Tumor Progression and Treatment-Related Changes: Radiological Diagnosis Challenges for the Evaluation of Post Treated Glioma
Abstract
Simple Summary
Abstract
1. Introduction
2. Characteristics of TRCs
2.1. Pseudoprogression (PsP)
2.2. Radiation Necrosis (RN)
2.3. Pseudoresponse
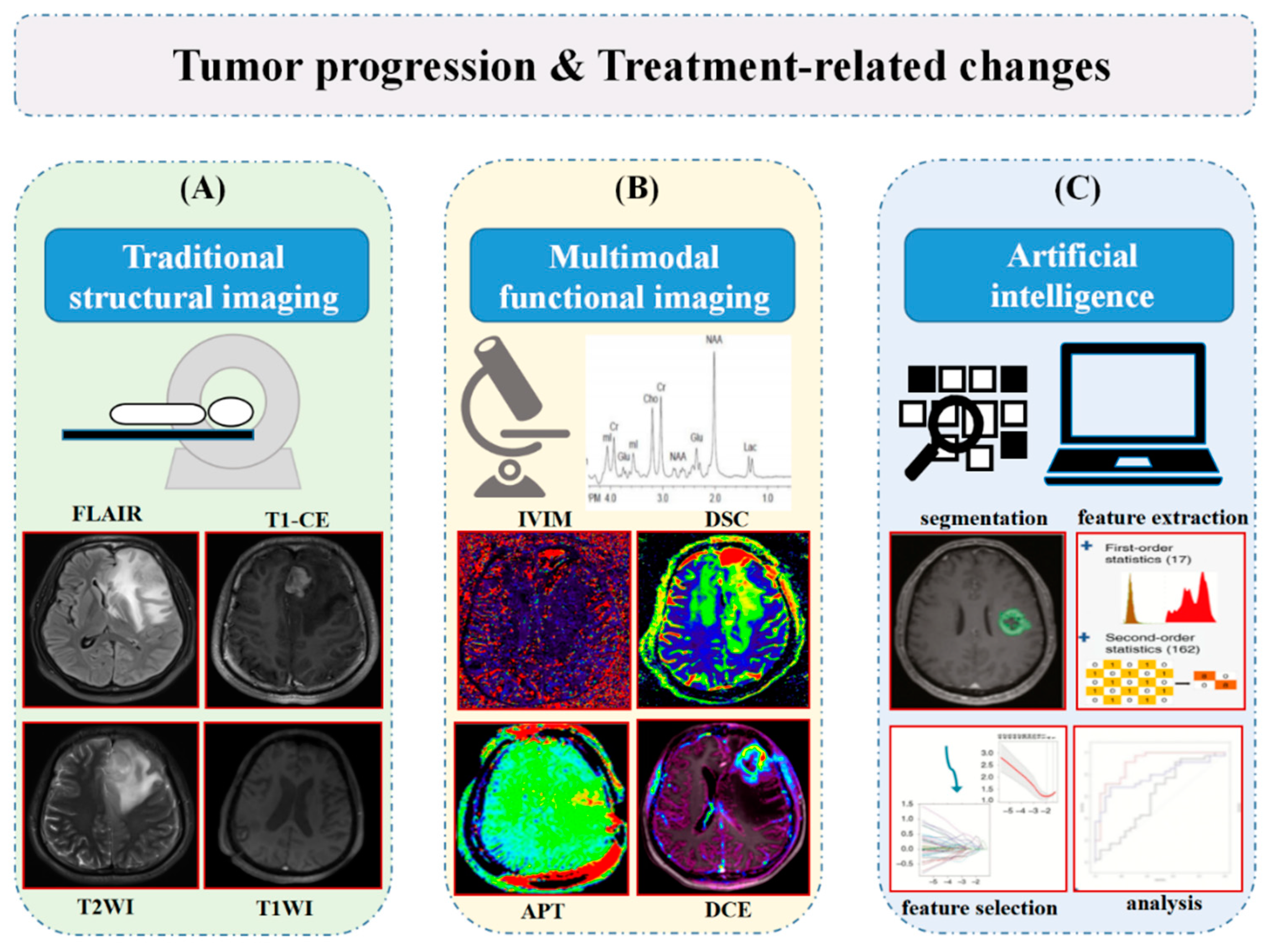
3. Diagnostic Imaging Modalities
3.1. Conventional MRI
3.2. Diffusion MRI
3.2.1. Diffusion-Weighted Imaging
3.2.2. Intravoxel Incoherent Motion
3.2.3. Diffusion Tensor Imaging
3.2.4. Diffusion Kurtosis Imaging
3.3. Perfusion MRI
3.3.1. Dynamic Susceptibility Contrast (DSC)
3.3.2. Dynamic Contrast-Enhanced
3.3.3. Arterial Spin Labeling (ASL)
3.4. Magnetic Resonance Spectroscopy (MRS)
3.5. Amide Proton Transfer Imaging
3.6. Positron Emission Tomography
3.7. Multi-Model Imaging Modality
4. Emerging Application of Artificial Intelligence
4.1. Grading and Molecular Information Prediction
4.2. Post-Treatment Follow-Up and Outcome Prediction
4.3. Future Challenges
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reason. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Riñones-Mena, E.; Corrales-García, E.M. Treatment-related changes in glioblastoma: A review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin. Transl. Oncol. 2017, 20, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yu, Z.; Liang, R. Effect of long-term adjuvant temozolomide chemotherapy on primary glioblastoma patient survival. BMC Neurol. 2021, 21, 424. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef] [PubMed]
- Prager, A.J.; Martinez, N.; Beal, K.; Omuro, A.; Zhang, Z.; Young, R.J. Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am. J. Neuroradiol. 2015, 36, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Bukhari, S.; Afridi, O.M.; Wang, S.; Yadav, S.K.; Akbari, H.; Verma, G.; Nath, K.; Haris, M.; Bagley, S.; et al. Metabolic and physiologic magnetic resonance imaging in distinguishing true progression from pseudoprogression in patients with glioblastoma. NMR Biomed. 2022, 35, e4719. [Google Scholar] [CrossRef]
- Bronk, J.K.; Guha-Thakurta, N.; Allen, P.K.; Mahajan, A.; Grosshans, D.R.; McGovern, S.L. Analysis of pseudoprogression after proton or photon therapy of 99 patients with low grade and anaplastic glioma. Clin. Transl. Radiat. Oncol. 2018, 9, 30–34. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Pession, A.; Tallini, G.; Bertorelle, R.; Bartolini, S.; Calbucci, F.; Andreoli, A.; et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 2008, 26, 2192–2197. [Google Scholar] [CrossRef]
- Abbasi, A.W.; Westerlaan, H.E.; Holtman, G.A.; Aden, K.M.; van Laar, P.J.; van der Hoorn, A. Incidence of Tumour Progression and Pseudoprogression in High-Grade Gliomas: A Systematic Review and Meta-Analysis. Clin. Neuroradiol. 2018, 28, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Motegi, H.; Kamoshima, Y.; Terasaka, S.; Kobayashi, H.; Yamaguchi, S.; Tanino, M.; Murata, J.; Houkin, K. IDH1 mutation as a potential novel biomarker for distinguishing pseudoprogression from true progression in patients with glioblastoma treated with temozolomide and radiotherapy. Brain Tumor. Pathol. 2012, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Pouleau, H.B.; Sadeghi, N.; Baleriaux, D.; Melot, C.; De Witte, O.; Lefranc, F. High levels of cellular proliferation predict pseudoprogression in glioblastoma patients. Int. J. Oncol. 2011, 40, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Kim, C.Y.; Han, J.H.; Choe, G.Y.; Kim, J.H.; Kim, J.H.; Kim, I.A. Pseudoprogression in patients with malignant gliomas treated with concurrent temozolomide and radiotherapy: Potential role of p53. J. Neurooncol. 2010, 102, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hygino da Cruz, L.C.; Rodriguez, I., Jr.; Domingues, R.C.; Gasparetto, E.L.; Sorensen, A.G. Pseudoprogression and pseudoresponse: Imaging challenges in the assessment of posttreatment glioma. AJNR Am. J. Neuroradiol. 2011, 32, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.B.; Meng, A.; Ebani, E.J.; Chiang, G.C. Imaging Glioblastoma Posttreatment: Progression, Pseudoprogression, Pseudoresponse, Radiation Necrosis. Neuroimaging Clin. N. Am. 2021, 31, 103–120. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neurooncol. 2017, 134, 495–504. [Google Scholar] [CrossRef]
- Rahmathulla, G.; Marko, N.F.; Weil, R.J. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J. Clin. Neurosci. 2013, 20, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.A.; Nagy, A.; Pintilie, M.; Wong, C.S. Hypoxia and Hypoxia-Inducible Factor-1 Target Genes in Central Nervous System Radiation Injury. Clin. Cancer Res. 2004, 10, 3342–3353. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C., Jr.; Cairncross, J.G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.J.; Leeds, N.E.; Fuller, G.N.; Van Tassel, P.; Maor, M.H.; Sawaya, R.E.; Levin, V.A. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000, 217, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Westerly, D.; Chen, C. MRI patterns of T1 enhancing radiation necrosis versus tumour recurrence in high-grade gliomas. J. Med. Imaging Radiat. Oncol. 2013, 57, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Zhang, Z.; Shi, W.; Holodny, A.I.; Omuro, A.M. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 2011, 76, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.E.; Choi, S.H.; Kim, T.M.; Lee, S.H.; Park, C.K.; Park, S.H.; Kim, I.H.; Yun, T.J.; Kim, J.H.; Sohn, C.H. Independent Poor Prognostic Factors for True Progression after Radiation Therapy and Concomitant Temozolomide in Patients with Glioblastoma: Subependymal Enhancement and Low ADC Value. AJNR Am. J. Neuroradiol. 2015, 36, 1846–1852. [Google Scholar] [CrossRef]
- Mullins, M.E.; Barest, G.D.; Schaefer, P.W.; Hochberg, F.H.; Gonzalez, R.G.; Lev, M.H. Radiation Necrosis Versus Glioma Recurrence: Conventional MR Imaging Clues to Diagnosis. AJNR Am. J. Neuroradiol. 2005, 26, 1967–1972. [Google Scholar]
- Agarwal, A.; Kumar, S.; Narang, J.; Schultz, L.; Mikkelsen, T.; Wang, S.; Siddiqui, S.; Poptani, H.; Jain, R. Morphologic MRI features, diffusion tensor imaging and radiation dosimetric analysis to differentiate pseudo-progression from early tumor progression. J. Neurooncol. 2013, 112, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef]
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.; Stoffel, M.; et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro. Oncol. 2015, 17, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Kebir, S.; Fimmers, R.; Galldiks, N.; Schafer, N.; Mack, F.; Schaub, C.; Stuplich, M.; Niessen, M.; Tzaridis, T.; Simon, M.; et al. Late Pseudoprogression in Glioblastoma: Diagnostic Value of Dynamic O-(2-[18F]fluoroethyl)-L-Tyrosine PET. Clin. Cancer Res. 2016, 22, 2190–2196. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, S.; Jha, A.; Damesha, N.K.; Negi, P.; Jadhav, G.K.; Verma, S.M.; Sogani, S.K. Multiparametric Evaluation in Differentiating Glioma Recurrence from Treatment-Induced Necrosis Using Simultaneous (18)F-FDG-PET/MRI: A Single-Institution Retrospective Study. AJNR Am. J. Neuroradiol. 2017, 38, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, C.; Kirchner, J.; Poeppel, T.D.; Schaarschmidt, B.; Kebir, S.; El Hindy, N.; Hense, J.; Quick, H.H.; Glas, M.; Herrmann, K.; et al. (11)C-MET PET/MRI for detection of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Lee, J.Y.; Kim, H.S.; Oh, J.-Y.; Jung, S.C.; Kim, S.J.; Keupp, J.; Oh, M.; Kim, J.S. Amide proton transfer imaging seems to provide higher diagnostic performance in post-treatment high-grade gliomas than methionine positron emission tomography. Eur. Radiol. 2018, 28, 3285–3295. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.M.; Stoffels, G.; Lichtenstein, T.; Borggrefe, J.; Lohmann, P.; Ceccon, G.; Shah, N.J.; Fink, G.R.; Langen, K.J.; Kabbasch, C.; et al. Differentiation of treatment-related changes from tumour progression: A direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1889–1901. [Google Scholar] [CrossRef]
- Maurer, G.D.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.J. (18)F-FET PET Imaging in Differentiating Glioma Progression from Treatment-Related Changes: A Single-Center Experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef]
- Pellerin, A.; Khalife, M.; Sanson, M.; Rozenblum-Beddok, L.; Bertaux, M.; Soret, M.; Galanaud, D.; Dormont, D.; Kas, A.; Pyatigorskaya, N. Simultaneously acquired PET and ASL imaging biomarkers may be helpful in differentiating progression from pseudo-progression in treated gliomas. Eur. Radiol. 2021, 31, 7395–7405. [Google Scholar] [CrossRef] [PubMed]
- Provenzale, J.M.; Mukundan, S.; Barboriak, D.P. Diffusion-weighted and Perfusion MR Imaging for Brain Tumor Characterization and Assessment of Treatment Response. Radiology 2006, 239, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Hein, P.A.; Eskey, C.J.; Dunn, J.F.; Hug, E.B. Diffusion-Weighted Imaging in the Follow-up of Treated High-Grade Gliomas: Tumor Recurrence versus Radiation Injury. AJNR Am. J. Neuroradiol. 2004, 25, 201–209. [Google Scholar] [PubMed]
- Asao, C.; Korogi, Y.; Kitajima, M.; Hirai, T.; Baba, Y.; Makino, K.; Kochi, M.; Morishita, S.; Yamashita, Y. Diffusion-Weighted Imaging of Radiation-Induced Brain Injury for Differentiation from Tumor Recurrence. AJNR Am. J. Neuroradiol. 2005, 26, 1455–1460. [Google Scholar]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Schweitzer, A.D.; Prager, A.; Shi, W.; Zhang, Z.; Huse, J.; Omuro, A.M. Potential role of preoperative conventional MRI including diffusion measurements in assessing epidermal growth factor receptor gene amplification status in patients with glioblastoma. AJNR Am. J. Neuroradiol. 2013, 34, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Choi, S.H.; Park, C.K.; Yi, K.S.; Kim, T.M.; Lee, S.H.; Kim, J.H.; Sohn, C.H.; Park, S.H.; Kim, I.H. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad. Radiol. 2012, 19, 1353–1361. [Google Scholar] [CrossRef]
- Kazda, T.; Bulik, M.; Pospisil, P.; Lakomy, R.; Smrcka, M.; Slampa, P.; Jancalek, R. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin. 2016, 11, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Choi, S.H.; Ryoo, I.; Kim, S.C.; Yeom, J.A.; Shin, H.; Shin, H.; Jung, S.C.; Lee, A.L.; Yoon, T.J.; et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: Comparison study of standard and high-b value diffusion—weighted imaging. Radiology 2013, 269, 831–840. [Google Scholar] [CrossRef]
- Song, Y.S.; Choi, S.H.; Park, C.K.; Yi, K.S.; Lee, W.J.; Yun, T.J.; Kim, T.M.; Lee, S.H.; Kim, J.H.; Sohn, C.H.; et al. True progression versus pseudoprogression in the treatment of glioblastomas: A comparison study of normalized cerebral blood volume and apparent diffusion coefficient by histogram analysis. Korean J. Radiol. 2013, 14, 662–672. [Google Scholar] [CrossRef]
- Reimer, C.; Deike, K.; Graf, M.; Reimer, P.; Wiestler, B.; Floca, R.O.; Kickingereder, P.; Schlemmer, H.P.; Wick, W.; Bendszus, M.; et al. Differentiation of pseudoprogression and real progression in glioblastoma using ADC parametric response maps. PLoS ONE 2017, 12, e0174620. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Shu, C.; Wang, Y.B.; Dong, L.Q. Diagnostic accuracy of diffusion MRI with quantitative ADC measurements in differentiating glioma recurrence from radiation necrosis. J. Neurol. Sci. 2015, 351, 65–71. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, B.R.J.; van Laar, P.J.; Holtman, G.A.; van der Hoorn, A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4129–4144. [Google Scholar] [CrossRef]
- Wu, C.C.; Jain, R.; Radmanesh, A.; Poisson, L.M.; Guo, W.Y.; Zagzag, D.; Snuderl, M.; Placantonakis, D.G.; Golfinos, J.; Chi, A.S. Predicting Genotype and Survival in Glioma Using Standard Clinical MR Imaging Apparent Diffusion Coefficient Images: A Pilot Study from The Cancer Genome Atlas. AJNR Am. J. Neuroradiol. 2018, 39, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Suh, C.H.; Kim, N.; Choi, C.G.; Kim, S.J. Histogram analysis of intravoxel incoherent motion for differentiating recurrent tumor from treatment effect in patients with glioblastoma: Initial clinical experience. AJNR Am. J. Neuroradiol. 2014, 35, 490–497. [Google Scholar] [CrossRef]
- Paschoal, A.M.; Leoni, R.F.; Dos Santos, A.C.; Paiva, F.F. Intravoxel incoherent motion MRI in neurological and cerebrovascular diseases. Neuroimage Clin. 2018, 20, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Yan, L.F.; Wu, L.; Du, P.; Chen, B.Y.; Wang, L.; Wang, S.M.; Han, Y.; Tian, Q.; Yu, Y.; et al. Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: Efficacy in preoperative grading. Sci. Rep. 2014, 4, 7208. [Google Scholar] [CrossRef]
- Federau, C.; Cerny, M.; Roux, M.; Mosimann, P.J.; Maeder, P.; Meuli, R.; Wintermark, M. IVIM perfusion fraction is prognostic for survival in brain glioma. Clin. Neuroradiol. 2017, 27, 485–492. [Google Scholar] [CrossRef]
- Puig, J.; Sanchez-Gonzalez, J.; Blasco, G.; Daunis, I.E.P.; Federau, C.; Alberich-Bayarri, A.; Biarnes, C.; Nael, K.; Essig, M.; Jain, R.; et al. Intravoxel Incoherent Motion Metrics as Potential Biomarkers for Survival in Glioblastoma. PLoS ONE 2016, 11, e0158887. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Yan, L.F.; Hu, Y.C.; Sun, Y.Z.; Tian, Q.; Nan, H.Y.; Yu, Y.; Sun, Q.; Wang, W.; Cui, G.B. Combination of IVIM-DWI and 3D-ASL for differentiating true progression from pseudoprogression of Glioblastoma multiforme after concurrent chemoradiotherapy: Study protocol of a prospective diagnostic trial. BMC Med. Imaging 2017, 17, 10. [Google Scholar] [CrossRef]
- Abdullah, K.G.; Lubelski, D.; Nucifora, P.G.; Brem, S. Use of diffusion tensor imaging in glioma resection. Neurosurg. Focus. 2013, 34(4), E1. [Google Scholar] [CrossRef]
- Razek, A.A.; El-Serougy, L.; Abdelsalam, M.; Gaballa, G.; Talaat, M. Differentiation of residual/recurrent gliomas from postradiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology 2018, 60, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Li, Y.L.; Lian, J.M.; Dou, S.W.; Yan, F.S.; Wu, H.; Shi, D.P. Distinction between postoperative recurrent glioma and radiation injury using MR diffusion tensor imaging. Neuroradiology 2010, 52, 1193–1199. [Google Scholar] [CrossRef]
- Wang, S.; Martinez-Lage, M.; Sakai, Y.; Chawla, S.; Kim, S.G.; Alonso-Basanta, M.; Lustig, R.A.; Brem, S.; Mohan, S.; Wolf, R.L.; et al. Differentiating Tumor Progression from Pseudoprogression in Patients with Glioblastomas Using Diffusion Tensor Imaging and Dynamic Susceptibility Contrast MRI. AJNR Am. J. Neuroradiol. 2016, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.J.; Zhuo, J.; Melhem, E.R. Diffusion kurtosis imaging: An emerging technique for evaluating the microstructural environment of the brain. AJR Am. J. Roentgenol. 2014, 202, W26–W33. [Google Scholar] [CrossRef]
- Pogosbekian, E.L.; Pronin, I.N.; Zakharova, N.E.; Batalov, A.I.; Turkin, A.M.; Konakova, T.A.; Maximov, I.I. Feasibility of generalised diffusion kurtosis imaging approach for brain glioma grading. Neuroradiology 2021, 63, 1241–1251. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, H.; Wang, X.; Qin, J.; Wang, L.; Yang, G.; Yan, H. Comparing the value of DKI and DTI in detecting isocitrate dehydrogenase genotype of astrocytomas. Clin. Radiol. 2019, 74, 314–320. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.C.; Zhang, H.; Wang, J.; Qin, J.B.; Wu, X.F.; Zhang, L.; Wang, L. Differentiation of high-grade-astrocytomas from solitary-brain-metastases: Comparing diffusion kurtosis imaging and diffusion tensor imaging. Eur. J. Radiol. 2015, 84, 2618–2624. [Google Scholar] [CrossRef]
- Wu, X.F.; Liang, X.; Wang, X.C.; Qin, J.B.; Zhang, L.; Tan, Y.; Zhang, H. Differentiating high-grade glioma recurrence from pseudoprogression: Comparing diffusion kurtosis imaging and diffusion tensor imaging. Eur. J. Radiol. 2021, 135, 109445. [Google Scholar] [CrossRef]
- Shi, W.; Qu, C.; Wang, X.; Liang, X.; Tan, Y.; Zhang, H. Diffusion kurtosis imaging combined with dynamic susceptibility contrast-enhanced MRI in differentiating high-grade glioma recurrence from pseudoprogression. Eur. J. Radiol. 2021, 144, 109941. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, B.R.J.; van Laar, P.J.; Smits, M.; Dankbaar, J.W.; Enting, R.H.; van der Hoorn, A. Perfusion MRI in treatment evaluation of glioblastomas: Clinical relevance of current and future techniques. J. Magn. Reason. Imaging 2019, 49, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Baradaran, H.; Delgado, D.; Askin, G.; Christos, P.; John Tsiouris, A.; Gupta, A. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro. Oncol. 2017, 19, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Wang, S.; Tu, M.; Wu, B.; Han, P.; Xu, H. The diagnostic performance of perfusion MRI for differentiating glioma recurrence from pseudoprogression: A meta-analysis. Medicine 2017, 96, e6333. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.S.; Kim, S.T.; Kim, E.H.; Lim, D.H.; Kim, W.S.; Suh, Y.L.; Lee, J.I.; Park, K.; Kim, J.H.; Nam, D.H. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: The role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am. J. Neuroradiol. 2011, 32, 382–387. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.-H.; Kim, S.-H.; Cho, K.-G.; Kim, S.Y. Posttreatment high-grade glioma: Usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence. Radiology 2010, 256, 906–915. [Google Scholar] [CrossRef][Green Version]
- Baek, H.J.; Kim, H.S.; Kim, N.; Choi, Y.J.; Kim, Y.J. Percent change of perfusion skewness and kurtosis: A potential imaging biomarker for early treatment response in patients with newly diagnosed glioblastomas. Radiology 2012, 264, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Boxerman, J.L.; Ellingson, B.M.; Jeyapalan, S.; Elinzano, H.; Harris, R.J.; Rogg, J.M.; Pope, W.B.; Safran, H. Longitudinal DSC-MRI for Distinguishing Tumor Recurrence from Pseudoprogression in Patients with a High-grade Glioma. Am. J. Clin. Oncol. 2017, 40, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Kim, H.S.; Kim, N.; Choi, C.G.; Kim, S.J. Recurrent Glioblastoma: Optimum Area under the Curve Method Derived from Dynamic Contrast-enhanced T1-weighted Perfusion MR Imaging. Radiology 2013, 269, 561–568. [Google Scholar] [CrossRef]
- Seeger, A.; Braun, C.; Skardelly, M.; Paulsen, F.; Schittenhelm, J.; Ernemann, U.; Bisdas, S. Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad. Radiol. 2013, 20, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Kim, S.T.; Kim, H.J.; Kim, B.J.; Kim, Y.K.; Lee, J.Y.; Jeon, P.; Kim, K.H.; Kong, D.S.; Nam, D.H. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am. J. Neuroradiol. 2014, 35, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.E.; Choi, S.H.; Kim, T.M.; Park, C.K.; Park, S.H.; Won, J.K.; Kim, I.H.; Lee, S.T.; Choi, H.J.; You, S.H.; et al. Dynamic contrast-enhanced MR imaging in predicting progression of enhancing lesions persisting after standard treatment in glioblastoma patients: A prospective study. Eur. Radiol. 2017, 27, 3156–3166. [Google Scholar] [CrossRef]
- Suh, C.H.; Kim, H.S.; Choi, Y.J.; Kim, N.; Kim, S.J. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. AJNR Am. J. Neuroradiol. 2013, 34, 2278–2286. [Google Scholar] [CrossRef]
- Thomas, A.A.; Arevalo-Perez, J.; Kaley, T.; Lyo, J.; Peck, K.K.; Shi, W.; Zhang, Z.; Young, R.J. Dynamic contrast enhanced T1 MRI perfusion differentiates pseudoprogression from recurrent glioblastoma. J. Neurooncol. 2015, 125, 183–190. [Google Scholar] [CrossRef]
- Bisdas, S.; Naegele, T.; Ritz, R.; Dimostheni, A.; Pfannenberg, C.; Reimold, M.; Koh, T.S.; Ernemann, U. Distinguishing recurrent high-grade gliomas from radiation injury: A pilot study using dynamic contrast-enhanced MR imaging. Acad. Radiol. 2011, 18, 575–583. [Google Scholar] [CrossRef]
- Yun, T.J.; Park, C.K.; Kim, T.M.; Lee, S.H.; Kim, J.H.; Sohn, C.H.; Park, S.H.; Kim, I.H.; Choi, S.H. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: Differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology 2015, 274, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Zaharchuk, G.; Thomas, D.L.; Lovblad, K.O.; Barkhof, F.; Golay, X. Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology 2016, 281, 337–356. [Google Scholar] [CrossRef]
- Ye, J.; Bhagat, S.K.; Li, H.; Luo, X.; Wang, B.; Liu, L.; Yang, G. Differentiation between recurrent gliomas and radiation necrosis using arterial spin labeling perfusion imaging. Exp. Ther. Med. 2016, 11, 2432–2436. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, H.S.; Jahng, G.H.; Kim, S.J.; Suh, D.C. Pseudoprogression in patients with glioblastoma: Added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta. Radiol. 2013, 54, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ozsunar, Y.; Mullins, M.E.; Kwong, K.; Hochberg, F.H.; Ament, C.; Schaefer, P.W.; Gonzalez, R.G.; Lev, M.H. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad. Radiol. 2010, 17, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chen, S.; Xiao, H.F.; Li, Y.; Wang, Y.; Liu, G.; Lou, X.; Ma, L. Differentiation between radiation-induced brain injury and glioma recurrence using 3D pCASL and dynamic susceptibility contrast-enhanced perfusion-weighted imaging. Radiother. Oncol. 2018, 129, 68–74. [Google Scholar] [CrossRef]
- Fatterpekar, G.M.; Galheigo, D.; Narayana, A.; Johnson, G.; Knopp, E. Treatment-Related Change Versus Tumor Recurrence in High-Grade Gliomas: A Diagnostic Conundrum—Use of Dynamic Susceptibility Contrast-Enhanced (DSC) Perfusion MRI. AJR Am. J. Roentgenol. 2012, 198, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Telischak, N.A.; Detre, J.A.; Zaharchuk, G. Arterial spin labeling MRI: Clinical applications in the brain. J. Magn. Reason. Imaging 2015, 41, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Bulik, M.; Kazda, T.; Slampa, P.; Jancalek, R. The Diagnostic Ability of Follow-Up Imaging Biomarkers after Treatment of Glioblastoma in the Temozolomide Era: Implications from Proton MR Spectroscopy and Apparent Diffusion Coefficient Mapping. Biomed. Res. Int. 2015, 2015, 641023. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Chawla, S.; Mohan, S.; Wang, S.; Nasrallah, M.; Sheriff, S.; Desai, A.; Brem, S.; O’Rourke, D.M.; Wolf, R.L.; et al. Three-dimensional echo planar spectroscopic imaging for differentiation of true progression from pseudoprogression in patients with glioblastoma. NMR Biomed. 2019, 32, e4042. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Kim, H.S.; Jung, S.C.; Choi, C.G.; Kim, S.J. Multiparametric MRI as a potential surrogate endpoint for decision-making in early treatment response following concurrent chemoradiotherapy in patients with newly diagnosed glioblastoma: A systematic review and meta-analysis. Eur. Radiol. 2018, 28, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lal, B.; Wilson, D.A.; Laterra, J.; van Zijl, P.C. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reason. Med. 2003, 50, 1120–1126. [Google Scholar] [CrossRef]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.V.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reason. Med 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Zhou, J.; Tryggestad, E.; Wen, Z.; Lal, B.; Zhou, T.; Grossman, R.; Wang, S.; Yan, K.; Fu, D.X.; Ford, E.; et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 2011, 17, 130–134. [Google Scholar] [CrossRef]
- Ma, B.; Blakeley, J.O.; Hong, X.; Zhang, H.; Jiang, S.; Blair, L.; Zhang, Y.; Heo, H.Y.; Zhang, M.; van Zijl, P.C.; et al. Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J. Magn. Reason Imaging 2016, 44, 456–462. [Google Scholar] [CrossRef]
- Park, K.J.; Kim, H.S.; Park, J.E.; Shim, W.H.; Kim, S.J.; Smith, S.A. Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur. Radiol. 2016, 26, 4390–4403. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Park, K.J.; Kim, S.J.; Kim, J.H.; Smith, S.A. Pre-and posttreatment glioma:comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology 2016, 278, 514–523. [Google Scholar] [CrossRef]
- Jiang, S.; Eberhart, C.G.; Lim, M.; Heo, H.Y.; Zhang, Y.; Blair, L.; Wen, Z.; Holdhoff, M.; Lin, D.; Huang, P.; et al. Identifying Recurrent Malignant Glioma after Treatment Using Amide Proton Transfer-Weighted MR Imaging: A Validation Study with Image-Guided Stereotactic Biopsy. Clin. Cancer Res. 2019, 25, 552–561. [Google Scholar] [CrossRef]
- Zhou, J.; Payen, J.-F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C.M. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Heo, H.Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef]
- Kertels, O.; Mihovilovic, M.I.; Linsenmann, T.; Kessler, A.F.; Tran-Gia, J.; Kircher, M.; Brumberg, J.; Monoranu, C.M.; Samnick, S.; Ernestus, R.I.; et al. Clinical Utility of Different Approaches for Detection of Late Pseudoprogression in Glioblastoma With O-(2-[18F]Fluoroethyl)-L-Tyrosine PET. Clin. Nucl. Med. 2019, 44, 695–701. [Google Scholar] [CrossRef]
- Grosu, A.L.; Astner, S.T.; Riedel, E.; Nieder, C.; Wiedenmann, N.; Heinemann, F.; Schwaiger, M.; Molls, M.; Wester, H.J.; Weber, W.A. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int. J. Radiat Oncol. Biol. Phys. 2011, 81, 1049–1058. [Google Scholar] [CrossRef]
- Nael, K.; Bauer, A.H.; Hormigo, A.; Lemole, M.; Germano, I.M.; Puig, J.; Stea, B. Multiparametric MRI for Differentiation of Radiation Necrosis from Recurrent Tumor in Patients With Treated Glioblastoma. AJR Am. J. Roentgenol. 2018, 210, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Goh, M.J.; Kim, S.J.; Kim, J.H. Pseudoprogression in Patients with Glioblastoma: Assessment by Using Volume-weighted Voxel-based Multiparametric Clustering of MR Imaging Data in an Independent Test Set. Radiology 2015, 275, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Yoon, R.G.; Kim, H.S.; Koh, M.J.; Shim, W.H.; Jung, S.C.; Kim, S.J.; Kim, J.H. Differentiation of Recurrent Glioblastoma from Delayed Radiation Necrosis by Using Voxel-based Multiparametric Analysis of MR Imaging Data. Radiology 2017, 285, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, H.; Shafaat, O.; Bernstock, J.D.; Brooks, M.D.; Elsayed, G.A.; Chen, J.A.; Szerip, P.; Chagoya, G.; Gessler, F.; Sotoudeh, E.; et al. Artificial Intelligence in the Management of Glioma: Era of Personalized Medicine. Front. Oncol. 2019, 9, 768. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, L.F.; Zhang, X.; Zhang, X.; Hu, Y.C.; Han, Y.; Liu, Z.C.; Nan, H.Y.; Sun, Q.; Sun, Y.Z.; et al. Radiomics strategy for glioma grading using texture features from multiparametric MRI. J Magn. Reason. Imaging 2018, 48, 1518–1528. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, L.F.; Zhang, X.; Han, Y.; Nan, H.Y.; Hu, Y.C.; Hu, B.; Yan, S.L.; Zhang, J.; Cheng, D.L.; et al. Glioma Grading on Conventional MR Images: A Deep Learning Study with Transfer Learning. Front. Neurosci. 2018, 12, 804. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, S.T.; Wei, J.W.; Dong, D.; Wang, X.C.; Yang, G.Q.; Tian, J.; Zhang, H. A radiomics nomogram may improve the prediction of IDH genotype for astrocytoma before surgery. Eur. Radiol. 2019, 29, 3325–3337. [Google Scholar] [CrossRef]
- Wei, J.; Yang, G.; Hao, X.; Gu, D.; Tan, Y.; Wang, X.; Dong, D.; Zhang, S.; Wang, L.; Zhang, H.; et al. A multi-sequence and habitat-based MRI radiomics signature for preoperative prediction of MGMT promoter methylation in astrocytomas with prognostic implication. Eur. Radiol. 2019, 29, 877–888. [Google Scholar] [CrossRef]
- Fang, S.; Fan, Z.; Sun, Z.; Li, Y.; Liu, X.; Liang, Y.; Liu, Y.; Zhou, C.; Zhu, Q.; Zhang, H.; et al. Radiomics Features Predict Telomerase Reverse Transcriptase Promoter Mutations in World Health Organization Grade II Gliomas via a Machine-Learning Approach. Front. Oncol. 2021, 10, 606741. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, N.; Sun, H.; Liu, Y.; Yang, X.; Wang, W.; Zhang, S.; Tan, Q.; Su, J.; Gong, Q.; et al. Automated machine learning based on radiomics features predicts H3 K27M mutation in midline gliomas of the brain. Neuro. Oncol. 2020, 22, 393–401. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Q.; Wang, L.; Liu, Y.; Li, B.; Liang, Z.; Gao, P.; Zheng, K.; Zhao, B.; Lu, H. Radiomics Strategy for Molecular Subtype Stratification of Lower-Grade Glioma: Detecting IDH and TP53 Mutations Based on Multimodal MRI. J Magn. Reason. Imaging 2018, 48, 916–926. [Google Scholar] [CrossRef]
- Haubold, J.; Demircioglu, A.; Gratz, M.; Glas, M.; Wrede, K.; Sure, U.; Antoch, G.; Keyvani, K.; Nittka, M.; Kannengiesser, S.; et al. Non-invasive tumor decoding and phenotyping of cerebral gliomas utilizing multiparametric (18)F-FET PET-MRI and MR Fingerprinting. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Bai, H.X.; Zhou, H.; Su, C.; Bi, W.L.; Agbodza, E.; Kavouridis, V.K.; Senders, J.T.; Boaro, A.; Beers, A.; et al. Residual Convolutional Neural Network for the Determination of IDH Status in Low- and High-Grade Gliomas from MR Imaging. Clin. Cancer Res. 2018, 24, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Park, S.Y.; Nam, S.J.; Chun, S.M.; Jo, Y.; Kim, J.H. Prediction of Core Signaling Pathway by Using Diffusion- and Perfusion-based MRI Radiomics and Next-generation Sequencing in Isocitrate Dehydrogenase Wild-type Glioblastoma. Radiology 2020, 294, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wong, K.K.; Young, G.S.; Guo, L.; Wong, S.T. Support vector machine multiparametric MRI identification of pseudoprogression from tumor recurrence in patients with resected glioblastoma. J. Magn. Reason. Imaging 2011, 33, 296–305. [Google Scholar] [CrossRef]
- Tiwari, P.; Prasanna, P.; Wolansky, L.; Pinho, M.; Cohen, M.; Nayate, A.P.; Gupta, A.; Singh, G.; Hatanpaa, K.J.; Sloan, A.; et al. Computer-Extracted Texture Features to Distinguish Cerebral Radionecrosis from Recurrent Brain Tumors on Multiparametric MRI: A Feasibility Study. AJNR Am. J. Neuroradiol. 2016, 37, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, N.; Kotrotsou, A.; Hassan, A.; Elshafei, N.; Hassan, I.; Ahmed, S.; Abrol, S.; Agarwal, A.; El Salek, K.; Bergamaschi, S.; et al. Multicenter study demonstrates radiomic features derived from magnetic resonance perfusion images identify pseudoprogression in glioblastoma. Nat. Commun. 2019, 10, 3170. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Wang, Y.-D.; Wu, S.-M.; Rui, W.-T.; Ma, D.-N.; Duan, Y.; Zhang, A.-N.; Yao, Z.-W.; Yang, G.; Yu, Y.-P. Differentiation of Treatment-Related Effects from Glioma Recurrence Using Machine Learning Classifiers Based Upon Pre-and Post-Contrast T1WI and T2 FLAIR Subtraction Features: A Two-Center Study. Cancer Manag. Res. 2020, 12, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, X.; Zhang, Z.; Yang, R.; Zhu, Y.; Jiang, X. Differentiation of true-progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide by GLCM texture analysis of conventional MRI. Clin. Imaging 2015, 39, 775–780. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.E.; Jo, Y.; Shim, W.H.; Nam, S.J.; Kim, J.H.; Yoo, R.E.; Choi, S.H.; Kim, H.S. Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro. Oncol. 2019, 21, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.S.; Jeon, S.H.; Kim, I.H.; Kim, I.A. Prediction of Pseudoprogression versus Progression using Machine Learning Algorithm in Glioblastoma. Sci. Rep. 2018, 8, 12516. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Chen, Y.; Li, Z.C.; Li, Q.; Zhang, J.; Liu, J.; Zhai, G. A Deep Learning-Based Radiomics Model for Prediction of Survival in Glioblastoma Multiforme. Sci. Rep. 2017, 7, 10353. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Qian, Z.; Sun, Z.; Xu, K.; Wang, K.; Liu, S.; Fan, X.; Li, S.; Zhang, Z.; et al. A radiomic signature as a non-invasive predictor of progression-free survival in patients with lower-grade gliomas. Neuroimage Clin. 2018, 20, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Mu, W.; Wang, X.C.; Yang, G.Q.; Gillies, R.J.; Zhang, H. Improving survival prediction of high-grade glioma via machine learning techniques based on MRI radiomic, genetic and clinical risk factors. Eur. J. Radiol. 2019, 120, 108609. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Bendszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Krainak, D. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro. Oncol. 2015, 17, 1188–1198. [Google Scholar] [PubMed]
- Welker, K.; Boxerman, J.; Kalnin, A.; Kaufmann, T.; Shiroishi, M.; Wintermark, M.A. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. Am. J. Neuroradiol. 2015, 36, E41–E51. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Andronesi, O.; Bogner, W.; Choi, I.Y.; Coello, E.; Cudalbu, C. Minimum Reporting Standards for in vivo Magnetic Resonance Spectroscopy (MRSinMRS): Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4484. [Google Scholar] [CrossRef] [PubMed]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [PubMed]
| Study | TP | TRCs | Modality | Tracer | Parameter | Cutoff | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| Galldiks et al. [28] | 11 | 11 | PET | 18F-FET | TBRmax, | 2.3 | 100% | 91% | 96% |
| Galldiks et al. [29] | 121 | 11 | PET/MRI | 18F-FET | TBRmean | 2.0 | 93% | 100% | 93% |
| Kebir et al. [30] | 19 | 7 | PET | 18F-FET | TBRmax | 1.9 | 84% | 86% | 85% |
| Jena et al. [31] | 25 | 10 | PET/MRI | 18F-FDG | TBRmax TBRmean | 1.579 1.179 | 93.3% 90.0% | 72.7% 81.8% | 87.8% 87.8% |
| Deuschl et al. [32] | 35 | 15 | PET/MRI | 11C–MET | TBRmax TBRmean | 1.83 1.33 | 97.14% | 93.33% | 96% |
| Park et al. [33] | 38 | 5 | PET/MRI | 11C–MET | TBRmax | 1.40 | 82.1% | 66.7% | - |
| Werner et al. [34] | 38 | 10 | PET/MRI | 18F-FET | TBRmax TBRmean | 1.95 | 100% | 79% | 83% |
| Maurer et al. [35] | 94 | 33 | PET | 18F-FET | TBRmax | 1.95 | 70% | 71% | 70% |
| Pellerin et al. [36] | 34 | 24 | PET/MRI | 18F-DOPA | Tumor isocontour maps and T-maps | - | 100% | 94.1% | - |
| Study | TP | TRCs | Modality Imaging | Parameter | Cut-off for TP | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Lee et al. [41] | 10 | 12 | DWI | Mean ADC | 1200 × 10−6 mm2/s | 80.0% | 83.3% | 81.2% |
| Yoo et al. [25] | 24 | 18 | DWI | The 5th percentile of ADC (b = 1000) | 915 × 10−6 mm2/s | 83% | 67% | - |
| Chu et al. [43] | 15 | 15 | DWI | The 5th percentile of ADC (b = 3000) | 645 × 10−6 mm2/s | 93.33% | 100% | 88.9% |
| Kim et al. [49] | 31 | 20 | IVIM | Mean 90th percentile for perfusion (f) Mean 90th percentile for nCBV | 0.056 2.892 | 87.1% 83.9% | 95.0% 95.0% | - - |
| Kong et al. [68] | 33 | 26 | DSC | Mean rCBV | 1.47 | 81.5% | 77.8% | - |
| Baek et al. [70] | 42 | 37 | DSC | Skewness and kurtosis of normalized CBV | 1.27 | 85.7% | 89.2% | - |
| Yun et al. [79] | 17 | 16 | DCE | Mean Ktrans/mean Ve | 0.347/0.570 | 59%/88% | 94%/56% | - |
| Yoo et al. [75] | 16 | 8 | DCE | Mean Ve | 0.873 | 100% | 63% | 88% |
| Thomas et al. [77] | 24 | 13 | DCE | Vp90%/mean Vp/mean Ktrans | 3.9/3.7/3.6 | 92%/85%/69% | 85%/79%/79% | - |
| Bisdas et al. [78] | 12 | 6 | DCE | Ktrans/IAUC | 0.91/15.35 | 100%/75% | 83%/67% | - |
| Suh et al. [76] | 43 | 36 | DCE | mAUCRH/50thAUCR | 0.31/0.19 | 90.1%/87.2% | 82.9%/83.1% | - |
| Chung et al. [72] | 32 | 25 | DCE | mAUCRH/90thAUCR | 0.23/0.32 | 93.8%/90.6% | 88%/88% | - - |
| Ma et al. [93] | 20 | 12 | APT | APTmean/APTmax | 2.42/2.54 | 85.0%/95% | 100%/91.7% | - |
| Choi et al. [82] | 34 | 28 | ASL/DSC | CBF/normalized rCBV | - | 94.1% | 82.1% | 88.7% |
| Nael et al. [101] | 34 | 12 | DWI/DSC/DCE | rCBV/Ktrans | 2.2/0.08 | 94.1 | 91.6 | 92.8 |
| Razek et al. [56] | 24 | 18 | ASL/DTI | CBF/FA/MD | - | 93.8% | 95.8% | 95% |
| Seeger et al. [73] | 23 | 17 | DSC/DCE/ASL/MRS | normalized rCBV or rCBF /Ktrans/rCBF/Cho/Crn | rCBV ≥ 3.9 or rCBF ≥ 4.1, Ktrans ≥ 0.08, rCBF ≥ 2.5, Cho/Crn ≥ 1.89 | 82.6% | 100% | 90% |
| Wang et al. [58] | 21 | 20 | DSC/DTI | FA/CL/rCBVmax | 0.55 | 76% | 95% | - |
| Prager et al. [6] | 58 | 10 | DWI/DSC | ADC/normalized rCBV | ADC ≤ 1.49 × 10−3 mm2/s/rCBV ≥1.27 | 51.2% | 100% | - |
| Park et al. [102] | 45 | 63 | DWI/DSC/DCE | 10th percentileof ADC (ADC10)/ 90th percentile of normalized rCBV (nCBV90)/ 90th percentile of IAUC (IAUC90) | ADC10 < 1.14 × 10 mm2/s/ nCBV90 of 3.19/ IAUC90 of 19.42/ total cluster score of 5.91 | 91.1% | 90.5% | 90.7% |
| Imaging Method | Parameters | Pattern Associated with TP | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Conventional MRI and TI-CE | No | Corpus callosum involvement; Subependymal enhancement | Widely applied; | Overlapping images | [23,24] |
| DWI | ADC | Lower mean ADC value | Characterize tissues and pathologic processes at the microscopic level; reflect the high cellularity | Influenced by many factors, such as inflammatory; Ignore the effects of perfusion | [16,48] |
| IVIM | D D* f | Higher f and D* Lower D | No contrast required; repeatedly acquire images; simultaneous acquisition of diffusion and perfusion parameters | Low cerebral perfusion fraction; susceptibility artifacts; low signal to noise ratio | [49,52,53] |
| DTI | FA MD | Lower MD and higher FA values | Measured directional variation of water diffusivity | Affected by many factors Susceptibility artifacts b value setting long acquisition time | [27,58] |
| DSC | rCBV rCBF MTT | Higher rCBV or rCBF value | Widely available; fast acquisition speed and simple post-processing | Poorer spatial resolution; susceptibility artifacts; contrast agent leakage | [73,74] |
| DCE | Ktrans Ve Vp IAUC | Higher Ktrans, Ve and Vp value | Higher spatial resolution; less susceptible to artifacts | Longer scan time; decreased temporal resolution; complex pharmacokinetic modeling | [72,76,77] |
| ASL | rCBF | Higher CBF values | No contrast required; less susceptibility artifacts | Low signal-to-noise ratio; risk of movement artifacts | [80,84,86] |
| MRS | Cho/NAA NAA/Cr Cho/Cr | Higher Cho/NAA and Cho/Cr and lower NAA/Cr | Reflects tissue metabolism; higher diagnostic accuracy | Long scan times required; voxel selection; metabolic overlap | [47] |
| APT | APTw | Higher APTw signals | Reflect cell proliferation; guide biopsies | Signal weakness; further optimized | [97,98] |
| 18F-FDG PET | SUVTBR | Higher TBR | Widely available | High background signal | [5] |
| 11C-MET PET | SUVTBR | SUVs tend to be higher | Lower background activity | Short half-life; requires an on-site cyclotron | [32] |
| 18F-FET PET | SUVTBR | Higher TBR | High contrast longer half-life efficient synthesis | Requires more research | [28,99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, D.; Yang, G.; Jing, H.; Tan, Y.; Zhao, B.; Zhang, H. Tumor Progression and Treatment-Related Changes: Radiological Diagnosis Challenges for the Evaluation of Post Treated Glioma. Cancers 2022, 14, 3771. https://doi.org/10.3390/cancers14153771
Qin D, Yang G, Jing H, Tan Y, Zhao B, Zhang H. Tumor Progression and Treatment-Related Changes: Radiological Diagnosis Challenges for the Evaluation of Post Treated Glioma. Cancers. 2022; 14(15):3771. https://doi.org/10.3390/cancers14153771
Chicago/Turabian StyleQin, Danlei, Guoqiang Yang, Hui Jing, Yan Tan, Bin Zhao, and Hui Zhang. 2022. "Tumor Progression and Treatment-Related Changes: Radiological Diagnosis Challenges for the Evaluation of Post Treated Glioma" Cancers 14, no. 15: 3771. https://doi.org/10.3390/cancers14153771
APA StyleQin, D., Yang, G., Jing, H., Tan, Y., Zhao, B., & Zhang, H. (2022). Tumor Progression and Treatment-Related Changes: Radiological Diagnosis Challenges for the Evaluation of Post Treated Glioma. Cancers, 14(15), 3771. https://doi.org/10.3390/cancers14153771






