Clinical Impact of Measurable Residual Disease in Acute Myeloid Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. MRD Testing
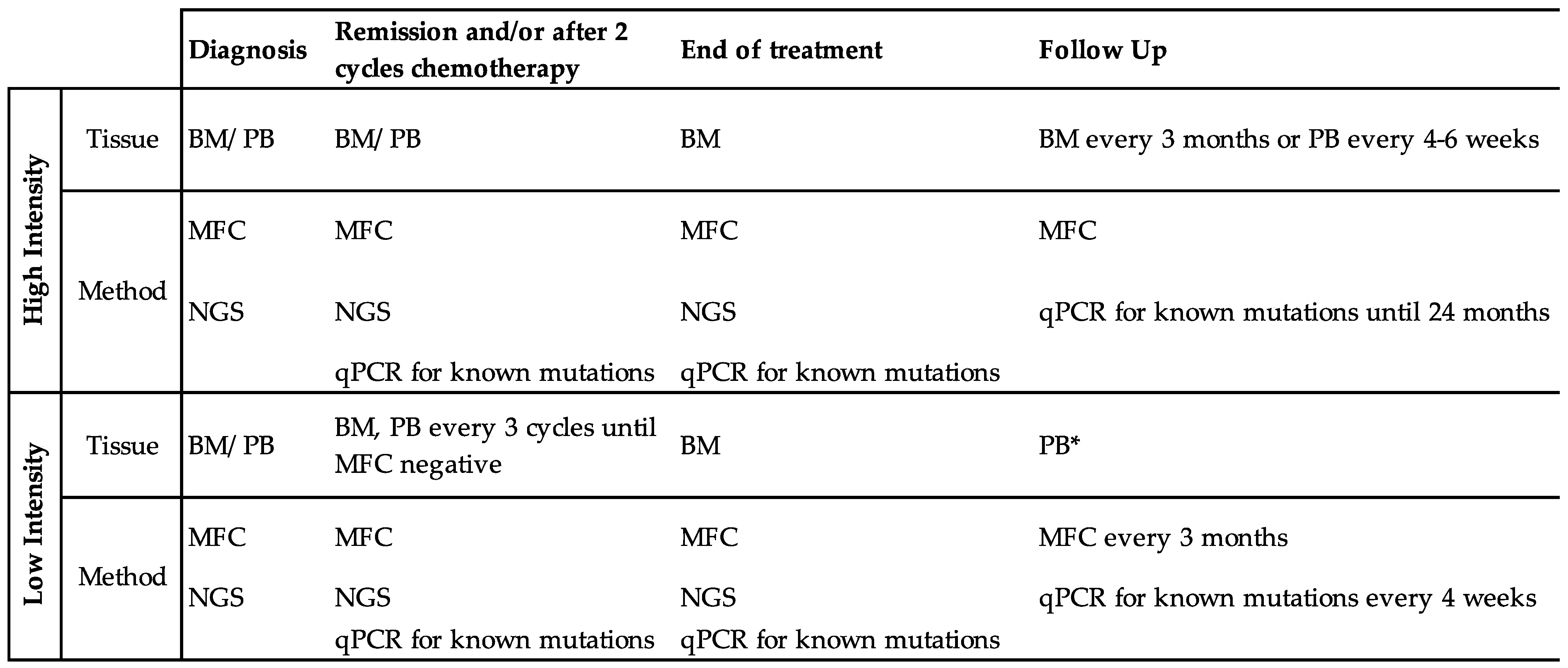
3.1. How Should We Test Patients for MRD?
3.2. At What Time Points Should We Test for MRD?
3.3. How Should We Account for Mutations Associated with Clonal Hematopoiesis?
4. MRD in Initial Therapy
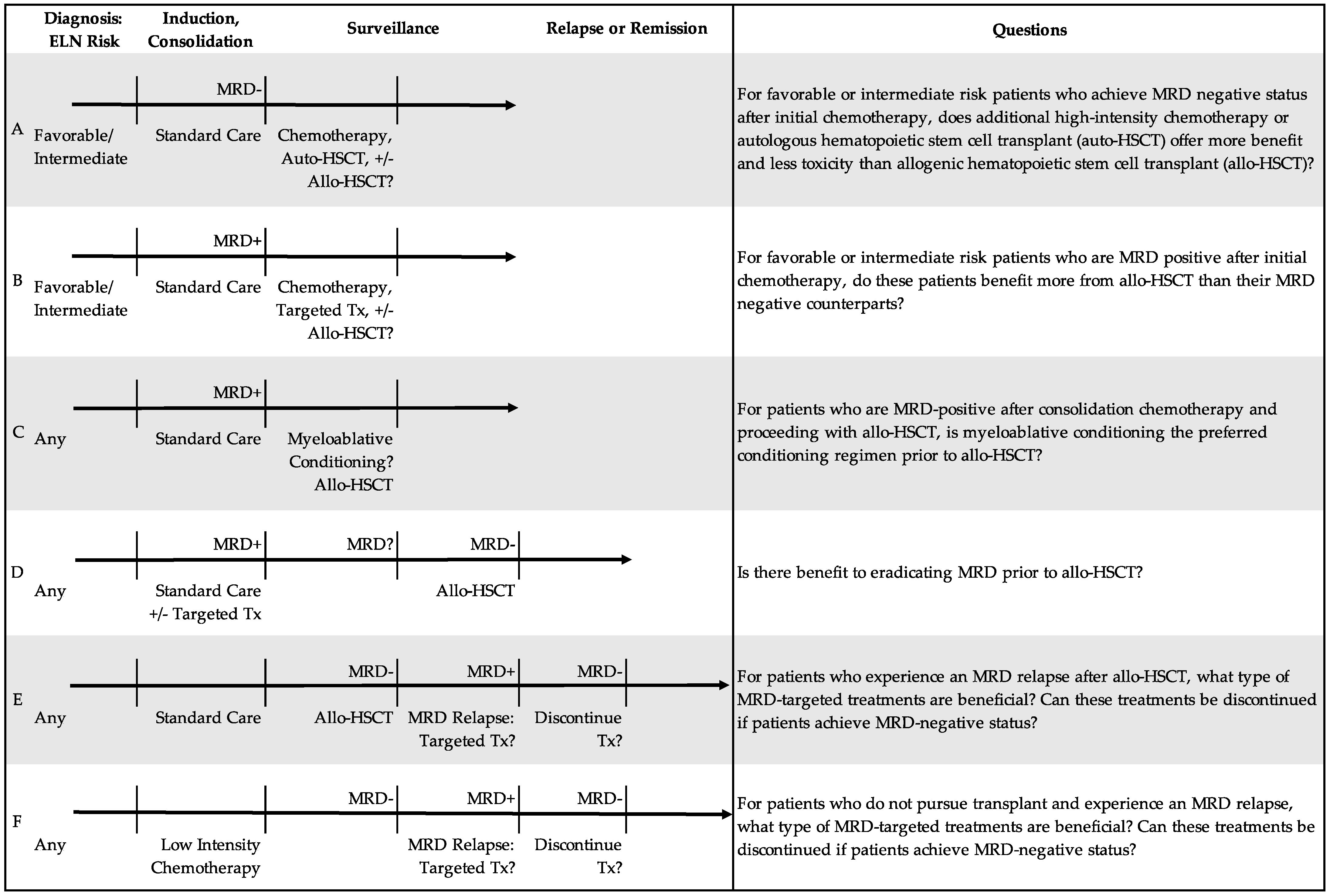
4.1. What Is the Significance of MRD-Positive Status at First Complete Remission?
4.2. What Treatment(s) Can Eradicate MRD?
4.3. Can We Use MRD-Directed Treatment to Prevent Relapse after Initial Chemotherapy?
5. MRD in Transplant and Beyond
5.1. Prognostic Value of MRD Prior to Transplant
5.2. For Whom Is Transplant Indicated?
5.3. Can We Use MRD to Determine Optimal Conditioning Regimen?
5.4. Can We Use MRD to Determine Optimal Donor Type?
5.5. How Should We Treat MRD Relapse during Post-Transplant Surveillance and/or Chronic Low Intensity Therapy?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morita, K.; Kantarjian, H.M.; Wang, F.; Yan, Y.; Bueso-Ramos, C.; Sasaki, K.; Issa, G.C.; Wang, S.; Jorgensen, J.; Song, X.; et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J. Clin. Oncol. 2018, 36, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef] [Green Version]
- Ngai, L.L.; Kelder, A.; Janssen, J.J.W.M.; Ossenkoppele, G.J.; Cloos, J. MRD tailored therapy in AML: What we have learned so far. Front. Oncol. 2020, 10, 603636. [Google Scholar] [CrossRef]
- Dillon, R.; Potter, N.; Freeman, S.; Russell, N. How we use molecular minimal residual disease (MRD) testing in acute myeloid leukaemia (AML). Br. J. Haematol. 2021, 193, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Ravandi, F. How close are we to incorporating measurable residual disease into clinical practice for acute myeloid leukemia? Haematologica 2019, 104, 1532–1541. [Google Scholar] [CrossRef] [Green Version]
- Gaut, D.; Mead, M. Measurable residual disease in hematopoietic stem cell transplantation-eligible patients with acute myeloid leukemia: Clinical significance and promising therapeutic strategies. Leuk. Lymphoma 2021, 62, 8–31. [Google Scholar] [CrossRef]
- Walter, R.B.; Ofran, Y.; Wierzbowska, A.; Ravandi, F.; Hourigan, C.S.; Ngai, L.L.; Venditti, A.; Buccisano, F.; Ossenkoppele, G.J.; Roboz, G.J. Measurable residual disease as a biomarker in acute myeloid leukemia: Theoretical and practical considerations. Leukemia 2021, 35, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (accessed on 30 May 2022).
- Curran, E.; Stock, W. Taking a “BiTE out of ALL”: Blinatumomab approval for MRD-positive ALL. Blood 2019, 133, 1715–1719. [Google Scholar] [CrossRef]
- De Haas, V.; Ismaila, N.; Advani, A.; Arber, D.A.; Dabney, R.S.; Patel-Donelly, D.; Kitlas, E.; Pieters, R.; Pui, C.-H.; Sweet, K.; et al. Initial Diagnostic Work-Up of Acute Leukemia: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists and American Society of Hematology Guideline. J. Clin. Oncol. 2019, 37, 239–253. [Google Scholar] [CrossRef]
- Patkar, N.; Kakirde, C.; Shaikh, A.F.; Salve, R.; Bhanshe, P.; Chatterjee, G.; Rajpal, S.; Joshi, S.; Chaudhary, S.; Kodgule, R.; et al. Clinical impact of panel-based error-corrected next generation sequencing versus flow cytometry to detect measurable residual disease (MRD) in acute myeloid leukemia (AML). Leukemia 2021, 35, 1392–1404. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; Al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular minimal residual disease in acute myeloid leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Othus, M.; Araki, D.; Wood, B.L.; Radich, J.P.; Halpern, A.B.; Mielcarek, M.; Estey, E.H.; Appelbaum, F.R.; Walter, R.B. Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 2016, 30, 1456–1464. [Google Scholar] [CrossRef]
- Araki, D.; Wood, B.L.; Othus, M.; Radich, J.P.; Halpern, A.B.; Zhou, Y.; Mielcarek, M.; Estey, E.H.; Appelbaum, F.R.; Walter, R.B. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? J. Clin. Oncol. 2016, 34, 329–336. [Google Scholar] [CrossRef]
- Walter, R.B.; Buckley, S.A.; Pagel, J.M.; Wood, B.L.; Storer, B.E.; Sandmaier, B.M.; Fang, M.; Gyurkocza, B.; Delaney, C.; Radich, J.P.; et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013, 122, 1813–1821. [Google Scholar] [CrossRef]
- Walter, R.B. Minimal residual disease testing after induction chemotherapy for acute myeloid leukemia: Moving beyond prognostication? J. Clin. Oncol. 2018, 36, 1463–1465. [Google Scholar] [CrossRef]
- Zeijlemaker, W.; Grob, T.; Meijer, R.; Hanekamp, D.; Kelder, A.; Carbaat-Ham, J.C.; Oussoren-Brockhoff, Y.J.M.; Snel, A.N.; Veldhuizen, D.; Scholten, W.J.; et al. CD34+CD38− leukemic stem cell frequency to predict outcome in acute myeloid leukemia. Leukemia 2019, 33, 1102–1112. [Google Scholar] [CrossRef]
- Venditti, A.; Piciocchi, A.; Candoni, A.; Melillo, L.; Calafiore, V.; Cairoli, R.; de Fabritiis, P.; Storti, G.; Salutari, P.; Lanza, F.; et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood 2019, 134, 935–945. [Google Scholar] [CrossRef]
- Tettero, J.M.; Freeman, S.; Buecklein, V.; Venditti, A.; Maurillo, L.; Kern, W.; Walter, R.B.; Wood, B.L.; Roumier, C.; Philippé, J.; et al. Technical Aspects of Flow Cytometry-based Measurable Residual Disease Quantification in Acute Myeloid Leukemia: Experience of the European LeukemiaNet MRD Working Party. HemaSphere 2022, 6, e676. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, P.; Borsani, O. Eradication of measurable residual disease in AML: A challenging clinical goal. Cancers 2021, 13, 3170. [Google Scholar] [CrossRef] [PubMed]
- Panuzzo, C.; Jovanovski, A.; Ali, M.S.; Cilloni, D.; Pergolizzi, B. Revealing the Mysteries of Acute Myeloid Leukemia: From Quantitative PCR through Next-Generation Sequencing and Systemic Metabolomic Profiling. J. Clin. Med. 2022, 11, 483. [Google Scholar] [CrossRef]
- Onecha, E.; Linares, M.; Rapado, I.; Ruiz-Heredia, Y.; Martinez-Sanchez, P.; Cedena, T.; Pratcorona, M.; Oteyza, J.P.; Herrera, P.; Barragan, E.; et al. A novel deep targeted sequencing method for minimal residual disease monitoring in acute myeloid leukemia. Haematologica 2019, 104, 288–296. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Gale, R.P.; Gormley, N.J.; Ossenkoppele, G.J.; Walter, R.B. Measurable residual disease testing in acute myeloid leukaemia. Leukemia 2017, 31, 1482–1490. [Google Scholar] [CrossRef]
- Lazzarotto, D.; Candoni, A. The role of wilms’ tumor gene (WT1) expression as a marker of minimal residual disease in acute myeloid leukemia. J. Clin. Med. 2022, 11, 3306. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef]
- Thol, F.; Gabdoulline, R.; Liebich, A.; Klement, P.; Schiller, J.; Kandziora, C.; Hambach, L.; Stadler, M.; Koenecke, C.; Flintrop, M.; et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018, 132, 1703–1713. [Google Scholar] [CrossRef] [Green Version]
- Onecha, E.; Rapado, I.; Morales, M.L.; Carreño-Tarragona, G.; Martinez-Sanchez, P.; Gutierrez, X.; Sáchez Pina, J.M.; Linares, M.; Gallardo, M.; Martinez-López, J.; et al. Monitoring of clonal evolution of acute myeloid leukemia identifies the leukemia subtype, clinical outcome and potential new drug targets for post-remission strategies or relapse. Haematologica 2020, 106, 2325. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Valk, P.J.M. Next-generation sequencing in the diagnosis and minimal residual disease assessment of acute myeloid leukemia. Haematologica 2019, 104, 868–871. [Google Scholar] [CrossRef]
- Juul-Dam, K.L.; Ommen, H.B.; Nyvold, C.G.; Walter, C.; Vålerhaugen, H.; Kairisto, V.; Abrahamsson, J.; Alm, S.J.; Jahnukainen, K.; Lausen, B.; et al. Measurable residual disease assessment by qPCR in peripheral blood is an informative tool for disease surveillance in childhood acute myeloid leukaemia. Br. J. Haematol. 2020, 190, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Malagola, M.; Bernardi, S.; Polverelli, N.; Russo, D. Minimal residual disease monitoring in acute myeloid leukaemia: Are we ready to move from bone marrow to peripheral blood? Br. J. Haematol. 2020, 190, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Godwin, C.D.; Zhou, Y.; Othus, M.; Asmuth, M.M.; Shaw, C.M.; Gardner, K.M.; Wood, B.L.; Walter, R.B.; Estey, E.H. Acute myeloid leukemia measurable residual disease detection by flow cytometry in peripheral blood vs bone marrow. Blood 2021, 137, 569–572. [Google Scholar] [CrossRef]
- Daga, S.; Rosenberger, A.; Kashofer, K.; Heitzer, E.; Quehenberger, F.; Halbwedl, I.; Graf, R.; Krisper, N.; Prietl, B.; Höfler, G.; et al. Sensitive and broadly applicable residual disease detection in acute myeloid leukemia using flow cytometry-based leukemic cell enrichment followed by mutational profiling. Am. J. Hematol. 2020, 95, 1148–1157. [Google Scholar] [CrossRef]
- Duncavage, E.J.; Schroeder, M.C.; O’Laughlin, M.; Wilson, R.; MacMillan, S.; Bohannon, A.; Kruchowski, S.; Garza, J.; Du, F.; Hughes, A.E.O.; et al. Genome sequencing as an alternative to cytogenetic analysis in myeloid cancers. N. Engl. J. Med. 2021, 384, 924–935. [Google Scholar] [CrossRef]
- Voso, M.T.; Ottone, T.; Lavorgna, S.; Venditti, A.; Maurillo, L.; Lo-Coco, F.; Buccisano, F. MRD in AML: The role of new techniques. Front. Oncol. 2019, 9, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Recher, C.; Schuh, A.C.; Thirman, M.J.; Garcia, J.S.; DiNardo, C.D.; Vorobyev, V.; Fracchiolla, N.S.; et al. Measurable Residual Disease Response and Prognosis in Treatment-Naïve Acute Myeloid Leukemia With Venetoclax and Azacitidine. J. Clin. Oncol. 2022, 40, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.A.L.; O’Brien, M.A.; Hills, R.K.; Daly, S.B.; Wheatley, K.; Burnett, A.K. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: Results of the United Kingdom MRC AML-15 trial. Blood 2012, 120, 2826–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rücker, F.G.; Agrawal, M.; Corbacioglu, A.; Weber, D.; Kapp-Schwoerer, S.; Gaidzik, V.I.; Jahn, N.; Schroeder, T.; Wattad, M.; Lübbert, M.; et al. Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): Results from the AML Study Group. Blood 2019, 134, 1608–1618. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Tang, J.-L.; Tien, F.-M.; Kuo, Y.-Y.; Wu, D.-C.; Lin, C.-C.; Tseng, M.-H.; Peng, Y.-L.; Hou, M.-F.; Chuang, Y.-K.; et al. Clinical implications of sequential MRD monitoring by NGS at 2 time points after chemotherapy in patients with AML. Blood Adv. 2021, 5, 2456–2466. [Google Scholar] [CrossRef]
- Puckrin, R.; Atenafu, E.G.; Claudio, J.O.; Chan, S.; Gupta, V.; Maze, D.; McNamara, C.; Murphy, T.; Shuh, A.C.; Yee, K.; et al. Measurable residual disease monitoring provides insufficient lead-time to prevent morphologic relapse in the majority of patients with core-binding factor acute myeloid leukemia. Haematologica 2021, 106, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasserjian, R.P.; Steensma, D.P.; Graubert, T.A.; Ebert, B.L. Clonal hematopoiesis and measurable residual disease assessment in acute myeloid leukemia. Blood 2020, 135, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Loghavi, S.; DiNardo, C.D.; Furudate, K.; Takahashi, K.; Tanaka, T.; Short, N.J.; Kadia, T.; Konopleva, M.; Kanagal-Shamanna, R.; Farnoud, N.R.; et al. Flow cytometric immunophenotypic alterations of persistent clonal haematopoiesis in remission bone marrows of patients with NPM1-mutated acute myeloid leukaemia. Br. J. Haematol. 2021, 192, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Godley, L.A. Germline mutations in MDS/AML predisposition disorders. Curr. Opin. Hematol. 2021, 28, 86–93. [Google Scholar] [CrossRef]
- Cappelli, L.V.; Meggendorfer, M.; Baer, C.; Nadarajah, N.; Hutter, S.; Jeromin, S.; Dicker, F.; Kern, W.; Haferlach, T.; Haferlach, C.; et al. Indeterminate and oncogenic potential: CHIP vs CHOP mutations in AML with NPM1 alteration. Leukemia 2022, 36, 394–402. [Google Scholar] [CrossRef]
- Grimm, J.; Jentzsch, M.; Bill, M.; Backhaus, D.; Brauer, D.; Küpper, J.; Schulz, J.; Franke, G.-N.; Vucinic, V.; Niederwieser, D.; et al. Clinical implications of SRSF2 mutations in AML patients undergoing allogeneic stem cell transplantation. Am. J. Hematol. 2021, 96, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.; Jentzsch, M.; Bischof, L.; Kohlschmidt, J.; Grimm, J.; Schmalbrock, L.K.; Backhaus, D.; Brauer, D.; Goldmann, K.; Franke, G.-N.; et al. Impact of IDH1 and IDH2 mutation detection at diagnosis and in remission in patients with AML receiving allogeneic transplantation. Blood Adv. 2022. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, N.J.; Zhou, S.; Fu, C.; Berry, D.A.; Walter, R.B.; Freeman, S.D.; Hourigan, C.S.; Huang, X.; Nogueras Gonzalez, G.; Hwang, H.; et al. Association of Measurable Residual Disease With Survival Outcomes in Patients With Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 1890–1899. [Google Scholar] [CrossRef]
- Simoes, C.; Paiva, B.; Martínez-Cuadrón, D.; Bergua, J.-M.; Vives, S.; Algarra, L.; Tormo, M.; Martinez, P.; Serrano, J.; Herrera, P.; et al. Measurable residual disease in elderly acute myeloid leukemia: Results from the PETHEMA-FLUGAZA phase 3 clinical trial. Blood Adv. 2021, 5, 760–770. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Wang, S.A.; Jorgensen, J.; Kadia, T.M.; Daver, N.G.; Short, N.J.; Yilmaz, M.; Pemmaraju, N.; Borthakur, G.; et al. Prognostic value of measurable residual disease after venetoclax and decitabine in acute myeloid leukemia. Blood Adv. 2021, 5, 1876–1883. [Google Scholar] [CrossRef]
- Roboz, G.J.; Ravandi, F.; Wei, A.H.; Dombret, H.; Thol, F.; Voso, M.T.; Schuh, A.C.; Porkka, K.; La Torre, I.; Skikne, B.; et al. Oral azacitidine prolongs survival of patients with AML in remission independently of measurable residual disease status. Blood 2022, 139, 2145–2155. [Google Scholar] [CrossRef]
- Short, N.J.; Rafei, H.; Daver, N.; Hwang, H.; Ning, J.; Jorgensen, J.L.; Kadia, T.M.; DiNardo, C.D.; Wang, S.A.; Jabbour, E.; et al. Prognostic impact of complete remission with MRD negativity in patients with relapsed or refractory AML. Blood Adv. 2020, 4, 6117–6126. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Knapper, S.; Freeman, S.; Huntly, B.; Clark, R.E.; Thomas, I.F.; Kjeldsen, L.; McMullin, M.F.; et al. Defining the optimal total number of chemotherapy courses in younger patients with acute myeloid leukemia: A comparison of three versus four courses. J. Clin. Oncol. 2021, 39, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.; Stein, E.M. Which are the most promising targets for minimal residual disease-directed therapy in acute myeloid leukemia prior to allogeneic stem cell transplant? Haematologica 2019, 104, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Tiong, I.S.; Dillon, R.; Ivey, A.; Teh, T.-C.; Nguyen, P.; Cummings, N.; Taussig, D.C.; Latif, A.-L.; Potter, N.E.; Runglall, M.; et al. Venetoclax induces rapid elimination of NPM1 mutant measurable residual disease in combination with low-intensity chemotherapy in acute myeloid leukaemia. Br. J. Haematol. 2021, 192, 1026–1030. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Kapp-Schwoerer, S.; Weber, D.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; Krönke, J.; Theis, F.; Rücker, F.G.; Teleanu, M.-V.; Panina, E.; et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in patients with NPM1-mutated AML: Results from the AMLSG 09-09 trial. Blood 2020, 136, 3041–3050. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am. J. Hematol. 2019, 94, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Platzbecker, U.; Middeke, J.M.; Sockel, K.; Herbst, R.; Wolf, D.; Baldus, C.D.; Oelschlägel, U.; Mütherig, A.; Fransecky, L.; Noppeney, R.; et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): An open-label, multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 1668–1679. [Google Scholar] [CrossRef]
- Bataller, A.; Oñate, G.; Diaz-Beyá, M.; Guijarro, F.; Garrido, A.; Vives, S.; Tormo, M.; Arnan, M.; Salamero, O.; Sampol, A.; et al. Acute myeloid leukemia with NPM1 mutation and favorable European LeukemiaNet category: Outcome after preemptive intervention based on measurable residual disease. Br. J. Haematol. 2020, 191, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Gooley, T.A.; Wood, B.L.; Milano, F.; Fang, M.; Sorror, M.L.; Estey, E.H.; Salter, A.I.; Lansverk, E.; Chien, J.W.; et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentzsch, M.; Grimm, J.; Bill, M.; Brauer, D.; Backhaus, D.; Schulz, J.; Goldmann, K.; Niederwieser, D.; Platzbecker, U.; Schwind, S. Prognostic relevance of remission and measurable residual disease status in AML patients prior to reduced intensity or non-myeloablative allogeneic stem cell transplantation. Blood Cancer J. 2021, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Gilleece, M.H.; Shimoni, A.; Labopin, M.; Robinson, S.; Beelen, D.; Socié, G.; Unal, A.; Ganser, A.; Vitek, A.; Sengeloev, H.; et al. Measurable residual disease status and outcome of transplant in acute myeloid leukemia in second complete remission: A study by the acute leukemia working party of the EBMT. Blood Cancer J. 2021, 11, 88. [Google Scholar] [CrossRef]
- Jentzsch, M.; Bischof, L.; Backhaus, D.; Brauer, D.; Schulz, J.; Franke, G.-N.; Vucinic, V.; Niederwieser, D.; Platzbecker, U.; Schwind, S. Impact of the MRD status in AML patients undergoing allogeneic stem cell transplantation in first vs second remission. Blood Adv. 2022. [Google Scholar] [CrossRef]
- Jentzsch, M.; Grimm, J.; Bill, M.; Brauer, D.; Backhaus, D.; Pointner, R.; Goldmann, K.; Schulz, J.; Niederwieser, D.; Platzbecker, U.; et al. Clinical value of the measurable residual disease status within the ELN2017 risk groups in AML patients undergoing allogeneic stem cell transplantation. Am. J. Hematol. 2021, 96, E237–E239. [Google Scholar] [CrossRef]
- Freeman, S.D.; Hills, R.K.; Virgo, P.; Khan, N.; Couzens, S.; Dillon, R.; Gilkes, A.; Upton, L.; Nielsen, O.J.; Cavenagh, J.D.; et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J. Clin. Oncol. 2018, 36, 1486–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsat, M.; Renneville, A.; Thomas, X.; de Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.-P.; Nibourel, O.; Berthon, C.; et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: A study by the acute leukemia french association group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-H.; Zhang, X.-H.; Qin, Y.-Z.; Liu, D.-H.; Jiang, H.; Chen, H.; Jiang, Q.; Xu, L.-P.; Lu, J.; Han, W.; et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: Results from the AML05 multicenter trial. Blood 2013, 121, 4056–4062. [Google Scholar] [CrossRef]
- Venditti, A.; Peter Gale, R.; Buccisano, F.; Ossenkoppele, G. Should persons with acute myeloid leukemia (AML) in 1st histological complete remission who are measurable residual disease (MRD) test positive receive an allotransplant? Leukemia 2020, 34, 963–965. [Google Scholar] [CrossRef]
- Estey, E.H. Acute myeloid leukemia: 2021 update on risk-stratification and management. Am. J. Hematol. 2020, 95, 1368–1398. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Gyurkocza, B.; Storer, B.E.; Godwin, C.D.; Pagel, J.M.; Buckley, S.A.; Sorror, M.L.; Wood, B.L.; Storb, R.; Appelbaum, F.R.; et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia 2015, 29, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsink, L.M.; Othus, M.; Bezerra, E.D.; Wood, B.L.; Fang, M.; Sandmaier, B.M.; Mielcarek, M.; Schoch, G.; Storb, R.; Deeg, H.J.; et al. Impact of pretransplant measurable residual disease on the outcome of allogeneic hematopoietic cell transplantation in adult monosomal karyotype AML. Leukemia 2020, 34, 1577–1587. [Google Scholar] [CrossRef]
- Morsink, L.M.; Bezerra, E.D.; Othus, M.; Wood, B.L.; Fang, M.; Sandmaier, B.M.; Mielcarek, M.B.; Deeg, H.J.; Schoch, G.; Appelbaum, F.R.; et al. Comparative analysis of total body irradiation (TBI)-based and non-TBI-based myeloablative conditioning for acute myeloid leukemia in remission with or without measurable residual disease. Leukemia 2020, 34, 1701–1705. [Google Scholar] [CrossRef]
- Freeman, S.; Craddock, C. Less is not necessarily more: Toward a rational selection of the conditioning regimen in acute myeloid leukemia. J. Clin. Oncol. 2020, 38, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.-F.; Xu, L.-P.; Liu, K.-Y.; Zhang, X.-H.; Ma, X.; Fan, Z.-P.; Wu, D.-P.; Huang, X.-J. Haploidentical vs identical-sibling transplant for AML in remission: A multicenter, prospective study. Blood 2015, 125, 3956–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidi, A.; Hamadani, M.; Zhang, M.-J.; Wang, H.-L.; Abdel-Azim, H.; Aljurf, M.; Assal, A.; Bajel, A.; Bashey, A.; Battiwalla, M.; et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019, 3, 1826–1836. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Wang, Y.; Liu, Y.-R.; Xu, L.-P.; Zhang, X.-H.; Chen, H.; Chen, Y.-H.; Wang, F.-R.; Han, W.; Sun, Y.-Q.; et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: A retrospective and prospective analysis. J. Hematol. Oncol. 2017, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Huang, F.; Wang, Y.; Xu, Y.; Yang, T.; Fan, Z.; Lin, R.; Xu, N.; Xuan, L.; Ye, J.; et al. Haploidentical transplantation might have superior graft-versus-leukemia effect than HLA-matched sibling transplantation for high-risk acute myeloid leukemia in first complete remission: A prospective multicentre cohort study. Leukemia 2020, 34, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Wang, S.; Kong, P.; Su, Y.; Hu, J.; Jiang, M.; Bai, H.; Lang, T.; Wang, J.; et al. Effect of rhG-CSF Combined With Decitabine Prophylaxis on Relapse of Patients With High-Risk MRD-Negative AML After HSCT: An Open-Label, Multicenter, Randomized Controlled Trial. J. Clin. Oncol. 2020, 38, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.M.; Knoth, H.; Röllig, C.; Schetelig, J.; et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Ambinder, A.J.; Levis, M. Potential targeting of FLT3 acute myeloid leukemia. Haematologica 2021, 106, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Khaled, S.; Martinelli, G.; Perl, A.E.; Ganguly, S.; Russell, N.; Krämer, A.; Dombret, H.; Hogge, D.; Jonas, B.A.; et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 984–997. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Bug, G.; Baron, F.; Brissot, E.; Ciceri, F.; Dalle, I.A.; Döhner, H.; Esteve, J.; Floisand, Y.; Giebel, S.; et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: A position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2020, 105, 1507–1516. [Google Scholar]
- Biederstädt, A.; Rezvani, K. How I treat high-risk acute myeloid leukemia using pre-emptive adoptive cellular immunotherapy. Blood 2022. [Google Scholar] [CrossRef]
- Dholaria, B.; Savani, B.N.; Labopin, M.; Luznik, L.; Ruggeri, A.; Mielke, S.; Al Malki, M.M.; Kongtim, P.; Fuchs, E.; Huang, X.-J.; et al. Clinical applications of donor lymphocyte infusion from an HLA-haploidentical donor: Consensus recommendations from the Acute Leukemia Working Party of the EBMT. Haematologica 2020, 105, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Spyridonidis, A. How I treat measurable (minimal) residual disease in acute leukemia after allogeneic hematopoietic cell transplantation. Blood 2020, 135, 1639–1649. [Google Scholar] [CrossRef]
- Jeyakumar, D.; O’Brien, S. Minimal residual disease in acute myeloid leukemia. JAMA Oncol. 2020, 6, 1899–1900. [Google Scholar] [CrossRef] [PubMed]
- Bassan, R. Using minimal residual disease to improve treatment response definitions and hematopoietic cell transplantation strategy in acute leukemia. J. Clin. Oncol. 2016, 34, 300–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossenkoppele, G.; Schuurhuis, G.J.; van de Loosdrecht, A.; Cloos, J. Can we incorporate MRD assessment into clinical practice in AML? Best Pract. Res. Clin. Haematol. 2019, 32, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Fenwarth, L.; Thomas, X.; de Botton, S.; Duployez, N.; Bourhis, J.-H.; Lesieur, A.; Fortin, G.; Meslin, P.-A.; Yakoub-Agha, I.; Sujobert, P.; et al. A Personalized Approach to Guide Allogeneic Stem Cell Transplantation in Younger Adults with Acute Myeloid Leukemia. Blood 2020, 137, 524–532. [Google Scholar] [CrossRef]
- Khera, N.; Deeg, H.J.; Kodish, E.; Rondelli, D.; Majhail, N. Allogeneic hematopoietic cell transplantation and other expensive cellular therapies: A miracle for the few but off limits to many? J. Clin. Oncol. 2020, 38, 1268–1272. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azenkot, T.; Jonas, B.A. Clinical Impact of Measurable Residual Disease in Acute Myeloid Leukemia. Cancers 2022, 14, 3634. https://doi.org/10.3390/cancers14153634
Azenkot T, Jonas BA. Clinical Impact of Measurable Residual Disease in Acute Myeloid Leukemia. Cancers. 2022; 14(15):3634. https://doi.org/10.3390/cancers14153634
Chicago/Turabian StyleAzenkot, Tali, and Brian A. Jonas. 2022. "Clinical Impact of Measurable Residual Disease in Acute Myeloid Leukemia" Cancers 14, no. 15: 3634. https://doi.org/10.3390/cancers14153634
APA StyleAzenkot, T., & Jonas, B. A. (2022). Clinical Impact of Measurable Residual Disease in Acute Myeloid Leukemia. Cancers, 14(15), 3634. https://doi.org/10.3390/cancers14153634





