Novel Drug Delivery Method Targeting Para-Aortic Lymph Nodes by Retrograde Infusion of Paclitaxel into Pigs’ Thoracic Duct
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Animals
2.2. Porcine Thoracic duct Cannulation Model
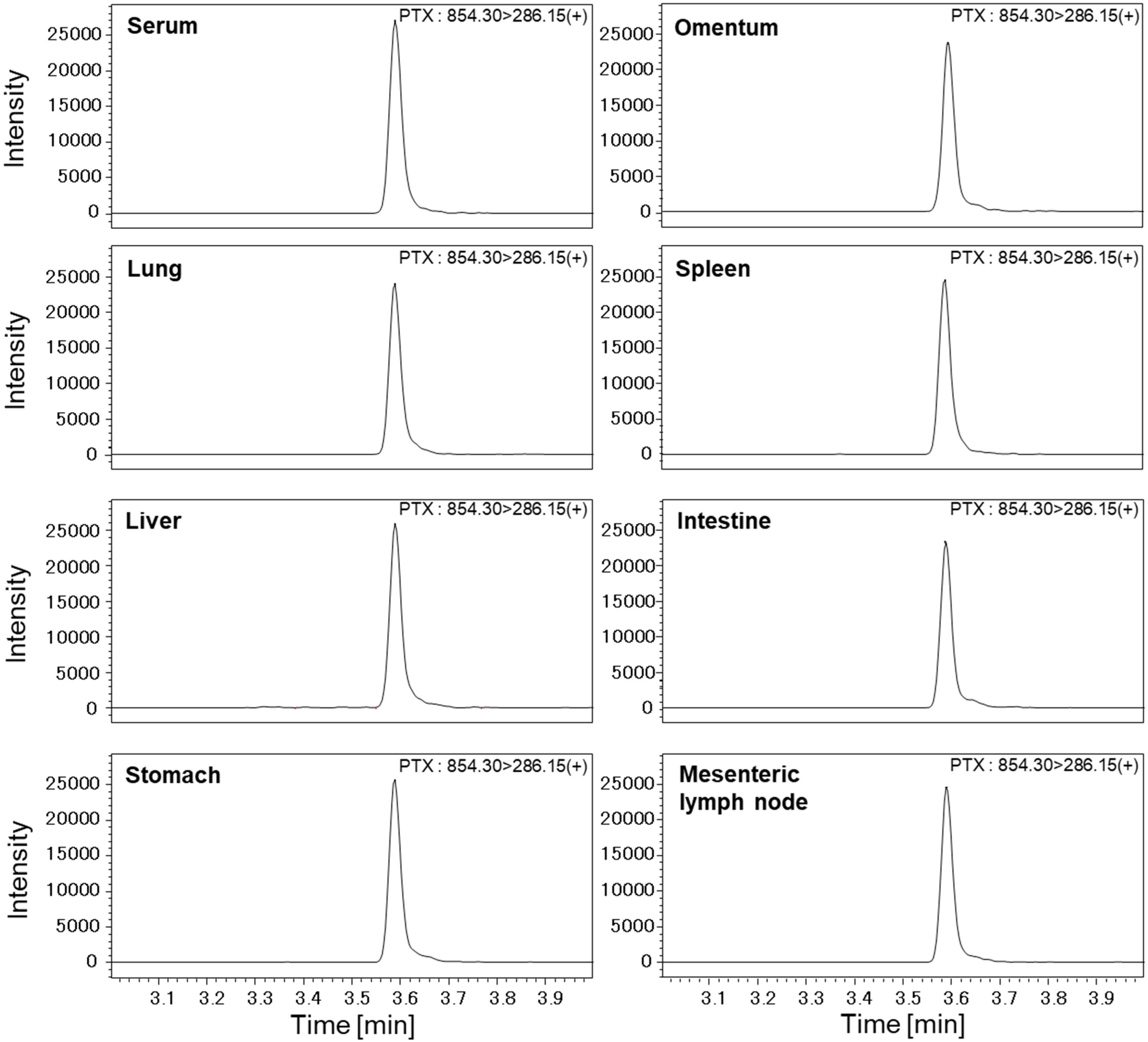
2.3. Measurement of PTX Concentrations in Various Organs with High-Performance Liquid Chromatography–Mass Spectrometry (LC-MS/MS)
2.4. Statistical Analysis
3. Results
3.1. Concentrations of PTX in Serum and Urine
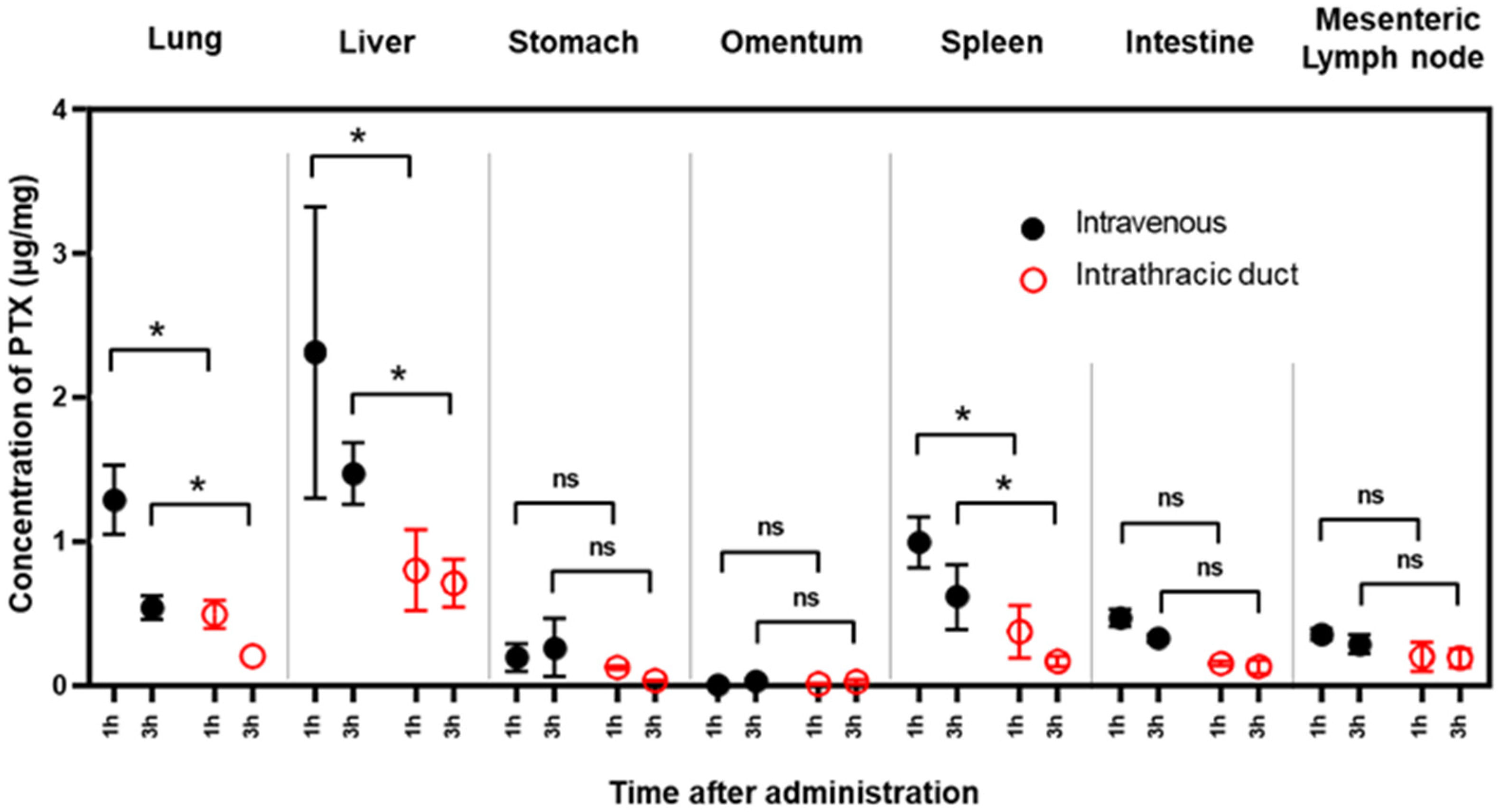
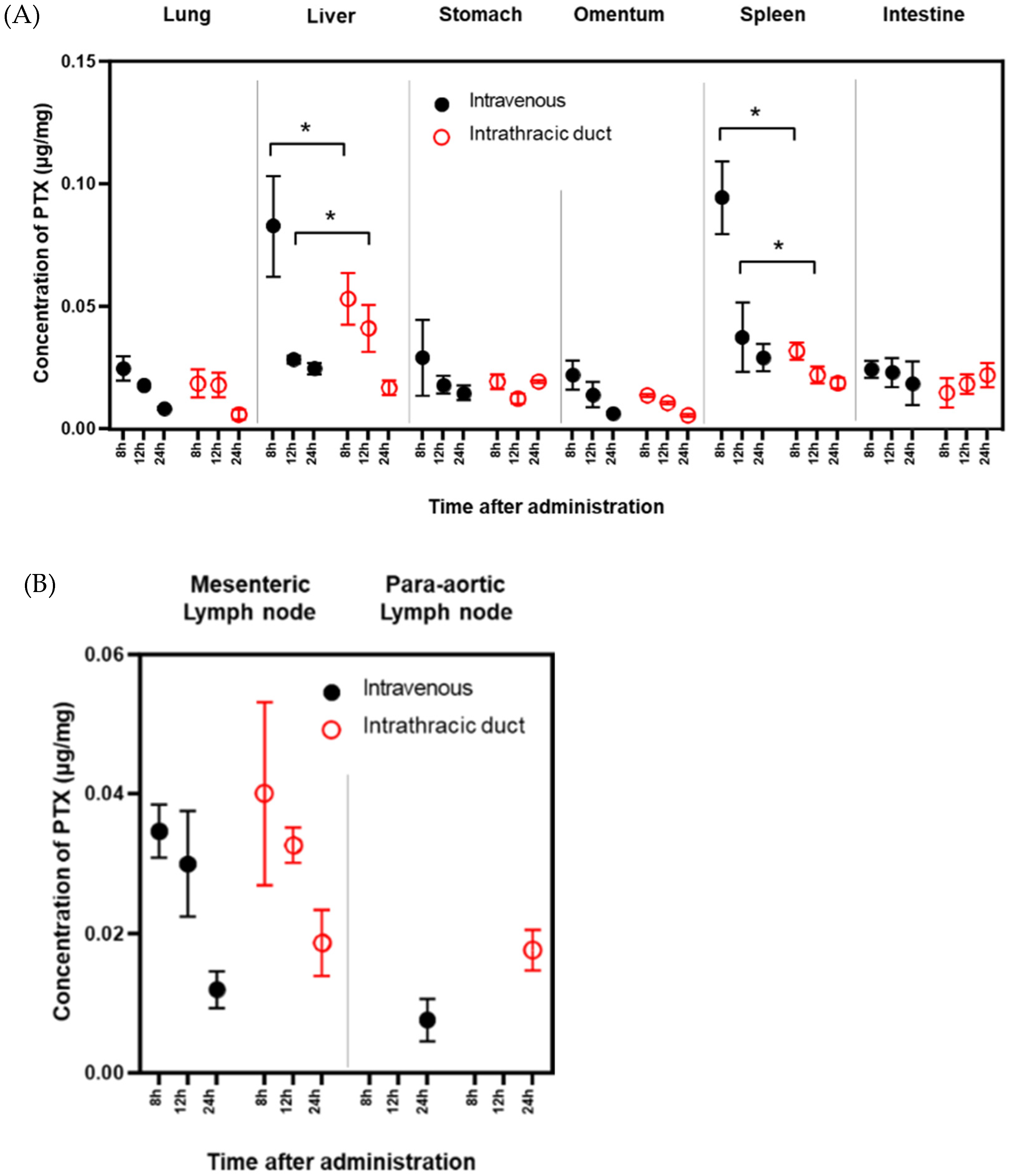
3.2. PTX Concentrations in Various Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sundriyal, D.; Kumar, N.; Dubey, S.K.; Walia, M. Virchow’s node. BMJ Case Rep. 2013, 2013, bcr2013200749. [Google Scholar] [CrossRef] [Green Version]
- Lopez, F.; Rodrigo, J.P.; Silver, C.E.; Haigentz, M., Jr.; Bishop, J.A.; Strojan, P.; Hartl, D.M.; Bradley, P.J.; Mendenhall, W.M.; Suarez, C.; et al. Cervical lymph node metastases from remote primary tumor sites. Head Neck 2016, 38 (Suppl. 1), E2374–E2385. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, Y.; Omori, T.; Demura, K.; Miyata, H.; Sugimura, K.; Ohue, M.; Kobatashi, S.; Takahashi, H.; Doki, Y.; Yano, M. A Multidisciplinary Approach for Advanced Gastric Cancer. Anticancer Res. 2015, 35, 6739–6746. [Google Scholar] [PubMed]
- Kim, J.H.; Park, S.R.; Ryu, M.H.; Ryoo, B.Y.; Kim, K.P.; Kim, B.S.; Yoo, M.W.; Yook, J.H.; Kim, B.S.; Kim, J.; et al. Phase II Study of Induction Chemotherapy with Docetaxel, Capecitabine, and Cisplatin Plus Bevacizumab for Initially Unresectable Gastric Cancer with Invasion of Adjacent Organs or Paraaortic Lymph Node Metastasis. Cancer Res. Treat. 2018, 50, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Morita, S.; Fukagawa, T.; Fujiwara, H.; Katai, H. The clinical significance of para-aortic nodal dissection for advanced gastric cancer. Eur. J. Surg. Oncol. 2016, 42, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Nakao, A.; Fujii, T.; Sugimoto, H.; Kanazumi, N.; Nomoto, S.; Kodera, Y.; Takeda, S. Pancreatic cancer with paraaortic lymph node metastasis: A contraindication for radical surgery? Pancreas 2009, 38, e13–e17. [Google Scholar] [CrossRef]
- Bae, S.U.; Han, Y.D.; Cho, M.S.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Oncologic Outcomes of Colon Cancer Patients with Extraregional Lymph Node Metastasis: Comparison of Isolated Paraaortic Lymph Node Metastasis with Resectable Liver Metastasis. Ann. Surg. Oncol. 2016, 23, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.S.; Tan, G.H.; Teo, M.C. Management of para-aortic lymph node metastasis in colorectal patients: A systemic review. Surg. Oncol. 2016, 25, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Pomel, C.; Filleron, T.; De Cuypere, M.; Mery, E.; Querleu, D.; Gladieff, L.; Poilblanc, M.; Ferron, G. Prognostic relevance of celiac lymph node involvement in ovarian cancer. Int. J. Gynecol. Cancer 2014, 24, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, H.; Fujiwara, Y.; Fukushima, R.; Nashimoto, A.; Yabusaki, H.; Imano, M.; Imamoto, H.; Kodera, Y.; Uenosono, Y.; Amagai, K.; et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J. Clin. Oncol. 2018, 36, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamaguchi, H.; Ohzawa, H.; Miyato, H.; Kanamaru, R.; Kurashina, K.; Hosoya, Y.; Lefor, A.K.; Sata, N.; Kitayama, J. Intraperitoneal administration of paclitaxel combined with S-1 plus oxaliplatin as induction therapy for patients with advanced gastric cancer with peritoneal metastases. Ann. Surg. Oncol. 2021, 28, 3863–3870. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Verma, A.; Teja, B.V.; Shukla, P.; Mishra, P.R. Development of stabilized Paclitaxel nanocrystals: In-vitro and in-vivo efficacy studies. Eur. J. Pharm. Sci. 2015, 69, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Szebeni, J.; Alving, C.R.; Savay, S.; Barenholz, Y.; Priev, A.; Danino, D.; Talmon, Y. Formation of complement-activating particles in aqueous solutions of Taxol: Possible role in hypersensitivity reactions. Int. Immunopharmacol. 2001, 1, 721–735. [Google Scholar] [CrossRef]
- Champion, S.; Cheung, V.L.S.; Wiseman, D. Isolated thoracic duct injury from blunt force trauma. J. Radiol. Case Rep. 2020, 14, 18–29. [Google Scholar] [CrossRef]
- Ilahi, M.; St Lucia, K.; Ilahi, T.B. Anatomy, Thorax, Thoracic Duct. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments—The ARRIVE guidelines. J. Cereb. Blood Flow Metab. 2011, 31, 991–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A El-Gendi, M.; A Zaky, H. Thoracic duct lymph flow: A theoretical concept. Surgery 1970, 68, 786–790. [Google Scholar]
- Oshiro, H.; Osaka, Y.; Tachibana, S.; Aoki, T.; Tsuchiya, T.; Nagao, T. Retrograde Lymphatic Spread of Esophageal Cancer: A Case Report. Medicine 2015, 94, e1139. [Google Scholar] [CrossRef]
- Ryu, W.; Lee, M.K.; Park, M.H.; Hyun, I.Y.; Lee, M.; No, E.J.; Yong, S.J.; Kim, J.S.; Lim, J.H.; Ryu, J.S. Abdominal lymph node metastasis by lymphatic spread through the thoracic duct in patients with non-small-cell lung cancer. Thorac. Cancer 2021, 12, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Soma, D.; Kitayama, J.; Ishigami, H.; Kaisaki, S.; Nagawa, H. Different tissue distribution of paclitaxel with intravenous and intraperitoneal administration. J. Surg. Res. 2009, 155, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.S.; Hong, J.; Windsor, J.A.; Itkin, M.; Phillips, A.R.J. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Front. Physiol. 2019, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.S.; Hong, J.; Windsor, J.A.; Itkin, M.; Phillips, A.R.J. The Influence of Thoracic Duct Resection on Postesophagectomy Hemodynamics. Recent Adv. Dis. Esophagus 1993. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, A.; Kimura, N.; Kaneda, Y.; Ohzawa, H.; Miyato, H.; Yamaguchi, H.; Lefor, A.K.; Nagai, R.; Sata, N.; Kitayama, J.; et al. Novel Drug Delivery Method Targeting Para-Aortic Lymph Nodes by Retrograde Infusion of Paclitaxel into Pigs’ Thoracic Duct. Cancers 2022, 14, 3753. https://doi.org/10.3390/cancers14153753
Saito A, Kimura N, Kaneda Y, Ohzawa H, Miyato H, Yamaguchi H, Lefor AK, Nagai R, Sata N, Kitayama J, et al. Novel Drug Delivery Method Targeting Para-Aortic Lymph Nodes by Retrograde Infusion of Paclitaxel into Pigs’ Thoracic Duct. Cancers. 2022; 14(15):3753. https://doi.org/10.3390/cancers14153753
Chicago/Turabian StyleSaito, Akira, Natsuka Kimura, Yuji Kaneda, Hideyuki Ohzawa, Hideyo Miyato, Hironori Yamaguchi, Alan Kawarai Lefor, Ryozo Nagai, Naohiro Sata, Joji Kitayama, and et al. 2022. "Novel Drug Delivery Method Targeting Para-Aortic Lymph Nodes by Retrograde Infusion of Paclitaxel into Pigs’ Thoracic Duct" Cancers 14, no. 15: 3753. https://doi.org/10.3390/cancers14153753
APA StyleSaito, A., Kimura, N., Kaneda, Y., Ohzawa, H., Miyato, H., Yamaguchi, H., Lefor, A. K., Nagai, R., Sata, N., Kitayama, J., & Aizawa, K. (2022). Novel Drug Delivery Method Targeting Para-Aortic Lymph Nodes by Retrograde Infusion of Paclitaxel into Pigs’ Thoracic Duct. Cancers, 14(15), 3753. https://doi.org/10.3390/cancers14153753






