao, Jian Zhang, Leiping Wang, Jun Cao, Yannan Zhao, Yizhao Xie, Xichun Hu, Zhongyi Yang, and et al. 2022. "Temporal Heterogeneity of HER2 Expression and Spatial Heterogeneity of 18F-FDG Uptake Predicts Treatment Outcome of Pyrotinib in Patients with HER2-Positive Metastatic Breast Cancer" Cancers 14, no. 16: 3973. https://doi.org/10.3390/cancers14163973
APA StyleGong, C., Liu, C., Tao, Z., Zhang, J., Wang, L., Cao, J., Zhao, Y., Xie, Y., Hu, X., Yang, Z., & Wang, B. (2022). Temporal Heterogeneity of HER2 Expression and Spatial Heterogeneity of 18F-FDG Uptake Predicts Treatment Outcome of Pyrotinib in Patients with HER2-Positive Metastatic Breast Cancer. Cancers, 14(16), 3973. https://doi.org/10.3390/cancers14163973






