Central Lymph Node Ratio Predicts Recurrence in Patients with N1b Papillary Thyroid Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Postoperative Management and Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Optimal Cutoff Values Determined by ROC Curve Analysis
3.2. Comparison of Baseline Clinicopathological Characteristics According to LNR
3.3. Comparison of Baseline Clinicopathological Characteristics According to CLNR
3.4. Comparison of Baseline Clinicopathological Characteristics According to LLNR
3.5. Univariate and Multivariate Analyses of the Risk Factors for Recurrence
3.6. Recurrence Patterns for the Study Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, E.D.; Pantanowitz, L.; Hornick, J.L. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021, 9, 193–194. [Google Scholar] [CrossRef]
- Ganly, I.; Nixon, I.J.; Wang, L.Y.; Palmer, F.L.; Migliacci, J.C.; Aniss, A.; Sywak, M.; Eskander, A.E.; Freeman, J.L.; Campbell, M.J. Survival from differentiated thyroid cancer: What has age got to do with it? Thyroid 2015, 25, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.J.; Kim, W.G.; Kim, T.H.; Kim, H.K.; Kim, B.H.; Yi, H.-S.; Kim, E.S.; Kim, H.; Kim, Y.N.; Kim, E.H. Disease-specific mortality of differentiated thyroid cancer patients in Korea: A multicenter cohort study. Endocrinol. Metab. 2017, 32, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.H.; Elashoff, D.A.; Abemayor, E.; St John, M.A. Surgery for papillary thyroid carcinoma: Is lobectomy enough? Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Radowsky, J.S.; Howard, R.S.; Burch, H.B.; Stojadinovic, A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014, 24, 241–244. [Google Scholar] [CrossRef]
- Gardner, R.E.; Tuttle, R.M.; Burman, K.D.; Haddady, S.; Truman, C.; Sparling, Y.H.; Wartofsky, L.; Sessions, R.B.; Ringel, M.D. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 309–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Kim, H.J.; Park, C.S.; Kim, B.W. Frozen section analysis of central lymph nodes in papillary thyroid cancer: The significance in determining the extent of surgery. Gland Surg. 2022, 11, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for recurrence of papillary thyroid carcinoma with clinically node-positive lateral neck. Ann. Surg. Oncol. 2015, 22, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Y.; Yan, T.; Qiu, W.W.; Fan, Y.B.; Yang, Z.L. The prognosis of skip metastasis in papillary thyroid microcarcinoma is better than that of continuous metastasis. J. Clin. Endocrinol. Metab. 2022, 107, 1589–1598. [Google Scholar] [CrossRef]
- Shaha, A.R. TNM classification of thyroid carcinoma. World J. Surg. 2007, 31, 879–887. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, M.J.; Oh, H.S.; Park, S.; Song, D.E.; Sung, T.Y.; Kim, T.Y.; Chung, K.W.; Kim, W.B.; Shong, Y.K.; et al. Prognostic implication of N1b classification in the eighth edition of the tumor-node-metastasis staging system of differentiated thyroid cancer. Thyroid 2018, 28, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American joint committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): What changed and why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Song, Y.; Xu, G.; Bai, Y.; Wang, T.; Fei, K.; Zhang, B. Level IIb neck dissection guided by fine-needle aspiration for N1b papillary thyroid carcinoma. Surg. Oncol. 2022, 40, 101705. [Google Scholar] [CrossRef]
- Nam, S.H.; Bae, M.R.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral. Oncol. 2018, 87, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwogu, C.E.; Groman, A.; Fahey, D.; Yendamuri, S.; Dexter, E.; Demmy, T.L.; Miller, A.; Reid, M. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann. Thorac. Surg. 2012, 93, 1614–1619; discussion 1619–1620. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, K.; Hosoda, K.; Ema, A.; Watanabe, M. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur. J. Surg. Oncol. 2016, 42, 1253–1260. [Google Scholar] [CrossRef]
- Sjo, O.H.; Merok, M.A.; Svindland, A.; Nesbakken, A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis. Colon Rectum 2012, 55, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Schneider, D.F.; Chen, H.; Sippel, R.S. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann. Surg. Oncol. 2013, 20, 1906–1911. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for recurrence after treatment of N1b papillary thyroid carcinoma. Ann. Surg. 2019, 269, 966–971. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, K.; Park, S.Y.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; Ahn, H.S.; et al. Refining the eighth edition AJCC TNM classification and prognostic groups for papillary thyroid cancer with lateral nodal metastasis. Oral. Oncol. 2018, 78, 80–86. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.H.; Lee, C.H.; Chen, H.A.; Loh, E.W.; Tam, K.W. Prophylactic central neck dissection for papillary thyroid carcinoma with clinically uninvolved central neck lymph nodes: A systematic review and meta-analysis. World J. Surg. 2018, 42, 2846–2857. [Google Scholar] [CrossRef]
- Liu, F.H.; Kuo, S.F.; Hsueh, C.; Chao, T.C.; Lin, J.D. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J. Surg. Oncol. 2015, 112, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Randolph, G.W.; Duh, Q.Y.; Heller, K.S.; LiVolsi, V.A.; Mandel, S.J.; Steward, D.L.; Tufano, R.P.; Tuttle, R.M.; American Thyroid Association Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal Surgery. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012, 22, 1144–1152. [Google Scholar] [CrossRef] [Green Version]
- Sakin, A.; Aldemir, M.N. Lymph node ratio predicts long-term survival in lymph node-positive breast cancer. Eur. J. Breast Health 2020, 16, 270–275. [Google Scholar] [CrossRef]
- Macedo, F.; Sequeira, H.; Ladeira, K.; Bonito, N.; Viana, C.; Martins, S. Metastatic lymph node ratio as a better prognostic tool than the TNM system in colorectal cancer. Future Oncol. 2021, 17, 1519–1532. [Google Scholar] [CrossRef]
- Khan, J.; Ullah, A.; Matolo, N.; Waheed, A.; Nama, N.; Sharma, N.; Ballur, K.; Gilstrap, L.; Singh, S.G.; Ghleilib, I.; et al. Prognostic value of lymph node ratio in cutaneous melanoma: A systematic review. Cureus 2021, 13, e19117. [Google Scholar] [CrossRef]
- Yip, J.; Orlov, S.; Orlov, D.; Vaisman, A.; Hernandez, K.G.; Etarsky, D.; Kak, I.; Parvinnejad, N.; Freeman, J.L.; Walfish, P.G. Predictive value of metastatic cervical lymph node ratio in papillary thyroid carcinoma recurrence. Head Neck 2013, 35, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.G.; Kim, K.; Yim, S.H.; Ryu, H.; Lee, C.R.; Kang, S.W.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; et al. Clinical value of lymph node ratio integration with the 8(th) edition of the UICC TNM classification and 2015 ATA risk stratification systems for recurrence prediction in papillary thyroid cancer. Sci. Rep. 2019, 9, 13361. [Google Scholar] [CrossRef]
- Parvathareddy, S.K.; Siraj, A.K.; Qadri, Z.; Ahmed, S.O.; DeVera, F.; Al-Sobhi, S.; Al-Dayel, F.; Al-Kuraya, K.S. Lymph node ratio is superior to AJCC N stage for predicting recurrence in papillary thyroid carcinoma. Endocr. Connect. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, U.M.; Turanli, S.; Acar, Y.; Berberoglu, U. The prognostic factors for clinical N1b patients in thyroid papillary carcinoma. J. Cancer Res. Ther. 2019, 15, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.W.; Son, S.H.; Hong, C.M.; Jeong, J.H.; Jeong, S.Y.; Ahn, B.C.; Lee, J. Prognostic value of lymph node uptake on pretreatment F-18 fdg pet/ct in patients with N1b papillary thyroid carcinoma. Endocr. Pract. 2019, 25, 787–793. [Google Scholar] [CrossRef]
- Park, Y.M.; Wang, S.G.; Shin, D.H.; Kim, I.J.; Son, S.M.; Lee, B.J. Lymph node status of lateral neck compartment in patients with N1b papillary thyroid carcinoma. Acta Otolaryngol. 2016, 136, 319–324. [Google Scholar] [CrossRef]
- Lee, Y.M.; Sung, T.Y.; Kim, W.B.; Chung, K.W.; Yoon, J.H.; Hong, S.J. Risk factors for recurrence in patients with papillary thyroid carcinoma undergoing modified radical neck dissection. Br. J. Surg. 2016, 103, 1020–1025. [Google Scholar] [CrossRef]
- Ryu, Y.J.; Cho, J.S.; Yoon, J.H.; Park, M.H. Identifying risk factors for recurrence of papillary thyroid cancer in patients who underwent modified radical neck dissection. World J. Surg. Oncol. 2018, 16, 205. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.W.; Dill, T.; Griffin, N.; Jobling, P.; Faulkner, S.; Paul, J.W.; King, S.; Smith, R.; Hondermarck, H. Innervation of papillary thyroid cancer and its association with extra-thyroidal invasion. Sci. Rep. 2020, 10, 1539. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, M.; Ito, Y.; Hirokawa, M.; Miya, A.; Shimizu, K.; Miyauchi, A. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World J. Surg. 2010, 34, 3007–3014. [Google Scholar] [CrossRef]
- Ito, Y.; Kudo, T.; Takamura, Y.; Kobayashi, K.; Miya, A.; Miyauchi, A. Lymph node recurrence in patients with N1b papillary thyroid carcinoma who underwent unilateral therapeutic modified radical neck dissection. World J. Surg. 2012, 36, 593–597. [Google Scholar] [CrossRef] [PubMed]
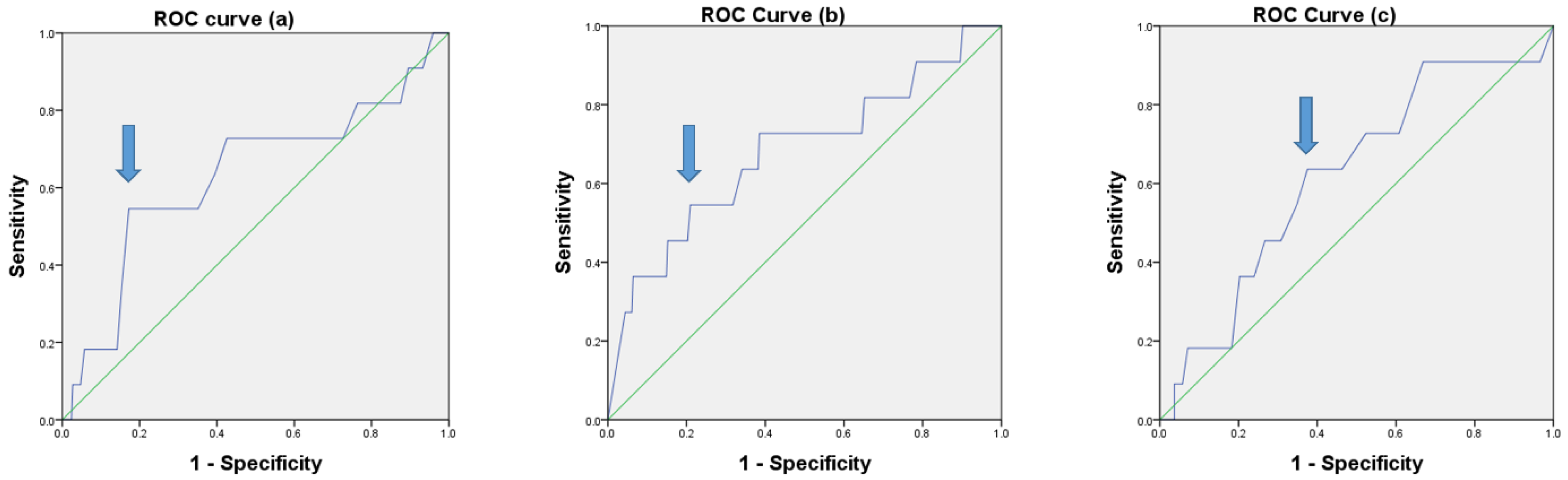
| Cutoff 0.23 | Low LNR (n = 178) | High LNR (n = 129) | p-Value |
|---|---|---|---|
| Age (years) | 44.8 ± 12.6 (range, 15–73) | 40.6 ± 14.1 (range, 15–78) | 0.006 |
| Female | 123 (69.1%) | 80 (62.0%) | 0.222 |
| Tumor size (cm) | 1.5 ± 1.0 (range, 0.3–6.7) | 1.8 ± 1.1 (range, 0.3–5.0) | 0.004 |
| Multifocality | 97 (54.5%) | 81 (62.8%) | 0.161 |
| Bilaterality | 59 (33.1%) | 48 (37.2%) | 0.469 |
| Gross ETE | 32 (18.0%) | 29 (22.5%) | 0.385 |
| Lymphatic invasion | 124 (69.7%) | 110 (85.3%) | 0.002 |
| Vascular invasion | 8 (4.5%) | 16 (12.4%) | 0.016 |
| Perineural invasion | 12 (6.7%) | 7 (5.4%) | 0.811 |
| BRAF positive | 121/134 (90.3%) | 75/94 (79.8%) | 0.033 |
| Harvested LNs | 59.3 ± 22.6 | 53.7 ± 22.9 | 0.033 |
| Central | 13.2 ± 7.5 | 14.3 ± 7.6 | 0.234 |
| Lateral | 46.1 ± 19.3 | 39.5 ± 18.6 | 0.003 |
| Positive LNs | 8.0 ± 4.7 | 17.9 ± 9.3 | <0.001 |
| Central | 3.6 ± 3.1 | 9.2 ± 6.3 | <0.001 |
| Lateral | 4.4 ± 3.0 | 8.7 ± 5.6 | <0.001 |
| T stage | 0.153 | ||
| T1 | 124 (69.7%) | 78 (60.5%) | |
| T2 | 18 (10.2%) | 20 (15.5%) | |
| T3a | 4 (2.2%) | 2 (1.6%) | |
| T3b | 26 (14.6%) | 23 (17.8%) | |
| T4a | 6 (3.4%) | 6 (4.7%) | |
| TNM stage | 0.081 | ||
| Stage I | 130 (73.0%) | 106 (82.2%) | |
| Stage II | 45 (25.3%) | 21 (16.3%) | |
| Stage III | 3 (1.7%) | 2 (1.6%) | |
| Recurrence | 3 (1.7%) | 8 (6.2%) | 0.058 |
| Cutoff 0.7 | Low CLNR (n = 239) | High CLNR (n = 68) | p-Value |
|---|---|---|---|
| Age (years) | 43.8 ± 13.6 (range, 15–78) | 40.4 ± 12.3 (range, 22–77) | 0.063 |
| Female | 170 (71.1%) | 33 (48.5%) | 0.001 |
| Tumor size (cm) | 1.5 ± 1.0 (range, 0.3–6.7) | 2.0 ± 1.2 (range, 0.5–5.0) | 0.001 |
| Multifocality | 140 (58.6%) | 38 (55.9%) | 0.781 |
| Bilaterality | 84 (35.1%) | 23 (33.8%) | 0.886 |
| Gross ETE | 48 (20.1%) | 13 (19.1%) | 1.000 |
| Lymphatic invasion | 178 (74.5%) | 56 (82.4%) | 0.200 |
| Vascular invasion | 15 (6.3%) | 9 (13.2%) | 0.073 |
| Perineural invasion | 15 (6.3%) | 4 (5.9%) | 1.000 |
| BRAF positive | 151/175 (86.3%) | 45/53 (84.9%) | 0.823 |
| Harvested LNs | 56.5 ± 22.8 | 58.8 ± 24.6 | 0.508 |
| Central | 13.9 ± 7.4 | 12.8 ± 8.0 | 0.303 |
| Lateral | 42.7 ± 19.4 | 45.7 ± 18.6 | 0.244 |
| Positive LNs | 10.3 ± 6.7 | 18.6 ± 10.7 | <0.001 |
| Central | 4.6 ± 4.1 | 10.6 ± 6.9 | <0.001 |
| Lateral | 5.7 ± 4.3 | 8.1 ± 5.8 | <0.001 |
| T stage | 0.062 | ||
| T1 | 164 (68.6%) | 38 (55.9%) | |
| T2 | 24 (10.0%) | 14 (20.6%) | |
| T3a | 3 (1.3%) | 3 (4.4%) | |
| T3b | 38 (15.9%) | 11 (16.2%) | |
| T4a | 10 (4.2%) | 2 (2.9%) | |
| TNM stage | 0.210 | ||
| Stage I | 179 (74.9%) | 57 (83.8%) | |
| Stage II | 55 (23.0%) | 11 (16.2%) | |
| Stage III | 5 (2.1%) | 0 (0.0%) | |
| Recurrence | 5 (2.1%) | 6 (8.8%) | 0.017 |
| Cutoff 0.16 | Low LLNR (n = 195) | High LLNR (n = 112) | p-Value |
|---|---|---|---|
| Age (years) | 43.2 ± 12.9 (range, 15–77) | 42.9 ± 14.2 (range, 15–78) | 0.852 |
| Female | 127 (65.1%) | 76 (67.9%) | 0.707 |
| Tumor size (cm) | 1.5 ± 1.0 (range, 0.3–6.7) | 1.8 ± 1.1 (range, 0.4–5.4) | 0.074 |
| Multifocality | 102 (52.3%) | 76 (67.9%) | 0.008 |
| Bilaterality | 56 (28.7%) | 51 (45.5%) | 0.004 |
| Gross ETE | 35 (17.9%) | 26 (23.2%) | 0.299 |
| Lymphatic invasion | 138 (70.8%) | 96 (85.7%) | 0.003 |
| Vascular invasion | 10 (5.1%) | 14 (12.5%) | 0.027 |
| Perineural invasion | 10 (5.1%) | 9 (8.0%) | 0.332 |
| BRAF positive | 128/144 (88.9%) | 41/53 (77.4%) | 0.115 |
| Harvested LNs | 59.1 ± 22.5 | 53.1 ± 23.1 | 0.027 |
| Central | 13.5 ± 7.0 | 13.8 ± 8.5 | 0.736 |
| Lateral | 45.6 ± 18.9 | 39.3 ± 19.2 | 0.005 |
| Positive LNs | 9.3 ± 5.9 | 17.1 ± 10.1 | <0.001 |
| Central | 5.3 ± 4.6 | 7.1 ± 6.6 | 0.010 |
| Lateral | 4.0 ± 2.5 | 10.0 ± 5.3 | <0.001 |
| T stage | 0.642 | ||
| T1 | 131 (67.2%) | 71 (63.4%) | |
| T2 | 24 (12.3%) | 14 (12.5%) | |
| T3a | 5 (2.6%) | 1 (0.9%) | |
| T3b | 29 (14.9%) | 20 (17.9%) | |
| T4a | 6 (3.1%) | 6 (5.4%) | |
| TNM stage | 0.840 | ||
| Stage I | 152 (77.9%) | 84 (75.0%) | |
| Stage II | 40 (20.5%) | 26 (23.2%) | |
| Stage III | 3 (1.5%) | 2 (1.8%) | |
| Recurrence | 4 (2.1%) | 7 (6.3%) | 0.106 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Tumor size | 1.707 (1.176–2.476) | 0.005 | ||
| Vascular invasion | 4.320 (1.145–16.302) | 0.031 | ||
| Perineural invasion | 5.588 (1.482–21.068) | 0.011 | 6.045 (1.593–22.937) | 0.008 |
| Positive LNs (whole) | 1.048 (1.009–1.090) | 0.017 | ||
| Positive LNs (lateral) | 1.095 (1.006–1.191) | 0.037 | ||
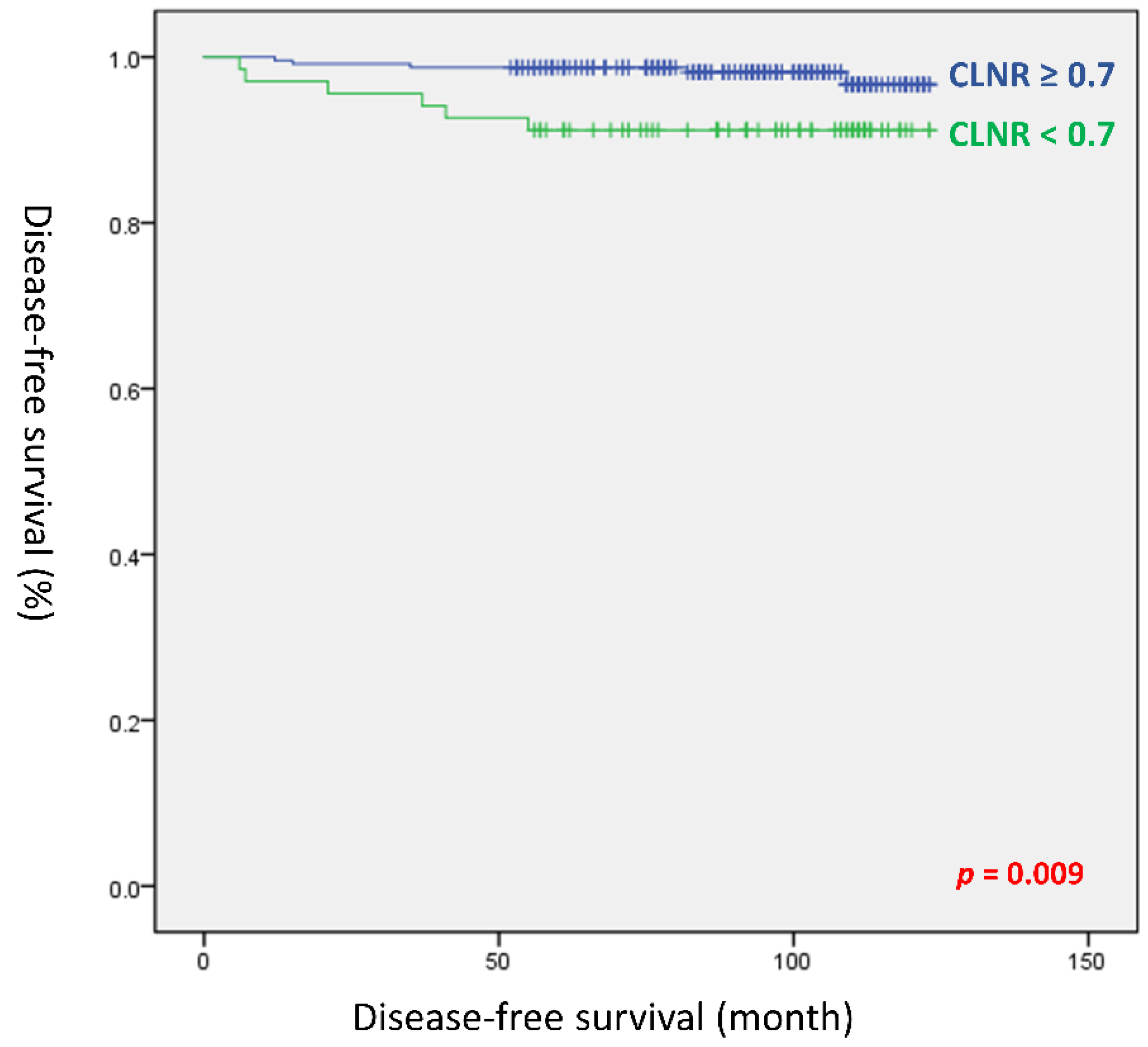
| CLNR | 11.026 (1.242–97.862) | 0.031 | ||
| CLNR < 0.7 | Ref. | Ref. | ||
| ≥0.7 | 4.238 (1.292–13.896) | 0.017 | 4.451 (1.356–14.613) | 0.014 |
| TNM stage | ||||
| I | Ref. | |||
| II | 0.382 (0.048–3.017) | 0.362 | ||
| III | 10.094 (1.262–80.753) | 0.029 | ||
| No. of Patients | Age | Sex | Tumor Size (cm) | Recurrence Site | DFS (Months) | |
|---|---|---|---|---|---|---|
| Low CLNR | 1 | 73 | Male | 2.5 | Contralateral Level-3, -4, -5 LNs | 109 |
| 2 | 30 | Female | 1.3 | Ipsilateral Level-3 LNs | 15 | |
| 3 | 68 | Female | 4.5 | Contralateral Strap muscle | 82 | |
| 4 | 24 | Female | 1.0 | Ipsilateral Level-6 LNs | 35 | |
| 5 | 27 | Female | 1.7 | Ipsilateral Level-4 LNs | 12 | |
| High CLNR | 1 | 49 | Male | 5.0 | Level-5 LNs, left | 55 |
| 2 | 37 | Male | 3.7 | Contralateral Level-2, -3 LNs | 21 | |
| 3 | 32 | Male | 4.0 | Contralateral Level-3, -4 LNs | 41 | |
| 4 | 35 | Female | 1.1 | Lung, left lower | 37 | |
| 5 | 38 | Male | 2.5 | Level-6 LNs, left | 7 | |
| 6 | 29 | Female | 2.7 | Contralateral Level-2, -4, -5 LNs | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, I.K.; Kim, K.; Park, J.; Bae, J.S.; Kim, J.S. Central Lymph Node Ratio Predicts Recurrence in Patients with N1b Papillary Thyroid Carcinoma. Cancers 2022, 14, 3677. https://doi.org/10.3390/cancers14153677
Kang IK, Kim K, Park J, Bae JS, Kim JS. Central Lymph Node Ratio Predicts Recurrence in Patients with N1b Papillary Thyroid Carcinoma. Cancers. 2022; 14(15):3677. https://doi.org/10.3390/cancers14153677
Chicago/Turabian StyleKang, Il Ku, Kwangsoon Kim, Joonseon Park, Ja Seong Bae, and Jeong Soo Kim. 2022. "Central Lymph Node Ratio Predicts Recurrence in Patients with N1b Papillary Thyroid Carcinoma" Cancers 14, no. 15: 3677. https://doi.org/10.3390/cancers14153677
APA StyleKang, I. K., Kim, K., Park, J., Bae, J. S., & Kim, J. S. (2022). Central Lymph Node Ratio Predicts Recurrence in Patients with N1b Papillary Thyroid Carcinoma. Cancers, 14(15), 3677. https://doi.org/10.3390/cancers14153677







