Clinical Outcome and Prognostic Factors of Pancreatic Adenosquamous Carcinoma Compared to Ductal Adenocarcinoma—Results from the German Cancer Registry Group
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Parameters
2.3. Statistics and Survival Analysis
3. Results
3.1. Patient Cohort and Baseline Characteristics
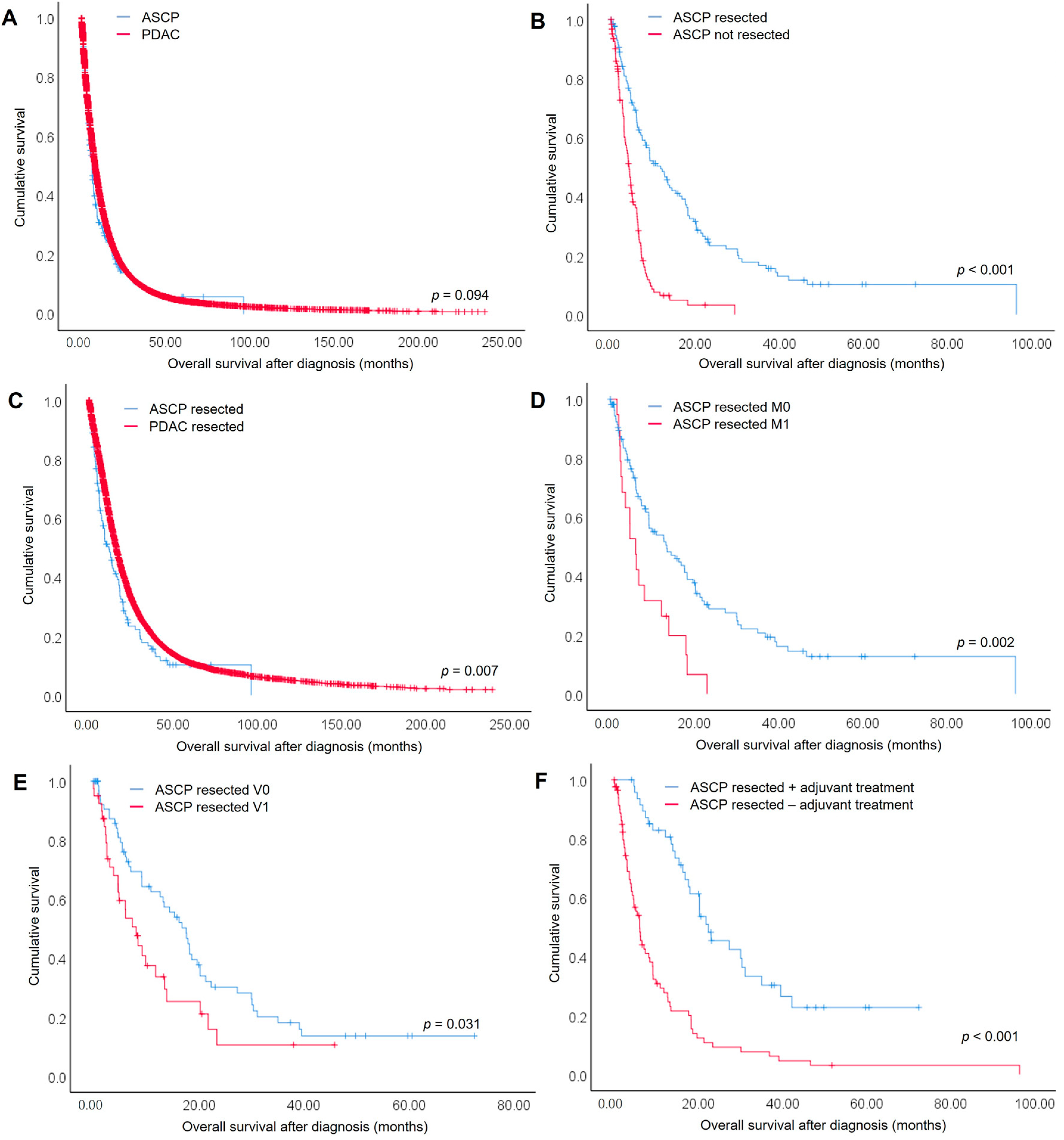
3.2. Overall Survival of ASCP Compared to PDAC Patients
3.3. Prognostic Factors for Overall Survival of Resected ASCP and PDAC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, R.A.; Marayati, R.; Flate, E.L.; Volmar, K.E.; Loeza, S.G.; Hoadley, K.A.; Rashid, N.U.; Williams, L.A.; Eaton, S.C.; Chung, A.H.; et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015, 47, 1168–1178. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Finetti, P.; Birnbaum, D.; Mamessier, E.; Bertucci, F. Validation and comparison of the molecular classifications of pancreatic carcinomas. Mol. Cancer 2017, 16, 168. [Google Scholar] [CrossRef]
- Kalimuthu, S.N.; Wilson, G.W.; Grant, R.C.; Seto, M.; O’Kane, G.; Vajpeyi, R.; Notta, F.; Gallinger, S.; Chetty, R. Morphological classification of pancreatic ductal adenocarcinoma that predicts molecular subtypes and correlates with clinical outcome. Gut 2020, 69, 317–328. [Google Scholar] [CrossRef]
- Sears, H.F.; Kim, Y.; Strawitz, J. Squamous cell carcinoma of the pancreas. J. Surg. Oncol. 1980, 14, 261–265. [Google Scholar] [CrossRef]
- Baylor, S.M.; Berg, J.W. Cross-classification and survival characteristics of 5000 cases of cancer of the pancreas. J. Surg. Oncol. 1973, 5, 335–358. [Google Scholar] [CrossRef]
- Hsu, J.T.; Yeh, C.N.; Chen, Y.R.; Chen, H.M.; Hwang, T.L.; Jan, Y.Y.; Chen, M.F. Adenosquamous carcinoma of the pancreas. Digestion 2005, 72, 104–108. [Google Scholar] [CrossRef]
- Rahemtullah, A.; Misdraji, J.; Pitman, M.B. Adenosquamous carcinoma of the pancreas: Cytologic features in 14 cases. Cancer 2003, 99, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Aranha, G.V.; Yong, S.; Olson, M. Adenosquamous carcinoma of the pancreas. Int. J. Pancreatol. 1999, 26, 85–91. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Classification of Tumours: Digestive System Tumours; World Health Organization (WHO): Lyon, France, 2019. [Google Scholar]
- Ishikawa, O.; Matsui, Y.; Aoki, I.; Iwanaga, T.; Terasawa, T.; Wada, A. Adenosquamous carcinoma of the pancreas: A clinicopathologic study and report of three cases. Cancer 1980, 46, 1192–1196. [Google Scholar] [CrossRef]
- Pour, P.M.; Sayed, S.; Sayed, G. Hyperplastic, preneoplastic and neoplastic lesions found in 83 human pancreases. Am. J. Clin. Pathol. 1982, 77, 137–152. [Google Scholar] [CrossRef]
- Mukada, T.; Yamada, S. Dysplasia and carcinoma in situ of the exocrine pancreas. Tohoku J. Exp. Med. 1982, 137, 115–124. [Google Scholar] [CrossRef]
- Hayashi, A.; Fan, J.; Chen, R.; Ho, Y.J.; Makohon-Moore, A.P.; Lecomte, N.; Zhong, Y.; Hong, J.; Huang, J.; Sakamoto, H.; et al. A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nat. Cancer 2020, 1, 59–74. [Google Scholar] [CrossRef]
- Katz, M.H.; Taylor, T.H.; Al-Refaie, W.B.; Hanna, M.H.; Imagawa, D.K.; Anton-Culver, H.; Zell, J.A. Adenosquamous versus adenocarcinoma of the pancreas: A population-based outcomes analysis. J. Gastrointest. Surg. 2011, 15, 165–174. [Google Scholar] [CrossRef]
- Boyd, C.A.; Benarroch-Gampel, J.; Sheffield, K.M.; Cooksley, C.D.; Riall, T.S. 415 patients with adenosquamous carcinoma of the pancreas: A population-based analysis of prognosis and survival. J. Surg. Res. 2012, 174, 12–19. [Google Scholar] [CrossRef]
- Okabayashi, T.; Hanazaki, K. Surgical outcome of adenosquamous carcinoma of the pancreas. World J. Gastroenterol. 2008, 14, 6765–6770. [Google Scholar] [CrossRef]
- Madura, J.A.; Jarman, B.T.; Doherty, M.G.; Yum, M.N.; Howard, T.J. Adenosquamous carcinoma of the pancreas. Arch. Surg. 1999, 134, 599–603. [Google Scholar] [CrossRef]
- Gruhl, J.D.; Garrido-Laguna, I.; Francis, S.R.; Affolter, K.; Tao, R.; Lloyd, S. The impact of squamous cell carcinoma histology on outcomes in nonmetastatic pancreatic cancer. Cancer Med. 2020, 9, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Heinemann, V.; Schmidberger, H. Duktales Adenokarzinom des Pankreas. Der Onkol. 2019, 25, 642–644. [Google Scholar] [CrossRef]
- Di Federico, A.; Tateo, V.; Parisi, C.; Formica, F.; Carloni, R.; Frega, G.; Rizzo, A.; Ricci, D.; Di Marco, M.; Palloni, A.; et al. Hacking Pancreatic Cancer: Present and Future of Personalized Medicine. Pharmaceuticals 2021, 14, 677. [Google Scholar] [CrossRef] [PubMed]
- Smoot, R.L.; Zhang, L.; Sebo, T.J.; Que, F.G. Adenosquamous carcinoma of the pancreas: A single-institution experience comparing resection and palliative care. J. Am. Coll. Surg. 2008, 207, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.T.; Dholakia, A.S.; Fan, K.Y.; Kumar, R.; Moningi, S.; Rosati, L.M.; Laheru, D.A.; Zheng, L.; De Jesus-Acosta, A.; Ellsworth, S.G.; et al. Efficacy of platinum chemotherapy agents in the adjuvant setting for adenosquamous carcinoma of the pancreas. J. Gastrointest. Oncol. 2015, 6, 115–125. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft Deutscher Tumorzentren (ADT). Einheitlicher Onkologischer Basisdatensatz. 2014. Available online: https://basisdatensatz.de/basisdatensatz/2014 (accessed on 3 March 2022).
- Fritz, A. International Classification of Diseases for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 4 March 2022).
- Kardon, D.E.; Thompson, L.D.; Przygodzki, R.M.; Heffess, C.S. Adenosquamous carcinoma of the pancreas: A clinicopathologic series of 25 cases. Mod. Pathol. 2001, 14, 443–451. [Google Scholar] [CrossRef]
- Kovi, J. Adenosquamous carcinoma of the pancreas: A light and electron microscopic study. Ultrastruct. Pathol. 1982, 3, 17–23. [Google Scholar] [CrossRef]
- Jamieson, J.D.; Ingber, D.E.; Muresan, V.; Hull, B.E.; Sarras, M.P., Jr.; Maylie-Pfenninger, M.F.; Iwanij, V. Cell surface properties of normal, differentiating, and neoplastic pancreatic acinar cells. Cancer 1981, 47, 1516–1527. [Google Scholar] [CrossRef]
- Hester, C.A.; Augustine, M.M.; Choti, M.A.; Mansour, J.C.; Minter, R.M.; Polanco, P.M.; Porembka, M.R.; Wang, S.C.; Yopp, A.C. Comparative outcomes of adenosquamous carcinoma of the pancreas: An analysis of the National Cancer Database. J. Surg. Oncol. 2018, 118, 21–30. [Google Scholar] [CrossRef]
- Kaiser, J.; Hinz, U.; Mayer, P.; Hank, T.; Niesen, W.; Hackert, T.; Gaida, M.M.; Buchler, M.W.; Strobel, O. Clinical presentation and prognosis of adenosquamous carcinoma of the pancreas-Matched-pair analysis with pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 2021, 47, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Deichmann, S.; Dworschak, O.; Kulemann, B.; Hoppner, J.; Bolm, L.; Klinkhammer-Schalke, M.; Zeissig, S.R.; Kleihus van Tol, K.; Braun, R.; Lapshyn, H.; et al. Incidence, Treatment and Survival in Pancreatic Cancer- Data of the Nationwide Oncological Quality Conference from a Surgical Perspective. Zent. Chir. 2022, 147, 147–154. [Google Scholar] [CrossRef]
- Swords, D.S.; Mulvihill, S.J.; Brooke, B.S.; Stoddard, G.J.; Firpo, M.A.; Scaife, C.L. County-level Variation in Use of Surgery and Cancer-specific Survival for Stage I-II Pancreatic Adenocarcinoma. Ann. Surg. 2020, 272, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Lenkiewicz, E.; Malasi, S.; Hogenson, T.L.; Flores, L.F.; Barham, W.; Phillips, W.J.; Roesler, A.S.; Chambers, K.R.; Rajbhandari, N.; Hayashi, A.; et al. Genomic and Epigenomic Landscaping Defines New Therapeutic Targets for Adenosquamous Carcinoma of the Pancreas. Cancer Res. 2020, 80, 4324–4334. [Google Scholar] [CrossRef]
- Pompella, L.; Tirino, G.; Pappalardo, A.; Caterino, M.; Ventriglia, A.; Nacca, V.; Orditura, M.; Ciardiello, F.; De Vita, F. Pancreatic Cancer Molecular Classifications: From Bulk Genomics to Single Cell Analysis. Int. J. Mol. Sci. 2020, 21, 2814. [Google Scholar] [CrossRef]
| Parameter | N (% of Total)/Median (Min–Max) | p-Value | ||
|---|---|---|---|---|
| ASCP | PDAC | |||
| A | All Patients | |||
| Total number | 278 (100) | 37,941 (100) | ||
| Sex | ||||
| male | 155 (55.8) | 20,226 (53.3) | 0.416 | |
| female | 123 (44.2) | 17,712 (46.7) | ||
| missing | 0 (0) | 3 (0.008) | ||
| Median age (years) | 70 (35–94) | 70 (17–102) | 0.385 | |
| Distant metastases | ||||
| M0 | 135 (48.6) | 12,964 (34.2) | <0.001 | |
| M1 | 88 (31.7) | 15,177 (40) | ||
| Mx | 55 (19.8) | 9800 (25.8) | ||
| Treatment | ||||
| No treatment reported; BSC | 92 (33.1) | 15,919 (42.0) | 0.003 | |
| Neoadjuvant treatment + resection | 4 (1.4) | 360 (0.9) | 0.402 | |
| Resection | 90 (32.4) | 7370 (19.4) | <0.001 | |
| Resection + adjuvant treatment | 48 (17.2) | 5038 (13.3) | 0.051 | |
| (Radio-)chemotherapy | 38 (13.7) | 8928 (23.5) | <0.001 | |
| Neoadjuvant treatment without resection | 6 (2.2) | 274 (0.7) | 0.005 | |
| Palliative surgery + (radio-)chemotherapy | 0 (0) | 52 (0.1) | 0.537 | |
| B | Surgically Resected Patients | |||
| Total number | 142 (100) | 12,768 (100) | ||
| Surgical procedure | ||||
| Partial pancreatoduodenectomy | 63 (44.4) | 6297 (49.3) | 0.240 | |
| Distal pancreatectomy | 33 (23.2) | 1211 (9.5) | <0.001 | |
| Total pancreatectomy | 10 (7.0) | 900 (7.0) | 0.998 | |
| Other | 36 (25.4) | 4360 (34.1) | ||
| Pathological classification of resected tumor | ||||
| Tumor stage | ||||
| pT0 | 0 (0) | 23 (0.2) | 0.072 | |
| pT1 | 0 (0) | 433 (3.4) | ||
| pT2/T3 | 134 (94.4) | 10,969 (85.9) | ||
| pT4 | 7 (4.9) | 926 (7.3) | ||
| pTx | 1 (0.7) | 417 (3.3) | ||
| Lymph node status | ||||
| pN0 | 44 (31.0) | 6868 (30.3) | 0.984 | |
| pN+ | 97 (68.3) | 8558 (67.0) | ||
| pNx | 1 (0.7) | 342 (2.7) | ||
| Grading | ||||
| G1 | 0 (0) | 510 (4.0) | <0.001 | |
| G2 | 40 (28.2) | 5895 (46.2) | ||
| G3 | 95 (66.9) | 5201 (40.7) | ||
| G4 | 0 (0) | 62 (0.5) | ||
| Gx | 7 (4.9) | 1100 (8.6) | ||
| Lymphatic vessel invasion | ||||
| L0 | 46 (32.4) | 3361 (26.3) | 0.885 | |
| L1 | 67 (47.2) | 5034 (39.4) | ||
| Lx | 29 (20.4) | 4373 (34.2) | ||
| Blood vessel invasion | ||||
| V0 | 72 (50.7) | 6086 (47.7) | 0.010 | |
| V1 | 40 (28.2) | 2037 (16.0) | ||
| Vx | 30 (21.1) | 4645 (36.4) | ||
| Resection status | ||||
| R0 | 77 (54.2) | 6278 (49.2) | 0.724 | |
| R1 | 24 (16.9) | 2191 (17.2) | ||
| R2 | 4 (2.8) | 241 (1.9) | ||
| Rx | 37 (26.1) | 4058 (31.8) | ||
| Distant metastasis | ||||
| M0 | 117 (82.4) | 10,101 (79.1) | 0.734 | |
| M1 | 21 (14.8) | 1966 (15.4) | ||
| Mx | 4 (2.8) | 701 (5.5) | ||
| Adjuvant chemotherapy | ||||
| Patients treated with adjuvant chemotherapy | 48 (33.8) | 5038 (39.5) | 0.170 | |
| Gemcitabine-based | 28 (19.7) | 3193 (25.0) | ||
| Platin-based | 5 (3.5) | 225 (1.8) | ||
| Fluoropyrimidine-based | 6 (4.2) | 466 (3.6) | ||
| Other | 9 (6.3) | 1154 (9.0) | ||
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ASCP | PDAC | |||||||
| Parameter | N | Deaths | Median Survival in Months (95% CI) | p-Value | N | Deaths | Median Survival in Months (95% CI) | p-Value |
| Overall | 134 | 100 | 11.80 (8.20–15.40) | 11,965 | 9136 | 16.17 (15.78–16.55) | ||
| Sex | ||||||||
| male | 76 | 61 | 9.16 (3.21–15.13) | 0.998 | 6374 | 4900 | 15.47 (14.99–15.95) | 0.051 |
| female | 58 | 39 | 12.73 (7.48–17.98) | 5591 | 4236 | 16.63 (16.07–17.20) | ||
| Age (years) | ||||||||
| ≤65 | 49 | 36 | 13.23 (3.75–22.72) | 0.497 | 4686 | 3517 | 18.40 (17.66–19.14) | <0.001 |
| >65 | 85 | 64 | 9.17 (3.57–14.77) | 7279 | 5619 | 14.43 (13.99–14.88) | ||
| Distant metastasis | ||||||||
| M0 | 111 | 78 | 13.43 (7.22–19.65) | 0.002 | 9499 | 6962 | 18.40 (17.89–18.91) | <0.001 |
| M1 | 19 | 18 | 6.03 (3.85–8.21) | 1784 | 1606 | 7.20 (6.69–7.71) | ||
| Adjuvant Therapy | ||||||||
| Yes | 48 | 30 | 22.37 (14.48–30.25) | <0.001 | 4973 | 3525 | 21.57 (20.92–22.22) | <0.001 |
| No | 86 | 70 | 6.03 (4.56–7.51) | 6992 | 5611 | 12.17 (11.71–12.62) | ||
| (b) | ||||||||
| ASCP | PDAC | |||||||
| Parameter | N | Deaths | Median Survival in Months (95% CI) | p-Value | N | Deaths | Median Survival in Months (95% CI) | p-Value |
| T-stage | ||||||||
| pT0 | 0 | 0 | 23 | 7 | 56.53 (0.00–126.69) | <0.001 | ||
| pT1 | 0 | 0 | 406 | 259 | 34.17 (28.38–39.95) | |||
| pT2/3 | 127 | 95 | 12.17 (8.60–15.73) | 0.979 | 10,303 | 7877 | 16.33 (15.92–16.75) | |
| pT4 | 6 | 4 | 6.07 (1.23–10.91) | 829 | 737 | 8.90 (8.15–9.65) | ||
| Lymph node metastases | ||||||||
| N0 | 40 | 28 | 14.43 (8.38–20.49) | 0.064 | 3610 | 2491 | 22.20 (21.18–23.22) | <0.001 |
| N+ | 93 | 71 | 9.17 (5.01–13.32) | 8020 | 6371 | 14.23 (13.82–14.64) | ||
| Lymph vessel invasion | ||||||||
| L0 | 45 | 29 | 18.67 (15.85–21.48) | 0.096 | 3252 | 1975 | 22.83 (21.79–23.88) | <0.001 |
| L1 | 66 | 48 | 9.23 (4.26–14.20) | 4850 | 3642 | 15.87 (15.30–16.43) | ||
| Blood vessel invasion | ||||||||
| V0 | 70 | 49 | 17.63 (13.43–21.84) | 0.031 | 5871 | 3947 | 19.93 (19.29–20.58) | <0.001 |
| V1 | 40 | 28 | 8.10 (4.89–11.306) | 1958 | 1440 | 13.47 (12.70–14.24) | ||
| Resection margin status | ||||||||
| R0 | 75 | 53 | 13.50 (9.97–17.03) | 0.369 | 6089 | 4162 | 21.00 (20.31–21.69) | <0.001 |
| R+ | 28 | 19 | 9.17 (0.00–28.13) | 2355 | 1793 | 13.23 (12.61–13.85) | ||
| Grading | ||||||||
| G1/G2 | 38 | 27 | 11.80 (2.26–21.34) | 0.508 | 6021 | 4397 | 19.87 (19.21–20.52) | <0.001 |
| G3/G4 | 89 | 66 | 10.90 (4.50–17.30) | 4943 | 3956 | 12.60 (12.16–13.04) | ||
| ASCP | PDAC | ASCP + PDAC | ||||
|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Histology (ASCP vs. PDAC) | 1.303 (1.013–1.677) | 0.039 | ||||
| Distant metastases (M1 vs. M0) | 3.436 (1.354–8.856) | 0.010 | 1.614 (1.453–1.793) | <0.001 | 1.625 (1.464–1.804) | <0.001 |
| Adjuvant therapy (no vs. yes) | 5.361 (2.858–10.055) | <0.001 | 1.871 (1.758–1.990) | <0.001 | 1.894 (1.781–2.014) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, R.; Klinkhammer-Schalke, M.; Zeissig, S.R.; Kleihus van Tol, K.; Bolm, L.; Honselmann, K.C.; Petrova, E.; Lapshyn, H.; Deichmann, S.; Abdalla, T.S.A.; et al. Clinical Outcome and Prognostic Factors of Pancreatic Adenosquamous Carcinoma Compared to Ductal Adenocarcinoma—Results from the German Cancer Registry Group. Cancers 2022, 14, 3946. https://doi.org/10.3390/cancers14163946
Braun R, Klinkhammer-Schalke M, Zeissig SR, Kleihus van Tol K, Bolm L, Honselmann KC, Petrova E, Lapshyn H, Deichmann S, Abdalla TSA, et al. Clinical Outcome and Prognostic Factors of Pancreatic Adenosquamous Carcinoma Compared to Ductal Adenocarcinoma—Results from the German Cancer Registry Group. Cancers. 2022; 14(16):3946. https://doi.org/10.3390/cancers14163946
Chicago/Turabian StyleBraun, Rüdiger, Monika Klinkhammer-Schalke, Sylke Ruth Zeissig, Kees Kleihus van Tol, Louisa Bolm, Kim C. Honselmann, Ekaterina Petrova, Hryhoriy Lapshyn, Steffen Deichmann, Thaer S. A. Abdalla, and et al. 2022. "Clinical Outcome and Prognostic Factors of Pancreatic Adenosquamous Carcinoma Compared to Ductal Adenocarcinoma—Results from the German Cancer Registry Group" Cancers 14, no. 16: 3946. https://doi.org/10.3390/cancers14163946
APA StyleBraun, R., Klinkhammer-Schalke, M., Zeissig, S. R., Kleihus van Tol, K., Bolm, L., Honselmann, K. C., Petrova, E., Lapshyn, H., Deichmann, S., Abdalla, T. S. A., Heckelmann, B., Bronsert, P., Zemskov, S., Hummel, R., Keck, T., & Wellner, U. F. (2022). Clinical Outcome and Prognostic Factors of Pancreatic Adenosquamous Carcinoma Compared to Ductal Adenocarcinoma—Results from the German Cancer Registry Group. Cancers, 14(16), 3946. https://doi.org/10.3390/cancers14163946







